Abstract
Background
Observational studies suggest that higher volumes of physical activity are associated with a lower risk of disease recurrence among colon cancer survivors. However, the feasibility and safety of prescribing higher volumes of physical activity to colon cancer survivors are unknown. Furthermore, the pathways through which exercise may reduce disease recurrence are unknown.
Patients and Methods
Stage I–III colon cancer survivors were randomized to usual-care control, 150 min·wk−1 of aerobic exercise (low-dose), or 300 min·wk−1 of aerobic exercise (high-dose). Changes in soluble intercellular adhesion molecule-1 (sICAM-1) and vascular adhesion molecule-1 (sVCAM-1) prognostic biomarkers were examined.
Results
From January 2015 to February 2016, 39 patients were enrolled (n=13 usual-care control; n=14 low-dose; n=12 high-dose) and 38 participants completed the study (97% follow-up). Over six-months, the low-dose group completed 142 min·wk−1 (92.8% adherence) and the high-dose group completed 247 min·wk−1 (89.0% adherence) of exercise. Compared to the control group, changes in sICAM-1 were −134.9 ng/mL (95% CI: −238.1 to −31.6) in the low-dose group and −114.8 ng/mL (95% CI: −222.5 to −7.1) in the high-dose group (linear Ptrend=0.023; nonlinear Ptrend=0.044). No changes were observed for sVCAM-1 (linear Ptrend=0.791; nonlinear Ptrend=0.604). Non-serious adverse events occurred at similar rates among randomized groups. No serious adverse events occurred.
Conclusion
Higher volumes of moderate-intensity aerobic exercise, up to 300 min·wk−1, are feasible, safe, and elicit favorable changes in prognostic biomarkers among patients recently treated for stage I–III colon cancer. These data can be used to guide clinical recommendations for patients, and inform future trials.
Keywords: physical activity, biomarkers, survivorship, lifestyle, energy balance
INTRODUCTION
Approximately 1 million people are diagnosed with colon cancer each year worldwide.1 Three-quarters of patients are diagnosed with disease that is localized to the primary site (stage I–II) or spread to regional lymph nodes (stage III). Despite surgical resection, either alone or in combination with adjuvant chemotherapy, five-year disease recurrence rates for stage I, II, and III colon cancer are 10%, 20%, and 30–50%, respectively.2–4 Consequently, there exists a need to identify additional adjuvant therapies that reduce the risk of recurrent disease in this population.
The prescription of physical activity or exercise is a potential adjuvant therapy that has been reported by prospective cohort studies to be associated with a reduction in the risk of recurrence and death among colon cancer survivors.5–7 The relationship between physical activity and disease outcomes is independent of known prognostic factors, and occurs in a dose-response fashion,8 such that larger volumes of physical activity or exercise, up to 300 min·wk−1, are associated with a lower risk of recurrence and premature mortality.5–7
However, it is unknown if doses of exercise as large as 300 min·wk−1 are behaviorally feasible and have tolerable safety profiles for colon cancer survivors when compared to smaller doses of exercise, such as 150 min·wk−1 as is currently recommended by various professional organizations including the National Comprehensive Cancer Network.9–11 The FITT-VP principle is the cornerstone of exercise prescription by specifying the frequency, intensity, time, type, volume and progression of exercise that is appropriate to achieve a desired health outcome.12 Prior trials of exercise among colon cancer survivors have not fully described FITT-VP principles, which may limit the specificity of guidance that oncology providers can offer to patients.13 In addition, the safety profile of exercise among colon cancer survivors has not been characterized.14 These limitations may hinder the prescription of exercise as an adjuvant therapy in clinical practice.15 Furthermore, the biological pathways through which exercise may reduce the risk of disease recurrence and premature mortality among colon cancer survivors are unknown. Soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular adhesion molecule-1 (sVCAM-1) are endothelial cell-adhesion molecules that promote the growth of existing micro-metastases and the formation of new micro-metastases.16 Concentrations of sICAM-1 and sVCAM-1 increase with disease stage,17,18 and elevated concentrations of sICAM-1 and sVCAM-1 are independently associated with disease recurrence and premature death among colon cancer survivors.17–21 sICAM-1 and sVCAM-1 have been recommended as therapeutic targets,22,23 and represent novel pathways through which exercise may reduce the risk of disease recurrence among colon cancer survivors.
The COURAGE trial was a randomized controlled trial with the primary objectives to test the feasibility, safety, and biological efficacy of two distinct doses of aerobic exercise compared to a usual-care control group among patients with stage I–III colon cancer.24 Our primary hypotheses were that both doses of exercise would be feasible and safe, and that exercise would induce dose-dependent improvements in sICAM-1 and sVCAM-1.
