Abstract
Boundary conditions enable cellular life through negentropy, chemiosmosis, and homeostasis as identifiable First Principles of Physiology. Self-referential awareness of status arises from this organized state to sustain homeostatic imperatives. Preferred homeostatic status is dependent upon the appraisal of information and its communication. However, among living entities, sources of information and their dissemination are always imprecise. Consequently, living systems exist within an innate state of ambiguity. It is presented that cellular life and evolutionary development are a self-organizing cellular response to uncertainty in iterative conformity with its basal initiating parameters. Viewing the life circumstance in this manner permits a reasoned unification between Western rational reductionism and Eastern holism.
Keywords: uncertainty, First Principles of Physiology, negentropy, chemiosmosis, homeostasis
Doubt is not a pleasant condition, but certainty is absurd.
Voltaire
1. Introduction: From the inanimate to the animate
A fully integrated approach to Biomathics that is sought in this Special Issue of Progress in Biophysics and Molecular Biology must account for both Eastern and Western approaches to the natural sciences. The following provides a path between Western reductionism and Eastern holism as they are applied to biology by recognizing a fundamental flaw in our way of thinking about the premise of our existence as self-referential organisms.
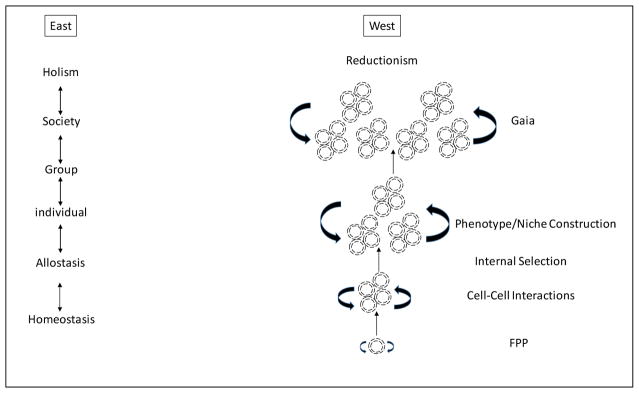
Life on Earth originated with the formation of protocells from asteroid-derived lipids floating in the primordial oceans (Deamer, 2017) [see Figure 1]. The ability to produce bioenergy through chemiosmosis was facilitated by the formation of endomembranes that partitioned negatively and positively charged ions on either side of them (Mitchell, 1966), initiating the first protocell/micelle (Deamer, 2017). Such constrained boundaries enabled life as a negentropic state that exists far from thermodynamic equilibrium, as first defined by Schrodinger (1944). This pseudo-physical state is sustained within the interior milieu of this membrane-bound compartment through homeostatic regulation (Torday, 2015a) as the reciprocating hub between the negentropic moment of the cellular interior and an ever-changing external environment. Therefore, homeostasis is a dynamic process realized through chemiosmosis that serves to maintain a range of far from equilibrium states within the boundary limits of the cell.
Fig. 1. Eastern versus Western Philosophy of Life.
The Eastern perspective on the origins and evolution of life is depicted on the left. The Western perspective is depicted on the right. FPP= First Principles of Physiology (Torday and Rehan, 2012); Gaia refers to the theory that the Earth is an organic whole.
When negentropy, chemiosmosis and homeostasis are achieved, the conditions are met under which self-referential awareness may arise (Torday and Rehan, 2012; Torday and Miller, 2016a). The origin of such self-awareness is unknown, although it has been considered a phase shift derivative of the thermodynamic scale expressed as a state function (a function that does not depend on the path taken to arrive at its present condition) (Miller, 2016). Though the actual dynamics are unknown, there is empiric evidence for physical self-organization based on observations of Ytterbium atoms that demonstrate coherent, spontaneous alignment. This phenomenon has been categorized as ‘time crystals’ (Vishwanath et al., 2017). It can be considered that that is the physical template for biological phenomenon (Torday, 2013).
It has further been maintained that life and its evolution are dependent upon communication (De Loof 2015a; Witzany, 2015). Plainly, communication is the transfer of information between ‘knowing’ entities. Therefore, as communicating entities, living organisms are information-dependent (Miller, 2016).
However, this requirement for information with regard to life raises a distinct paradox. Despite its overt material biological form, life is thoroughly imbued with ambiguities. Within biological media, neither the sender nor the receiver of information are necessarily known to each other (Czárán and Hoekstra, 2009). The communication between living organisms, whether through physical processes such as electrical signals, or by bioactive molecules is more akin to a general broadcast than definitely targeted information, such that highly integrated and ordered hierarchical systems must interact (Guenther, 2012). Furthermore, all communicative signals are subject to a range of physical parameters that render information ‘noisy’ by a series of time- or distance-related degradations. Therefore, any difference between the physical order within the internal milieu and the external environment is always a source of uncertainty since this depends on multiple interdependent components (Torday, 2015b). In such circumstances, life is always conditional self-organization in which the available information is equivocal (Torday and Miller, 2017; Miller, 2016).
2. The First Principles of Physiology and cell-cell communication
From life’s initial calcium burst at the time of conception (Antoine et al., 2001), to the synaptic neuronal activity of the brain, the cardinal properties of negentropy, chemiosmosis and homeostasis manifested by the first protocell can be advanced as continuously constituting the First Principles of Physiology (Torday and Rehan, 2012). Together, these processes mediate the communication mechanisms between the unicellular organisms that dominated Earth for its first four billion years (Cavalier-Smith, 2006). Cellular communication evolved into cell-cell interactions that accommodated multicellular metabolic drive as a means of coping with a continually shifting environment, most particularly, as a rise in environmental oxygen (Berner, Vandenbrooks, and Ward, 2007).
These communicative properties of cells form the key elements for any mechanistic understanding of the evolutionary process. Initially, primitive cells interacted with their environment in a limited manner in continuing compliance with the First Laws of Physiology (Torday and Rehan, 2012). Since evolution can be defined as the aggregate of its communications directed towards problem-solving (De Loof, 2015a), it might be surmised that the reciprocating complexity of cellular networks may have arisen as a defensive mechanism against the organized pseudo-multicellular behaviors of bacteria, such as quorum sensing (Castillo-Juárez et al., 2015) and biofilm (Majumdar and Pal, 2017). This coordinate problem solving by the three cellular forms (Bacteria, Archeae, Eukaryota), would have provided complementary, interdependent interchanges that gradually gave rise to the complex physiologic traits of multicellular organisms. This can only be enabled via cell-cell communication that permits a robust and reciprocating interplay with the environmental circumstances by fostering the collection of epigenetic marks (Torday and Miller, 2016d). This resultant and continuous stream of interactions would be used to provide critical flexibility to meet the endless cycles of environmental stress that characterize Earthly life, and ultimately determine macroscopic form (Torday and Rehan, 2011). An important aspect of the path of the first protocells from primitive organisms towards the enduring survival characteristics of prokaryotes was the assimilation of some features of the environment that have levels of toxicity that might have extinguished life. Long ago, some gases, ions, and heavy metals were biologically entrained, losing their biological toxicity, and instead became physiologic tools. The unique faculty of the protocell that permitted that transmutation was its ability to compartmentalize its internal milieu through endomembranes (Deamer, 2017). It can be asserted that it was this cellular boundary system that mobilized such physical entities for their biological use in metabolism (Morré and Mollenhauer, 1974). For example, the iron core of the hemoglobin molecule facilitates oxygen carrying capacity and thus promotes eukaryotic metabolism. By contrast, both free iron and oxygen are toxic. Yet, when each is integrated into the physiology of the organism, they constitute elements of intermediary metabolism. It can be asserted that this transition could only occur when such physical substrates are utilized as problem-solving elements. Importantly, in an information-dependent process in which environmental cues have uncertain meanings due to fluctuating conditions, and information is subject to its own sources of error, the boundary compartment can be seen as a zone in which equivocal information can best be realized. The default requirements include a self-organized entity that can deploy equivocal information as tools in immediate uncertain times based on a set of First Principles that delimits enduring life-sustaining parameters.
