Abstract
Background
Atrial Fibrillation (AF) symptoms are a major component of treatment decisions for AF patients and impact quality of life and functional ability, yet are poorly understood.
Objective
This review aimed to determine what is known about the prevalence of symptoms, and the association of symptoms to AF characteristics, psychological distress, sex, and race.
Methods
We performed a structured review of AF symptoms as of March 2016 using PubMed, EMBASE, and CINAHL, and reference searches of retrieved articles. Full-text, published, peer-reviewed, English language articles were examined. Articles were included if they reported original research data on symptom prevalence and type among patients with AF.
Results
The three most common symptoms were dyspnea, palpitations, and fatigue. The results suggested that while AF characteristics are not a significant predictor of symptoms, tachycardia, female sex, race and psychological distress have a positive association to symptoms.
Conclusions
There is a scarcity of research examining symptoms in AF. Furthermore, the inconsistency in measurement methods and the failure to include diverse populations in AF research makes it difficult to draw definitive conclusions from current literature. Given the prevalence of AF in the United States and the impact of symptoms on quality of life and healthcare utilization, further research examining predictors of symptoms and interventions to alleviate symptoms is crucial.
Keywords: Atrial Fibrillation, Symptoms, Psychology, Sex, Race
INTRODUCTION
Atrial Fibrillation (AF) is the most common adult cardiac rhythm disorder. It is estimated that there are 5.2 million cases of AF in the United States (US), and this number is projected to increase to 12.1 million cases by 2030.(1) Conservative estimates suggest that healthcare expenditures due to AF-specific care in the US cost $6 billion a year.(2) The main driver for seeking medical attention by persons with AF is the symptoms they experience.(3, 4) AF symptoms are associated with increased healthcare utilization, decreased quality of life (QOL), and poor health outcomes.(5–11) Clinical decisions for procedural symptom management strategies, including cardioversions, ablations, and rhythm and rate control medications are largely guided by patients’ symptom reports.(12, 13) The symptoms are highly variable. Between 25 to 30% of patients with AF are asymptomatic, while other patients report severe symptoms that affect their QOL.(4, 14) Among patients with symptomatic AF, a significant percentage has episodes that are asymptomatic and the specific symptoms reported vary by type of AF.(15)
The causal mechanism of AF symptoms and why symptoms are present in some patients and absent in others is unknown.(4, 16) There is limited understanding of the variability of symptoms with respect to psychosocial, race, and sex related differences. Females and non-whites are significantly underrepresented in cardiovascular clinical trials.(17–20) Since there are tremendous costs and potential complications related to AF treatment strategies, a thorough comprehension of symptoms caused by AF is essential. The purpose of this review is to improve understanding of what is known about the prevalence and type of symptoms in AF patients, and the association of AF symptoms to AF characteristics, psychological distress, sex, and race.
METHODS
A structured literature search of the PubMed, CINAHL, and EMBASE databases was conducted with the assistance of a university librarian in March 2016 (Figure 1). Search terms included the MeSH term “atrial fibrillation.” For symptom burden, the terms “symptom burden,” “functional impairment,” “functional capacity,” “symptom,” “asymptomatic” and “burden of symptoms” were used. Functional impairment and functional capacity were included because they are often measured in studies of symptoms’ effect on an individual. Reference lists were reviewed to identify relevant articles. No time limitations were imposed to ensure the inclusion of key, seminal articles examining AF symptoms. This search of online databases yielded a total of 4085 articles. 26 articles were removed after screening for duplicates. Abstracts and titles were reviewed for inclusion criteria. Articles that were potentially relevant were reviewed in full text. Where it was unclear if a study should be included, the co-author team was consulted. 11 articles were identified by hand review. After screening, 38 articles remained and were reviewed using the following inclusion and exclusion criteria. The inclusion criteria were: Full text, published, peer-reviewed, English language, 100% of participants diagnosed with AF, and data on symptom prevalence and type were collected and analyzed.
Figure 1.
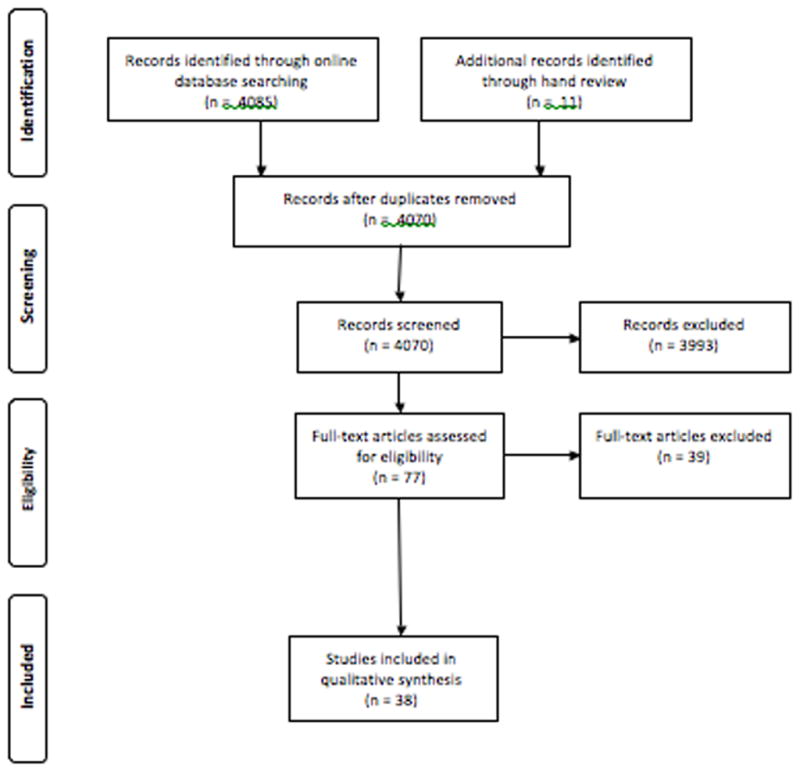
Flow Diagram
Data were extracted from each article included: prevalence and type of symptoms, the association between symptoms, sex, psychological distress, race, and AF characteristics, and instruments used. The extracted data are depicted in tables 1, 2, 3, and 4. Quality assessments of each quantitative study were conducted using the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) checklist.(21) The STROBE checklist does not include a scoring system.