MATERIALS and METHODS
Study design and patients
The COURAGE trial was a single-center, phase II, randomized, three-arm dose-response exercise trial. Detailed methods for the COURAGE trial are published.24 Patients were eligible if they were diagnosed with stage I–III colon cancer; completed surgical resection and adjuvant chemotherapy within 36-months of entering the study; self-reported <150 min·wk−1 of moderate or vigorous intensity physical activity using the Paffenbarger Physical Activity Questionnaire;25 age ≥18 years; provided written physician approval; had no additional surgery planned within the six-month intervention period; and had the ability to walk unaided for six-minutes. Patients were ineligible if they had a history of another primary cancer (other than non-melanoma skin cancer); had evidence of distant metastatic disease; were pregnant or breast feeding; were unable to provide a baseline blood sample; had a myocardial infarction or coronary revascularization procedure within the past three months; had uncontrolled hypertension; had high-risk or uncontrolled cardiac arrhythmias; had clinically significant heart valve disease; had decompensated heart failure; had a known aortic aneurysm; or had any other condition which, in the opinion of the investigator, may impede testing of study hypotheses or make it unsafe to engage in the exercise program. All participants provided written informed consent.
Randomization and blinding
Participants were randomly allocated to one of three groups: usual-care control, low-dose aerobic exercise (150 min·wk−1), or high-dose aerobic exercise (300 min·wk−1). Randomization was stratified by cancer stage. Participants were not blinded to treatment assignment. Outcome measures were obtained by assessors blinded to treatment assignment.
Exercise treatment plan
Aerobic exercise was performed for six-months using study-provided in-home treadmills (LifeSpan Fitness, TR1200i, Salt Lake City, UT, USA). Participants were provided with a heart rate monitor to objectively-record heart rate during each exercise session (Polar Electro Inc., RS400, Lake Success, NY, USA). The heart rate monitors had sufficient memory to record 8–12 weeks of exercise using a one-minute epoch. Participants also completed exercise logs to record the date, time, average heart rate, and exercise duration. Participants met with a clinical exercise physiologist to introduce the exercise prescription, and familiarize the participant with use of the treadmill, completion of exercise logs, use of the heart rate monitor, appropriate warm-up and cool-down, stretches, and proper footwear for aerobic exercise. Participants were encouraged to individualize their frequency (days/week), fractionation (sessions/day), and duration (minutes/session) of exercise according to a schedule that promoted a high level of adherence to the prescribed exercise volume. The exercise physiologist provided ongoing behavioral and clinical support and monitored exercise adherence throughout the duration of the study with the use of weekly telephone and email communications. Exercise intensity was prescribed at 50–70% of the age-predicted maximum heart rate. The low-dose and high-dose groups progressed towards of the goal of 150 or 300 min·wk−1 of exercise, respectively. Details of the exercise prescription are published.24 The study outcome of exercise feasibility was operationalized as the average weekly adherence to the prescribed exercise dose, defined using the completed number of minutes divided by the prescribed number of minutes.24 The outcome was capped at 100% each week to prevent the over estimation of exercise adherence.
Participants randomized into the usual-care control group were asked to maintain their pre-study levels of physical activity and follow the recommendations provided by their physician. After completing six-month measures, control group participants were provided with an in-home treadmill and individualized exercise program, similar to that prescribed to the two exercise groups. Upon study completion, all participants were allowed to keep their study provided treadmills.
Measurements
Demographic characteristics were self-reported. Smoking status was obtained from a standardized questionnaire.26 Caloric intake was quantified using three-day food records. Moderate to vigorous intensity physical activity was quantified using an accelerometer (ActiGraph GT3X+).27 Functional status was quantified using the six-minute walk test.28 Body mass index (BMI; kg/m2) was calculated using height (m) and weight (kg). Clinical information including cancer stage, treatment with chemotherapy, and performance status were obtained from cancer registry reports, pathology reports, or physician records. Comorbid health conditions were assessed using a self-reported questionnaire and physician records. Five-year predicted overall survival was calculated using a validated nomogram.29 All participants underwent a fasting blood draw at baseline and six-months. EDTA-preserved plasma was stored at −80°C. Concentrations of sICAM-1 and sVCAM-1 were quantified using an enzyme-linked immunosorbent assay (EMD Millipore, Bellerica, MA, USA). Baseline and six-month plasma samples were assayed simultaneously and in duplicate at the end of the study. The coefficients of variation for the sICAM-1 and sVCAM-1 assays were 9.4% and 4.3%, respectively. Participants in all study groups were asked each week by the study coordinator via telephone or email about incident adverse events. All adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.30
Statistical analysis
Categorical baseline characteristics were compared among the three groups using Fisher’s exact test, and continuous baseline characteristics were compared among the three study groups using the Kruskal-Wallis test. Based on prior studies (described elsewhere in detail),24 against the hypothesis of a dose-response relationship, 39 participants provided 80% power for two tests for trend (one for sICAM-1 and one for sVCAM-1), each tested at a type I error rate of 0.025, to maintain the experiment-wise overall error rate of 0.05 for the two biologic endpoints. All inferential analyses were conducted on an intention-to-treat basis. sICAM-1 and sVCAM-1 concentrations were log transformed in the inferential analysis to improve normality and back transformed to facilitate interpretation. Changes in outcomes were evaluated from baseline to six-months among the three groups using repeated-measures mixed-effects regression models. This statistical approach includes all available data and accounts for the correlation between repeated measures. The baseline value of the dependent variable and cancer stage (randomization stratification factor) were included as covariates in the regression models.31 Group-by-time interaction terms were estimated as fixed-effects. Results from the regression models are presented as least-square means (LS Mean) ± standard error (SE) or 95% confidence interval (CI). To evaluate the presence of a dose-response relationship across randomized groups, a test for trend was conducted by examining linear and nonlinear (quadratic) contrasts.
