Abstract
Mucormycosis is a fungal infection with fulminant angioinvasion leading to high morbidity and mortality in susceptible individuals. The major predisposing conditions are uncontrolled diabetes, neutropenia, malignancies, receipt of a transplant and traumatic injury [1]. Over the past decade, mucormycosis has become an emerging fungal infection due to the increase in patient groups presenting with these pre-disposing conditions and our medical advances in diagnosing the infection [2-4]. Yet, we currently lack clinical interventions to treat mucormycosis effectively. This in turn is due to a lack of understanding of mucormycosis pathogenesis.
Here, we discuss our current understanding of selected aspects of interactions at the mucormycete-host interface. We will highlight open questions that might guide future research directions for investigations into the pathogenesis of mucormycosis and potential innovative therapeutic approaches.
Innate immune responses during mucormycosis
Once a pathogen has overcome our non-specific barriers (e.g. skin and mucosal layers), innate immune effectors such as macrophages and neutrophils are our first cellular response against the foreign attack. Many fungal pathogens (e.g. Cryptococcus, Candida, Coccidioides species and Histoplasma capsulatum) have been recognized as intracellular pathogens of phagocytes (reviewed in [5]). Similarly, there is growing evidence that pathogenic Mucorales species can adapt an intracellular life style within these innate immune effectors.
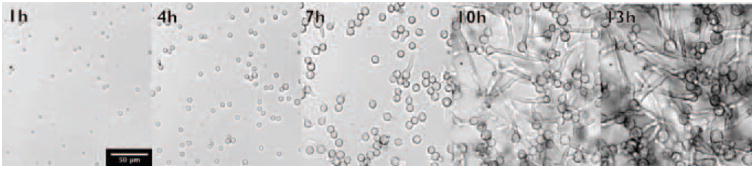
The infectious particles for mucormycosis are asexual sporangiospores found ubiquitously within the environment. These resting spores can swell and germinate to produce fast-growing hyphae during their natural life cycle (Figure 1) [6]. Germination and filamentous growth within a host causes angioinvasion, vessel thrombosis and necrosis [7-9].
Figure 1. Spore germination and filamentous growth of Rhizopus microsporus.
Resting spores start to swell shortly after incubation in rich media. First germ tubes are produced after approximately 7 hours incubation with a hyphal network established at 13 hours incubation. Scale bar 50 μm.
Monocytes, macrophages and natural killer (NK) cells can recognize and damage, but are unable to kill, hyphae. Conversely, filamentous forms are effectively killed by human polymorphonuclear leukocytes (PMNs) in vitro [10-13]. Invasive fungal growth activates pro-inflammatory signaling. Hyphae interact with TLR-2 on the surface of human PMNs inducing transcription of the proinflammatory cytokines TNF-α and IL-1β [14]. Human monocyte derived dendritic cells recognize β-glucan exclusively expressed on the hyphal surface through the pattern recognition receptor dectin-1 to induce IL-23, IL-1 and TNF-α [15]. Damage and killing is mediated by oxidative means after monocyte or neutrophil attachment to fungal filaments [16-18], through degranulation, and release of cationic peptides or perforin by rabbit and human neutrophils or NK cells, respectively [12,18-20]. Hydrocortisone treatment inhibits neutrophil or macrophage induced hyphal damage [18,21] and macrophages from diabetic mouse have reduced ability to adhere to hyphae [17]. Even in healthy hosts, the extent of hyphal damage depends on the extent of fungal biomass [12,22].
Mucormycetes are extremely fast-growing fungi and thus are likely to outcompete our immune response when it is in state of suppression. Hyphal growth is essential for virulence in yeast-locked mutants of Mucor circinelloides. Inhibition of the calcineurin pathway that regulates hyphal growth chemically through the calcineurin inhibitor FK506 or by mutation of the calcineurin regulatory subunit cnbR significantly reduced virulence of M. circinelloides in wax moth larvae [23]. Mucorales species with fast germination rates (e.g. Cunninghamella bertholletiae) are significantly more virulent than species with slower germination rates (e.g. Rhizopus oryzae, R. microspores, M. circinelloides) in a neutropenic rabbit model of pulmonary mucormycosis. The increased virulence is characterized by higher lung burden, amplified angioinvasion and lower survival [24]. Likewise, M. circinelloides isolates with larger spores germinate faster and are more virulent in the wax moth larva and a murine intraperitoneal infection model [25]. Thus, a protective immune response might require spore clearance before onset of filamentous growth.
