Abstract
Background
Data regarding long-term outcomes following percutaneous cholecystostomy (PC) are limited, and comparisons to cholecystectomy (CCY) are lacking. We hypothesized that chronic disease burden would predict 1-year mortality following PC, and that outcomes following PC and CCY would be similar when controlling for preprocedural risk factors.
Methods
We performed a 10-year retrospective cohort analysis of patients with acute cholecystitis managed by PC (n = 114) or CCY (n = 234). Treatment response was assessed by systemic inflammatory response syndrome (SIRS) criteria at PC/CCY and 72 h later. Logistic regression identified predictors of 30-day and 1-year mortality following PC. PC and CCY patients were matched by age, Tokyo Guidelines (TG13) cholecystitis severity grade, and VASQIP calculator predicted mortality (n = 42/group).
Results
The presence of SIRS at 72 h following PC was associated with 30-day mortality [OR 8.9 (95% CI 2.6–30)]. SIRS at 72 h was present in and 21.4% of all PC patients, significantly higher than unmatched CCY patients (4.7%, p = 0.048). Independent predictors of 1-year mortality following PC were DNR status [19.7 (2.1–186)], disseminated cancer [7.5 (2.1–26)], and congestive heart failure [3.9 (1.4–11)]. PC patients with none of these risk factors had 17.9% 90-day mortality and no deaths after 90 days; late deaths continued to occur among patients with DNR, CHF, or disseminated cancer. At baseline, PC patients had greater acute and chronic disease burden than CCY patients. After matching, PC and CCY patients had similar age (69 vs. 70 years), TG13 grade (2.4 vs. 2.4), and predicted 30-day mortality (5.5 vs. 6.8%). Matched PC patients had higher 30-day mortality (14.3 vs. 2.4%, p = 0.109) and 180-day mortality (28.6 vs. 7.1%, p = 0.048).
Conclusions
Treatment response to PC predicted 30-day mortality; DNR status, and chronic diseases predicted 1-year mortality. Although the matching procedure did not eliminate selection bias, PC was associated with persistent systemic inflammation and higher long-term mortality than CCY.
Keywords: Cholecystitis, Percutaneous cholecystostomy, Cholecystectomy, Prognosis, Outcomes, Mortality
Percutaneous cholecystostomy (PC) may be performed for high-risk operative candidates with acute cholecystitis as an alternative to cholecystectomy (CCY). PC may function as a temporizing measure for patients with calculous cholecystitis, or as definitive therapy for patients with no gallstones who are at low risk for recurrence [1, 2]. Incidence of PC has increased over time despite controversy fueled by the paucity of direct comparisons to CCY and limited prognostic data for outcomes following PC [3–6]. The purposes of this study were to compare outcomes following PC versus CCY and to identify predictors of 30-day and 1-year mortality following PC. We hypothesized that chronic disease burden would predict 1-year mortality following PC, and that outcomes following PC and CCY would be similar when controlling for preprocedural risk factors.
Methods
Study population
We performed a retrospective cohort analysis of 348 consecutive patients with acute cholecystitis managed by PC (n = 114) or CCY (n = 234) at a Veterans Affairs hospital from January 1, 2004 through October 1, 2014. Institutional Review Board approval was obtained. Our radiology and surgery databases were searched for patients with acute cholecystitis and procedural codes for PC and CCY. Patients who underwent PC and subsequent CCY were analyzed in the PC cohort. Pregnant women, patients with concomitant cholangitis or gallstone pancreatitis, and subjects with less than 30-day follow-up were excluded. Although subtotal CCY may be a safe and effective option under certain circumstances [7], these patients were excluded to decrease heterogeneity in the CCY cohort. Treatment response was assessed by systemic inflammatory response syndrome (SIRS) criteria [8] at the time of the procedure and 72 h later. The primary endpoints were mortality at 30, 180 days, and 1 year. 1-year follow-up within the VA system was 96%.