RESULTS
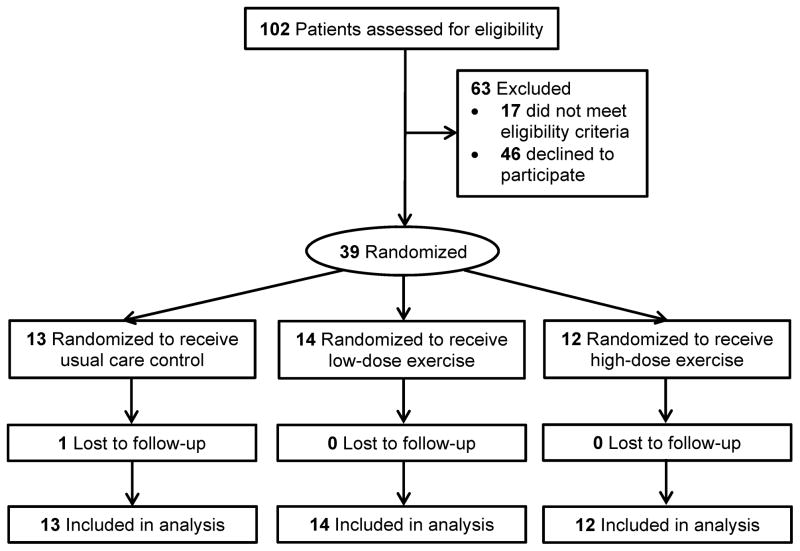
Between January 2015 and August 2015, 39 colon cancer survivors were recruited and randomized with data collection ending in February 2016. Baseline characteristics of study participants are presented in Table 1. Figure 1 shows the flow of the 39 randomized participants through the study. One participant was lost to follow-up (97% follow-up rate).
Table 1.
Baseline characteristics of the participantsa
| Characteristic | Total (n=39) | Control (n=13) | Low-Dose (n=14) | High-Dose (n=12) |
|---|---|---|---|---|
| Age, years | 56.5±10.0 | 57.9±9.7 | 58.2±9.8 | 53.1±10.5 |
| Sex, % | ||||
| Male | 15 (38%) | 4 (31%) | 7 (50%) | 4 (33%) |
| Female | 24 (62%) | 9 (69%) | 7 (50%) | 8 (67%) |
| Race, % | ||||
| White | 31 (80%) | 8 (62%) | 12 (86%) | 11 (92%) |
| Black | 6 (15%) | 3 (23%) | 2 (14%) | 1 (8%) |
| Other | 2 (5%) | 2 (15%) | 0 (0%) | 0 (0%) |
| Education, % | ||||
| High School or Less | 7 (18%) | 1 (8%) | 4 (29%) | 2 (17%) |
| Some College | 8 (20%) | 3 (23%) | 2 (14%) | 3 (25%) |
| College Degree or More | 24 (62%) | 9 (69%) | 8 (57%) | 7 (58%) |
| Smoking History, % | ||||
| Never | 23 (59%) | 10 (77%) | 6 (43%) | 7 (58%) |
| Former | 14 (36%) | 3 (23%) | 7 (50%) | 4 (33%) |
| Current | 2 (5%) | 0 (0%) | 1 (7%) | 1 (8%) |
| Caloric Consumption, kcal·d−1 | 1747±542 | 1749±545 | 1816±569 | 1665±543 |
| Moderate or Vigorous Physical Activity, min·d−1 | 15.7±8.7 | 12.2±8.1 | 18.8±9.6 | 15.7±7.3 |
| Body Mass Index, kg/m2 | 30.3±5.8 | 29.2±6.0 | 29.5±4.3 | 32.4±6.9 |
| Stage, % | ||||
| I | 5 (13%) | 1 (8%) | 2 (14%) | 2 (17%) |
| II | 14 (36%) | 5 (38%) | 5 (36%) | 4 (33%) |
| III | 20 (51%) | 7 (54%) | 7 (50%) | 6 (50%) |
| Chemotherapy, % | 28 (72%) | 10 (77%) | 10 (71%) | 8 (67%) |
| Time Since Treatment Completion, Months | 10.9±6.1 | 11.3±6.7 | 8.8±5.8 | 11.3±5.7 |
| ECOG Performance Status, % | ||||
| 0, Fully active | 29 (74%) | 10 (77%) | 9 (64%) | 10 (83%) |
| 1, Ambulatory, but restricted in strenuous activity | 10 (26%) | 3 (23%) | 5 (36%) | 2 (17%) |
| Six Minute Walk, m | 495.1±82.9 | 497.1±116.9 | 479.5±60.1 | 511.1±62.7 |
| Comorbid Conditions, % | ||||
| Hypertension | 13 (33%) | 4 (31%) | 6 (43%) | 3 (25%) |
| Hyperlipidemia | 6 (15%) | 1 (8%) | 2 (14%) | 3 (25%) |
| Type 2 Diabetes | 5 (13%) | 1 (8%) | 1 (7%) | 3 (25%) |