3. Physical phenomena and cellular requirements
The physiology of a number of organs- lung, kidney, bone, uterus- is under mechanical control (Torday and Rehan, 2003; Bosch et al., 1999; Pirola et al., 1994), likely ultimately due to evolutionary adaptation to gravity (Torday, 2003). Gravity is the oldest, constant, unidirectional effector of biology. Studies of the effect of microgravity on Parathyroid Hormone-related Protein (PTHrP) signaling have elucidated the effects of gravity on lung (Torday, 2003), kidney (Bosch et al., 1999), bone (Torday, 2003) and uterus (Daifotis et al., 1992) alike, all of which are dependent upon PTHrP’s physiologic functions. Evidence for the primacy of PTHrP comes from the pathophysiologic effects of over-distension on these organs, and protection by stimulating the PTHrP down-stream signaling target gene, Peroxisome Proliferator Activator Receptor gamma (PPARγ) (Rehan and Torday, 2007), preventing or curing the loss of mechanical signaling.
Further, PPARγ is the determinant of Peroxisome formation (Farmer, 2005). These structures are small, membrane-enclosed organelles, discovered by Christian De Duve (De Duve, 1969) that employ enzymes involved in a variety of energetic and metabolic reactions (Speijer (2011) BioEssays 33:88; Bolte et al. (2015) BioEssays 37:195; Moore (2015) BioEssays 37:113). De Duve hypothesized that peroxisomes originated due to endoplasmic reticulum (ER) stress caused by rising levels of oxygen in the environment (Berner et al., 2000). The stress caused leakage of calcium from the ER into the cytoplasm, and the peroxisomes exploited lipids to ‘buffer’ that intracellular calcium (Case et al., 2007). It can thereby be advanced that the ubiquitous ability of PPARγ agonists to prevent fibrosis (Deng, Xiong and Cheng, 2012) characterizes an ancient homeostatic problem-solving mechanism in accord with the First Principle of Physiology (Torday, 2015a).
Within the complex dynamics of cellular life, the Target of Rapamycin (TOR) gene has demonstrated itself to be a master regulator of virtually all aspects of metabolic function, such as ion flux (Lang and Pierce, 2016), mechanotransduction (Goodman, 2014), oxygenation (Cam and Houghton, 2011), and nutrient availability (Ben-Sahra and Manning, 2017). Crucially, the TOR gene is bound to the cytoskeleton (Torres et al., 2002), where it monitors these diverse traits that have been regarded as independent in origin, but are each and together directed towards the maintenance of equipoise within the cell. The significance of this gene is exemplified by the cytoprotective effects of the antibiotic Rapamycin, which is manifested by its ability to lengthen the life span of mice (Bitto et al., 2015). Clearly, then, the cell can only truly be understood as a completely integrated mechanism that has its own basal core, to which every aspect of the cell contributes with fidelity. The cytoskeleton of the cell can then be appreciated as a significant determinant of the physiologic status of the cell, and further yet, must itself adhere to a consistent set of First Principles against which a range of ambiguous external cues can be judged. Physiology can thereby be viewed in an integrated manner that extends far beyond a description of metabolic intermediates, yielding to a finite set of initiating First Principles upon which any cellular component, including the crucial conformation of the cytoskeleton, depends on continuous reaction to contemporary stresses built on lifetimes of experience phylogenetically. The cytoskeleton has long been viewed purely as a cellular architectural element (Hardin, Bertoni and Kleinsmith, 2015), but in fact additionally aids and abets the collection of epigenetic marks (Trerotola et al., 2015), just as every other aspect of the cell participates (Torday and Rehan, 2017), including its prime importance for the functioning of the cognitive memory system according to De Loof (2016). These proximate markers of environmental stress must be purposed towards the long-term preservation of homeostatic status according to the ultimate primordial rules embodied within the First Principles of Physiology. The cytoskeleton can therefore be considered a governing aspect of homeostasis in addition to mitotic and meiotic division (Prosser and Pelletier, 2017; Touati and Wasserman, 2016), quite in opposition to convention. For example, when yeast, a simple unicellular eukaryote, is put into microgravity, it loses its ability to polarize and bud (Purevdorj-Gage, Sheehan and Hyman, 2006). The effects of microgravity inhibit calcium flux, and then, through complex cellular connections, its metabolism and ability to reproduce. In each instance, this fluctuation can be attributed to the loss of cytoskeletal control (Najrana and Sanchez-Esteban, 2016). Hence, all aspects of cellular organization and function are linked through reciprocating pathways. For example, TOR, as an essential metabolic regulator, also controls actin polymerization, and is therefore an important determinant of cytoskeletal conformation (Johnson et al., 2015). If yeast is deprived of the normal environmental cues for polarity and budding, the effects reverberate across the cell through the cytoskeleton, resulting in changes in TOR-mediated metabolism and reproduction (Najrana T, Sanchez-Esteban, 2016).
Therefore, every cellular structure serves the situation of the cell and participates in evaluating the stream of information upon which all cells depend. Importantly, the informational landscape for all living things is filled with conflicting sources. Cells must meet a requirement of flexible adjustment within any single transient cycle of stress so as to assure immediate relief and ensure current survival. However, the extent of any such adjustment must be weighed against a concurrent and also periodic long-term environmental scale. Over-adjusting to a current temporary stress might mean significant loss in a subsequent environmental cycle.
Conceptually, this is no more complex than understanding that long-term moving averages of prices in any modern market economy exhibit greater stability over time than short-term impulses. The means by which organisms cope appropriately with current stresses within its range of ambivalent cues is through the countervailing limits established via First Principles as a perpetuating set of cellular constraints.
4. Resolving ambiguities: Information and problem-solving
Within living organisms, uncertainties are best appraised as a bandwidth of potential reactions, which Bohm termed a range of potential implicates and explicates (Bohm, 1980). As such, all living things exist within a spectrum comprised by the superimposition of their full potential reactions to stress, which can be based upon their self-referential assessment of available information despite its ambiguities. The solutions to problems for any living entity become its specific attachment to its available sources of information that it might direct towards achieving preferred homeostatic states by discriminating among physiological cues (Takada and Jameson, 2009). The sorting and resolution of the total range of ambiguities that confront living things is appropriately considered its tool kit for problem-solving. Therefore, communication among organisms is simply a problem-solving tool that has its benefits and attendant limitations (De Loof, 2015a; Torday and Miller, 2016a).
Environmental stress, with its range of uncertainties, is met through the cellular interrogation of information space as the cell is challenged to assess its state of being (Miller, 2016). This is the process that forces a commitment towards explicit, albeit transient, biological expression. Any real permanent change would limit the range of potential solutions to stress, raising the odds of extinction, given the ever-changing environment.
As an important codicil, the centrality of problem-solving as the essential aspect of living things explains why holobionts emerged as the exclusive form of eukaryotic multicellular life (Miller, 2013). Holobionts are the optimized cellular solution to uncertain information when confronting a widely varying external environment. It is the ‘wisdom of crowds’, in which the cells get smarter as they work collectively since the quality of information is improved (Ben-Jacob and Levine, 2006).
Recent research confirms that this is the manner in which neuronal cell networks make collective decisions. They operate upon two levels (Daniels et al., 2017).
Initially, when neurons are confronted with an unsure decision set, they ‘poll’ the collective network, in summation, akin to crowdsourcing, to develop an effective conjoint consensus. As they collectively process the available information, they begin to shift towards an explicit action potential as part of a dual or biphasic mechanism, in which the individual responses achieve high levels of homogeneity. In this way, the individual cellular differences merge into a coordinate pattern that permits the resolution of a range of implicates into explicate biologic action, which must always be effected through individual cellular actions. Necessarily then, all aspects of any cell, be it genes, or lipids or calcium flux, are tools in service to the assessment of a complex, overlapping information space and the maintenance of a preferred homeostatic moment (Miller, 2016; Torday and Miller, 2016b; Torday and Miller, 2016c).
