Abstract
Chronic kidney disease (CKD) has become a significant public health concern as it is associated with substantial morbidity. Prior research has evaluated multiple novel CKD biomarkers to supplement serum creatinine and proteinuria. The ultimate goal of this research is to find biomarkers that can be used to accurately predict CKD progression and to better time outpatient follow-up, and referral for transplant. Also, an optimal panel of biomarkers can augment the predictive value of proteinuria and serum creatinine by enriching patient enrollment in clinical trials. In this review, we discuss salient findings on 12 candidate plasma and urine biomarkers and their reported association with CKD. We explore the common pathways of CKD progression and the pathophysiologic processes of tubulointerstitial injury, inflammation, repair, and fibrosis that are potentially classified by specific biomarkers. We describe both pediatric and adult findings and highlight the paucity of pediatric research in CKD progression. It will be important for future research in cohorts with longitudinal follow-up to evaluate these CKD biomarkers in children for use in pediatric clinical trials and routine CKD management.
Keywords: CKD progression, CKD, ESRD, pediatrics, biomarker
Introduction
Chronic Kidney Disease (CKD) in children is characterized by kidney damage or a decrease in glomerular filtration rate (GFR) lasting for at least 3 months [1]. CKD in children is associated with high morbidity and mortality [2]. Additionally, pediatric CKD strikes during a vulnerable time and negatively impacts a child’s growth and development. Despite poor outcomes, there has only been one clinical trial demonstrating efficacy in limiting CKD progression in children [3]. New treatments to slow CKD progression in children are sorely needed.
A barrier to developing new CKD therapies is the limitations of our conventional biomarkers, proteinuria and serum creatinine. Although elevated serum creatinine and proteinuria are the most frequently utilized biomarkers of CKD, creatinine and proteinuria increase relatively late in the course of kidney damage and progression in CKD [4]. Children with underlying structural changes in the kidney may not have changes in serum creatinine due to the underlying ‘renal reserve’ – the capacity of nephrons to maintain GFR by hyperfiltration and compensatory hypertrophy until substantial injury has occurred. Serum creatinine levels may be affected by the race, sex, muscle mass, hydration status and medications; hence changes in creatinine may not represent true changes in kidney function [5]. Additionally, the kidney may undergo significant structural changes in the glomerulus and tubulointerstitial compartments before proteinuria is measureable. Proteinuria and serum creatinine only explain 32% of the variability of measured GFR decline among children in the Chronic Kidney Disease in Children (CKiD) study [6]. Additional biomarkers of CKD progression may capture the variability left unexplained by our traditional CKD biomarkers. An ideal biomarker should be non-invasive, easy to measure, plausible, sensitive, and serve to indicate targeted therapies likely to be effective [7].
A biomarker is any molecular, histologic, radiographic, and physiologic characteristic that can be measured as an indicator or predictor of a normal biologic process, pathologic process, or response to a therapeutic intervention [8]. The Food and Drug Administration-National Institute of Health Biomarker Working Group has used the terms diagnostic, prognostic, predictive, monitoring, pharmacodynamics/response, safety, or susceptibility/risk to classify different types of biomarkers [9]. A prognostic biomarker is used to identify the likelihood of a clinical event, disease recurrence, or progression of a disease [9]. In children with CKD, a prognostic biomarker may allow us to identify patients at high risk of CKD progression. Prognostic biomarkers may be useful to enhance enrollment in pediatric CKD clinical trials and improve the efficiency of trial design.
Although there are several causes of CKD in children, there is one common pathway of CKD progression involving kidney injury, maladaptive repair, and progressive nephron loss [10]. This is supported by common histologic findings in advanced CKD regardless of primary disease [11]. Our overall conceptual model is presented in Figure 1, with the associated putative pathophysiologic process that may yield biomarkers [12]. CKD progression is strongly influenced by these multiple pathophysiologic processes including systemic and glomerular hypertension, proteinuria, tubulointerstitial injury and fibrosis, inflammation, and capacity for renal repair [13]. Novel biomarkers can be leveraged to characterize these various mechanisms involved in CKD progression. A better understanding of the diverse proteins and pathways that predict renal outcomes may augment clinical research efforts and lead to the development of therapies to limit CKD progression.
Figure 1.