Table 1.
Prevalence of Symptoms
| Study | Sample | Prevalence of Symptoms | Symptom Type | Instrument | Important to Note |
|---|---|---|---|---|---|
| Diker, 2015, Turkey(22) | N=510, 56% permanent, 21% persistent, 13% paroxysmal | 56% had symptoms in past 7 days, 78.8% had symptoms in past year | 34.5% dyspnea, 34.4% palpitations, and 32.5% fatigue | EHRA | EKG findings indicated that 88.4% of patients were in AF at time data was collected |
| Dorian, 2000, US, Canada, Germany and England(29) | N=152, 60% paroxysmal, 40% persistent | 90% reported symptoms during AF | 68% palpitations, 62% fatigue, 60% shortness of breath | AFSS | Permanent AF patients were excluded |
| Golwala, 2016, US(7) | N=9542, 51% paroxysmal, 45% persistent/permanent, 4% Undetermined | 62% reported symptoms | 32% palpitations, 28% dyspnea, 26% fatigue | EHRA, AFEQT | The EHRA classification, done by physicians, and AFEQT, done by patients, were highly correlated, though findings suggest physicians may underestimate mild AF symptoms |
| Ha, 2014, 21 countries(8) | N=2439, 52% paroxysmal, 43% persistent, 5% Undetermined | Baseline score of 8.7 SD 7 | Not described | AFSS | Cohort consists of recent-onset AF patients |
| Patel, 2014, US(31) | N=286, 40% persistent, 60% paroxysmal | 25% reported severe AF symptoms | Not described | AFSS | Stable outpatient sample |
| Kupper, 2013, Netherlands(32) | N=52, persistent | 9 symptoms on average with an average frequency of 2.5 during AF | 92% tiredness 76% dyspnea, 70% heart racing | ATSSS | Persistent AF sample that was a substudy, pre-ablation data examined |
| Murin, 2014, 26 countries(33) | N=4869,
permanent N=6622, Nonpermanent |
Permanent: 25% reported severe
symptoms Nonpermanent: 19.7% EHRA Class III reported severe symptoms |
Not reported | EHRA | 50% of permanent AF patients did not have controlled AF in this sample |
| Nieuwlatt, 2005, 35 countries(23) | N=5333, 28% paroxysmal, 21% persistent, 29% permanent | 69% currently symptomatic | 75% palpitations and/or syncope | Not described | Majority of the patients were recruited from university and specialized centers |
| Patten, 2006, Germany(34) | N=1033, Symptomatic paroxysmal | Palpitations, tachycardia, and dyspnea most prevalent in EKG-confirmed AF episodes, palpitations, angina, and vertigo most common in perceived AF episodes in SR | Self-monitoring event recorder system | Study reviewed of patient-reported symptomatic AF events against the EKG-report | |
| Rienestra, 2005(26) | N=522, Recurrent persistent | 70% reported symptoms | 39% fatigue, 35% dyspnea, 27% palpitations | Not described | No data of how symptom information was collected, and how patients were questioned about symptoms |
| Sears, 2005, US(27) | N=96 | Average of 8.89 AF symptoms across participants | Tiredness and difficulty sleeping two most frequently experienced | ATSSS | Sample consisted of symptomatic, drug refractory AF patients |
| Singh, 2006, US(25) | N=496, Persistent | 63% perceived symptomatic AF | AF symptom burden score 13.4, SCL frequency 18.15, SCL severity of 14.6 | SCL, AFSS | Symptom prevalence reported as baseline data from a longitudinal study on AF control |
| Siontis, 2016, US(35) | N=476 | 66% reported symptoms | 40% palpitations, 26% symptoms other than palpitations | No standardized measurement of symptoms, data was based on information from chart review | |
| Steg, 2012, 26 countries located in Europe, the Middle East, Africa, Asia & America (10) | N=9665, | 61% reported symptoms in past 7 days, 22% reported severe symptoms | 39.4% dyspnea, 35.5% fatigue, 33.8% palpitations | EHRA | Study has unprecedented geographical relevance, includes developing low- and middle-income countries |
| Vermond, 2014, Netherlands(11) | N=558, Permanent | 35% reported a high symptom severity, 34% moderate symptom severity | 76% dyspnea, 75% fatigue, 53% palpitations | AFSS | Self-reported AFSS scores were more sensitive than the history taking by treating physician, 70% of patients who reported mild symptoms were classified as asymptomatic by physician |
Abbreviations: AF, atrial fibrillation; EKG, electrocardiogram; EHRA, European Heart Rhythm Association score of AF-related symptoms; AFSS, Atrial Fibrillation Symptom Severity Scale; SCL, Symptom Checklist; SR, sinus rhythm; ATSSS, Atrial Tachyarrhythmia Symptom Severity Scale, AFEQT Atrial Fibrillation Effect on QualiTy of Life
Table 2.