5. Life cycles, ambiguity, and the unicell
It has previously been advanced that the macroscopic phase of eukaryotes is purposed towards the optimal accumulation of epigenetic marks to deal with a shifting environment and its stresses (Torday and Miller, 2016d). In such circumstances, any understanding of phenotype requires reconsideration. If the imperative of any organism is understood as its continual requirement to adjust to a stream of epigenetic inputs and the concomitant environmental stresses that are thereby imposed, then phenotype becomes an agent for environmental responsiveness (Torday and Miller, 2016b,c,d). This requires a complete change in perspective compared to any conventional Darwinian narrative. Phenotype can be deconstructed from its material form and instead understood in its role as a means of remaining in concert with external environmental shifts. In ambiguous informational circumstances, the multicellular, multi-species eukaryotic form uses phenotype as an agent to monitor its changing environment. Through that experience, information acquired from the phenotypic phase as epigenetic marks are carried back to the unicellular zygotic form for the reproductive cycle. This is the multicellular eukaryotic means that effects a reconciliation of current environmental information with its long-term circumstances in which the subsequent macro-organic elaboration represents its prediction (Torday and Miller, 2016b,c,d). Importantly, it experiences this transition within an explicit context that measures short-term impulses in accordance with the essential First Principles of cellular life. Put within another equivalency, all aspects of evolutionary conservation represent a reliance on sources of information in which that information is least equivocal, e.g. gravity, as measured against a full range of others that are more uncertain. In consequence, the unicellular zygotic recapitulating form and function represents the best means by which cells interpolate their ambiguous circumstances. An understanding of evolution in this manner moves Darwinian selection from descriptive observational study towards testable and refutable processes, rendering it scientifically efficacious rather than mere belief (Schermer, 2013).
When the eukaryotic cellular form is thereby understood as a means of accumulating epigenetic cues from the environment towards the larger imperative of sustaining the zygotic unicellular form vis-a-vis transient stresses, otherwise untenable aspects of the life cycle of various organisms can be clarified. For example, it is our instinct to view longevity as the length of the life cycle of a macroscopic form, varying widely from twenty-four hours for the May Fly, to thousands of years for the Giant Sequoia. In conventional biological terms, there is no specific logic to such huge variations in life span. However, when the life span is considered as a means of acquiring epigenetic marks from the external environment (Torday and Miller, 2016d), a mechanistic rationale emerges. If the life cycle is in service to the interactions between the organism and its environment in perpetual service to the eukaryotic unicellular form, life spans become dependent variables for unicellular needs (Torday and Miller, 2016c), acting in a way that is consonant with the organism’s long-term environmental niche within its cellular frame, which needs no rationale through our macroscopic bias.
For example, the slime mold can exist in two different states, a free-swimming amoeboid form or a sessile colonial form, directly dependent on the abundance of nutrients in the environment (Gilbert, 2006). In such an instance, it is clear that the acquisition of epigenetic marks is dictating the phenotype, and then, as a derivative, the relative life span of the organism.
The effect of food abundance on the slime mold Dictyostelium, which can switch phenotypes between the freely moving amoeboid structure or the sessile colonial form has been shown to be mediated by TOR expression that depends on environmental cues (Rosel et al., 2012). Therefore, phenotypic influences can be acquired under conditions of food abundance or starvation, but still remain centered within unicellular proscriptions and an underlying set of reactions to information that require a dependable set of principles.
Consequently, any synchronic snapshot of any current organic form in its presently evolved state is only a moment within an active and diachronically transcendent process that continuously extends forward from the unicellular state (Torday and Miller, 2016b). This perspective disabuses viewing life as endless levels of complexity (Valentine, 2004). Instead, it leads towards a unifying realization that life is simple insofar as it adheres to a few sustaining tenets (Torday, 2016a). Biology can consequently be understood as the self-referential application of mechanistic tools originating within First Principles of Physiology, dynamically purposed towards pragmatic solutions set against an ever-changing environment (Torday, 2016b). All living phenomena that have been individually categorized as separable processes of homeostasis (Torday, 2015a), pleiotropy (Torday, 2015b), or heterochrony (Torday, 2016) can finally be appraised as means as opposed to ends in coping with the ambiguity that characterizes the circumstances of all living things. Within a self-referential construct, problem solving is life’s imperative, and the information that it has available has its conspicuous limitations.
As a pertinent example of this perspective, a hydrozoan, Turitopsis dohrnii, can change from its jellyfish phenotype to its developmentally immature polyp form under stressful conditions. This latter phenomenon is conventionally assessed as an example of ‘reverse development’ or ‘immortality’ based on an apparent reversion to an earlier stage of the jellyfish life cycle (Piraino et al., 1996). A better explanation can be offered as a reversion to an atavistic phenotype for the commensurate collection of epigenetic marks relevant to an ever-changing environment (Torday and Rehan, 2011). Therefore, it can be assessed that the jellyfish Turitopsis reverts to its immature stage under physiologic stress as the form that permits the most advantageous weighing of adverse environmental cues.
In a similar manner, our own understanding of our human life cycle might be productively reconsidered. It has been suggested that human primates have a protracted childhood to accommodate the growth and differentiation of our large brains. This is the perspective that the developmental psychologist Piaget advocated (Gardner, 1981). Yet, this strategy could be considered alternatively as the prolonged passage through the developmental sequence from infancy, to crawling, toddling, adolescence, puberty and adulthood as the requisite stages for the collection of condition-related epigenetic marks, including its microbiome, that better prepare the human organism to deal with its full range of environmental stresses when no longer under parental protective judgment.
6. Exaptations, epigenetic stress and conformity to First Principles
When eukaryotic life is recognized as centered within cellular requirements and necessary adjustments to environmental stresses, with their attendant uncertainties, evolutionary development can be premised as a continuous series of pre-adaptations, or exaptations, to meet those otherwise equivocal conditions, employing the epigenetic tools at hand. As an example, by using the synthesis of cholesterol as a ‘molecular fossil’ as had been envisioned by Konrad Bloch (Bloch, 1992), the arc of vertebrate evolution can be surmised (Torday and Rehan, 2012). Bloch reasoned that since it takes 11 atoms of oxygen to synthesize one molecule of cholesterol, the primordial atmosphere must have been relatively rich in oxygen when cholesterol synthesis began. As cholesterol was crucially important for vertebrate evolution, facilitating metabolism, oxygenation and locomotion (Perry and Carrier, 2006) by thinning out the phospholipid cell membrane, that event must have been a pre-adaptation or exaptation (Pievani and Serrelli, 2011). With that scenario in mind, it can be hypothesized that lipids were the originating crucial substrate for the origin of life and the formation of the first cell. (Torday and Rehan, 2012; Deamer, 2017). Thereafter, lipids provided the so-called ‘rafts’ for the receptors that enabled cell-cell communication (Golub, Wacha, and Caroni, 2004) as a manifestation of the self-referential use of information. Over time and in continuous reciprocation with environmental stresses in which informational cues are always uncertain, cholesterol became the substrate tool for the responsive steroid hormone system (Wollman and Antebi, 2011). In turn, Vitamin D emerged to serve the endocrine system to maintain whole organism homeostasis and allostasis (Pinto and Cooper, 2014). In this manner, the endocrine system that determines the length and depth of the various stages of the life cycle under epigenetic influences (Crews and McLachlan, 2006) constitutes an essential part of the continuous complementarity between the organism and its environment that propels niche construction at varying scales (Torday, 2016). This integral interrelationship between cholesterol and homeostasis has been demonstrated experimentally. This allostatic principle is exemplified by the deletion of SCAP-1, which is necessary for cholesterol synthesis, specifically in lung alveolar epithelial cells (Besnard et al., 2009). Without the consequent ability to synthesize cholesterol, it was hypothesized that lung surfactant surface tension reducing activity would be degraded (Orgeig et al., 2003), causing pathophysiologic effects, but such an effect was not realized. However, upon histologic examination of the lung tissue, there was an excess number of lipofibroblasts in the walls of the alveoli of SCAP-1 −/− homozygous mice, compensating for the inferior functional quality of the surfactant. Importantly, it could be shown that this response was an exaptation of the phase in lung evolution when lipofibroblasts first appeared in the mammalian lung (Torday and Rehan, 2016b) based on the increased activity of PPARγ in the SCAP-1 −/− lung tissue, the latter being necessary for mesenchymal lipofibroblast (LIF) expression (Rehan et al., 2007). This atavism can be connected back in time phylogenetically to the advent of cholesterol in unicellular eukaryotes that facilitated oxygen exchange (Torday and Rehan, 2012). This vertical integration of physiologic gas exchange mediated by cholesterol (Torday and Rehan, 2012) is a prime example of the fidelity of cellular life towards the First Principles of Physiology. Thereafter, evolution can be regarded as working through a set of pre-exaptations through which such epigenetic connections can be amply advanced. There are other examples of such physiologic vertical integrations, such as the role of gravity in mechanotransduction (Torday and Rehan, 2003), and of mTOR (Torday, 2016c), statins (Spindler et al., 2012), and PPARγ (Argmann et al., 2009) in overall cellular homeostasis. In each case, short-term responses to stress represent an attempt at resolution when both the environmental stressor fluxes and the sources of pertinent information have limits of accuracy for the living conditions. Adherence to a set of vital, enduring First Principles is mandated by evolution, without which organisms would fatally go askew.