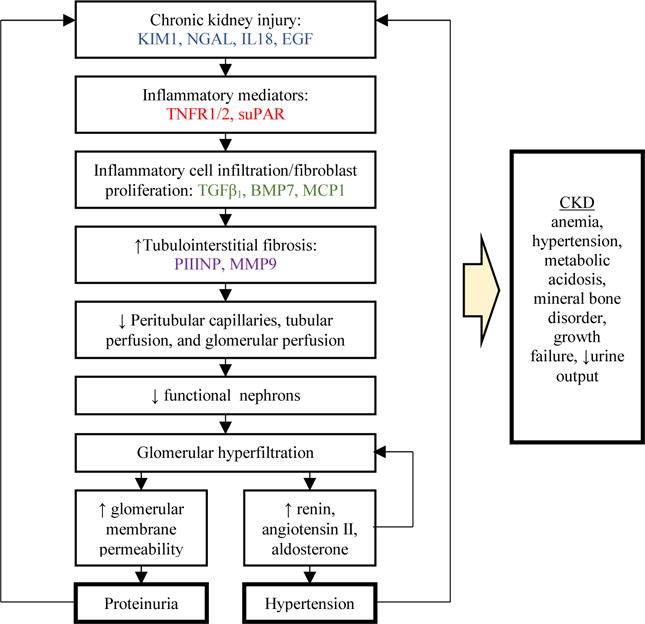
Novel biomarkers of chronic kidney disease (CKD) progression: mediators or byproducts associated with the pathophysiology of glomerular filtration rate (GFR) decline
We summarized the existing literature on candidate biomarkers of CKD and CKD progression with an emphasis on research in children. We categorized biomarkers by the associated pathophysiologic pathways. We searched the PubMed database to identify a comprehensive list of research studies that evaluated biomarkers of CKD. Search terms included ‘biomarker’ or ‘biologic marker’ in conjunction with ‘CKD’, ‘CKD progression’, ‘Chronic Renal Insufficiency’, or ‘Chronic Renal Failure’. Studies were eligible for inclusion if they were published in English language from January 1985 onward, produced quantitative statistics, and were retrospective or prospective cohort studies, case-control studies, or randomized controlled trials. Studies were excluded if they reported on fewer than 20 patients at the time of follow-up. We focused on novel biomarkers of CKD that had been evaluated in both pediatric and adult populations. We found 794 possible articles, 719 in adult cohorts and 75 of which were focused on children. Full text review identified a total of 56 studies for inclusion, 39 in adults and 17 in children. Table 1 lists specific biomarkers in categories of putative pathways. The results of prior pediatric research on biomarkers of CKD are summarized in Table 2. Our search revealed that there is a relative dearth of information on biomarkers in pediatric CKD. Virtually all the studies published in children were cross-sectional, and only 1 had more than 100 pediatric patients studied. Much of the existing knowledge concerning biomarkers, therefore is derived from studies in adults, who have a very different set of pathologies leading to CKD.
Table 1.
Candidate biomarkers of chronic kidney disease in children
| Biomarker | Biospecimen | |
|---|---|---|
| Tubulointerstitial injury | NGAL | Urine |
| IL-18 | Urine | |
| KIM-1 | Urine/Blood | |
| EGF | Urine | |
| Tubulointerstitial fibrosis | MMP-9 | Urine/Blood |
| PIIINP | Urine | |
| TGF-β1 | Blood | |
| BMP-7 | Blood | |
| Inflammation | MCP-1 | Urine |
| TNFR 1 & 2 | Blood | |
| suPAR | Blood |
Table 2.
Summary of Pediatric Research Studying Novel chronic kidney disease (CKD) Biomarkers
| Biomarker | Sample Source | Author, Year | Study type | Number of Patients (controls) | Mean Age (years) | Type of Kidney Disease | Study Outcome |
|---|---|---|---|---|---|---|---|
| NGAL | Urine | Brunner et al., 2006 | Cross-sectional | 43 (8) | 11.6 | SLE | Increased NGAL was associated with biopsy-proven SLE |
| Urine | Trachtman et al. 2006 | Prospective Cohort | 34 | 5.9 | HUS | High NGAL was associated with a need for acute dialysis | |
| IL-18 | Urine | Zubowska et al. 2013 | Cross-sectional | 85 | 8.8 | Postchemotherapy nephropathy | IL-18 was associated with nephropathy* |
| KIM-1 | Urine | Bienias et al. 2015 | Cross-sectional | 59 (20) | 13 | Nephrotic syndrome | KIM-1 was higher in patients with nephrotic syndrome |
| Urine | Ucaturk et al. 2016 | Cross-sectional | 120 (60) | 13 | Type 1 Diabetes | KIM-1 was high in normoalbuminuric patients with DM1 | |
| EGF | Urine | Tsau et al. 1998 | Cross-sectional | 42 (12) | 12.0 | CKD | EGF is correlated with GFR in pediatric CKD |
| MMP-9 | Urine | Korzeniecka-Kozerska et al. 2013 | Cross-sectional | 36 (16) | 9 | FSGS vs. MCNS | MMP-9 was higher in FSGS than MCNS |
| Blood | Musial et al. 2011 | Cross-sectional | 61 (24) | 10.5 | CKD | MMP9 was higher in children with CKD | |
| PIIINP | Urine | Jianguo et al. 2014 | Cross-sectional | 59 (30) | 5.4 | UPJ Obstruction | PIIINP is directly correlated with worsening obstruction |
| MCP-1 | Urine | Vianna et al. 2013 | Cross-sectional | 79 (37) | 12 | CKD | MCP-1 was higher in CKD patients |
| Urine | Ghobrial et al. 2015 | Cross-sectional | 75 (15) | 9.8 | SLE | MCP-1 was higher in active vs inactive SLE | |
| TGF-β1 | Urine | Woroniecki et al. 2008 | Cross-sectional | 36 (12) | 13.1 | FSGS vs. MCNS | TGF-β1 was higher in FSGS than MCNS |
| Urine | Zieg et al. 2011 | Cross-sectional | 51 (21) | 1.0 | Obstructive uropathy | TGF-β1 was higher in obstructive uropathy than non-obstructive uropathy | |
| BMP-7 | Blood | Musial et al. 2008 | Cross-sectional | 30 (12) | NA | CKD | BMP-7 was higher in CKD patients |
| TNFR 1/2 | Blood | Jutley et al. 2000 | Cross-sectional | 29 (14) | NA | Reflux nephropathy | TNFR1 was higher in reflux nephropathy |
| suPAR | Blood | Wei et al. 2012 | Cross-sectional | 70 (150) | 17.9 | FSGS | suPAR was higher in patients with FSGS and a decrease in suPAR was associated with remission |
| Blood | Gellerman et al. 2014 | Prospective Cohort | 60 | 9 | Minimal Change Disease | MMF and CSA modulate suPAR levels |