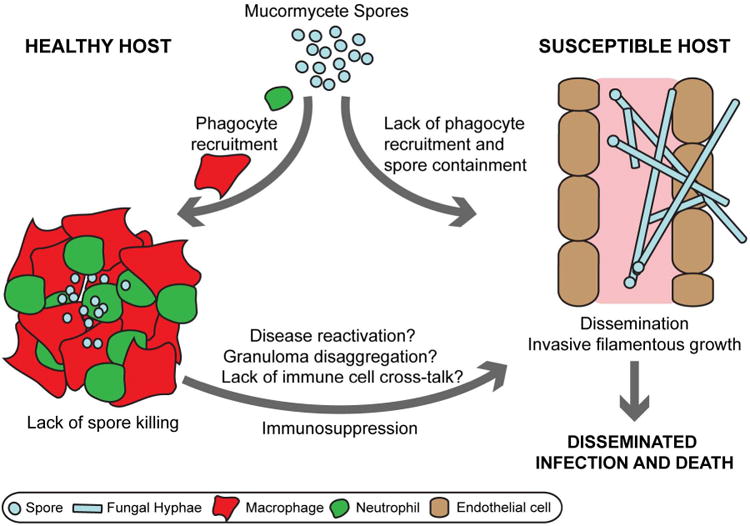
After infection with mucormycete spores, phagocytes are recruited rapidly to the site of infection to internalize and form tight clusters around spores in rabbit [26,27], mouse [9,28,29] and zebrafish larval models of disease [30]. A lack or delay of this early inflammatory response renders diabetic hosts susceptible to infection leading to disease dissemination [17,27,30]. Yet, phagocytes are not able to kill resting spores in vitro or in vivo in vertebrate [9,29,30] and non-vertebrate model systems [31]. To establish within the phagocytic niche, mucormycete spores must either withstand the harsh environment or subvert phagocyte anti-microbial mechanisms. It has been demonstrated that Rhizopus oryzae downregulates the transcription of host defense genes (e.g. immune-inducible peptides) in infected fruit flies [31]. Resting spores are not able to elicit a pro-inflammatory cytokine response in dendritic cells [15] whilst hyphae also inhibit IFN-γ expression by IL-2 stimulated human natural killer cells [12,13]. The human macrophage-like cell line THP-1 failed to express proinflammatory cytokines in response to M. circinelloides or R. oryzae compared to A. fumigatus or C. albicans [32]. The oxidative burst elicited from PMNs by mucormycete spores is strain dependent and reflects the virulence potential. For example, intermediate virulent strains belonging to the Rhizopus genus induce a smaller reactive burst than the low virulence strain Lichtheimia corymbifera [33,34]. Resting spores are resistant to cationic peptides released from neutrophils in vitro [19]. Although phagocytes fail to kill spores, they effectively prevent spore germination in healthy murine hosts [17,35,36]. Rat alveolar macrophages, but not the human macrophage cell line THP-1, inhibit spore germination through nitric oxide [37]. In susceptible mice with induced diabetes or treated with corticosteroids, inhibition of spore germination by bronchoalveolar macrophages fails allowing for filamentous growth [17,36].
Interestingly, disease can be reactivated from granulomatous clusters during acute diabetic acidosis in rabbits [26]. This opens the possibility of latent infections with Mucorales and disease reactivation in previously healthy hosts after acquired immunosuppression. Yet, we have little knowledge on the virulence factors that enable Mucorales spores to reside insight phagocytes and granulomas. At the same time, the unique enhanced susceptibility of uncontrolled diabetics and DKA patients indicates that immune responses to Mucorales are distinct from other fungal pathogens and/or Mucorales possess virulence traits that enable them to thrive in such hosts (Table 1). Thus, we need a better understanding of the mechanisms employed to establish intracellular survival within phagocytes and the phagocytic defects induced by predisposing conditions that allow spore germination.