Description of Commonly Used AF Symptom Assessment Instruments
| Measure, Year Created | Description | Symptoms Included | Time Period |
|---|---|---|---|
| European Heart Rhythm Association symptom classification (EHRA), EHRA, 2007(36) | Provider rating of patients’ symptom severity and symptoms’ impact on daily activities | Does not assess specific symptoms, provider rates severity and impact on daily activities of symptoms attributable to AF | At time of visit, past 7 days, and past 12 months excluding previous 7 days |
| Symptom Checklist-Frequency and Severity Scale (SCL), Bubien et al., 1996(37) | Score based on severity and frequency of symptoms | Tiredness/lack of energy, heart fluttering/skipping, heart racing, lightheadedness/dizziness, hard-to-catch breath, shortness of breath, chest pain, pressure, or fullness when the heart is racing or fluttering, and chest pain, pressure, or fullness, when heart is not racing or fluttering, headache, trouble concentrating, feeling warm/flushed, sweating, weakness, poor appetite, nausea, difficulty sleeping | Present |
| AF Effect on Quality of Life (AFEQT), Spertus et al., 2011(38) | A measure of quality of life based on self-report of extent symptoms have bothered patient and interfered with activities of daily living | Palpitations (described as heart fluttering, skipping, or racing), irregular heart best, pause in heart activity, lightheadedness or dizziness | Over the past 4 weeks |
| University of Toronto AF Severity Scale (AFSS), Dorian et al., 2000(29) | Subjective and objective ratings of AF disease burden, including frequency, duration, and patient-perceived severity of episodes, and healthcare use | Palpitations, shortness of breath at rest, shortness of breath with activity, exercise intolerance, fatigue at rest, lightheadedness/dizziness, and chest pain/pressure | Present |
| Canadian Cardiovascular Society Severity of AF Scale (CCS-SAF), Dorian et al., 2006(39) | Scored by providers in collaboration with patients, score determined by identification of major AF-related symptoms, determination of symptom-rhythm correlation, and assessment of symptom impact of daily activities and quality of life | Palpitations, dyspnea, dizziness/syncope, chest pain, weakness/fatigue | Present |
| Quality of Life in AF patients (QLAF), Braganca et al., 2010(40) | Score determined by symptoms and treatments | Palpitations, breathlessness, chest pain, dizziness | Present, asks patient to specify how frequent palpitations are |
Table 3.
AF Characteristics’ Association to Symptoms
| Study | Samples | Design | Symptom Measurement | Assessment of cardiac rhythm | Findings | Limitations |
|---|---|---|---|---|---|---|
| Bhandari (1992), US(41) | N=113, 100% Paroxysmal, Age 50 (±15), 69% Female | Substudy of a randomized-controlled trial | Patient report | Transtelephonic EKG monitoring | Symptomatic calls significantly associated with documented AF, 91% sensitivity | Symptomatic AF was an inclusion criteria |
| Manganiello (2014), Italy(42) | N=113, 9% paroxysmal, 91% persistent, Age 64 (±20), 78% Male | Prospective cohort | Symptom diary | Insertable cardiac monitor | 47% of patients had asymptomatic AF episodes, Significant association of symptoms to AF rhythm (P<0.01) | Symptomatic AF was inclusion criteria for the sample, all participants had undergone ablation, univariate analysis |
| Mehall, (2007), US(43) | N=50, Age 69, 74% Male | Prospective cohort | Manual activator when symptomatic episode perceived | Continuous home EKG monitoring | 15% sensitivity, No significant association between symptoms and AF episode, 52% PPV | Long periods of AF were sometimes interrupted by EKG artifact, caused the monitor to record a new episode of AF |
| Quirino (2009), Italy(44) | N=102, 100% Paroxysmal AF, Age 73 (±7), 58% Male | Prospective Cohort | Symptom diary | Pacemaker | 81% of device-stored episodes were asymptomatic, sensitivity was 19% and 21% PPV, duration of episodes was significantly related to symptomatic episode | Participants each had a pacemaker, symptoms were measured through self-report rather than a standardized tool |
| Sears (2005), US(27) | N=96, Age 62 (±12) 72% Male | Prospective cohort | ATSSS | Implantable cardioverter defibrillator | Insignificant association between symptoms and AF rhythm | All patients had an implantable cardioverter defibrillator |
| Silbauer (2009), UK(45) | N=79, 100% paroxysmal, Age 70 (±9), 57% Male | Prospective cohort substudy | Symptom diary | Pacemaker | Sensitivity of 13% and PPV of 63%, no significant difference in AF episode frequency between symptomatic and symptomatic follow-up periods, Heart rate and length of episodes significantly related to symptomatic episodes (p<0.001) | Symptomatic AF sample, relied on patients to complete symptom diaries, the participants each had a pacemaker |
| Strickberger (2005), US(46) | N=48, Age 76 (±10), 58% Male | Prospective randomized single-blind parallel study | Manual activator when symptomatic episode perceived, & symptom diary | Pacemaker | 6% of AF episodes were symptomatic, 17% PPV, did not find a significant difference in ventricular rate and symptoms | Relied on patients to use the activator to identify symptomatic episodes over a 12-month period, sample consisted of patients with symptomatic bradycardia |
| Tondo (2014), Italy(47) | N=143, Age 59 (±9), 85% Male, 55% paroxysmal, 45% persistent | Prospective cohort | Manual activator when symptomatic episode perceived, & symptom diary | Implanted cardiac monitor | 53% sensitivity, 89% PPV | Study was performed following catheter ablation |
| Patel (2014), US(31) | N=286, Age 62 (±13), 65% Male, 60% paroxysmal, 40% persistent | Prospective single-center cohort study | AFSS | Continuous looping monitor | No AF monitor characteristic was predictive of severe AF symptom burden, including HR and AF burden | Since symptoms were measured using the AFSS prior to the 7-day cardiac monitoring period, symptoms experienced when during the monitoring period were not captured |
| Patten (2006), Germany(34) | N=1033, Symptomatic paroxysmal | Longitudinal cohort | Self-monitoring event recorder system | Tele-EKG recorder | 46% documented AF associated with specific symptoms, Symptoms were significantly correlated with heart rate (p<0.001), 37% perceived symptomatic AF episodes were genuine EKG-episodes, 69% of patients reported symptoms when in SR | Inclusion criteria was a history of symptomatic paroxysmal AF, the Tele-EKG monitoring did not collect data about the onset and duration of episodes because it a maximal EKG recording time of 1 minute |
| Verma (2013), Canada(48) | N=50, 80% paroxysmal, 10% persistent | Prospective cohort | Standardized symptoms diary | Continuous implanted cardiac monitoring | Symptoms had a 87% PPV, 69% of all AF episodes were asymptomatic | Inclusion criteria was first-time ablation procedure |
Abbreviations: EKG, electrocardiogram; PPV, positive predictive value; SR, sinus rhythm; ATSSS, Atrial Tachyarrhythmia Symptom Severity Scale; AFSS, University of Toronto Atrial Fibrillation Severity Scale
Table 4.