Abbreviations: SLE, Systemic Lupus Erythematosus, HUS, Hemolytic Uremic Syndrome, *, GFR < 75 mL/min/1.73 m2, microalbuminuria > 15 micrograms/min, or proteinuria (>0.15 g/24 h), MCNS, minimal chance nephrotic syndrome, FSGS, focal segmental glomerulosclerosis, UPJ, ureteropelvic junction, NA, not available, MMF, mycophenolate mofetil, CSA, cyclosporine
Biomarkers of Tubulointerstitial injury and Fibrosis
Regardless of the primary cause of CKD, tubulointerstitial disease is an important step that leads to nephron loss and is an accurate predictor of progression to ESRD [14]. Damage in the tubulointerstitium develops as a consequence of a number of processes involving the tubular lumen, tubular epithelial cells, peritubular capillaries, interstitial cells, and extracellular matrix. In response to injury, tubular epithelial cells undergo a complex series of structural and functional changes and produce cytokines, which in turn lead to interstitial inflammation and fibrosis [15]. Knowledge of the mechanisms and interactions leading to these changes has increased exponentially over the past decade, and has defined a number of new inflammatory mediators and targets for treatment. Biomarkers of tubulointerstitial injury as well as the subsequent fibrosis may serve as appropriate markers of prior and ongoing CKD progression [16–18].
Biomarkers of tubular injury
Urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL)
Urinary Neutrophil Gelatinase-Associated Lipocalin (uNGAL) is a protein produced by neutrophils and kidney tubular cells with increased synthesis occurring in states of tubular injury. The production of uNGAL in the distal segments of the nephron is upregulated in patients with CKD. It has been established as a sensitive marker of early renal tubular injury in children [19]. uNGAL has also been shown to be a predictor of disease severity, as demonstrated in two pediatric studies of childhood lupus and hemolytic uremic syndrome [20, 21]. In a study by Smith et al. including 158 adults with stage 3 or 4 CKD, baseline uNGAL was associated with a rapid decline in renal function within 1 year and ESRD within 2 years [16]. Liu et al. assessed the performance of baseline uNGAL for predicting GFR decline in 3386 adults in the Chronic Renal Insufficiency Cohort Study [18]. At 3 years of follow-up, patients with the highest quintile of uNGAL were 70% more likely to experience a 50% reduction in estimated GFR (eGFR) or ESRD. However, when controlled for known CKD progression risk factors, including eGFR and proteinuria, uNGAL did not significantly improve risk prediction of progression outcomes. Pre-clinical as well as human studies of kidney disease have shown that uNGAL decreases after effective therapeutic interventions [22, 23].
Urinary Interleukin-18 (IL-18)
Urinary Interleukin-18 (IL-18) is an inflammatory cytokine produced by macrophages and proximal tubular cells in response to injury. Zubowska et al. studied the role of IL-18 in identifying subclinical renal dysfunction in 85 pediatric oncology patients [24]. At 4.6 years of follow-up, nephrectomy and previous exposure to chemotherapy were associated with significantly higher levels of urinary IL-18. In 153 transplant recipients, higher levels of urinary IL-18 measured on the first post-transplant day predicted a faster decline in renal allograft function at 1 year post-transplant [25]. In a cohort of 908 HIV-infected patients, baseline IL-18 was associated with a decline in kidney function and higher mortality, even when controlled for eGFR and albuminuria [26, 27].
Urinary and Blood Kidney Injury Molecule-1 (KIM-1)
Urinary and Blood Kidney Injury Molecule-1 (KIM-1) is a tubular protein that is undetectable in a healthy kidney but is markedly elevated with renal injury [28]. Urinary KIM-1 has been shown to be a marker of disease severity in children with nephrotic syndrome as levels were higher in steroid resistant nephrotic syndrome as compared with steroid dependent nephrotic syndrome [29]. Children with type 1 diabetes and without albuminuria had higher levels of urinary KIM-1 when compared to non-diabetic controls [30]. Urinary KIM-1 expression was significantly increased on kidney biopsies within areas of fibrosis or inflammation [31]. Urine KIM-1 was also found to be elevated in all patients with kidney disease except those with minimal change disease [31]. In a study of 145 renal transplant patients, compared to the first tertile, the highest tertile of urine KIM-1 predicted future GFR decline and graft loss at 5 years of follow-up with an adjusted hazard ratio of 5.1 (95% CI: 1.5–17.8) [32]. In a study by Szeto et al. on 63 kidney transplant recipients, each log-unit increase in urinary KIM-1 conferred a 2.9-fold higher risk (95% CI: 1.3–6.2) of developing graft failure at 48 months [33]. Sabbisetti et al. studied 107 adults with CKD stages 1–3, type 1 diabetes, and proteinuria and displayed how baseline blood KIM-1 predicted GFR decline and incident ESRD [34].