Table 1. Proven virulence traits of Mucorales.
| Virulence trait | Function | References |
|---|---|---|
| High affinity iron permease (Ftr1p) | Acquisition of host iron | [51,57] |
| Ferrioxamine receptors (Fob1 and Fob2) | Acquisition of iron from ferrioxamine | [52] |
| Fungal Spore coating protein (CotH) | Invasion of the endothelium | [47] |
| Host Glucose regulated protein 78 (GRP78) | Invasion of the endothelium | [46,49] |
| Host Platelet-derived growth factor receptor (PDGFR) | Invasion of host cells | [48] |
| Spore size | Faster germination | [25] |
| calcineurin pathway | Regulation of hyphal growth | [23,58] |
| Uncharacterized toxins | Host cell damage and possible induction of inflammatory response | [32,44] |
Platelets are known to play a role in antimicrobial host defense against several pathogens by secretion of platelet microbicidal proteins [38]. Platelets were shown to adhere to Mucorales, induce time dependent damage to fungal hyphae and suppress hyphal elongation through a granule dependent mechanism [39].
Taken together, protection from mucormycosis by the innate immune system relies on the control of spores residing in phagocytes and granulomatous clusters to inhibit spore germination. In susceptible individuals, this control is lost leading to filamentous fungal growth. Increasing evidence, supporting Mucorales as intracellular pathogens within granulomas, poses the possibility of latent infections. This might offer new therapeutic strategies targeting resting spores before onset of fulminant hyphal growth in prophylactic approaches.
Adaptive immunity during mucormycosis
There is limited evidence for a major role of the adaptive immune system in combating mucormycosis. HIV alone is not a predisposing condition for disease, though cases have been reported in this patient population in association with intravenous drug or corticosteroid use and neutropenia [40]. Similarly, T-lymphocyte depletion in mice does not increase susceptibility to mucormycosis [41].
As with the innate immune response, CD4+ and CD8+ T-cell are only produced in response to hyphae [42] and during invasive mucormycosis [43]. However, these T-cells are lost soon after resolution of infection [43]. Both sets of T-cells produce a range of cytokines including IL-4, IFN-γ, IL-10 and IL-17 [43]. CD4+ cells are predominant and show cross-reactivity with a range of other fungal pathogens (Aspergillus fumigatus, Penicillum chrysogenum and C. albicans) in healthy individuals [42]. Although spores can persist in hosts, clearance of R. pusillus from lungs of infected mice has been reported after approximately 30 days [35]. This indicates some relevance of an adaptive immune response that warrants further investigation and might be relevant for future development of immunotherapeutic approaches against the disease.
The mucormycete-epithelial and mucormycete-endothelial interface
There has not been much work conducted on studying the interactions of mucormycetes and epithelial cells, despite these interactions representing some of the earliest events during infection. A study linked outbreak of food poisoning due to intake of yogurt to contamination with Mucor circinelloides [32]. This study demonstrated that Mucorales produce secondary metabolites that are toxic to the gastrointestinal mucosa. Similarly, dead Mucorales can cause considerable host cell damage in vitro supporting the presence of toxins [44]. It is possible that these toxic substances are responsible for the clinical feature of extensive tissue necrosis. It is also known that Rhizopus spores can adhere to extracellular matrix proteins such as laminin and type IV collagen [45] that embeds epithelial or endothelial cells.
Unlike epithelial cells, considerable work has been conducted on interactions of Mucorales and endothelial cells because of the angioinvasive nature of the disease. It was found that Mucorales adhere to, and invade human umbilical vein endothelial cells through specific and unique binding capacity to the heat shock glucose-regulated protein 78 (GRP78) [46]. This interaction occurs via the unique cell surface CotH invasins (Figure 2) [47] and results in a substantial injury to the endothelium in vitro [46]. CotH proteins are universally present in Mucorales and absent from other pathogens [48]. Interestingly, elevated glucose, iron, and β-Hydroxy butyrate (BHB) concentrations (relevant to levels seen in diabetic ketoacidosis patients) induces endothelial cell invasion and damage by Rhizopus and promotes virulence in mice due to surface overexpression of both GRP78 and CotH proteins [46,47,49]. It appears that during these interactions acquisition of host iron via several mechanisms (e.g. high affinity iron permease, and ferrioxamine receptors) is critical in determining the fate of infection [50-52]. Importantly, antibodies targeting GRP78/CotH interactions reduce Mucorales-induced invasion and injury of endothelial cells and protect mice from mucormycosis [46,49]. These results provide insights into why patients with diabetic ketoacidosis are uniquely predisposed to mucormycosis infections and point to potentially novel immunotherapeutic interventions.