| Cardiovascular Disease | 4 (10%) | 2 (15%) | 1 (7%) | 1 (8%) |
| 5-Year Predicted Survival,b % | 68 [60–87] | 68 [61–85] | 71 [60–88] | 65 [60–83] |
Data are mean ± standard deviation or N (%) unless otherwise noted.
All baseline characteristics were balanced across groups.
Median [interquartile 25–75% range].
Figure 1.
Flow of participants through the study
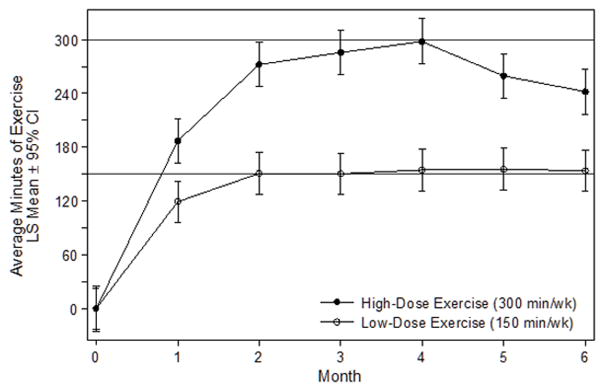
Exercise prescription program variables are presented in Table 2. Over six-months including the dose-titration period, the average exercise volume in the low-dose and high-dose groups were 141.5 min·wk−1 (95% CI: 122.0–160.9) and 247.2 min·wk−1 (95% CI: 226.2–268.2), respectively (P<0.001, Figure 2). Excluding the period during which the exercise dose was being titrated, the average exercise volume in the low-dose and high-dose groups were 153.3 min·wk−1 (95% CI: 133.4–173.7) and 271.3 min·wk−1 (95% CI: 249.6–293.1), respectively (P<0.001). Over six-months, adherence to the prescribed volumes of exercise in the low-dose and high-dose groups were 92.8% (95% CI: 88.0–97.6) and 89.0% (95% CI: 83.8–94.2), respectively (P=0.287). The high-dose group exercised on more days per week (P=0.001), more sessions per day (P=0.001), longer duration per session (P<0.001), and required more weeks to progress to the full dose of prescribed exercise (P=0.007), as compared with the low-dose group. Exercise intensity was 70.7% (95% CI: 69.0–72.4) of the age-predicted maximal heart rate, the proportion of exercise sessions using the study-provided treadmill was 76.5% (95% CI: 58.2–94.9), and the proportion of exercise sessions validated with objective heart rate data was 96.8% (95% CI: 95.6–98.0), all of which did not differ between the two exercise groups. Compared to the control group, over six-months accelerometer quantified moderate to vigorous intensity physical activity increased by 22.3 min·d−1 (95% CI: 4.7–39.8) in the low-dose group, and 28.8 min·d−1 (95% CI: 10.8–47.0) in the high-dose group, respectively (Ptrend<0.001). Compared to the control group, over six-months the distance walked in six-minutes increased by 11.4 m (95% CI: −24.4–47.2) in the low-dose group, and 40.9 m (95% CI: 3.7–78.1) in the high-dose group, respectively (Ptrend=0.002).
Table 2.