Urinary Epidermal Growth Factor (EGF)
Urinary Epidermal Growth Factor (EGF) is implicated in tubular cell repair and renal recovery after tubulointerstitial injury. EGF is implicated in key pathways of CKD progression across several etiologies. EGF has a high degree of tissue specificity as it is derived from the ascending loop of Henle and distal convoluted tubule. uEGF has been measured in a cohort of children with CKD and found to have a strong correlation with GFR [35]. In three independent cohorts of glomerular disease, addition of uEGF to a conventional model including eGFR and albuminuria, prediction of CKD progression was improved [36].
Biomarkers of Interstitial Fibrosis
Blood Matrix Metalloproteinase-9 (MMP-9)
Blood Matrix Metalloproteinase-9 (MMP-9) is a protease responsible for degrading extracellular matrix and is a candidate biomarker of renal fibrosis, cardiovascular outcomes, and progressive renal injury [37]. In children with FSGS, urinary MMP-9 was found to be substantially higher than children with MCNS and children without kidney disease [38]. In a cohort of pediatric patients with CKD, plasma MMP-9 was significantly higher in children with CKD compared to children without CKD [39]. In a cohort of 251 adults, elevated plasma MMP-9 predicted CKD progression after 8.5 years of follow-up with a hazard ratio of 4.7 (95% CI: 2.1–10.4) [40].
Blood Transforming Growth Factor Beta 1 (TGF-β1)
Blood Transforming Growth Factor Beta 1 (TGF-β1) promotes cell migration, inflammation, extracellular matrix formation, and irreversible tissue damage. TGF-β1 is released from tubular epithelial cells, fibroblasts, and infiltrating inflammatory cells. In children with idiopathic nephrotic syndrome, urinary TGF-β1 was higher in patients with FSGS as compared to minimal change disease [41]. Children with obstructive uropathy had higher urinary TGF-β1 than children with non-obstructive uropathy [42]. In a study of 281 patients with type 2 diabetes, high TGF-β1 was a strong predictor of doubling of serum creatinine and ESRD. The cumulative risk of doubling of serum creatinine and ESRD in the fourth quartile of TGF-β1 was 8.4 times that of the lowest quartile [43].
Blood Bone Morphogenetic Protein-7 (BMP-7)
Blood Bone Morphogenetic Protein-7 (BMP-7) is a TGF-β1 antagonist, with anti-fibrotic and anti-inflammatory properties. Blood BMP-7 was higher in pediatric CKD patients as compared with controls [44]. Recombinant human BMP-7 infusion decreases fibrosis and inflammation in models of obstructive uropathy and slows GFR decline in mouse models of lupus nephritis and Goodpasture’s disease [45, 46]. In a study of 281 patients with type 2 diabetes, low levels of BMP-7 was a strong predictor of doubling of serum creatinine and ESRD. The cumulative risk of renal endpoints in the lowest quartile of BMP-7 was 24 times that of the highest quartile [43].
Urinary Procollagen III N-Terminal Propeptide (PIIINP)
Urinary Procollagen III N-Terminal Propeptide (PIIINP) is a propeptide byproduct of collagen 3 deposition and is used as a marker of renal fibrosis [47]. In a study on 29 children with UPJ obstruction and 30 healthy controls urine PIIINP levels were found to be associated with worsening obstruction [48]. Urine PIIINP has been found to correlate with interstitial fibrosis on the renal biopsy of CKD patients [49]. Additionally, in a study on renal transplant recipients, urine PIIINP was found to be a marker of CKD progression [50]. In a study evaluating 958 adults in the Cardiovascular Health Study, each doubling of urine PIIINP was associated with a 22% higher odds of CKD progression [51].
Inflammatory Biomarkers
A complex interaction between cytokines and immunologic cells play an essential role in mediating inflammation. Chronic low grade inflammation appears to play an important role in the initiation and progression of CKD. Inflammation is due to the initial, continuous, or recurrent kidney injury from the primary disease, hypoalbuminemia, and reduced clearance of cytokines and metabolites. Furthermore, proteinuria, which is very common in CKD, appears to contribute to renal injury and a pro-inflammatory state. Several candidate inflammatory biomarkers have been hypothesized to not only predict GFR decline but also contribute directly to renal injury and CKD progression [52, 53].
Blood Soluble Urokinase-type Plasminogen Activator Receptor (suPAR)
Blood Soluble Urokinase-type Plasminogen Activator Receptor (suPAR) is directly involved in the regulation of cell adhesion and migration through binding of integrins. suPAR interferes with podocyte migration, induces apoptosis, and is implicated in the pathogenesis of kidney disease. Wei et al. studied 70 children and adults with biopsy proven FSGS and found that a reduction of circulating suPAR concentration was positively associated with complete remission as well as a reduction of proteinuria [54]. This research suggests that suPAR may be a biomarker of future decline in GFR, as proteinuria is an established risk factor for CKD progression. Additionally, plasma suPAR concentration was higher in pediatric patients with steroid resistant nephrotic syndrome versus steroid sensitive nephrotic syndrome (3,744.1 ± 2,226.0 vs. 2,153.5 ± 1,167.0, p < 0.05) [55]. In a cohort of 3683 adults from the Emory Cardiovascular Biobank, higher plasma suPAR predicted incident CKD such that the patients with the lowest quartile of suPAR levels had an annual eGFR change of −0.9 ml/min/1.73m2 compared with −4.2 ml/min/1.73m2 in the highest quartile [53].