Matching procedure
Cholecystitis was characterized by Tokyo (TG13) severity grade (1 = mild uncomplicated cholecystitis, 2 = moderate cholecystitis characterized marked local inflammation or systemic toxicity, and 3 = severe cholecystitis characterized by evidence of organ dysfunction) [9]. The response to PC was assessed by systemic inflammatory response syndrome (SIRS) criteria [8]. PC patients were matched to CCY patients 1:1 based on VASQIP predicted 30-day mortality. For General Surgery operations, the VASQIP Fiscal Year 2015 Model uses 42 variables to predict 30-day and 180-day postoperative mortality. For each PC patient, the CCY cohort was searched for the subject with the closest corresponding predicted mortality. If this subject had predicted mortality within 0.99% of the PC patient, both subjects were set aside into matched groups. In this manner, 90 PC patients were matched to 90 CCY patients. Next, patients were matched by TG 13 grade. Of the 90 PC patients, 36 were grade III, 24 were grade II, and 30 were grade I. Of the 90 CCY patients, 18 were grade III, 39 were grade II, and 33 were grade I. The 18 grade III CCY patients were matched to 18 grade III PC patients according to age by the greedy nearest-neighbor method (the first patient was paired with the closest age match, both were set aside, and the process was repeated). Grade II patients were matched by the same method. Grade I patients were excluded, as PC is not recommended for this population [10, 11]. This process matched 42 PC patients to 42 CCY patients with similar baseline characteristics (Table 1).
Table 1.
Patient characteristics at the time of percutaneous cholecystostomy (PC) or cholecystectomy (CCY)
| Patient characteristics | PC n = 114 | CCY n = 234 | p | Matched PC n = 42 | Matched CCY n = 42 | p |
|---|---|---|---|---|---|---|
| Age (years) | 71 ± 12 | 66 ± 11 | <0.001 | 69 ± 11 | 70 ± 9 | 0.708 |
| Male | 110 (96%) | 210 (90%) | 0.035 | 40 (95%) | 40 (95%) | – |
| ASA physical status classification | 3.5 ± 0.7 | 3.4 ± 0.8 | 0.560 | 3.5 ± 0.5 | 3.6 ± 0.5 | 0.388 |
| Calculous cholecystitisa | 85 (75%) | 210 (90%) | <0.001 | 32 (76%) | 40 (95%) | 0.026 |
| TG13 severity gradea | 2.1 ± 0.9 | 1.6 ± 0.7 | <0.001 | 2.4 ± 0.5 | 2.4 ± 0.5 | >0.999 |
| Temperature (°C)a | 37.4 ± 0.9 | 37.1 ± 0.8 | 0.014 | 37.4 ± 0.8 | 37.3 ± 0.9 | 0.877 |
| White blood cell count (x109/L) | 14.0 ± 7.1 | 12.9 ± 4.6 | 0.082 | 14.7 ± 7.0 | 15.0 ± 4.6 | 0.881 |
| Creatinine (mg/dL) | 1.7 ± 1.9 | 1.0 ± 0.3 | <0.001 | 1.5 ± 1.2 | 1.2 ± 0.5 | 0.097 |
| International normalized ratio | 1.4 ± 0.6 | 1.1 ± 0.1 | <0.001 | 1.4 ± 0.5 | 1.2 ± 0.2 | 0.039 |
| Myocardial infarctionb | 6 (5%) | 1 (0.4%) | 0.006 | 2 (5%) | 0 | 0.494 |
| Congestive heart failure | 23 (20%) | 39 (17%) | 0.457 | 5 (12%) | 11 (26%) | 0.164 |
| COPD | 30 (26%) | 54 (23%) | 0.594 | 8 (19%) | 17 (41%) | 0.055 |
| Ascites | 6 (5%) | 5 (2%) | 0.187 | 3 (7%) | 3 (7%) | – |
| On dialysis | 5 (5%) | 0 | 0.004 | 1 (2%) | 0 | > 0.999 |
| Active smoker | 27 (24%) | 67 (29%) | 0.369 | 8 (19%) | 15 (36%) | 0.141 |
| Chronic steroid use | 7 (6%) | 11 (5%) | 0.609 | 2 (5%) | 1 (2%) | >0.999 |
| Outpatient oral hypoglycemic use | 18 (16%) | 34 (15%) | 0.751 | 8 (19%) | 7 (17%) | >0.999 |
| Outpatient insulin use | 38 (33%) | 58 (25%) | 0.098 | 12 (29%) | 14 (33%) | 0.814 |
| Disseminated cancer | 15 (13%) | 1 (0.4%) | <0.001 | 1 (2%) | 1 (2%) | >0.999 |
| Chemotherapy within 30 days | 9 (8%) | 1 (0.4%) | <0.001 | 1 (2%) | 0 | >0.999 |
| Radiotherapy within 90 days | 4 (4%) | 0 | 0.011 | 0 | 0 | – |
| Weight loss >10% over 6 months | 10 (9%) | 12 (5%) | 0.240 | 1 (2%) | 4 (10%) | 0.360 |
| Body mass index | 28 ± 6 | 30 ± 6 | 0.003 | 30 ± 7 | 29 ± 6 | 0.360 |
| Do-not-resuscitate order | 7 (6%) | 0 | <0.001 | 2 (5%) | 0 | 0.494 |
Data are presented as mean ± standard deviation or n (%)
TG13 Tokyo criteria grade for severity of acute cholecystitis, ASA American Society of Anesthesiologists, COPD chronic obstructive pulmonary disease
Not a VASQIP surgical risk calculator input variable
Within six months.