Exercise prescription program variablesa
| Characteristic | Low-Dose (n=14) | High-Dose (n=12) | Δ Between Groups (LS Mean ± SE) | P |
|---|---|---|---|---|
| Volume (minutes of exercise per week) | 141.5±9.92 | 247.2±10.71 | 105.7±14.60 | <0.001 |
| % adherence to prescribed exercise dose | 92.8±2.44 | 89.0±2.64 | −3.8±3.60 | 0.287 |
| Frequency (days of exercise per week) | 3.5±0.15 | 4.3±0.16 | 0.75±0.22 | 0.001 |
| Fractionation (sessions of exercise per day) | 1.1±0.07 | 1.4±0.07 | 0.31±0.10 | 0.001 |
| Intensity (% of heart rate maximum) | 71.6±1.16 | 69.6±1.27 | −1.89±1.72 | 0.272 |
| Time (minutes of exercise per day) | 41.6±2.38 | 59.1±2.57 | 17.4±3.50 | <0.001 |
| Type (% of exercise sessions using treadmill) | 72.3±12.9 | 81.3±15.5 | 8.0±18.71 | 0.669 |
| Progression (weeks to full dose of exercise)b | 4 [4–5] | 8 [7–10] | 4 [2–6] | 0.007 |
| % of exercise confirmed with heart rate monitor | 97.3±0.81 | 96.2±0.87 | −1.1±1.19 | 0.344 |
| % with ≥80% adherence, n (%) | 12 (86%) | 9 (75%) | −10.6±15.2 | 0.488 |
| MET hours per weekc | 13.7±0.96 | 23.9±1.03 | 10.2±1.41 | <0.001 |
Data are least squares mean (LS Mean) ± standard error (SE) unless otherwise noted.
Median [interquartile 25–75% range].
Calculated using treadmill speed and incline, and averaged across all exercise sessions.
Figure 2.
Average minutes of exercise each month, stratified by group
Endothelial cell-adhesion outcomes are presented in Table 3. At baseline, no differences among the three groups were observed for sICAM-1 (P=0.180) and sVCAM-1 (P=0.148). Exercise reduced sICAM-1 in dose-response fashion (linear Ptrend=0.023; nonlinear Ptrend=0.044). Compared to the control group, over six-months sICAM-1 decreased 134.9 ng/mL (95% CI: −238.1 to −31.6) in the low-dose group, and 114.8 ng/mL (95% CI: −222.5 to −7.1) in the high dose group. No effects of exercise were seen for sVCAM-1 (linear Ptrend=0.791; nonlinear Ptrend=0.604).
Table 3.
Endothelial cell-adhesion outcomes at baseline and change during six-months
| Outcome | Baseline (Mean ± SD) | Δ Baseline to Month 6 (LS Mean ± SE) | Δ from Control (LS Mean ± SE) |
|---|---|---|---|
| sICAM-1, ng/mL | |||
| Control | 301.1±195.2 | −0.4±39.2 | — |
| Low-Dose | 416.6±140.1 | −135.3±35.2a | −134.9±52.7b |
| High-Dose | 431.7±268.3 | −115.2±38.5a | −114.8±54.9b |
| Test for trend | Linear Ptrend=0.023 Nonlinear Ptrend=0.044 |
||
| sVCAM-1, ng/mL | |||
| Control | 931.4±250.8 | 404.8±95.8a | — |
| Low-Dose | 1245.3±504.7 | 329.6±89.4a | −75.1±131.1 |
| High-Dose | 895.6±173.2 | 429.3±98.6a | 24.5±137.2 |
| Test for trend | Linear Ptrend=0.791 Nonlinear Ptrend=0.604 |
||
SD, standard deviation; LS Mean, least squares mean; SE, standard error.
Signifcantly different from baseline (within-group), P<0.005.
Significantly different from control, P<0.05.
Adverse events are presented in Table 4. Common non-serious (grade 1–2) adverse events included arthralgia (n=16 [42%]), back pain (n=12 [31%]), generalized flu-like symptoms (n=8 [21%]), foot blisters (n=7 [18%]), and myalgia (n=6 [16%]). The anatomical sites of arthralgia included the knees (n=8 [50%]), ankles (n=5 [31%]), hips (n=2 [13%]), and shoulders (n=1 [6%]). The anatomical sites of myalgia included the feet (n=4 [67%]) and calves (n=2 [33%]). No serious (grade ≥3) adverse events occurred.
Table 4.