7. Discussion
It has been advanced that the obligatory recapitulation through the unicellular zygotic form is a mechanism for sorting epigenetic marks so that there is consistent adherence to an identifiable set of First Principles (Torday and Rehan, 2012). Among the uncertain informational cues that impact a multicellular organism, gravity would be among the few physical cues that could be interrogated in the most reliable manner. Therefore, any information that indicates that the loss of this most basic and reliable informational constituency should illustrate the cascade of inferential errors that occur in living organisms that must always assess information with varying degrees of equivocation. Such an example is the reaction of yeast cells to microgravity in which they experience a loss of polarity, abnormal clumping and aberrant budding (Purevdorj-Gage, Sheehan and Hyman, 2006). It has also been shown that changes in microgravity in yeast initiate a transcriptomic cascade that changes the coordinated regulation of the genes that control polarity, which can continue over many generations (Sheehan et al., 2007). In Candida albicans, microgravity induces an increase in random budding, differences in antifungal and general stress resistance, and down-regulation of genes controlling the actin cytoskeleton (Crabbe et al., 2013).
From this base of emerging experimental data, it can be argued that the precondition for the cellular confrontation of imprecise information exists within a balanced interplay between a deeply conserved set of cellular requisites and a flexible response to contemporary exigencies. Unbounded deviation from either could initiate a disruptive cascade with a diverse array of reshaped cellular responses. It should be noted that although such responses might be properly considered aberrant compared to normal cellular development, the term ‘adaptive’ might equally apply. The salient difference is in its explicit context. Conserved pathways are those that meet realistic long-term environmental exigencies in the most reliable long-term manner when contemporaneous environmental cues are always equivocal.
In any circumstance in which information is considered imprecise among biological participants, it might be considered how any deviation from a set of random variables might relate to a probabilistic quantum system. One such quantum model indicates that randomness need not be an irreducible condition, in a quantum sense, when it applies to physical systems. In that situation, it can be alternatively framed as incomplete information about that system (Khrennikov, 2007). A derivative for cognitive processes can follow. In biological terms, ambiguity can be construed as an expression of apprehension and judgment by any self-aware entity that involves quantum-like inferences in its conditional approach to biological problem solving. In effect, decision-making involves a representational matrix for the upper and lower boundaries of expectational status of an object or of any group of stimuli. In circumstances of informational insecurity, these delimiting approximations can be represented as an orthomodular lattice that conforms to a set of quantum inferences. (Gunji, Sonoda and Basios, 2016). In biological terms, the boundaries define the effective set of implicates that apply to that living entity as it encounters environmental uncertainties. Ultimately then, other than in exceptional circumstances, biological expression settles within those boundaries. It might be considered that it is those excursions beyond prior expectational boundaries that constitute the active zone of evolutionary novelty. Put differently, living systems are dependent upon predictable space-time information sets. Only a few are baseline persistent physical forces, such as gravity. As a derivative, the impulse for living organisms that must deal with informational ambiguity is to utilize a perception pattern as an internalized quantum measurement system to support mechanisms that minimize measurement discrepancies. Evidence does exist that these are actual operating characteristics of the cell. The transmission of signals via the cytoskeleton conforms to shaping these kinds of perception patterns in this specific manner (Igamberdiev and Shklovskiy-Kordi, 2017). This phenomenon has been termed the ‘principle of optimality’, which recognizes that spatiotemporal patterns in organisms have been established to achieve maximal predictability in space-time (Igamberdiev and Shkolvskiy-Kordi, 2017).
That living entities exist within this convention of boundaries is not conjectural. There is a sizable body of evidence that cell-based molecular and chemical interactions, such as those that are protein-mediated, are inherently probabilistic and equivocal, but still constrained within limiting boundaries (Kurakin, 2007). These reactions depend upon the specific cellular situations in which adaptive plasticity must meet an uncertain environment along pathways that contain many ambiguities but still maintains the requirement of some essential conformities (Kurakin, 2007).
8. Epistemic and ontological implications of our natural state of ambiguity
In any attempt to reconcile Western thought with Eastern beliefs, the essential disagreement between Einstein and Eddington provides an historical perspective. Einstein retained a steadfast commitment to determinism. For him, there is an objective reality that can be observed and accurately measured if there was sufficient completeness in quantum mechanics. (Landsman, 2009). Eddington believed differently. He concluded that beyond any measured understanding, there is both more and less to any physical object. The actual natural state always remains hidden within indeterminate variables. (Durham, 2003).
Certainly, quantum theory points towards indeterminism. It is underpinned by Heisenberg uncertainty and the Born Rule (Landsman, 2009). In the quantum frame, the outcome of any event is probabilistic and uncertain. When that concept was scaled to complex biological systems, both Prigogine and Monod argued for indeterminism. (Prigogine and Stengers, 1997; Merlin F, 2015).
Yet, it can be contended that any dichotomous perception of reality embodied within Western dualism and Eastern holism can be productively unified through a cellular-molecular model of physiology. As that model has been presented, biology is both a simultaneous synchronic ‘snapshot’ and a vertical diachronic integration across space-time. When combined, both offer an opportunity to synthesize both schools of thought in a testable/refutable manner (Bacon, 2016).
In a Western tradition, the direct assumption is that any investigation of reality should be based on observation, reasoning, and experiment. The intent is to assess subjective phenomena in such a manner as to yield an objective ‘third person’ understanding of reality. However, this issue reaches an acute difficulty with the subject of consciousness (Lakoff and Johnson, 1999). In Western thought, if consciousness is granted to exist, it is a ‘knowing’ whose etiology is itself unknown.
Eastern philosophy differs, perhaps as a product of its multiple backgrounds. Eastern philosophy remains centered on a proper life through spiritual self-realization, in which the search for knowledge is a means of liberation of individuals from false materiality, attachments and beliefs (Rao, 1998). In Eastern terms, consciousness is considered an Ultimate reality, related to an outer and inner ‘spirit’, which can expand as we actualize ourselves through focused actions, such as meditation.
With this background, what might we do, then, with a proper appreciation of our multi-cellular eukaryotic selves? We are vast collaborative enterprises between trillions of microbes and intrinsic cells? (Miller, 2013) Can this genuine substance be a validation of the Eastern way of thinking, in which our context consists of a “ multi-dimensional impression formed from the superimposition of single impressions from different points of view”? (Simeone and Ehresmann, 2017; Capra, 2010). Are we then material beings that produce consciousness or, instead, spiritual entities whose ‘being’ is directly embedded within the matter that is required to produce ourselves? In consequence, is the mechanistic-particle world based on empiric observations sufficient to understand our reality, or is it necessary to accept that there is no immutable ‘objective reality’ that can ever be achieved?