The Association of Gender and Symptoms
| Study | Study Design | Significant Association with Sex and AF Symptoms (p<0.05) | Measurement Tool | Other Variables in Analysis | Women Were More Symptomatic |
|---|---|---|---|---|---|
| Boriani (2015)(57) | Prospective cohort, n=3119, 60% Male | Yes | EHRA | Age, previous myocardial infarction | Yes |
| Clair (1993)(59) | Prospective cohort, N=150, 53% Male | No | Patient report | Age | |
| Golwala (2016)(7) | Prospective cohort, N=9542 | Yes | EHRA, AFEQT | Not specified | Yes |
| Henry (2013)(58) | Prospective cohort, N=540, 66% Male | Yes | SCL | Age, left atrial size, duration of AF (months), coronary artery bypass graft surgery, valve surgery, CHF, EuroSCORE | Yes |
| Manganiello (2014)(42) | Prospective cohort, N=113, 78% Male | No | Symptom diary | Univariate comparisons | |
| Kang (2006)(60) | Prospective cohort, n=81, 51% Male | No | SCL | Not specified | |
| Kupper (2013)(32) | Prospective cohort, N=52, 61% Male | No | ATSSS | Age, emotional distress, cardiac disease, BMI, physical activity | |
| Patel (2014)(31) | Prospective cohort, N=286, 65% Male | Yes | AFSS | Age, comorbidities, heart rate, AF characteristics, PVC and PAC burden | Yes |
| Rienstra (2005)(26) | Randomized control trial, N=522, 63% Male | Yes | Report of: Palpitations, dyspnea, or fatigue | Age, underlying heart disease, DM, left ventricular function | Yes |
| Sears (2005)(27) | Prospective cohort, N=96, 72% Male | No | ATSSS | Age, AF characteristics, negative emotion | |
| Vermond (2014)(11) | Post hoc analysis, N=558, 67% Male | Yes | AFSS | Not specified | Yes |
| Siontis, 2016(35) | Retrospective evaluation, N=476, 53% Male | Yes | Not specified | Age, CHADS score, comorbidities | Yes |
Abbreviations: EHRA, European Heart Rhythm Association score of AF-related symptoms; AFEQT, Atrial Fibrillation Effect on Quality of Life; SCL, Symptom Checklist; ATSSS, The Atrial Tachyarrhythmia Symptom Severity Scale; BMI, Body Mass Index; PVC, Premature ventricular contractions; PAC, Premature atrial contractions; DM, Diabetes Mellitus
Our review is not intended to be all-inclusive or systematic. It represents the main studies examining psychological distress, sex, race, AF characteristics, and AF symptoms. This review is organized by discussing the prevalence and type of symptoms in AF, and associations among AF characteristics, psychological distress, sex, race, and AF symptoms.
RESULTS
Prevalence and Type of Symptoms
Fourteen studies that reported data on the prevalence and type of symptoms experienced by individuals with AF were examined (Table 1). AF symptom measurement instruments typically include palpitations, dyspnea, fatigue, chest pain, and dizziness. However, there is variance in the type and number of symptoms, time frame, and wording included in commonly used instruments (Table 2). In three prospective, cross-sectional studies that used the European Heart Rhythm Association (EHRA) symptom classification for AF, between 56 to 62% of patients had one or more AF symptoms in the past seven days.(7, 10, 22) While one study used a sample (n=510) consisting only of symptomatic AF patients,(22) the other two had larger samples (n=9665 and 9542) that included asymptomatic and symptomatic patients and had comparable findings.(7, 10) Three studies similarly reported that 63% to 70% of study participants reported AF symptoms in the baseline data collection prior to tests of rhythm control interventions.(23–25) Two of these studies did not describe the use of any instrument to measure AF symptoms, which may explain their findings of a slightly higher prevalence of current AF symptoms.(4, 23, 24)
Dyspnea, fatigue, and palpitations were the three most commonly reported symptoms.(6, 7, 10, 11, 22, 26–29) Results from one qualitative study supported the reports of symptoms experienced in the quantitative studies.(30) Participants (n=15) reported distress from abrupt occurrences of palpitations often accompanied by fatigue and shortness of breath, which they perceived as an AF episode. Most of the participants reported fatigue during AF episodes that lasted for a few days following the episode.