Statistical analysis
The analytic plan followed the STROBE recommendations for observational cohort studies [12]. Multiple logistic regression was performed to identify predictors of 30-day and 1-year mortality following PC with SPSS (version 23, IBM, Armonk, NY). All conditions present on admission listed in Table 1 were considered for inclusion in the prediction models. Univariate regression was performed to identify variables associated with the outcome of interest. Variables with significant collinearity (|r| ≥ 0.20, p < 0.05) to other variables in the model were eliminated, and all remaining variables were entered into the regression equation. Models were compared by area under the receiver operating characteristic curve (AUROC) [13, 14]. AUROC for different models were compared as described by DeLong et al. [15] using SAS (version 9.3, SAS Institute, Cary, NC). Kaplan–Meier curves were generated in GraphPad Prism (version 6.05, GraphPad Software, La Jolla, CA) and compared using the Mantel-Cox log-rank test. Significance was set at α = 0.05.
Results
Patient characteristics
At baseline, PC patients were older, had higher incidence of acalculous cholecystitis, and had greater acute and chronic disease burden (Table 1). Predicted 30-day mortality was significantly higher in the PC cohort (10.9 vs. 2.7%, p < 0.001). After matching, PC and CCY patients had similar predicted 30-day mortality (5.5 vs. 6.8%), TG grade (2.4 vs. 2.4), and age (69 vs. 70 years). Matched CCY patients had significantly higher rates of calculous cholecystitis (95 vs. 76%, p = 0.026) as well as higher incidence of chronic obstructive pulmonary disease, although this difference was not statistically significant (41 vs. 19%, p = 0.055). The matching procedure eliminated significant differences between groups for each of the following variables: age, TG13 severity grade, temperature, creatinine, dialysis status, disseminated cancer, preprocedural chemotherapy, preprocedural radiotherapy, and body mass index, and do-not-resuscitate status.
Short-term outcomes
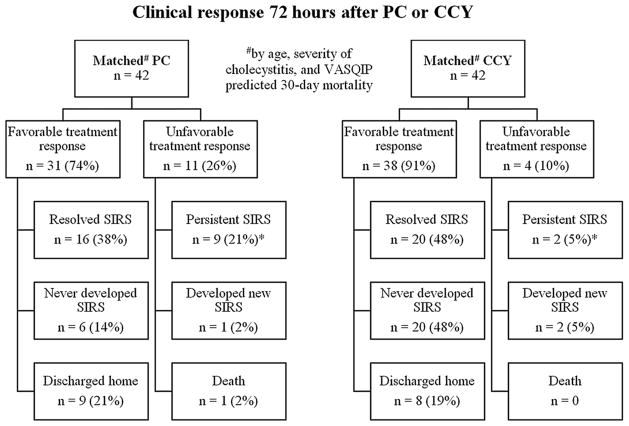
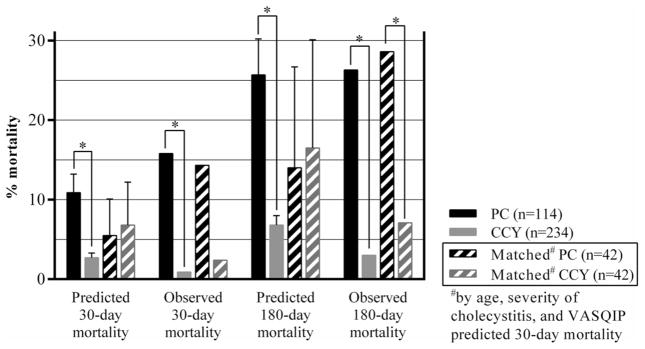
Clinical response 72 h after PC and CCY is illustrated for matched cohorts in Fig. 1. Matched PC patients were more likely to have a persistent SIRS response 72 h following their procedure (21.4 vs. 4.7%, p = 0.048). The presence of SIRS 72 h after PC was associated with 30-day mortality (Table 2). Integrating SIRS criteria with the VASQIP calculator increased the sensitivity and specificity of model predictions, but the observed increase in AUROC did not reach statistical significance (VASQIP calculator AUROC: 0.72 (95% CI 