Certainly, Western thought can accommodate this latter point of view beyond any search for objectivism. Consider the Heisenberg Uncertainty Principle (1927) and the inability to simultaneously determine the momentum and direction of a particle. Or reflect on Bohr’s explanation for the duality of light, which is referred to as complementarity (Hall, 1997). That dyad is caused by the nature of the ‘measurement’ of a physical system and its ambiguous status when there is an attempt to assess its reality. It can be contended by analogy that life is always struggling to appraise its own reality, approximating it within its own Receiver-Operating Curve (Green and Swets, 1966). After all, life can never be a perfect fit with its environment; it is asymptotic to it, retaining a certain number of ‘degrees of freedom’ rather than foolishly committing itself within an ever-changing environment
The initially surprising findings of quantum phenomena has yielded substantive physical advances, such as quantum computing and communication. But, it has also interjected a fundamental wave-particle dualism that now reverberates into the biological sciences and carries fresh questions. How can local connections be reconciled with non-local correlations? What should be made of the presence of energetic fields (electromagnetic, mechanical, electrical, unexplained action at a distance) that impact cellular lives and then, our own? Hodgson (2002) noted several specific features of quantum mechanics that might pertain to biology including indeterminism, non-locality, and observer-participation. Yet, any acceptance of such an overlap requires life to be seen to fit under its own aegis of ambiguity as a manifestation of its an inherent complementarity that derives from obligatory physical underpinnings.
Nonetheless, there are pathways that permit a reconciliation. It has been said by Fritjof Capra (2010) in ‘The Tao of Physics’ that “ Science does not need mysticism and mysticism does not need science; but man needs both. In Eastern philosophical terms, understanding the inherent imperfection of the life is consistent with striving for Karmic perfection. The paradox is that perfection per se, as congruence, is not an ideal in biological terms just as it is not realistic within a physicist’s realm. In biology, a perpetual state of flux is needed to cope with a similarly variable environment. However, if Karmic perfection is instead re-framed as biological iterations towards achieving perpetual balance with the outward environment, congruence becomes a dynamic that maintains a consistent internal connection with a self-referential center with which it has achieved a desired internal confluence that ensconces its connectedness to the outward. This iterative approach to life as pre-adaptations is not unlike the Hindu concept of Karma, acting to hone the individual from one generation to the next through serial pre-adaptations. However, as within Western thought, such adaptations need not be colored as ‘good or bad’, but merely as the manner in which an organism copes with an ever-changing environment.
Just as there is the potential for reconciliation with Eastern sensibilities, there is a concurrent opportunity to do the same within the Western canon. For example, Leibniz’s Law, as the Identity of Indiscernibles, is considered an exemplar of Western thought. That ontological principle holds that there cannot be separate objects or entities that have all their properties in common (French and Redhead, 1988). However, it can be maintained that this relationship may not hold if regarded within a context of ambiguity that devolves from a set of superimposed Bohmian implicates that might not yield a consistent explicit biological resolution. When ambiguity rules, then identical entities can have differing outcomes that relate to both immediate impacts and history. We may never be able to discern that difference with precision. Indeed, there is objective evidence that the principle of the Identity of Indiscernibles is flawed through the examination of the action of photons (Cortes and Leibniz, 1976).
When Western quantum mechanics is considered, it has been hypothesized that quantum physics does not represent an ultimate theory of nature since it describes phenomena that are only manifested under specific conditions. (Josephson, 2002). Such phenomena are intimately related to an observer that lies outside of the ‘descriptive capacities of quantum mechanics.’ It is the interrogation by that observer that yields the wave function collapse. In such terms, our quantum duality is part of our existence. It is the overlapping and interrelated product of our own introspective intervention and an implicit dimension of an external observer that participates in the suspension of implicates that characterizes and precedes biological expression. Who is that external observer? Perhaps, it might be an Eastern cosmic Oneness, or as an alternative, it might be our own discursive inner dialogue conducted at the multi-dimensional levels that characterize all multicellular eukaryotic organisms. Certainly, it has been noted that “Matter and meaning are not separate elements” (Bard, 2007). Yet, that need not mean any one to one correspondence. Therein, within that gap, lies ambiguity.
There can be a further step towards reconciliation though a contemporary deconstruction of our macro-organic selves as perpetually cellular beings. It has been noted that life and its evolution proceeds without any privileged level of causation (Noble, 2012). This is the essential condition of our holobionic form (Miller, 2016). Cells attempt to find their own reality within the context of their circumstances in which ambiguity reigns. That pursuit is through cellular alliances, cooperation, co-dependence, co-linkage, and mutual competition. The yield is the resolution of ambiguity, according to scale, to affect biological decisions upon which their lives depend. Therefore, the natural bridge between our competing impulses towards Eastern and Western philosophies is typified by the nature of the interaction of living things as they are truly constituted compared to the illusion of our senses. That bridge is the cell. As Goswami (1997) suggested, “ The self-organization in a cell that enables it to distinguish itself from its environment, the breakdown of the whole into living system plus environment, is a tangled interplay of choice from the superposition (of possibilities) and the arena of living in which the ‘chosen’ facet manifests”.
Other contributors to this Special Issue have offered commentary that is especially pertinent to these viewpoints. Ford’s visionary recognition of the single cell as volitional and self-regulated with a significant degree of autonomy is shared. (Ford reference) As he states, cells have “ single-minded purpose” and demonstrate faculties of prediction, planned responses, and memory. Therefore, they must be credited as problem-solving agencies. His emphasis on their ingenuity mirrors our regard for their creative capacities in response to environmental stresses.
Tozzi et. al. offer a biological gauge theory in elegant correspondence with the cosmological doctrine of Zhu Xi. (Tozzi et al.) Xi’s cosmological organizing principle of li and its coexistent material or physical force, qi, can be considered abstract proxies of scientific dualism: organized signaling information within global symmetry that must assimilate both the cellular expenditure of metabolic energy and the environmental induction of symmetry breaks. In combination, these emulate Taji as an Ultimate creative energy force. The result, according to Tozzi et al., is an “existential” cell. Our work aligns. The cell is an organized signaling system that seeks to maintain li (homeostasis) in self-referential juxtaposition with qi (energy dissipation as work and environmental perturbations).
Nakajima’s insightful contribution in this Special Issue defends the insufficiency of the standard Darwinian model and emphasizes the direct and indirect interdependence of all living things (Nakajima reference). This, too, is exactly our point, though bolstered by our clarifying explanatory assertion that such collaborating inter-relationships are derivative of the uncertain circumstances of all living things as they seek to sustain individual equipoise.
How then to reconcile Eastern and Western philosophy in the context in which ambiguity is the living circumstance ? The resolution is plain. We, at our scale, debate and argue among ourselves to satisfy. The cell, at its scale, settles those inherent conflicts to survive. Through this path lies the connection between a Western reductionist approach to evolution and Eastern philosophy in which both can coexist as exemplars of life’s simplicity (Torday, 2016a) and unity (Torday and Miller, 2016b) when seen from a cellular molecular vantage point [Refer to Table 1].
Table 1.
Compatibility of cellular biological ambiguity with Western and Eastern philosophies
| Western Philosophy | Eastern Philosophy | |
|---|---|---|
| Materialism, objective reality | (+) Material forms exist as objects of study | (+) The material world of our human senses is ‘unreal’ |
| Self-reference, consciousness | (+) Embedded, to an uncertain extent in all living things; of unknown origin | (+) Embedded in the cosmos |
| Self-realization | (+) Maintenance of Homeostasis | (+) Being that transcends things |
| Macroorganisms | (−) End points | (+) Way stations |
| Quantum phenomena | (+) Used to improve predictions from space-time information sets i.e. quantum measurement | (+) Superimposition of possibilities settled by self referential consciousness |
| Unicellular recapitulation | (−) Intermediary step between crucial macro-organic forms | (+) Continual transcendent self- referential cosmic Being |
| Phenotype | (−) An attribute of a macro-organic form to assist in survival | (+) A form of continuous environmental-biologic complementarity |
| First Principles of Physiology | (+) Subject to random reinforcement | (+) An aspect of a universal life force |
| Genes | (+) Essential code as the primary script of an organism | (+) Tools of self-awareness |
| Evolution | (+) random and necessarily gradual | (+) An aspect of an Ultimate that can proceed through significant discontinuities |
It can be entertained that Western science is largely a directed search towards an ultimate testable reality. It has been suggested by others that ‘mysticism’ is also a science of an Ultimate whose reality is not directly self-evident through first-hand knowledge or ‘reasoned about’ through typical means (Guilford, 2011). Heidegger acknowledged that a quest to reveal Being must proceed along at least two levels as an entanglement between rational consideration and transcendent experiences. The one is calculating and the other meditative (Guilford, 2011). The wellspring for this search is our anxiety, an awareness of our finitude within a surrounding context of observed physical permanence (Hatab, 2000). In this sense, there is direct connection between Neo-platonic Western mysticism and its Eastern cousin, that can be channeled through Heidegger’s Truth of Being. That connection is between the quantum-realm and its superimposition of possibilities, towards the biological requisite of action to survive amidst incertitude. This larger truth must proceed through ‘knowing’ life’s actual Truth in Being. Being is Doubt.