Association of AF Characteristics and Symptoms
Eleven studies examining the association of AF characteristics to symptoms were reviewed (Table 3). AF characteristics, including AF burden, heart rate when in AF, sinus rhythm rate, and frequency and severity of pauses, were measured through a pacemaker, continuous looping monitor, or an electrocardiogram (EKG) recorder. Seven studies found essentially no association between the number and frequency of symptoms of AF reported by patients and AF characteristics measured by a continuous monitor.(27, 28, 31, 43–46) In contrast, two studies found a significantly greater AF burden in patients who reported symptoms in comparison to asymptomatic patients.(41, 42) In the study by Manganiello and colleagues, continuous subcutaneous EKG monitoring was utilized to measure AF burden, and the sample (n=113) consisted of participants at a single center who had undergone ablation for AF, which may explain the differing results.(42)
Eight studies examining the association between heart rate and AF symptoms were reviewed.(11, 31, 34, 41, 42, 45, 48, 49) The findings of seven studies support that there is a significant association of tachycardia to prevalence of symptomatic AF episodes (P<0.001).(11, 34, 41, 42, 45, 48, 49) A heart rate greater than 100 beats per minute (bpm) was associated with increased symptoms.(11, 42) One study did not find a significant association between heart rate and AF symptoms by measuring symptoms prior to applying a continuous monitor for seven days to measure AF characteristics.(31) This data collection timing contrasts with the other studies described, where data were collected on the patients’ reported symptoms in parallel with the monitoring period. Seven studies analyzed the sensitivity and positive predictive value of self-reported symptoms to detect the pacemaker’s report of an AF episode. The reported findings of four studies suggested a similar sensitivity of 19% or lower,(43–46) with the exception of two studies that reported a sensitivity of 53%(47) and 91%.(41) However, the studies reported divergent positive predictive values of symptoms, ranging from values of a positive predictive 21% or lower,(44, 46) to 63% and higher.(41, 43, 45, 47, 48) A weight reduction and cardiometabolic risk factor management intervention study found that AF symptoms, the number of episodes, and the cumulative duration of episodes were significantly reduced following the intervention, though whether the reduction in symptoms was correlated to the reduction in number of episodes and duration was not examined.(50)
Association of Psychological Distress and AF Symptoms
Seven studies examining the association of psychological distress to AF symptom burden were reviewed.(14, 27, 32, 51–54) The findings from five studies supported a significant (p<0.05) association between depression and anxiety and AF symptoms.(27, 32, 51, 53, 54) The studies measured AF characteristics through EKGs, continuous looping monitors and implanted cardioverter defibrillators (ICD), and included these measurements in the analyses. The majority of the studies used different measurements of psychological distress: the State Trait Anxiety Inventory (STAI), Profile of Mood States (POMS), participant report of psychological stress, Beck’s Depression Inventory (BDI), Type D personality (DS14), and the Perceived Stress Scale (PSS-10), the hospital anxiety and depression scale (HADS-A), and the Patient Health Questionnaire (PHQ-9). Three of the studies adjusted for sex and age in the analysis; one study also adjusted for ethnicity, working status, education, smoking and congestive heart failure(53) and another study additionally adjusted for physical activity and body mass index (BMI).(32) A study that examined emotional response to symptoms found that 67% of participants (n=150) reported that their symptoms worried them to some degree, and 50% reported being frustrated by their symptoms.(55)
A randomized-controlled trial (n=49) examined the effects of yoga on AF measured both pre- and post- anxiety and depression scores using the Zung self-assessment anxiety score (56) and Zung self-assessment depression score (SDS) in addition to AF symptoms using a symptom diary and cardiac nonlooping event monitors and found that changes in anxiety and depression did not significantly correlate with changes in AF symptom experience, though the findings indicated a significant improvement in AF symptom burden, anxiety and depression following the intervention.(52) Findings from the one qualitative study supported the quantitative results in that most of the participants (n=15) reported that emotional distress could precipitate an AF episode.(30)
Association of Sex and AF Symptoms
The majority of studies reported a significant, positive association between symptomatic AF and female sex (p<0.05)(7, 11, 26, 31, 35, 57, 58) with the exception of five studies that found no association between symptomatic AF and sex (Table 4).(27, 32, 42, 59, 60) The studies that found a significant association had larger sample sizes than the studies without findings indicative of a significant association. With the exception of one,(26) all of the studies that examined sex differences in symptoms were prospective cohort studies.
Association of Race and AF Symptoms
Notably, only three of the studies reviewed examined racial differences in symptom experience.(7, 22, 31) In each of these studies’ samples, over 85% of the participants were Caucasian. The studies found that nonwhites were significantly more likely to experience symptoms than Caucasians (p<0.05).(7, 31)
DISCUSSION
The findings indicate that there is limited research examining the associations between AF symptoms and AF characteristics, psychological distress, race, and sex. The lack of standardized assessment of AF symptoms, homogenous samples, and observational designs limits most prior research.(7) The research reviewed was mainly observational studies, and the findings were largely inconclusive. Few of the studies in this review were designed for the exclusive purpose of examining symptoms in AF.(49, 50, 52, 61) Many of the articles reported on symptoms through subsample analysis where part of the data and sample was taken from a larger study.(5, 8, 57, 62)
One of the key methodological weaknesses of the prior research is the inconsistent measurement of AF symptoms. This variation in measurement complicates drawing conclusions from the literature because the studies used instruments with differing symptoms and time periods.(10, 46, 53, 62) For example, the EHRA includes a time frame of one week and one year to survey patients on symptoms, whereas ATSSS surveys about symptoms experienced in that past month.(63) Some instruments include as many as sixteen symptoms whereas others include four.(37, 38) Differences in instruments display a lack of agreement among AF researchers over which symptoms can be attributed to AF and the appropriate time period to understand patients’ experiences with AF symptom burden. Additionally, all commonly used instruments for the measurement of AF symptoms have limited reported evidence of reliability and validity for use among different race and ethnicity groups.(29, 38, 39, 64) The majority of the tools to measure symptoms were tested on predominantly Caucasian male samples,(29, 38, 39) making the applicability of the tools to females and non-Caucasian populations questionable.
AF symptoms are the primary reason patients seek medical treatment.(4) They are known to negatively affect quality of life.(65, 66) Patients’ report of symptoms is a major component of the treatment path for AF.(12) Yet, there is a universal inadequate understanding of AF symptoms.(4) The differences that exist among the AF symptom measurement tools are both reflective of the lack of a thorough comprehension of AF symptom burden and an impediment to developing a deeper understanding of AF symptoms. There is a notable lack of qualitative studies,(30) which are necessary to understanding the subjective and highly variable experience of AF symptom burden. Qualitative studies could also be used to better inform AF symptom measurement instruments, which currently vary in their measurements of the number, type, and time-frame of symptoms,(38, 40, 64) and to enrich understanding of the different predictors of AF symptom burden.(30)
In an examination of the existing literature on sex differences in AF symptoms, it is important to note that the majority of the sample was male in each study described in this review. While estimates suggest that a male’s lifetime risk for AF at age 40 is 26% while a female’s lifetime risk of 23% at age 40, the difference is not large enough to account for the lack of female representation in the study samples.(67) Since the limited research investigating sex differences in symptom burden suggests that females may be more symptomatic than males, the prevalence of symptoms may not be generalizable to women since females were not equally represented in the samples. Findings from Rienstra and colleagues suggest that asymptomatic patients have better outcomes;(24) differences in symptoms may be contributing to disparate outcomes in marginalized populations.(68)
While the limited research examining race and AF symptoms suggests non-whites experience more symptoms, the majority of research in AF is done on largely Caucasian samples. Efforts must be made to determine the potential causes that contribute to non-whites being more symptomatic. The current findings reflect a need for improved symptom management for minorities.