0.58–0.85); integrated model AUROC: 0.83 (95% CI 0.72–0.93); difference between AUROC: 0.11 (95% CI 0.01–0.24, p = 0.062). Although matched PC and CCY patients had similar predicted 30-day mortality (5.5 vs. 6.8%, p = 0.228) and 180-day mortality (14.0 vs. 16.5%, p = 0.382), PC patients had somewhat higher 30-day mortality (14.3 vs. 2.4%, p = 0.109) and significantly higher 180-day mortality (28.6 vs. 7.1%, p = 0.048) (Fig. 2, exact values listed in Table 3). Causes of death for matched PC patients who died within 30 days are listed in Table 4.
Fig. 1.
Outcomes 72 h after percutaneous cholecystostomy (PC) and cholecystectomy (CCY) (SIRS systemic inflammatory response syndrome, *p = 0.048)
Table 2.
Multivariate predictors of 30-day and 1-year mortality after percutaneous cholecystostomy (PC)
| Outcome factor(s) | OR | 95% CI | p |
|---|---|---|---|
| 30-day mortality | |||
| SIRS 72 h after PC | 8.9 | 2.6–30 | <0.001 |
| 1-year mortality | |||
| Do-not-resuscitate status | 19.7 | 2.1–186 | 0.009 |
| Disseminated cancer | 7.5 | 2.1–26 | 0.002 |
| Congestive heart failure | 3.9 | 1.4–11 | 0.010 |
OR odds ratio, CI confidence interval, SIRS systemic inflammatory response syndrome
Fig. 2.
Predicted and observed mortality rates 30 and 180 days following percutaneous cholecystostomy (PC) and cholecystectomy (CCY) (*p < 0.05)
Table 3.
Predicted and observed mortality rates for unmatched and matched groups of patients who underwent percutaneous cholecystostomy (PC) or cholecystectomy (CCY) as initial management of acute cholecystitis
| Outcome | Analysis | PC | CCY | p |
|---|---|---|---|---|
| Predicted 30-day mortality | Unmatched | 10.9 ± 0.6 | 2.7 ± 4.3 | <0.001 |
| Matched | 5.5 ± 4.6 | 6.8 ± 5.4 | 0.228 | |
| Observed 30-day mortality | Unmatched | 18 (15.8) | 2 (0.9) | <0.001 |
| Matched | 6 (14.3) | 1 (2.4) | 0.109 | |
| Predicted 180-day mortality | Unmatched | 25.7 ± 4.5 | 6.8 ± 1.2 | <0.001 |
| Matched | 14.0 ± 12.7 | 16.5 ± 13.6 | 0.382 | |
| Observed 180-day mortality | Unmatched | 30 (26.3) | 7 (3.0) | <0.001 |
| Matched | 12 (28.6) | 3 (7.1) | 0.020 |
Matched by age, severity of cholecystitis, and VASQIP predicted 30-day mortality. Data are presented as mean ± SD or n (%)
Table 4.
Causes of death for matched percutaneous cholecystostomy (PC) patients with mortality within 30 days of PC
| Age | Sex | Cause of death |
|---|---|---|
| 85 | Male | Pneumonia, ARDS |
| 57 | Male | Stroke/hospice |
| 59 | Male | Diffuse lymphoma/hospice |
| 65 | Male | Myocardial infarction |
| 63 | Male | Pulmonary embolism |
| 75 | Male | PEA arrest |
Matched to cholecystectomy patients by age, severity of cholecystitis, and VASQIP predicted 30-day mortality.