Figure 2. Colocalization of host cell GRP78 and R. delemar CotH during invasion of human umbilical vein endothelial cells.
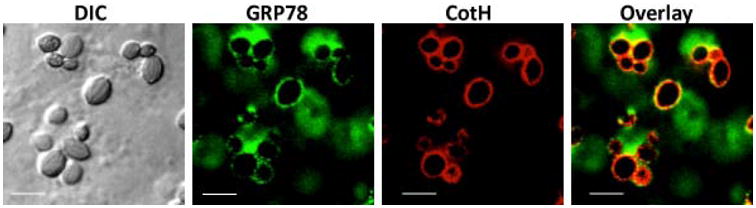
GRP78 (green) is labeled with Alexa Fluor 488, CotH (red) is labeled with Alexa Fluor 658. Merged image show colocolization (yellow) of endocytosed fungal swollen spores ∼60 min after incubation. Scale bar 10 μm.
Clinical relevance and application
Much of the focus in understanding the immune responses to mucormycosis is focused on invasive disease. While this knowledge is critical in our understanding on how mucormycosis progressively develops into a disseminated infection and ultimately will help in designing adjunctive therapies to improve outcome, understanding early events in the course of infection is likely to add therapeutic strategies that act synergistically with strategies targeting angioinvasion. Further, understanding early infection events can develop preventative measures in targeted populations. For example, this review highlights the inability of innate immune effectors in susceptible hosts to inhibit the transition to filamentous growth and the quick growing nature of Mucorales hyphae as the main contributors to the high mortality during mucormycosis. Together with the possibility of latent infections of this emerging intracellular pathogen, development of new treatments can focus on either inhibiting the fungal ability to undergo germination or enable protective immunity targeting spores before onset of invasive disease.
Although we know a range of environmental factors that initiate spore germination (e.g. pH, nutrient availability, hydrophobicity), we currently lack an understanding of the genetic regulation of this developmental process. Likewise, we have little information on the virulence determinants enabling spores to survive within phagocytes. Whilst research has been hindered by lack of genetic tractability of Mucorales, a range of tools has become available in recent years. Whole genome projects and comparative genomics have revealed a genome wide duplication and gene family expansions for ergosterol synthesis pathway (e.g. lanosterol 14α-demethylase), GTPases, secreted proteases and cell wall synthesis enzymes that could support resistance to antifungals and adaptation to changing environments [48,53]. In addition, targeted gene attenuation in Rhizopus can reliably be achieved using RNAi techniques [47,51,52]. Finally, the community will benefit from a recently published RNAi-based knock out library of M. circinelloides enabling screens for genes involved in germination and virulence [54].
Protective immunity could be achieved by correcting immune deficiencies in susceptible patients or inhibition of virulence strategies employed by Mucorales (e.g. neutralization of CotH with antibodies [47]). In the context of mucormycosis, adjuvant cytokine treatments have proven some efficacy. GM-CSF and GM-CSF in combination with IFN-γ increase antifungal activity of PMNs by increasing the oxidative burst in vitro [33,34], whilst GM-CSF in combination with liposomal amphotericin B improved the survival of mice with systemic mucormycosis [55]. Recovery of normal blood pH in mice with β-Hydroxy butyrate (BHB) induced acidosis through bicarbonate treatment significantly increased survival of mucormycosis in prophylaxis or therapeutic mouse models [49]. Lastly, isolation and proliferation of T-cells increased phagocytic capacity and reactive oxygen burst in response to mucormycetes in vitro and might offer the possibility of adoptive immune cell transfer in the future [42,56]. The timing of any clinical intervention and immunomodulation should be considered carefully in the context of mucormycosis.