Grade 1–2 adverse events, overall and by randomized groupa
| Characteristic | Total (n=39) | Control (n=13) | Low-Dose (n=14) | High-Dose (n=12) |
|---|---|---|---|---|
| Blood and Lymphatic System Disorders | ||||
| Lymph Node Pain | 1 (3%) | — | 1 (7%) | — |
| Cardiac Disorders | ||||
| Palpitations | 6 (15%) | 1 (8%) | 2 (14%) | 3 (25%) |
| Ear and Labyrinth Disorders | ||||
| External Ear Inflammation | 2 (5%) | — | 2 (14%) | — |
| Endocrine Disorders | ||||
| Hyperthyroidism | 1 (3%) | 1 (8%) | — | — |
| Eye Disorders | ||||
| Blurred Vision | 1 (3%) | — | 1 (7%) | — |
| Dry Eye | 1 (3%) | 1 (8%) | — | — |
| Gastrointestinal Disorders | ||||
| Abdominal Pain | 4 (10%) | 3 (23%) | — | 1 (8%) |
| Constipation | 1 (3%) | — | 1 (7%) | — |
| Diarrhea | 5 (13%) | 2 (15%) | 3 (21%) | — |
| Dry Mouth | 4 (10%) | 1 (8%) | 1 (7%) | 2 (17%) |
| Gastroesophageal Reflux Disease | 2 (5%) | 1 (8%) | 1 (7%) | — |
| Lip Pain | 1 (3%) | — | — | 1 (8%) |
| Toothache | 3 (8%) | — | 2 (14%) | 1 (8%) |
| Vomiting | 2 (5%) | 1 (8%) | 1 (7%) | — |
| General Disorders | ||||
| Chills | 1 (3%) | 1 (8%) | — | — |
| Edema Limbs | 2 (5%) | 1 (8%) | 1 (7%) | — |
| Fatigue | 1 (3%) | 1 (8%) | — | — |
| Flu Like Symptoms | 8 (21%) | 2 (15%) | 2 (14%) | 4 (33%) |
| Non-Cardiac Chest Pain | 2 (5%) | 1 (8%) | 1 (7%) | — |
| Infections and Infestations | ||||
| Otitis Media | 3 (8%) | — | 1 (7%) | 2 (17%) |
| Upper Respiratory Infection | 1 (3%) | — | 1 (7%) | — |
| Injury | ||||
| Bruising | 7 (18%) | 2 (15%) | 2 (14%) | 3 (25%) |
| Fall | 4 (10%) | 1 (8%) | 2 (14%) | 1 (8%) |
| Metabolism and Nutrition Disorders | ||||
| Hyperglycemia | 1 (3%) | — | — | 1 (8%) |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Arthralgia | 16 (42%) | 5 (38%) | 6 (47%) | 5 (42%) |
| Arthritis | 1 (3%) | 1 (8%) | — | — |
| Back Pain | 12 (31%) | 4 (31%) | 5 (35%) | 3 (25%) |
| Muscle Weakness of Lower Limb | 5 (13%) | 4 (31%) | 1 (7%) | — |
| Myalgia | 6 (16%) | — | 2 (14%) | 4 (33%) |
| Neck Pain | 2 (5%) | — | 2 (14%) | — |
| Pain in Extremity | 3 (8%) | 1 (8%) | 2 (14%) | — |
| Nervous System Disorders | ||||
| Dizziness | 6 (16%) | 2 (15%) | 1 (7%) | 3 (25%) |
| Headache | 5 (13%) | 1 (8%) | 1 (7%) | 3 (25%) |
| Stroke | 1 (3%) | — | 1 (7%) | — |
| Neuralgia | 3 (8%) | 1 (8%) | 2 (14%) | — |
| Sinus Pain | 4 (10%) | 1 (8%) | 1 (7%) | 2 (17%) |
| Psychiatric Disorders | ||||
| Insomnia | 6 (15%) | 4 (31%) | 2 (14%) | — |
| Restlessness | 2 (5%) | 1 (8%) | — | 1 (8%) |
| Reproductive System and Breast Disorders | ||||
| Breast Pain | 1 (3%) | — | — | 1 (8%) |
| Respiratory, Thoracic and Mediastinal Disorders | ||||
| Sneezing | 2 (5%) | 1 (8%) | — | 1 (8%) |
| Skin and Subcutaneous Disorders | ||||
| Alopecia | 1 (3%) | 1 (8%) | — | — |
| Other – Foot Blisters | 7 (18%) | 2 (15%) | 1 (7%) | 4 (33%) |
| Vascular Disorders | ||||
| Hot Flashes | 1 (3%) | — | — | 1 (8%) |
| Hypertension | 2 (5%) | — | 1 (7%) | 1 (8%) |
Data are N (%).