It is difficult to draw conclusions based on the studies included in this review on the association of psychological distress and symptoms. Individuals with high levels of psychological distress prior to diagnosis of AF may be more likely to experience symptoms, psychological distress may precipitate a symptomatic AF episode, and the symptoms of AF may result in high levels of psychological distress.(14, 30, 53, 54) The randomized-controlled trial examining the effects of yoga on AF symptoms and found that changes in anxiety and depression did not significantly correlate with changes in AF symptom experience. While a causal relationship cannot be determined from the studies in this review, the relationship may be bidirectional. However, future studies specifically designed to examine psychological distress and AF symptoms are needed to draw definitive conclusions.
There are limitations to the findings of this review. It is possible that informative articles were not found in the review due to the lack of consistency and broadly defined term “symptoms.” The review was not systematic and it is possible articles with valuable symptoms data were not found. There were limited studies that were designed for the specific purpose of examining symptoms and the associated variables found in the literature. The results from this review remain valuable in highlighting knowledge gaps and methodological shortcomings that restrict the ability to draw evidence-based conclusions.
Research gaps exist in understanding the association between race and ethnicity, sex, psychological distress and symptoms. The few studies that did include data on race and ethnicity reported a largely homogenous patient population. Findings suggest that psychological distress may be associated with symptoms. Improved understanding of AF patients’ symptom experience and reliable, valid, and clinically-feasible methods to measure symptom experience may lead to effective, tailored strategies to improve the quality and outcomes of AF care.
Supplementary Material
Acknowledgments
Funding Sources
Kelly T. Gleason received support from predoctoral fellowship in Interdisciplinary
Training in Cardiovascular Health Research, T32 NR012704, and Predoctoral Clinical Research Training Program, TL1 TR001078.
Footnotes
Disclosures
Dr. Nazarian is a scientific advisor to Biosense Webster Inc. and CardioSolv Inc. and principal investigator for research funding to Johns Hopkins from Biosense Webster, Inc. (Diamond Bar, California). All other authors have no conflicts of interest
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–7. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 3.Pinter A, Dorian P. New approaches to atrial fibrillation management: treat the patient, not the ECG. Cardiovasc Ther. 2010;28(5):302–10. doi: 10.1111/j.1755-5922.2010.00135.x. [DOI] [PubMed] [Google Scholar]
- 4.Rienstra M, Lubitz SA, Mahida S, Magnani JW, Fontes JD, Sinner MF, et al. Symptoms and functional status of patients with atrial fibrillation: state of the art and future research opportunities. Circulation. 2012;125(23):2933–43. doi: 10.1161/CIRCULATIONAHA.111.069450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y, Bai R, Wu JH, Zhang T, Liu N, Shi XB, et al. Differences in Quality of Life Between Atrial Fibrillation Patients with Low Stroke Risk Treated With and Without Catheter Ablation. J Am Heart Assoc. 2015;4(9):e002130. doi: 10.1161/JAHA.115.002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, et al. Association Between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results From the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Circ Cardiovasc Qual Outcomes. 2015;8(4):393–402. doi: 10.1161/CIRCOUTCOMES.114.001303. [DOI] [PubMed] [Google Scholar]
- 7.Golwala H, Jackson LR, 2nd, Simon DN, Piccini JP, Gersh B, Go AS, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: Insights from Outcomes Registry for Better Informed Treatment for Atrial Fibrillation Registry. Am Heart J. 2016;174:29–36. doi: 10.1016/j.ahj.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Ha AC, Breithardt G, Camm AJ, Crijns HJ, Fitzmaurice GM, Kowey PR, et al. Health-related quality of life in patients with atrial fibrillation treated with rhythm control versus rate control: insights from a prospective international registry (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation: RECORD-AF) Circ Cardiovasc Qual Outcomes. 2014;7(6):896–904. doi: 10.1161/HCQ.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds MR, Morais E, Zimetbaum P. Impact of hospitalization on health-related quality of life in atrial fibrillation patients in Canada and the United States: results from an observational registry. Am Heart J. 2010;160(4):752–8. doi: 10.1016/j.ahj.2010.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Steg PG, Alam S, Chiang CE, Gamra H, Goethals M, Inoue H, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98(3):195–201. doi: 10.1136/heartjnl-2011-300550. [DOI] [PubMed] [Google Scholar]
- 11.Vermond RA, Crijns HJ, Tijssen JG, Alings AM, Van den Berg MP, Hillege HL, et al. Symptom severity is associated with cardiovascular outcome in patients with permanent atrial fibrillation in the RACE II study. Europace. 2014;16(10):1417–25. doi: 10.1093/europace/euu151. [DOI] [PubMed] [Google Scholar]
- 12.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071–104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 13.Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of Atrial Fibrillation. JAMA. 2015;314(3):278–88. doi: 10.1001/jama.2015.7505. [DOI] [PubMed] [Google Scholar]
- 14.McCabe PJ, Barnason SA. Illness perceptions, coping strategies, and symptoms contribute to psychological distress in patients with recurrent symptomatic atrial fibrillation. J Cardiovasc Nurs. 2012;27(5):431–44. doi: 10.1097/JCN.0b013e31821e7ab1. [DOI] [PubMed] [Google Scholar]