ARDS acute respiratory distress syndrome, PEA pulseless electrical activity
Long-term outcomes
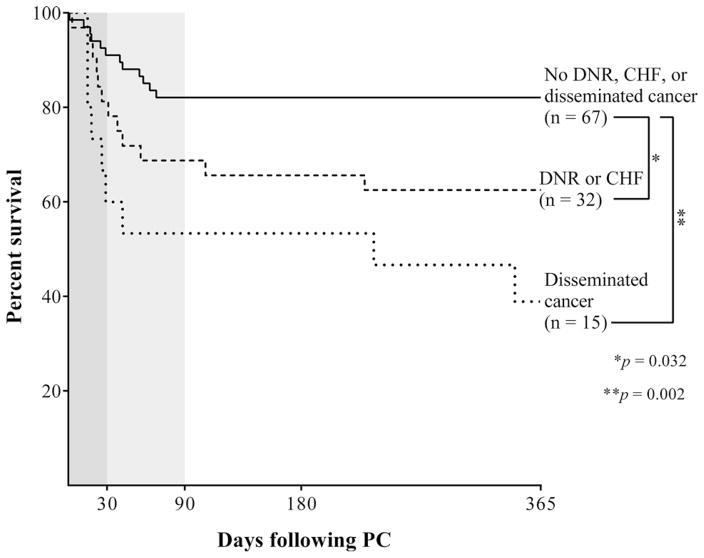
Ninety-day mortality following PC was 24.4%, 180-day mortality was 25.7%, and 1-year mortality was 29.8%. Do-not-resuscitate (DNR) status was the strongest predictor of 1-year mortality, followed by disseminated cancer and congestive heart failure (CHF) (Table 2). Patients with disseminated cancer had 60% 1-year mortality and were analyzed as a separate group. Of the remaining 99 patients, those with DNR status or CHF had significantly higher 1-year mortality following PC (37.5%) than patients without DNR status or CHF (17.9%) (Fig. 3).
Fig. 3.
Kaplan–Meier survival curves following percutaneous cholecystostomy (PC) (DNR do-not-resuscitate status, CHF congestive heart failure)
Interval CCY was performed in 38 patients at an average 104 days following PC. Twenty-three cases were performed laparoscopically, eight were converted from laparoscopic to open, and seven were planned open procedures. None of the gallbladder specimens in the interval CCY group contained cancer; three patients in the primary CCY group (1.3%) had gallbladder cancer found incidentally on pathologic examination. Among interval CCY patients, there was one mortality which occurred on postoperative day 21 in a 72 year old Veteran with coronary artery disease, CHF, peripheral vascular disease, and chronic renal insufficiency. His postoperative course was complicated by non-ST elevation myocardial infarction, unplanned reintubation, pneumonia, and acute on chronic renal insufficiency requiring hemodialysis. The cohort of 76 patients who underwent PC without interval CCY had 23.7% 30-day mortality and 42.1% 1-year mortality. 6 of these patients had a DNR order, 14 had disseminated cancer, and 21 had CHF. Forty-two patients in this cohort had their PC tube removed without replacement.
Discussion
Patients who underwent PC carried a substantial burden of acute and chronic disease, hindering direct comparison to CCY. The matching procedure generated PC and CCY groups with similar characteristics, but statistically and clinically significant differences in the incidence of acalculous cholecystitis remained. In addition, the PC group may have had risk factors that were not included in the VASQIP surgical risk calculator, rendering calculator predictions inaccurate. However, several studies have demonstrated that the VASQIP calculator performs well in predicting mortality [16–18]. Ashfar et al. [19] validated the VASQIP calculator in predicting 30-day postoperative mortality for a cohort of 1618 Veterans age >80 who underwent major general, cardiac, orthopedic, urologic, or vascular surgery, reporting AUROC 0.82. Although the VASQIP surgical risk calculator has not been validated specifically for CCY patients, the weight of evidence supports its accuracy.
Therefore, the possibility that CCY is more effective than PC for high-risk surgical candidates with acute cholecystitis deserves further attention. A Cochrane Database systematic review by Gurusamy et al. [1] was intended to establish the role for PC in high-risk surgical patients but included only two randomized clinical trials: one trial comparing early CCY after PC with late CCY after PC [20] and one trial comparing PC with conservative management [21]. Analysis of these studies was inadequate to provide recommendations for the use of PC in high-risk patients. Several authors have established the safety and efficacy of early CCY for patients with acute cholecystitis, including high-risk populations [6, 22, 23]. In addition, a recent propensity-matched analysis by Dimou et al. [24] found that cholecystostomy tube placement was associated with increased mortality among elderly patients with Grade III cholecystitis. However, level I evidence comparing PC to CCY is lacking. Efforts to address these issues are ongoing [25].