DISCUSSION
Our data indicate that colon cancer survivors are able to complete high volumes (300 min·wk−1) of aerobic exercise. To our knowledge, 300 min·wk−1 is the largest volume of exercise that has been prescribed in this population. Both 150 and 300 min·wk−1 doses of exercise elicited physiologic effects on sICAM-1, a prognostic biomarker and potential biologic mediator of the effects of exercise in this population. Exercise was well-tolerated. No serious (grade ≥3) adverse events occurred, and the profile of non-serious (grade 1–2) adverse events is consistent with what would be expected with exercise: arthralgia, myalgia, back pain, and foot blisters, which can be mitigated with appropriate warm-up and cool down, proper progression of exercise, and consultation with an exercise physiologist to tailor the prescribed exercise dose in response to adverse events. These data can be used to guide clinical recommendations for patients, and inform future phase II and III trials.
The biologic pathways through which exercise may reduce the risk for disease recurrence and premature mortality have not been elucidated. Endothelial cell-adhesion molecules including sICAM-1 and sVCAM-1 have emerged as therapeutic targets,22,23 given their implication in promoting the growth of existing micro-metastases and the formation of new micro-metastases through circulating tumor cell differentiation, cell-cell adhesion and dissemination via activation of the PI3K-Akt-mTOR pathway.16 We hypothesized that exercise would reduce sICAM-1 and sVCAM-1. Our hypothesis was supported, in part, as both doses of exercise produced significant reductions in sICAM-1, but not in sVCAM-1. It is not clear why sVCAM-1 was not reduced with exercise. These data are consistent with the hypothesis that sICAM-1 may be involved in the anti-cancer effects of exercise. Our findings build upon prior studies among colon cancer survivors that have demonstrated exercise favorably alters oxidative DNA damage and immune parameters.14
There are several limitations to this trial. The main limitation is the small sample size which limits the generalizability of our findings. We have reported that trial participants were younger than the population from which they were recruited,24 which may help oncology providers to circumscribe the patient population these findings may be applicable. The exercise intervention was six-months, which limits our ability to understand if colon cancer survivors are able to sustain the observed changes in behavior over longer periods such as 24- or 36-months.
There are several strengths to this trial. The use of two distinct doses of exercise is novel in this setting, and allowed us to understand the feasibility, safety, and physiologic effects along the exercise dose curve. Exercise adherence was excellent in both groups, which is likely a result of providing in-home treadmills, allowing participants to individualize the exercise prescription according to a schedule of their choosing, and providing regular encounters with an exercise physiologist to deliver behavioral and clinical support. Participants in this trial were more likely to be treated with chemotherapy than the population from which they were recruited,24 demonstrating the willingness of colon cancer survivors at high risk for disease recurrence to engage in behavior modification practices. Participants were racially diverse, with 20% of the study sample reporting non-white race. Participants reported a variety of comorbid health conditions that are common among colon cancer survivors including hypertension, hyperlipidemia, diabetes, and cardiovascular disease. Endpoint data collection was excellent (97% complete).
Conclusion
The findings from this randomized trial establish the feasibility, safety, and biological efficacy of 150 and 300 min·wk−1 of moderate-intensity aerobic exercise among patients with stage I–III colon cancer. It is noteworthy that both doses of exercise produced reductions in sICAM-1. Additional research is necessary to understand how exercise intensity may alter biomarker outcomes in this population. The findings from this randomized trial are useful to clinical researchers to begin to understand the biologic pathways that are hypothesized to mediate the relationship between exercise and disease recurrence in this population. The findings from this randomized trial may also be useful to oncology providers to improve the specificity of exercise prescriptions, delineate the risk to benefit ratio of prescribing exercise, and promote the prescription of exercise as a potential adjuvant therapy to colon cancer survivors.
Clinical Practice Points.
Prospective cohort studies that demonstrate that participation in physical activity after a diagnosis of stage I–III colon cancer is associated with a 50% lower risk of cancer recurrence and mortality. A consistent finding is that post-diagnosis physical activity is associated with disease outcomes in a dose-response fashion, suggesting that larger volumes of physical activity or exercise, up to approximately 300 minutes per week (min·wk−1), are necessary to maximally reduce the risk of disease recurrence and mortality. However, it is unknown if prescribing doses of exercise as large as 300 min·wk−1 are behaviorally feasible and have tolerable safety profiles for colon cancer survivors when compared to smaller doses of exercise, such as 150 min·wk−1 as is currently recommended by various professional organizations. Furthermore, the biological pathways through which exercise may reduce the risk of disease recurrence and mortality among colon cancer survivors are unknown. Thirty-nine stage I–III colon cancer survivors were randomized to usual-care control, 150 min·wk−1 of aerobic exercise (low-dose), or 300 min·wk−1 of aerobic exercise (high-dose). Both intervention groups maintained excellent adherence to the exercise program over six-months: the low-dose group completed 142 min·wk−1 (92.8% adherence) and the high-dose group completed 247 min·wk−1 (89.0% adherence). Both doses of exercise lowered sICAM-1, which is a biomarker that has been associated with disease recurrence and mortality. This may partially explain the anti-cancer effects of exercise. Non-serious adverse events occurred at similar rates among randomized groups. These data can be used to guide clinical recommendations for patients, and inform future trials.