- 15.Reiffel JA, Kowey PR, Myerburg R, Naccarelli GV, Packer DL, Pratt CM, et al. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry) Am J Cardiol. 2010;105(8):1122–9. doi: 10.1016/j.amjcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 16.MacRae CA. Symptoms in atrial fibrillation: why keep score? Circ Arrhythm Electrophysiol. 2009;2(3):215–7. doi: 10.1161/CIRCEP.109.878355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narasimha D, Curtis AB. Sex Differences in Utilisation and Response to Implantable Device Therapy. Arrhythm Electrophysiol Rev. 2015;4(2):129–35. doi: 10.15420/aer.2015.04.02.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zusterzeel R, Selzman KA, Sanders WE, Canos DA, O’Callaghan KM, Carpenter JL, et al. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174(8):1340–8. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 19.Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol. 2009;29(3):279–83. doi: 10.1161/ATVBAHA.108.179796. [DOI] [PubMed] [Google Scholar]
- 20.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67(20):2419–40. doi: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diker E, Bellur G, Yildiz N, Izgi C, Naditch-Brule L. Evaluation of atrial fibrillation (AF) management and cardiovascular risk profile in AF patients: data from Turkish patients in the international observational cross-sectional REALISE AF trial. Turk Kardiyol Dern Ars. 2015;43(1):60–74. doi: 10.5543/tkda.2015.93530. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–34. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 24.Rienstra M, Vermond RA, Crijns HJ, Tijssen JG, Van Gelder IC Investigators R. Asymptomatic persistent atrial fibrillation and outcome: results of the RACE study. Heart Rhythm. 2014;11(6):939–45. doi: 10.1016/j.hrthm.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Singh SN, Tang XC, Singh BN, Dorian P, Reda DJ, Harris CL, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program Substudy. J Am Coll Cardiol. 2006;48(4):721–30. doi: 10.1016/j.jacc.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 26.Rienstra M, Van Veldhuisen DJ, Hagens VE, Ranchor AV, Veeger NJ, Crijns HJ, et al. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46(7):1298–306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 27.Sears SF, Serber ER, Alvarez LG, Schwartzman DS, Hoyt RH, Ujhelyi MR. Understanding atrial symptom reports: objective versus subjective predictors. Pacing Clin Electrophysiol. 2005;28(8):801–7. doi: 10.1111/j.1540-8159.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 28.Walfridsson H, Walfridsson U, Nielsen JC, Johannessen A, Raatikainen P, Janzon M, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation: results on health-related quality of life and symptom burden. The MANTRA-PAF trial. Europace. 2015;17(2):215–21. doi: 10.1093/europace/euu342. [DOI] [PubMed] [Google Scholar]
- 29.Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36(4):1303–9. doi: 10.1016/s0735-1097(00)00886-x. [DOI] [PubMed] [Google Scholar]
- 30.McCabe PJ, Schumacher K, Barnason SA. Living with atrial fibrillation: a qualitative study. J Cardiovasc Nurs. 2011;26(4):336–44. doi: 10.1097/JCN.0b013e31820019b9. [DOI] [PubMed] [Google Scholar]
- 31.Patel N, Chung EH, Mounsey JP, Schwartz JD, Pursell I, Gehi AK. Effectiveness of atrial fibrillation monitor characteristics to predict severity of symptoms of atrial fibrillation. Am J Cardiol. 2014;113(10):1674–8. doi: 10.1016/j.amjcard.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Kupper N, van den Broek KC, Widdershoven J, Denollet J. Subjectively reported symptoms in patients with persistent atrial fibrillation and emotional distress. Front Psychol. 2013;4:192. doi: 10.3389/fpsyg.2013.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murin J, Naditch-Brule L, Brette S, Chiang CE, O’Neill J, Steg PG. Clinical characteristics, management, and control of permanent vs. nonpermanent atrial fibrillation: insights from the RealiseAF survey. PLoS One. 2014;9(1):e86443. doi: 10.1371/journal.pone.0086443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patten M, Maas R, Karim A, Muller HW, Simonovsky R, Meinertz T. Event-recorder monitoring in the diagnosis of atrial fibrillation in symptomatic patients: subanalysis of the SOPAT trial. J Cardiovasc Electrophysiol. 2006;17(11):1216–20. doi: 10.1111/j.1540-8167.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 35.Siontis KC, Gersh BJ, Killian JM, Noseworthy PA, McCabe P, Weston SA, et al. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: Characteristics and prognostic implications. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC, et al. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9(11):1006–23. doi: 10.1093/europace/eum191. [DOI] [PubMed] [Google Scholar]
- 37.Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation. 1996;94(7):1585–91. doi: 10.1161/01.cir.94.7.1585. [DOI] [PubMed] [Google Scholar]
- 38.Spertus J, Dorian P, Bubien R, Lewis S, Godejohn D, Reynolds MR, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-Life (AFEQT) Questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4(1):15–25. doi: 10.1161/CIRCEP.110.958033. [DOI] [PubMed] [Google Scholar]
- 39.Dorian P, Cvitkovic SS, Kerr CR, Crystal E, Gillis AM, Guerra PG, et al. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS-SAF scale. Can J Cardiol. 2006;22(5):383–6. doi: 10.1016/s0828-282x(06)70922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braganca EO, Filho BL, Maria VH, Levy D, de Paola AA. Validating a new quality of life questionnaire for atrial fibrillation patients. Int J Cardiol. 2010;143(3):391–8. doi: 10.1016/j.ijcard.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 41.Bhandari AK, Anderson JL, Gilbert EM, Alpert BL, Henthorn RW, Waldo AL, et al. Correlation of symptoms with occurrence of paroxysmal supraventricular tachycardia or atrial fibrillation: a transtelephonic monitoring study. The Flecainide Supraventricular Tachycardia Study Group. Am Heart J. 1992;124(2):381–6. doi: 10.1016/0002-8703(92)90601-q. [DOI] [PubMed] [Google Scholar]
- 42.Manganiello S, Anselmino M, Amellone C, Pelissero E, Giuggia M, Trapani G, et al. Symptomatic and asymptomatic long-term recurrences following transcatheter atrial fibrillation ablation. Pacing Clin Electrophysiol. 2014;37(6):697–702. doi: 10.1111/pace.12387. [DOI] [PubMed] [Google Scholar]