On systematic review of 53 studies incorporating 1918 PC patients, Winbladh et al. [2] reported that 85.2% had clinical improvement within 72 h, consistent with our results. The strength of the SIRS response at 72 h in predicting 30-day mortality may be attributable to a variety of factors. Patients who improved after PC may represent a cohort whose acute illness was primarily or entirely attributable to acute cholecystitis rather than a comorbid inflammatory process. Alternatively, patients who did not improve following PC may have been disproportionately affected by ongoing inflammation of a necrotic gallbladder wall, rendering biliary decompression and drainage insufficient to address the source of ongoing inflammation. Finally, drain clogging or dislodgement within the peritoneal cavity may have played a role, although it is impossible to accurately assess these events retrospectively. Whatever the cause, the presence of SIRS 72 h after PC was strongly associated with 30-day mortality. Persistent SIRS appears to be a useful metric to gauge the therapeutic efficacy and prognosis following PC. These findings gain relevance in the context of recent work by Smith et al. [26] which reinforced the validity of measuring 30-day mortality by demonstrating significant correlation to long-term outcomes. While 30-day mortality remains an important benchmark, our survival curves suggest that measuring 90-day mortality captures a substantial number of deaths which appear to be temporally related to the inciting event.
Notably, there were 30 patients with Grade I TG13 cholecystitis who underwent PC during the study period. These patients had acute cholecystitis in conjunction with another disease process posing increased risk for perioperative and postoperative morbidity and mortality, but did not qualify as Grade II or III cholecystitis. For example, a patient with a 48-h history of acute cholecystitis, white blood cell count 17 × 109/L, a nonpalpable gallbladder, and no imaging evidence of gallbladder gangrene or empyema has Grade I cholecystitis, even if they also have a pulmonary embolism with preserved PaO2/FiO2 ratio, a recent myocardial infarction in the absence of hypotension and vasopressor requirements, or a significant chronic disease burden that does not meet criteria for organ failure. However, because Grade I cholecystitis is not an indication for PC per TG13 guidelines, such patients from excluded from the matched analysis.
The major limitations of this study are its retrospective design, propensity to introduce selection bias, a paucity of female subjects, and a tendency toward late presentation at Veterans Health Administration facilities [27]. The high proportion of interval cholecystectomies performed open (18%) or converted to open (21%) underscores the unique nature of our patient population and practice patterns. Therefore, these findings may not be generalizable for many practices and clinical scenarios. The effects of selection bias were minimized by matching PC and CCY patients based on VASQIP predicted 30-day mortality, severity of cholecystitis, and age. Although the VAS-QIP surgical risk calculator is well validated, it cannot account for clinical gestalt and surgeon judgement, does not include several disease-specific variables, and was not designed to predict mortality for nonsurgical procedures like PC. In addition, the VASQIP calculator was designed for 30- and 180-day outcome predictions, and may not be suitable for 1-year outcomes. Finally, risk calculators and disease-severity scoring systems should not replace clinical judgement, and experienced clinicians often recognize situations in which PC is prudent for a high-risk patient with acute cholecystitis, and situations in which algorithms may underestimate the likelihoods of perioperative morbidity and mortality. Therefore, randomized trials are needed to provide level I evidence supporting complex management decisions for high-risk surgical candidates with acute cholecystitis [28].
Conclusions
Among PC patients, the presence of SIRS 72 h following PC was associated with the increased 30-day mortality, and may be a useful indicator of therapeutic efficacy and prognosis. When controlling for VASQIP predicted 30-day mortality, TG13 severity of cholecystitis, and age, PC was associated with persistent systemic inflammation and increased long-term mortality compared with CCY. These findings must be interpreted in the context that matching procedures do not eliminate selection bias, the VASQIP calculator was not designed for percutaneous procedures like PC, and the practice setting limits generalizability. Therefore, randomized trials comparing PC with CCY for high-risk patients with acute cholecystitis are needed.