Blood Tumor Necrosis Factor receptor 1 & 2 (TNFR1 &TNFR2)
Tumor necrosis factor (TNF) is a central mediator of inflammation, cell proliferation, cellular differentiation, and cell death [56]. TNF exerts its effect on intracellular signaling pathways through interaction with two cell membrane receptors, TNFR1 and TNFR2. A pediatric study implicated the TNF pathway in the recurrence of FSGS and showed improvement in proteinuria after TNF antibodies were administered [57]. Serum TNFR1 was studied in children with vesico-ureteric reflux and higher levels were able to distinguish children with reflux nephropathy versus those without [58]. Patel et al. studied 25 children with systemic lupus erythematous and 20 healthy controls and displayed that TNFR2 is higher in children with SLE, and highest in children with lupus nephritis [34]. The TNF pathway has been implicated in several causes of CKD including diabetic nephropathy, obstructive nephropathy, lupus nephritis, non-diabetic CKD, and ANCA induced nephritis [56, 59–61]. In a study of 410 patients with type 2 diabetes, TNFR1 was a strong predictor of progression to ESRD [52]. The cumulative risk of ESRD in the fourth quartile of TNFR1 was 54% after 12 years versus 3% for all other patients. The hazard ratios for ESRD show that non-proteinuric patients in the fourth quartile of TNFR1 are over 7 times more likely to progress to ESRD than all other patients. TNFR1 and TNFR2 were also found to predict incident stage 3 CKD in type 1 diabetics [17]. Animal models of obstructive uropathy, a common cause of CKD in children, showed that renal tubular TNFR2 was markedly increased [62]. Furthermore, TNFR2 deficient mice were found to have significantly less tubulointerstitial fibrosis in models of obstructive uropathy [63].
Urinary Monocyte Chemoattractant Protein-1 (MCP-1)
Urinary Monocyte Chemoattractant Protein-1 (MCP-1) is a chemokine which recruits monocytes and promotes their transformation into macrophages. Urinary levels of MCP-1 were significantly higher in pediatric CKD patients compared to controls. Additionally, patients with glomerular disease had higher MCP-1 as compared with non-glomerular disease patients [64]. Urinary MCP-1 was identified as a marker of kidney disease severity in children with SLE [65]. In animal models of lupus nephritis, MCP-1 antagonists have limited kidney disease progression, suggesting MCP-1 has a primary role in disease activity [66]. In patients with diabetic nephropathy, urine MCP-1 levels correlate with kidney macrophage accumulation, fibrosis, and GFR decline [67, 68]. Urine MCP-1 levels also correlate with the rate of decline in GFR in non-diabetic CKD [69]. MCP-1 has been identified as a potential therapeutic target in patients with CKD with multiple ongoing MCP-1 inhibitor trials [70].
Conclusion
Numerous longitudinal studies in adults describe the association between novel biomarkers and CKD progression. However, none of these biomarkers have yet been qualified by the FDA for clinical use. In the field of pediatric medicine, studies in CKD biomarkers are mostly restricted to cross-sectional analyses, and only six studies have included more than 50 children. CKD biomarkers that more precisely identify chronic injury and act as surrogates of CKD progression are sought for advancing pediatric clinical trials. In particular, if prognostic biomarkers could be identified, strategies to enrich recruited subjects at high risk of meeting study endpoints could be employed which may decrease the cost and sample size necessary to identify effective treatments. In the clinical setting, these biomarkers may help to predict response to therapy and to predict long-term outcomes in patients with CKD. When using biomarkers of therapeutic response, consideration should be given to biomarkers that correlate with a proposed therapy, depending on the targeted pathway of CKD progression. Although there is a paucity of treatments for pediatric CKD progression, clinical trials in adult CKD participants targeting inflammatory, hemodynamic, or fibrotic pathways may soon yield candidate therapies that can be trialed in children [71].
Importantly, novel biomarkers must first be shown to enhance prediction of CKD progression when added to a model that includes serum creatinine and proteinuria. Liu et al. demonstrated that urine NGAL predicted CKD progression, but did not significantly improve upon a clinical model of CKD risk factors including eGFR and proteinuria [18]. However, TNFR1 and TNFR2 were found to have a strong association with progression to ESRD even after controlling for albuminuria and eGFR [52].
Novel biomarkers of CKD may be more readily identified studying pediatric cohorts as adults have more comorbidities and concurrent disease, which may affect biomarker concentrations and modify CKD progression. Additionally, CKD in children is most commonly caused by isolated congenital anomalies of the kidney or urinary tract (CAKUT), which is not a systemic multi-organ disease such as diabetic nephropathy. The CAKUT model of CKD progression may be easier to characterize with less systemic and inflammatory influences. With additional research we will learn whether these novel biomarkers have an associative relationship with GFR decline or whether they also have true mechanistic roles in CKD progression.