- 43.Mehall JR, Kohut RM, Jr, Schneeberger EW, Merrill WH, Wolf RK. Absence of correlation between symptoms and rhythm in “symptomatic” atrial fibrillation. Ann Thorac Surg. 2007;83(6):2118–21. doi: 10.1016/j.athoracsur.2007.02.084. [DOI] [PubMed] [Google Scholar]
- 44.Quirino G, Giammaria M, Corbucci G, Pistelli P, Turri E, Mazza A, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32(1):91–8. doi: 10.1111/j.1540-8159.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 45.Silberbauer J, Veasey RA, Cheek E, Maddekar N, Sulke N. Electrophysiological characteristics associated with symptoms in pacemaker patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2009;26(1):31–40. doi: 10.1007/s10840-009-9411-x. [DOI] [PubMed] [Google Scholar]
- 46.Strickberger SA, Ip J, Saksena S, Curry K, Bahnson TD, Ziegler PD. Relationship between atrial tachyarrhythmias and symptoms. Heart Rhythm. 2005;2(2):125–31. doi: 10.1016/j.hrthm.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 47.Tondo C, Tritto M, Landolina M, PDEG, Bencardino G, Moltrasio M, et al. Rhythm-symptom correlation in patients on continuous monitoring after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25(2):154–60. doi: 10.1111/jce.12292. [DOI] [PubMed] [Google Scholar]
- 48.Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173(2):149–56. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 49.Connolly SJ, Schnell DJ, Page RL, Wilkinson WE, Marcello SR, Pritchett EL, et al. Symptoms at the time of arrhythmia recurrence in patients receiving azimilide for control of atrial fibrillation or flutter: results from randomized trials. Am Heart J. 2003;146(3):489–93. doi: 10.1016/S0002-8703(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 50.Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middeldorp ME, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. 2013;310(19):2050–60. doi: 10.1001/jama.2013.280521. [DOI] [PubMed] [Google Scholar]
- 51.Hansson A, Madsen-Hardig B, Olsson SB. Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord. 2004;4:13. doi: 10.1186/1471-2261-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, et al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. 2013;61(11):1177–82. doi: 10.1016/j.jacc.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 53.Thompson TS, Barksdale DJ, Sears SF, Mounsey JP, Pursell I, Gehi AK. The effect of anxiety and depression on symptoms attributed to atrial fibrillation. Pacing Clin Electrophysiol. 2014;37(4):439–46. doi: 10.1111/pace.12292. [DOI] [PubMed] [Google Scholar]
- 54.Gehi AK, Sears S, Goli N, Walker TJ, Chung E, Schwartz J, et al. Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol. 2012;23(5):473–8. doi: 10.1111/j.1540-8167.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 55.McCabe PJ, Rhudy LM, DeVon HA. Patients’ experiences from symptom onset to initial treatment for atrial fibrillation. J Clin Nurs. 2015;24(5–6):786–96. doi: 10.1111/jocn.12708. [DOI] [PubMed] [Google Scholar]
- 56.Efremidis M, Letsas KP, Lioni L, Giannopoulos G, Korantzopoulos P, Vlachos K, et al. Association of quality of life, anxiety, and depression with left atrial ablation outcomes. Pacing Clin Electrophysiol. 2014;37(6):703–11. doi: 10.1111/pace.12420. [DOI] [PubMed] [Google Scholar]
- 57.Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, et al. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am J Med. 2015;128(5):509–18. e2. doi: 10.1016/j.amjmed.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 58.Henry L, Hunt S, Holmes SD, Martin LM, Ad N. Are there gender differences in outcomes after the Cox-Maze procedure for atrial fibrillation? Innovations (Phila) 2013;8(3):190–8. doi: 10.1097/IMI.0b013e3182a2306c. [DOI] [PubMed] [Google Scholar]
- 59.Clair WK, Wilkinson WE, McCarthy EA, Page RL, Pritchett EL. Spontaneous occurrence of symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia in untreated patients. Circulation. 1993;87(4):1114–22. doi: 10.1161/01.cir.87.4.1114. [DOI] [PubMed] [Google Scholar]
- 60.Kang Y. Relation of atrial arrhythmia-related symptoms to health-related quality of life in patients with newly diagnosed atrial fibrillation: a community hospital-based cohort. Heart Lung. 2006;35(3):170–7. doi: 10.1016/j.hrtlng.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 61.Alegret JM, Vinolas X, Martinez-Rubio A, Pedrote A, Beiras X, Garcia-Sacristan JF, et al. Gender Differences in Patients with Atrial Fibrillation Undergoing Electrical Cardioversion. J Womens Health (Larchmt) 2015;24(6):466–70. doi: 10.1089/jwh.2014.5014. [DOI] [PubMed] [Google Scholar]
- 62.van Gelder IC, Hagens VE, Kingma JH, Bosker HA, Kamp O, Kingma T, et al. Rate control versus electrical cardioversion for atrial fibrillation: A randomised comparison of two treatment strategies concerning morbidity, mortality, quality of life and cost-benefit - the RACE study design. Neth Heart J. 2002;10(3):118–24. [PMC free article] [PubMed] [Google Scholar]
- 63.Maglio CSJ, Paquette M, Dorian P, Bygrave A, Wood K, Ayers G. Measuring quality of life and symptom severity in patients with atrial fibrillation. Pacing Clinical Electrophysiology. 1998;21(839) [Google Scholar]
- 64.Berti DMP, Isegham MAV, Heidbuchel H. Assessing atrial fbrillation-related symptoms: Do we have a validated tool? Acta Cardiol. 2011;66(1):93–4. [Google Scholar]
- 65.Akintade BF, Chapa D, Friedmann E, Thomas SA. The influence of depression and anxiety symptoms on health-related quality of life in patients with atrial fibrillation and atrial flutter. J Cardiovasc Nurs. 2015;30(1):66–73. doi: 10.1097/JCN.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 66.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448e1–19. doi: 10.1016/j.amjmed.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 67.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 68.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132(9):873–98. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