Acknowledgments
The authors thank Dr. Loretta Coady-Fariborzian for her assistance in obtaining IRB approval.
Funding Tyler J. Loftus was supported by post-graduate training grant (Grant No. T32 GM-08721) from the National Institute of General Medical Sciences (NIMGS). Alicia M. Mohr was supported by (Grant No. P50 GM111152–010) and (Grant No. R01 GM105893-01) from the NIGMS.
Footnotes
Author contributions TJL, EMC, and WJZ contributed to the study design. TJL, EMC, CGD, and ANH contributed to data collection and analysis. AMM, RMT, and CEH contributed to data analysis and critical revisions. GAS and WJZ contributed to the study design, data analysis, and critical revisions.
Compliance with Ethical Standards
Disclosures Tyler J. Loftus, Elisha M. Collins, Camille G. Dessaigne, Amber N. Himmler, Alicia M. Mohr, Ryan M. Thomas, Charles E. Hobson, George A. Sarosi Jr. and William J. Zingarelli have no conflicts of interest to disclose.
Portions of this work were presented at the 2016 Association of VA Surgeons Annual Meeting.
References
- 1.Gurusamy KS, Rossi M, Davidson BR. Percutaneous cholecystostomy for high-risk surgical patients with acute calculous cholecystitis. Cochrane Database Syst Rev. 2013;8:CD007088. doi: 10.1002/14651858.CD007088.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Winbladh A, Gullstrand P, Svanvik J, Sandstrom P. Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB. 2009;11:183–193. doi: 10.1111/j.1477-2574.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duszak R, Jr, Behrman SW. National trends in percutaneous cholecystostomy between 1994 and 2009: perspectives from medicare provider claims. J Am Coll Radiol. 2012;9:474–479. doi: 10.1016/j.jacr.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Patel PP, Daly SC, Velasco JM. Training vs practice: a tale of opposition in acute cholecystitis. World J Hepatol. 2015;7:2470–2473. doi: 10.4254/wjh.v7.i23.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cull JD, Velasco JM, Czubak A, Rice D, Brown EC. Management of acute cholecystitis: prevalence of percutaneous cholecystostomy and delayed cholecystectomy in the elderly. J Gastrointest Surg. 2014;18:328–333. doi: 10.1007/s11605-013-2341-z. [DOI] [PubMed] [Google Scholar]
- 6.Kamalapurkar D, Pang TC, Siriwardhane M, Hollands M, Johnston E, Pleass H, Richardson A, Lam VW. Index cholecystectomy in grade II and III acute calculous cholecystitis is feasible and safe. ANZ J Surg. 2015;85:854–859. doi: 10.1111/ans.12986. [DOI] [PubMed] [Google Scholar]
- 7.Strasberg SM, Pucci MJ, Brunt LM, Deziel DJ. Subtotal cholecystectomy-“fenestrating” vs “reconstituting” subtypes and the prevention of bile duct injury: definition of the optimal procedure in difficult operative conditions. J Am Coll Surg. 2016;222:89–96. doi: 10.1016/j.jamcollsurg.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Davies MG, Hagen PO. Systemic inflammatory response syndrome. Br J Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 9.Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Buchler MW, Kiriyama S, Kimura Y, Tsuyuguchi T, Itoi T, Yoshida M, Miura F, Yamashita Y, Okamoto K, Gabata T, Hata J, Higuchi R, Windsor JA, Bornman PC, Fan ST, Singh H, de Santibanes E, Kusachi S, Murata A, Chen XP, Jagannath P, Lee S, Padbury R, Chen MF Tokyo Guidelines Revision C. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:578–585. doi: 10.1007/s00534-012-0548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokyo Guidelines for the management of acute cholangitis and cholecystitis. J Hepatobiliary Pancreat Surg; Proceedings of a consensus meeting; April 2006; Tokyo, Japan. 2007. pp. 1–121. [PubMed] [Google Scholar]