Acknowledgments
This study was supported by grants from the National Cancer Institute (R21-CA182767, F31-CA192560) and the National Center for Advancing Translational Science (UL1-TR000003). LifeSpan Fitness, LLC provided discounts for treadmills.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Clinicaltrials.gov Identifier: NCT02250053
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. The Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: Findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 8.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: A systematic review and meta-analysis. Ann Oncol. 2014;25(7):1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 9.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 11.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: Healthy lifestyles, version 2.2014. J Natl Compr Canc Netw. 2014;12(9):1222–1237. doi: 10.6004/jnccn.2014.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 8. Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 13.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: A review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2013;48(12):987–95. doi: 10.1136/bjsports-2012-091732. [DOI] [PubMed] [Google Scholar]
- 14.Cramer H, Lauche R, Klose P, Dobos G, Langhorst J. A systematic review and meta-analysis of exercise interventions for colorectal cancer patients. European journal of cancer care. 2014;23(1):3–14. doi: 10.1111/ecc.12093. [DOI] [PubMed] [Google Scholar]
- 15.Brown JC, Schmitz KH. The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc. 2014;46(12):2202–2209. doi: 10.1249/MSS.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paschos KA, Canovas D, Bird NC. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal. 2009;21(5):665–674. doi: 10.1016/j.cellsig.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Alexiou D, Karayiannakis AJ, Syrigos KN, et al. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: Correlations with clinicopathological features, patient survival and tumour surgery. Eur J Cancer. 2001;37(18):2392–2397. doi: 10.1016/s0959-8049(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 18.Okugawa Y, Miki C, Toiyama Y, Koike Y, Inoue Y, Kusunoki M. Serum level of soluble vascular cell adhesion molecule 1 is a valuable prognostic marker in colorectal carcinoma. Dis Colon Rectum. 2009;52(7):1330–1336. doi: 10.1007/DCR.0b013e3181a0d144. [DOI] [PubMed] [Google Scholar]
- 19.Toiyama Y, Miki C, Inoue Y, et al. Soluble intercellular adhesion molecule-1 as a prognostic marker for stage II colorectal cancer patients. Ann Surg Oncol. 2008;15(6):1617–1624. doi: 10.1245/s10434-008-9874-5. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Arao T, Matsumoto K, et al. Plasma concentrations of VCAM-1 and PAI-1: A predictive biomarker for post-operative recurrence in colorectal cancer. Cancer Sci. 2010;101(8):1886–1890. doi: 10.1111/j.1349-7006.2010.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toiyama Y, Miki C, Inoue Y, Kawamoto A, Kusunoki M. Circulating form of human vascular adhesion protein-1 (VAP-1): Decreased serum levels in progression of colorectal cancer and predictive marker of lymphatic and hepatic metastasis. J Surg Oncol. 2009;99(6):368–372. doi: 10.1002/jso.21246. [DOI] [PubMed] [Google Scholar]
- 22.Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)—An increasing insight into its role in tumorigenicity and metastasis. International Journal of Cancer. 2015;136(11):2504–2514. doi: 10.1002/ijc.28927. [DOI] [PubMed] [Google Scholar]
- 23.Howard K, Lo KK, Ao L, et al. Intercellular adhesion molecule-1 mediates murine colon adenocarcinoma invasion. J Surg Res. 2014;187(1):19–23. doi: 10.1016/j.jss.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JC, Troxel AB, Ky B, et al. A randomized phase II dose-respnse exercise trial among colon cancer survivors: Purpose, study design, methods, and recruitment results. Contemp Clin Trials. 2016;47:366–375. doi: 10.1016/j.cct.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paffenbarger R, Wing A, Hyde R. Paffenbarger physical activity questionnaire. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 26.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National health interview survey, 2008. Vital Health Stat. 2009;10(242):1–157. [PubMed] [Google Scholar]
- 27.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the united states measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Weiser MR, Gonen M, Chou JF, Kattan MW, Schrag D. Predicting survival after curative colectomy for cancer: Individualizing colon cancer staging. J Clin Oncol. 2011;29(36):4796–4802. doi: 10.1200/JCO.2011.36.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0. 03 Vol. 4. National Institutes of Health, National Cancer Institute; 2009. [Google Scholar]
- 31.Fitzmaurice G, Laird N, Ware J. Applied longitudinal analysis. Hoboken, New Jersey: Wiley; 2004. [Google Scholar]