Novel biomarkers may identify children with the earliest stages of injury and repair, before proteinuria or serum creatinine identifies irreversible injury and nephron loss. Since CKD progression involves multiple processes in the kidney, it is unlikely that one biomarker will best predict GFR decline. It is likely that panels of biomarkers which leverage the additive properties of each individual biomarker will be the most useful clinical tools in identifying risk for CKD progression in children. A panel of biomarkers that represent the multifactorial pathophysiologic mechanisms leading to CKD progression including tubulointerstitial injury, fibrosis, inflammation, and repair may prove useful for predicting GFR decline in children. Further pediatric research, preferably prospective longitudinal studies, will be needed to assess whether these novel biomarkers can be used to predict CKD progression. In addition to research applications, novel biomarkers could help guide the prognosis given to families as well as the timing of follow-up visits and referral for transplant evaluation. The application of novel biomarkers has the potential to revolutionize clinical decision-making as well as clinical trials of CKD progression.
Acknowledgments
Funding: This review was supported by the NIH career development grant K08DK110536 to J.H.G. C.R.P. is supported by the NIH K24DK090203, R01HL085757, U01DK082185, and P30DK079310-07 O’Brien Center Grant. S.L.F. is supported by the NIH K24DK078737 and U01DK66174.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.NKF KDOQI Guidelines. National Kidney Foundation; 2002. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Group ET. Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Moller K, Wigger M, Peruzzi L, Mehls O, Schaefer F. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, Kasiske B, Hostetter T. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54:205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Parikh CR, Lu JC, Coca SG, Devarajan P. Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. Ann Clin Biochem. 2010;47:301–312. doi: 10.1258/acb.2010.010076. [DOI] [PubMed] [Google Scholar]
- 6.Munoz A, Townsend K. Use of Generalized Gamma Regression Models to assess explained variance of decline in GFR by UPCr and SCr. Johns Hopkins; Bloomberg School of Public Health: 2015. p. 7. [Google Scholar]
- 7.Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis. 2010;17:469–479. doi: 10.1053/j.ackd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biomarkers Definitions Working G. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 9.2016 BEST (Biomarkers, EndpointS, and other Tools) Resource, Maryland.
- 10.Schnaper HW. Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol. 2014;29:193–202. doi: 10.1007/s00467-013-2494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avner ED, Harmon WE, Niaudet P, Yoshikawa N. Pediatric Nephrology. Williams & Wilkins; Baltimore: 2009. [Google Scholar]
- 12.Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774–1777. doi: 10.1038/ki.1996.265. [DOI] [PubMed] [Google Scholar]
- 13.Fogo AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol. 2007;22:2011–2022. doi: 10.1007/s00467-007-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 15.Harris DC. Tubulointerstitial renal disease. Curr Opin Nephrol Hypertens. 2001;10:303–313. doi: 10.1097/00041552-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, Holt SG. Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD) Nephrol Dial Transplant. 2013;28:1569–1579. doi: 10.1093/ndt/gfs586. [DOI] [PubMed] [Google Scholar]
- 17.Gohda T, Niewczas MA, Ficociello LH, Walker WH, Skupien J, Rosetti F, Cullere X, Johnson AC, Crabtree G, Smiles AM, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soca Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY, Chronic Renal Insufficiency Cohort study i Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int. 2013;83:909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zappitelli M, Washburn KK, Arikan AA, Loftis L, Ma Q, Devarajan P, Parikh CR, Goldstein SL. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunner HI, Mueller M, Rutherford C, Passo MH, Witte D, Grom A, Mishra J, Devarajan P. Urinary neutrophil gelatinase-associated lipocalin as a biomarker of nephritis in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2006;54:2577–2584. doi: 10.1002/art.22008. [DOI] [PubMed] [Google Scholar]
- 21.Trachtman H, Christen E, Cnaan A, Patrick J, Mai V, Mishra J, Jain A, Bullington N, Devarajan P, Investigators of the HUSSPMCT Urinary neutrophil gelatinase-associated lipocalcin in D+HUS: a novel marker of renal injury. Pediat Nephrol. 2006;21:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 22.Kasahara M, Mori K, Satoh N, Kuwabara T, Yokoi H, Shimatsu A, Sugawara A, Mukoyama M, Nakao K. Reduction in urinary excretion of neutrophil gelatinase-associated lipocalin by angiotensin receptor blockers in hypertensive patients. Nephrol Dial Transplant. 2009;24:2608–2609. doi: 10.1093/ndt/gfp238. author reply 2609–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwabara T, Mori K, Mukoyama M, Kasahara M, Yokoi H, Saito Y, Yoshioka T, Ogawa Y, Imamaki H, Kusakabe T, Ebihara K, Omata M, Satoh N, Sugawara A, Barasch J, Nakao K. Urinary neutrophil gelatinase-associated lipocalin levels reflect damage to glomeruli, proximal tubules, and distal nephrons. Kidney Int. 2009;75:285–294. doi: 10.1038/ki.2008.499. [DOI] [PubMed] [Google Scholar]