Before any calculus can be conducted, any mathematical formulation must be based upon an appraisal of what things to associate and the manner by which they are connected. Such mathematics would therefore require an expansive Category Theory that is inherently relational (Simeone and Ehresmann, 2017) and might encompass the superimposition of possibilities that can become biologic explicates. The difficulties are plain. Simeone and Ehresmann (2017) quote the philosopher Henri Bortoft’s explanation of Goethe’s concept of morphogenesis: “ “The whole emerges simultaneously with the accumulation of the parts, not because it is sum of the parts, but because it is immanent within them”. It is argued herein that this vital fundament is the product of overarching self-referential consciousness that was first instantiated within the cellular form and remains ever-centered within it at that level. Therefore, the parts cannot be accurately summed. At any moment, the conditional relationship between the parts and their outward environment is always cloaked in self-referential uncertainty. Under these circumstances, reality might be best assessed through both objective and subjective internal means. This dualism can be regarded as ‘relational holism’, wherein, a Western idealism of relation does not necessarily reduce to another series of non-relational properties on any surface inspection, yet does so through a deeper and more profound ‘inherent relationalism’ (Teller, 1986). Inner reality thereby adduces to a natural inherency in which the co-existent non-relational properties remain active.
If the preceding can be accepted as a Central Relationality, in which Being is Doubt, and then, as its necessary derivative, that life must be viewed as the continuous resolution of ambiguity, an effective unification of Eastern holism with Western objectivism unfolds that combines testables and refutables with a self-referential Ultimate to be explored.
9. Conclusion
It is proposed that life is determined through its reactions to ambiguities at every scale. Its success is based on its capacity to confront a range of stressful uncertainties and effect heritable biological resolutions. For any such success, it depends upon self-referential assessment of status within a stream of equivocal information. It is clear that life would not be present except through those specific conditions in which problem solving is required (De Loof, 2015b; Miller, 2016; Torday, 2015c). Directly put, without environmental uncertainties, there would be no problems to solve. In cellular circumstances that become evolutionary outcomes, macroscopic forms can be appraised as consensual solutions to environmental problems under conditions in which uncertainty dominates. Cell-cell communications permit those solutions. Resulting physiological mechanisms defend those imperative connections that affect continued success through physiological and phenotypic paths that are continually channeled through a narrowing set of First Principles, and are the permanent anchors of cellular life (Torday and Rehan, 2012).
It might further be considered that the ability to discriminate among a conflicting assembly of equivocal informational cues can be considered the conventionally referenced ‘life force’ in non-mechanistic descriptive terms (Torday and Miller, 2016). It accomplishes this through its own dualisms. It achieves its macro-organic form through the maintenance of its cellular inherency and sustains it re-elaborating wholeness by its perpetual and obligatory reproductive recapitulations through its originating oneness.
Although life is generally seen as a self-organizing process, it is only so if it is dealing with its uncertain circumstances to advantage. It can plainly be asserted that self-organization actually requires ambiguity. Self-organization can only exist if discriminated against a background state in which its prior fuller range of implicates can be circumscribed. When so considered, an ultimate paradox for the living condition can be speculated. Does life simply cope with ambivalence or is uncertitude its absolute requirement? In answer, life’s circumstance is deeply apparent. Life exists within a persistent state of ambiguity, and anything that is alive must likewise comply.
Acknowledgments
JST was supported by NIH Grant HL055268
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John S. Torday, Department of Pediatrics, Harbor-UCLA Medical Center, Torrance, CA 90502
William B. Miller, Jr., Paradise Valley, AZ
References Cited
- Antoine AF, Faure JE, Dumas C, Feijó JA. Differential contribution of cytoplasmic Ca2+ and Ca2+ influx to gamete fusion and egg activation in maize. Nat Cell Biol. 2001;3:1120–1123. doi: 10.1038/ncb1201-1120. [DOI] [PubMed] [Google Scholar]
- Argmann C, Dobrin R, Heikkinen S, Auburtin A, Pouilly L, Cock TA, Koutnikova H, Zhu J, Schadt EE, Auwerx J. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon F. Stanford Encyclopedia of Philosophy. Stanford: 2016. Francis Bacon. [Google Scholar]
- Bard K. Meeting the universe halfway: Quantum physics and the entanglement of matter and meaning. Duke University Press; Durham: 2007. [Google Scholar]
- Ben-Jacob E, Levine H. Self-engineering capabilities of bacteria. J R Soc Interface. 2006;3:197–214. doi: 10.1098/rsif.2005.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol. 2017;45:72–82. doi: 10.1016/j.ceb.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner RA, Petsch ST, Lake JA, Beerling DJ, Popp BN, Lane RS, Laws EA, Westley MB, Cassar N, Woodward FI, Quick WP. Isotope fractionation and atmospheric oxygen: implications for phanerozoic O(2) evolution. Science. 2000;287:1630–1633. doi: 10.1126/science.287.5458.1630. [DOI] [PubMed] [Google Scholar]
- Berner RA, Vandenbrooks JM, Ward PD. Evolution. Oxygen and evolution Science. 2007;316:557–558. doi: 10.1126/science.1140273. [DOI] [PubMed] [Google Scholar]
- Besnard V, Wert SE, Stahlman MT, Postle AD, Xu Y, Ikegami M, Whitsett JA. Deletion of Scap in alveolar type II cells influences lung lipid homeostasis and identifies a compensatory role for pulmonary lipofibroblasts. J Biol Chem. 2009;284:4018–4030. doi: 10.1074/jbc.M805388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Wang AM, Bennett CF, Kaeberlein M. Biochemical Genetic Pathways that Modulate Aging in Multiple Species. Cold Spring Harb Perspect Med. 2015:5. doi: 10.1101/cshperspect.a025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch K. Sterol molecule: structure, biosynthesis, and function. Steroids. 1992;57:378–383. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- Bohm D. Wholeness and the Implicate Order. Routledge&Keagan; London: 2002. [Google Scholar]
- Bolte K, Rensing SA, Maier UG. The evolution of eukaryotic cells from the perspective of peroxisomes: phylogenetic analyses of peroxisomal beta-oxidation enzymes support mitochondria-first models of eukaryotic cell evolution. Bioessays. 2015 Feb;37(2):195–203. doi: 10.1002/bies.201400151. [DOI] [PubMed] [Google Scholar]
- Bosch RJ, Rodríguez-Puyol D, Bover J, Rodríguez-Puyol M. Parathyroid hormone-related protein: roles in the glomerulus. Exp Nephrol. 1999;7:212–216. doi: 10.1159/000020604. [DOI] [PubMed] [Google Scholar]
- Cam H, Houghton PJ. Regulation of mammalian target of rapamycin complex 1 (mTORC1) by hypoxia: causes and consequences. Target Oncol. 2011;6:95–102. doi: 10.1007/s11523-011-0173-x. [DOI] [PubMed] [Google Scholar]
- Capra F. The Tao of Physics. Shambhala Publications; Boston: 1975. [Google Scholar]
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signaling system. Cell Calcium. 2007;42:345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Castillo-Juárez I, Maeda T, Mandujano-Tinoco EA, Tomás M, Pérez-Eretza B, García-Contreras SJ, Wood TK, García-Contreras R. Role of quorum sensing in bacterial infections. World J Clin Cases. 2015;3:575–598. doi: 10.12998/wjcc.v3.i7.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Cell evolution and Earth history: stasis and revolution. Philos Trans R Soc Lond B Biol Sci. 2006;361:969–1006. doi: 10.1098/rstb.2006.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes A. Leibniz’s principle of the identity of indiscernibles: A false principle. Phil Sci. 1976;43:491–505. [Google Scholar]