- 11.Mayumi T, Takada T, Kawarada Y, Nimura Y, Yoshida M, Sekimoto M, Miura F, Wada K, Hirota M, Yamashita Y, Nagino M, Tsuyuguchi T, Tanaka A, Gomi H, Pitt HA. Results of the Tokyo Consensus Meeting Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:114–121. doi: 10.1007/s00534-006-1163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159(6):1638–1645. doi: 10.1016/j.surg.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Lusted LB. Signal detectability and medical decision-making. Science. 1971;171:1217–1219. doi: 10.1126/science.171.3977.1217. [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 16.McCloskey CA, Wilson MA, Hughes SJ, Eid GM. Laparoscopic colorectal surgery is safe in the high-risk patient: a NSQIP risk-adjusted analysis. Surgery. 2007;142:594–597. doi: 10.1016/j.surg.2007.07.020. discussion 597 e591–592. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer JG, Reynoso JF, Seevers GA, Schmid KK, Muralidhar P, Konigsberg B, Lynch TG, Johanning JM. Assessing pre-operative frailty utilizing validated geriatric mortality calculators and their association with postoperative hip fracture mortality risk. Geriatr Orthop Surg Rehabil. 2014;5:109–115. doi: 10.1177/2151458514537272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MS, Bailey TL, Schmid KK, Lydiatt WM, Johanning JM. A frailty index identifies patients at high risk of mortality after tracheostomy. Otolaryngol Head Neck Surg. 2014;150:568–573. doi: 10.1177/0194599813519749. [DOI] [PubMed] [Google Scholar]
- 19.Afshar AH, Virk N, Porhomayon J, Pourafkari L, Dosluoglu HH, Nader ND. The validity of the VA surgical risk tool in predicting postoperative mortality among octogenarians. Am J Surg. 2015;209:274–279. doi: 10.1016/j.amjsurg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Akyurek N, Salman B, Yuksel O, Tezcaner T, Irkorucu O, Yucel C, Oktar S, Tatlicioglu E. Management of acute calculous cholecystitis in high-risk patients: percutaneous cholecystotomy followed by early laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:315–320. doi: 10.1097/01.sle.0000191619.02145.c0. [DOI] [PubMed] [Google Scholar]
- 21.Hatzidakis AA, Prassopoulos P, Petinarakis I, Sanidas E, Chrysos E, Chalkiadakis G, Tsiftsis D, Gourtsoyiannis NC. Acute cholecystitis in high-risk patients: percutaneous cholecystostomy vs conservative treatment. Eur Radiol. 2002;12:1778–1784. doi: 10.1007/s00330-001-1247-4. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy K, Samraj K, Gluud C, Wilson E, Davidson BR. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–150. doi: 10.1002/bjs.6870. [DOI] [PubMed] [Google Scholar]
- 23.Zafar SN, Obirieze A, Adesibikan B, Cornwell EE, 3rd, Fullum TM, Tran DD. Optimal time for early laparoscopic cholecystectomy for acute cholecystitis. JAMA Surg. 2015;150:129–136. doi: 10.1001/jamasurg.2014.2339. [DOI] [PubMed] [Google Scholar]
- 24.Dimou FM, Adhikari D, Mehta HB, Riall TS. Outcomes in older patients with grade III cholecystitis and cholecystostomy tube placement: a propensity score analysis. J Am Coll Surg. 2017 doi: 10.1016/j.jamcollsurg.2016.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambe PC, Kaptanis S, Papadakis M, Weber SA, Zirngibl H. Cholecystectomy vs. percutaneous cholecystostomy for the management of critically ill patients with acute cholecystitis: a protocol for a systematic review. Syst Rev. 2015;4:77. doi: 10.1186/s13643-015-0065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T, Li X, Nylander W, Gunnar W. Thirty-day postoperative mortality risk estimates and 1-year survival in veterans health administration surgery patients. JAMA Surg. 2016;151(5):417–422. doi: 10.1001/jamasurg.2015.4882. [DOI] [PubMed] [Google Scholar]
- 27.McDermott K, Maynard C, Trivedi R, Lowy E, Fihn S. Factors associated with presenting > 12 hours after symptom onset of acute myocardial infarction among Veteran men. BMC Cardiovasc Disord. 2012;12:82. doi: 10.1186/1471-2261-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kortram K, van Ramshorst B, Bollen TL, Besselink MG, Gouma DJ, Karsten T, Kruyt PM, Nieuwenhuijzen GA, Kelder JC, Tromp E, Boerma D. Acute cholecystitis in high risk surgical patients: percutaneous cholecystostomy versus laparoscopic cholecystectomy (CHOCOLATE trial): study protocol for a randomized controlled trial. Trials. 2012;13:7. doi: 10.1186/1745-6215-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]