- 24.Zubowska M, Wyka K, Fendler W, Mlynarski W, Zalewska-Szewczyk B. Interleukin 18 as a marker of chronic nephropathy in children after anticancer treatment. Dis Markers. 2013;35:811–818. doi: 10.1155/2013/369784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR. Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol. 2012;7:1224–1233. doi: 10.2215/CJN.00310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women: Biomarkers and Kidney Decline. J Acquir Immune Defic Syndr. 2012;61:565–573. doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, Bennett M, Butch A, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C, Shlipak M. Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS) HIV Med. 2014;15:291–300. doi: 10.1111/hiv.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG, Consortium T-A Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8:1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bienias B, Zajaczkowska M, Borzecka H, Sikora P, Wieczorkiewicz-Plaza A, Wilczynska B. Early Markers of Tubulointerstitial Fibrosis in Children With Idiopathic Nephrotic Syndrome: Preliminary Report. Medicine. 2015;94:e1746. doi: 10.1097/MD.0000000000001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ucakturk A, Avci B, Genc G, Ozkaya O, Aydin M. Kidney injury molecule-1 and neutrophil gelatinase associated lipocalin in normoalbuminuric diabetic children. J Pediatr Endocrinol Metab. 2016;29:145–151. doi: 10.1515/jpem-2015-0138. [DOI] [PubMed] [Google Scholar]
- 31.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 32.van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007;84:1625–1630. doi: 10.1097/01.tp.0000295982.78039.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szeto CC, Kwan BC, Lai KB, Lai FM, Chow KM, Wang G, Luk CC, Li PK. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol. 2010;5:2329–2337. doi: 10.2215/CJN.01910310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsau Y, Chen C. Urinary epidermal growth factor excretion in children with chronic renal failure. Am J Nephrol. 1999;19:400–404. doi: 10.1159/000013485. [DOI] [PubMed] [Google Scholar]
- 36.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PX, Mariani LH, Eichinger FH, Berthier CC, Randolph A, Lai JY, Zhou Y, Hawkins JJ, Bitzer M, Sampson MG, Thier M, Solier C, Duran-Pacheco GC, Duchateau-Nguyen G, Essioux L, Schott B, Formentini I, Magnone MC, Bobadilla M, Cohen CD, Bagnasco SM, Barisoni L, Lv J, Zhang H, Wang HY, Brosius FC, Gadegbeku CA, Kretzler M, Ercb CPN, Consortium PK-I Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 2006;20:1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- 38.Korzeniecka-Kozerska A, Wasilewska A, Tenderenda E, Sulik A, Cybulski K. Urinary MMP-9/NGAL ratio as a potential marker of FSGS in nephrotic children. Dis Markers. 2013;34:357–362. doi: 10.3233/DMA-130980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musial K, Zwolinska D. Matrix metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2) as novel markers of stress response and atherogenesis in children with chronic kidney disease (CKD) on conservative treatment. Cell Stress Chaperones. 2011;16:97–103. doi: 10.1007/s12192-010-0214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu TW, Kuo KL, Hung SC, Huang PH, Chen JW, Tarng DC. Progression of kidney disease in non-diabetic patients with coronary artery disease: predictive role of circulating matrix metalloproteinase-2, -3, and -9. PloS One. 2013;8:e70132. doi: 10.1371/journal.pone.0070132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woroniecki RP, Shatat IF, Supe K, Du Z, Kaskel FJ. Urinary cytokines and steroid responsiveness in idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2008;28:83–90. doi: 10.1159/000109396. [DOI] [PubMed] [Google Scholar]
- 42.Zieg J, Blahova K, Seeman T, Bronsky J, Dvorakova H, Pechova M, Janda J, Matousovic K. Urinary transforming growth factor-beta1 in children with obstructive uropathy. Nephrology. 2011;16:595–598. doi: 10.1111/j.1440-1797.2011.01459.x. [DOI] [PubMed] [Google Scholar]
- 43.Wong MG, Perkovic V, Woodward M, Chalmers J, Li Q, Hillis GS, Yaghobian Azari D, Jun M, Poulter N, Hamet P, Williams B, Neal B, Mancia G, Cooper M, Pollock CA. Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013;83:278–284. doi: 10.1038/ki.2012.383. [DOI] [PubMed] [Google Scholar]
- 44.Musial K, Fornalczyk K, Zwolinska D. Osteopontin (OPN), PDGF-BB (platelet-derived growth factor) and BMP-7 (bone morphogenetic protein) as markers of atherogenesis in children with chronic kidney disease (CKD) treated conservatively–preliminary results. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2008;24(Suppl 4):25–27. [PubMed] [Google Scholar]