- Crabbé A, Nielsen-Preiss SM, Woolley CM, Barrila J, Buchanan K, McCracken J, Inglis DO, Searles SC, Nelman-Gonzalez MA, Ott CM, Wilson JW. Spaceflight enhances cell aggregation and random budding in Candida albicans. PLoS One. 2013;8:e80677. doi: 10.1371/journal.pone.0080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, McLachlan JA. Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology. 2006;147:S4–S10. doi: 10.1210/en.2005-1122. [DOI] [PubMed] [Google Scholar]
- Czárán T, Hoekstra RF. Microbial communication, cooperation and cheating: quorum sensing drives the evolution of cooperation in bacteria. PLoS One. 2009;4:e6655. doi: 10.1371/journal.pone.0006655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daifotis AG, Weir EC, Dreyer BE, Broadus AE. Stretch-induced parathyroid hormone-related peptide gene expression in the rat uterus. J Biol Chem. 1992;267:23455–23458. [PubMed] [Google Scholar]
- Daniels BC, Flack JC, Krakauer DC. Dual Coding Theory Explains Biphasic Collective Computation in Neural Decision-Making. Frontiers Neurosci. 2017;11 doi: 10.3389/fnins.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C. Evolution of the peroxisome. Ann N Y Acad Sci. 1969;168:369–381. doi: 10.1111/j.1749-6632.1969.tb43124.x. [DOI] [PubMed] [Google Scholar]
- De Loof A. How to deduce and teach the logical and unambiguous answer, namely L = ΣC, to “What is Life?” using the principles of communication? Commun Integr Biol. 2015;8:e1059977. doi: 10.1080/19420889.2015.1059977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loof A. The cell’s self-generated electrome: The biophysical essence of the immaterial dimension of Life? Commun Integr Biol. 2016;9:e1197446. doi: 10.1080/19420889.2016.1197446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. The Role of Lipid Membranes in Life’s Origin. Biology (Basel) 2017:7. doi: 10.3390/life7010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YL, Xiong XZ, Cheng NS. Organ fibrosis inhibited by blocking transforming growth factor-β signaling via peroxisome proliferator-activated receptor γ agonists. Hepatobiliary Pancreat Dis Int. 2012;11:467–478. doi: 10.1016/s1499-3872(12)60210-0. [DOI] [PubMed] [Google Scholar]
- Durham IT. Eddington and uncertainty. Physics in Perspective. 2003;5:398–418. [Google Scholar]
- Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29:S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- French S, Redhead M. Quantum physics and the identity of indiscernibles. Br J Phil Sci. 1988;39:233–246. [Google Scholar]
- Gardner H. The Quest for Mind: Piaget, Levi-Strauss and the Structuralist Movement. University of Chicago Press; Chicago: 1981. [Google Scholar]
- Gilbert SF. Developmental Biology. Sinauer; Sunderland: 2006. [Google Scholar]
- Golub T, Wacha S, Caroni P. Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol. 2004;14:542–550. doi: 10.1016/j.conb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Goodman CA. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev Physiol Biochem Pharmacol. 2014;166:43–95. doi: 10.1007/112_2013_17. [DOI] [PubMed] [Google Scholar]
- Goswami A. Consciousness and biological order: Toward a quantum theory of life and its evolution. Integr Physiol Behav Sci. 1997;32:86–101. doi: 10.1007/BF02688616. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. John Wiley and Sons; New York: 1966. [Google Scholar]
- Guenther W. Biocommunication of Plants. Springer; Berlin Heidelberg: 2012. [Google Scholar]
- Guilford J. Was Heidegger a Mystic. Explorations. 2011;6:86–94. [Google Scholar]
- Gunji YP, Sonoda K, Basios V. Quantum cognition based on an ambiguous representation derived from a rough set approximation. Biosystems. 2016;141:55–66. doi: 10.1016/j.biosystems.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Hall GM. The Ingenious Mind of Nature: Deciphering the Patterns of Man, Society, and the Universe. Springer; New York: 1997. [Google Scholar]
- Hardin J, Bertoni G, Kleinsmith LJ. Becker’s World of the Cell. Pearson; New York: 2015. [Google Scholar]
- Hatab LJ. Why Heidegger is not an existentialist: interpreting authenticity and historicity in Being and Time. Florida Philosoph Rev. 2003;3:5–22. [Google Scholar]
- Heisenberg W. ber den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik. Zeitschrift für Physik. 1927;43:172–198. [Google Scholar]
- Hodgson D. Quantum physics, consciousness, and free will. The Oxford Handbook of Free Will; Oxford: 2002. [Google Scholar]
- Igamberdiev AU, Shklovskiy-Kordi NE. The quantum basis of spatiotemporality in perception and consciousness. Prog Biophys Molec Biol. 2017 doi: 10.1016/j.pbiomolbio.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Huang W, Roman G, Costa-Mattioli M. TORC2: a novel target for treating age-associated memory impairment. Sci Rep. 2015;5:15193. doi: 10.1038/srep15193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson BD. ‘Beyond quantum theory: a realist psycho-biological interpretation of reality’revisited. BioSystems. 2002;64:43–45. doi: 10.1016/s0303-2647(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Khrennikov A. Can quantum information be processed by macroscopic systems? Quantum Information Processing. 2007;6:401–429. [Google Scholar]
- Kurakin A. Self-organization versus Watchmaker: ambiguity of molecular recognition and design charts of cellular circuitry. J Mol Recog. 2007;20:205–214. doi: 10.1002/jmr.839. [DOI] [PubMed] [Google Scholar]
- Lakoff G, Johnson M. Philosophy in the flesh: The embodied mind and its challenge to western thought. Basic books; New York: 1999. [Google Scholar]
- Landsman NP. Compendium of quantum physics. Springer; Berlin: 2009. Born rule and its interpretation. [Google Scholar]
- Lang F, Pearce D. Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway. Nephrol Dial Transplant. 2016;31:200–205. doi: 10.1093/ndt/gfv270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Pal S. Bacterial intelligence: imitation games, time-sharing, and long-range quantum coherence. J Cell Commun Signal. 2017 doi: 10.1007/s12079-017-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin F. Monod’s conception of chance: Its diversity and relevance today. Comptes rendus biologies. 2015;338:406–412. doi: 10.1016/j.crvi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Miller WB., Jr Cognition, Information Fields and Hologenomic Entanglement: Evolution in Light and Shadow. Biology (Basel) 2016:5. doi: 10.3390/biology5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WB., Jr . The Microcosm Within. Universal; Boca Raton: 2013. [Google Scholar]
- Miller WB., Jr Cognition, Information Fields and Hologenomic Entanglement: Evolution in Light and Shadow. Biology (Basel) 2016:5. doi: 10.3390/biology5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic Coupling in Oxidative and Photosynthetic Phosphorylation. Biol Rev. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Moore A. Peroxisomes: a small step from mitochondria but a giant leap for eukaryotes. Bioessays. 2015;37:113. doi: 10.1002/bies.201500003. [DOI] [PubMed] [Google Scholar]
- Morré DJ, Mollenhauer HH. Dynamic Aspects of Plant infrastructure. McGraw-Hill; London, New York: 1974. [Google Scholar]
- Najrana T, Sanchez-Esteban J. Mechanotransduction as an Adaptation to Gravity. Front Pediatr. 2016;4:140. doi: 10.3389/fped.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. A theory of biological relativity: no privileged level of causation. Interface focus. 2012;2:55–64. doi: 10.1098/rsfs.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgeig S, Daniels CB, Johnston SD, Sullivan LC. The pattern of surfactant cholesterol during vertebrate evolution and development: does ontogeny recapitulate phylogeny? Reprod Fertil Dev. 2003;15:55–73. doi: 10.1071/rd02087. [DOI] [PubMed] [Google Scholar]
- Perry SF, Carrier DR. The coupled evolution of breathing and locomotion as a game of leapfrog. Physiol Biochem Zool. 2006;79:997–999. doi: 10.1086/507657. [DOI] [PubMed] [Google Scholar]
- Pievani T, Serrelli E. Exaptation in human evolution: how to test adaptive vs exaptive evolutionary hypotheses. J Anthropol Sci. 2011;89:9–23. doi: 10.4436/jass.89015. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Cooper AJ. From cholesterogenesis to steroidogenesis: role of riboflavin and flavoenzymes in the biosynthesis of vitamin D. Adv Nutr. 2014;5:144–163. doi: 10.3945/an.113.005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piraino S, Boero F, Aeschbach B, Schmid V. Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa) University of Chicago Press; Chicago: 1996. [DOI] [PubMed] [Google Scholar]
- Pirola CJ, Wang HM, Strgacich MI, Kamyar A, Cercek B, Forrester JS, Clemens TL, Fagin JA. Mechanical stimuli induce vascular parathyroid hormone-related protein gene expression in vivo and in vitro. Endocrinology. 1994;134:2230–2236. doi: 10.1210/endo.134.5.8156926. [DOI] [PubMed] [Google Scholar]
- Prigogine I, Stengers I. The End of Certainty. The Free Press; New York: 1997. [Google Scholar]
- Prosser SL, Pelletier L. Mitotic spindle assembly in animal cells: a fine balancing act. Nat Rev Mol Cell Biol. 2017;18:187–201. doi: 10.1038/nrm.2016.162. [DOI] [PubMed] [Google Scholar]
- Purevdorj-Gage B, Sheehan KB, Hyman LE. Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:4569–4575. doi: 10.1128/AEM.03050-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purevdorj-Gage B, Sheehan KB, Hyman LE. Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol. 2006;72:4569–4575. doi: 10.1128/AEM.03050-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KR. Two faces of consciousness: A look at eastern and western perspectives. J Consciousness Studies. 1998;5:309–327. [Google Scholar]
- Rehan VK, Sakurai R, Wang Y, Santos J, Huynh K, Torday JS. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation bystimulants of parathyroid hormone-related protein signaling. Lung. 2007;185:151–159. doi: 10.1007/s00408-007-9007-0. [DOI] [PubMed] [Google Scholar]
- Rehan VK, Torday JS. Exploiting the PTHrP signaling pathway to treat chronic lung disease. Drugs Today (Barc) 2007;43:317–331. doi: 10.1358/dot.2007.43.5.1062665. [DOI] [PubMed] [Google Scholar]
- Rosel D, Khurana T, Majithia A, Huang X, Bhandari R, Kimmel AR. TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing. J Cell Sci. 2012;125:37–48. doi: 10.1242/jcs.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer M. Why we should choose science over beliefs. Sci Am. 2013:135. [Google Scholar]
- Schrodinger E. What is Life? Cambridge University Press; Cambridge: 1944. [Google Scholar]
- Sheehan KB, McInnerney K, Purevdorj-Gage B, Altenburg SD, Hyman LE. Yeast genomic expression patterns in response to low-shear modeled microgravity. BMC Genomics. 2007;8:3. doi: 10.1186/1471-2164-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone PL, Ehresmann AC. Some resonances between Eastern thought and Integral Biomathics in the framework of the WLIMES formalism for modeling living systems. Prog Biophys Mol Biol. 2017 doi: 10.1016/j.pbiomolbio.2017.05.014. (in press) [DOI] [PubMed] [Google Scholar]
- Speijer D. Oxygen radicals shaping evolution: why fatty acid catabolism leads to peroxisomes while neurons do without it: FADH2/NADH flux ratios determining mitochondrial radical formation were crucial for the eukaryotic invention of peroxisomes and catabolic tissue differentiation. Bioessays. 2011;33:88–94. doi: 10.1002/bies.201000097. [DOI] [PubMed] [Google Scholar]
- Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS One. 2012;7:e39581. doi: 10.1371/journal.pone.0039581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- Teller P. Relational holism and quantum mechanics. British Journal for the Philosophy of Science. 1986;37:71–81. [Google Scholar]
- Torday JS. Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology. Adv Space Res. 2003;32(8):1569–76. doi: 10.1016/S0273-1177(03)90397-8. [DOI] [PubMed] [Google Scholar]
- Torday JS. Homeostasis as the Mechanism of Evolution. Biology (Basel) 2015a;4:573–590. doi: 10.3390/biology4030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS. Pleiotropy as the Mechanism for Evolving Novelty: Same Signal, Different Result. Biology (Basel) 2015b;4:443–459. doi: 10.3390/biology4020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS. The cell as the mechanistic basis for evolution. Wiley Interdiscip Rev Syst Biol Med. 2015c;7:275–284. doi: 10.1002/wsbm.1305. [DOI] [PubMed] [Google Scholar]
- Torday JS. The Cell as the First Niche Construction. Biology (Basel) 2016:5. doi: 10.3390/biology5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS. Life Is Simple-Biologic Complexity Is an Epiphenomenon. Biology (Basel) 2016a:5. doi: 10.3390/biology5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS. Heterochrony as Diachronically Modified Cell-Cell Interactions. Biology (Basel) 2016b:5. doi: 10.3390/biology5010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS. The Emergence of Physiology and Form: Natural Selection Revisited. Biology (Basel) 2016:5. doi: 10.3390/biology5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr On the Evolution of the Mammalian Brain. Front Syst Neurosci. 2016a;10:31. doi: 10.3389/fnsys.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr The Unicellular State as a Point Source in a Quantum Biological System. Biology (Basel) 2016b:5. doi: 10.3390/biology5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr Life is determined by its environment. Int J Astrobiology. 2016c;15:345–350. doi: 10.1017/S1473550415000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr Phenotype as Agent for Epigenetic Inheritance. Biology (Basel) 2016d:5. doi: 10.3390/biology5030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr Biologic relativity: Who is the observer and what is being observed? Prog Biophys Mol Biol. 2016e;121:29–34. doi: 10.1016/j.pbiomolbio.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Miller WB., Jr A systems approach to physiologic evolution: From micelles to consciousness. J Cell Physiol. 2017 Jan 23; doi: 10.1002/jcp.25820. [DOI] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. Mechanotransduction determines the structure and function of lung and bone: a theoretical model for the pathophysiology of chronic disease. Cell Biochem Biophys. 2003;37:235–46. doi: 10.1385/cbb:37:3:235. [DOI] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. A cell-molecular approach predicts vertebrate evolution. Mol Biol Evol. 2011;28:2973–2981. doi: 10.1093/molbev/msr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. Evolutionary Biology, Cell-Cell Communication and Complex Disease. Wiley; Hoboken: 2012. [Google Scholar]
- Today JS, Rehan VK. On the evolution of the pulmonary alveolar lipofibroblast. Exp Cell Res. 2016b;340:215–219. doi: 10.1016/j.yexcr.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, Rehan VK. Evolution, the Logic of Biology. Wiley-Blackwell; London: 2017. [Google Scholar]
- Torres J, Di Como CJ, Herrero E, De La Torre-Ruiz MA. Regulation of the cell integrity pathway by rapamycin-sensitive TOR function in budding yeast. J Biol Chem. 2002;277:43495–43504. doi: 10.1074/jbc.M205408200. [DOI] [PubMed] [Google Scholar]
- Touati SA, Wassmann K. How oocytes try to get it right: spindle checkpoint control in meiosis. Chromosoma. 2016;125:321–335. doi: 10.1007/s00412-015-0536-7. [DOI] [PubMed] [Google Scholar]
- Trerotola M, Relli V, Simeone P, Alberti S. Epigenetic inheritance and the missing heritability. Hum Genomics. 2015;9:17. doi: 10.1186/s40246-015-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine J. Phyla. University of Chicago Press; Chicago: 2004. [Google Scholar]
- Vishwanath A, Yao NY, Monroe C. Observation of a discrete time crystal. Nature. 2017;543:217–220. doi: 10.1038/nature21413. [DOI] [PubMed] [Google Scholar]
- Witzany G. Life is physics and chemistry and communication. Ann N Y Acad Sci. 2015;1341:1–9. doi: 10.1111/nyas.12570. [DOI] [PubMed] [Google Scholar]
- Wollam J, Antebi A. Sterol regulation of metabolism, homeostasis, and development. Annu Rev Biochem. 2011;80:885–916. doi: 10.1146/annurev-biochem-081308-165917. [DOI] [PMC free article] [PubMed] [Google Scholar]