- 45.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 46.Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol. 2000;279:F130–143. doi: 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jianguo W, Zhenzhen L, Xianghua L, Zhanzheng Z, Suke S, Suyun W. Serum and urinary procollagen III aminoterminal propeptide as a biomarker of obstructive nephropathy in children. Clin Chim Acta. 2014;434:29–33. doi: 10.1016/j.cca.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Ghoul BE, Squalli T, Servais A, Elie C, Meas-Yedid V, Trivint C, Vanmassenhove J, Grunfeld JP, Olivo-Marin JC, Thervet E, Noel LH, Prie D, Fakhouri F. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin Am Soc Nephrol. 2010;5:205–210. doi: 10.2215/CJN.06610909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75:2113–2119. doi: 10.1097/01.TP.0000066809.60389.48. [DOI] [PubMed] [Google Scholar]
- 51.Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, Katz R, Delaney JA, Chaves P, Rifkin DE, Hughes-Austin JM, Garimella PS, Sarnak MJ, Shlipak MG, Kizer JR. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. J Am Soc Nephrol. 2015;26:2494–503. doi: 10.1681/ASN.2014070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niewczas MA, Gohda T, Skupien J, Smiles AM, Walker WH, Rosetti F, Cullere X, Eckfeldt JH, Doria A, Mayadas TN, Warram JH, Krolewski AS. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J. Soluble Urokinase Receptor and Chronic Kidney Disease. New Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei C, Trachtman H, Li J, Dong C, Friedman AL, Gassman JJ, McMahan JL, Radeva M, Heil KM, Trautmann A, Anarat A, Emre S, Ghiggeri GM, Ozaltin F, Haffner D, Gipson DS, Kaskel F, Fischer DC, Schaefer F, Reiser J, PodoNet, Consortia FCS Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051–2059. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gellermann J, Schaefer F, Querfeld U. Serum suPAR levels are modulated by immunosuppressive therapy of minimal change nephrotic syndrome. Pediatr Nephrol. 2014;29:2411–2414. doi: 10.1007/s00467-014-2913-5. [DOI] [PubMed] [Google Scholar]
- 56.Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015;87:281–296. doi: 10.1038/ki.2014.285. [DOI] [PubMed] [Google Scholar]
- 57.Bitzan M, Babayeva S, Vasudevan A, Goodyer P, Torban E. TNFalpha pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and beta3 integrin activation. Pediatr Nephrol. 2012;27:2217–2226. doi: 10.1007/s00467-012-2163-3. [DOI] [PubMed] [Google Scholar]
- 58.Jutley RS, Youngson GG, Eremin O, Ninan GK. Serum cytokine profile in reflux nephropathy. Pediatr Surg Int. 2000;16:64–68. doi: 10.1007/s003830050017. [DOI] [PubMed] [Google Scholar]
- 59.Ernandez T, Mayadas TN. Immunoregulatory role of TNFalpha in inflammatory kidney diseases. Kidney Int. 2009;76:262–276. doi: 10.1038/ki.2009.142. [DOI] [PubMed] [Google Scholar]
- 60.Shankar A, Sun L, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dounousi E, Koliousi E, Papagianni A, Ioannou K, Zikou X, Katopodis K, Kelesidis A, Tsakiris D, Siamopoulos KC. Mononuclear leukocyte apoptosis and inflammatory markers in patients with chronic kidney disease. A J Nephrol. 2012;36:531–536. doi: 10.1159/000345352. [DOI] [PubMed] [Google Scholar]
- 62.Misaki T, Yamamoto T, Suzuki S, Fukasawa H, Togawa A, Ohashi N, Suzuki H, Fujigaki Y, Oda T, Uchida C, Kitagawa K, Hattori T, Kitagawa M, Hishida A. Decrease in tumor necrosis factor-alpha receptor-associated death domain results from ubiquitin-dependent degradation in obstructive renal injury in rats. Am J Pathol. 2009;175:74–83. doi: 10.2353/ajpath.2009.080884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol. 1999;277:F766–772. doi: 10.1152/ajprenal.1999.277.5.F766. [DOI] [PubMed] [Google Scholar]
- 64.Vianna HR, Soares CM, Silveira KD, Elmiro GS, Mendes PM, de Sousa Tavares M, Teixeira MM, Miranda DM, Simoes ESAC. Cytokines in chronic kidney disease: potential link of MCP-1 and dyslipidemia in glomerular diseases. Pediatr Nephrol. 2013;28:463–469. doi: 10.1007/s00467-012-2363-x. [DOI] [PubMed] [Google Scholar]
- 65.Ghobrial EE, El Hamshary AA, Mohamed AG, Abd El Raheim YA, Talaat AA. Urinary monocyte chemoattractant protein-1 as a biomarker of lupus nephritis activity in children. Saudi J Kidney Dis Transpl. 2015;26:507–515. doi: 10.4103/1319-2442.157350. [DOI] [PubMed] [Google Scholar]
- 66.Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, Hieshima K, Maruyama H, Miyazaki J, Yoshie O, Nose M, Fujita S. Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice. Arthritis Rheum. 2003;48:2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- 67.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2008;294:F697–701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 68.Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, Cantley L, Devarajan P, Parikh CR, Coca SG. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol. 2016;11:1343–1352. doi: 10.2215/CJN.12051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camilla R, Brachemi S, Pichette V, Cartier P, Laforest-Renald A, MacRae T, Madore F, Troyanov S. Urinary monocyte chemotactic protein 1: marker of renal function decline in diabetic and nondiabetic proteinuric renal disease. J Nephrol. 2011;24:60–67. doi: 10.5301/jn.2010.1458. [DOI] [PubMed] [Google Scholar]
- 70.de Zeeuw D, Bekker P, Henkel E, Hasslacher C, Gouni-Berthold I, Mehling H, Potarca A, Tesar V, Heerspink HJ, Schall TJ, Group CBDNS The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 2015;3:687–696. doi: 10.1016/S2213-8587(15)00261-2. [DOI] [PubMed] [Google Scholar]
- 71.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15:568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]