Conclusion and Future Research Directions
The rise of the number of susceptible individuals together with current lack of effective treatment requires further research into the host-pathogen interactions during mucormycosis and will enable us to devise new and more effective treatments for this debilitating disease.
Highlights.
The innate immune system controls mucormycete spores by inhibiting spore germination
There is limited evidence for adaptive immunity in combating mucormycosis
Host iron acquisition is the determining factor for progression of mucormycosis on the endothelial interface
Acknowledgments
ASI was supported in part by Public Health Service grant R01 AI063503. KV was supported by a Wellcome Seed Award (108387/Z/15/Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 2.Bitar D, Lortholary O, Le Strat Y, Nicolau J, Coignard B, Tattevin P, Che D, Dromer F. Population-based analysis of invasive fungal infections, france, 2001-2010. Emerg Infect Dis. 2014;20:1163–1169. doi: 10.3201/eid2007.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 4.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne JR, Ganesan A, Li P, Bradley W, Gaskins LJ, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012;55:1441–1449. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith LM, May RC. Mechanisms of microbial escape from phagocyte killing. Biochem Soc Trans. 2013;41:475–490. doi: 10.1042/BST20130014. [DOI] [PubMed] [Google Scholar]
- 6.Medwid RD, Grant DW. Germination of Rhizopus oligosporus Sporangiospores. Appl Environ Microbiol. 1984;48:1067–1071. doi: 10.1128/aem.48.6.1067-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125:375–378. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 8.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JM. In vivo development of spores of Absidia ramosa. Sabouraudia. 1976;14:11–15. [PubMed] [Google Scholar]
- 10.Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986;78:511–524. doi: 10.1172/JCI112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simitsopoulou M, Roilides E, Maloukou A, Gil-Lamaignere C, Walsh TJ. Interaction of amphotericin B lipid formulations and triazoles with human polymorphonuclear leucocytes for antifungal activity against Zygomycetes. Mycoses. 2008;51:147–154. doi: 10.1111/j.1439-0507.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt S, Schneider A, Demir A, Lass-Florl C, Lehrnbecher T. Natural killer cell-mediated damage of clinical isolates of mucormycetes. Mycoses. 2016;59:34–38. doi: 10.1111/myc.12431. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt S, Tramsen L, Perkhofer S, Lass-Florl C, Hanisch M, Roger F, Klingebiel T, Koehl U, Lehrnbecher T. Rhizopus oryzae hyphae are damaged by human natural killer (NK) cells, but suppress NK cell mediated immunity. Immunobiology. 2013;218:939–944. doi: 10.1016/j.imbio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. 2008;52:722–724. doi: 10.1128/AAC.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamilos G, Ganguly D, Lande R, Gregorio J, Meller S, Goldman WE, Gilliet M, Kontoyiannis DP. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One. 2010;5:e12955. doi: 10.1371/journal.pone.0012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond RD, Haudenschild CC, Erickson NF., 3rd Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982;38:292–297. doi: 10.1128/iai.38.1.292-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldorf AR, Levitz SM, Diamond RD. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984;150:752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- 18.Diamond RD, Clark RA. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982;38:487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levitz SM, Selsted ME, Ganz T, Lehrer RI, Diamond RD. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154:483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- 20.Diamond RD, Krzesicki R, Epstein B, Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978;91:313–328. [PMC free article] [PubMed] [Google Scholar]
- 21.Diamond RD. Inhibition of monocyte-mediated damage to fungal hyphae by steroid hormones. J Infect Dis. 1983;147:160. doi: 10.1093/infdis/147.1.160. [DOI] [PubMed] [Google Scholar]
- 22.Antachopoulos C, Demchok JP, Roilides E, Walsh TJ. Fungal biomass is a key factor affecting polymorphonuclear leucocyte-induced hyphal damage of filamentous fungi. Mycoses. 2010;53:321–328. doi: 10.1111/j.1439-0507.2009.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SC, Li A, Calo S, Heitman J. Calcineurin Plays Key Roles in the Dimorphic Transition and Virulence of the Human Pathogenic Zygomycete Mucor circinelloides. PLoS Pathog. 2013;9:e1003625. doi: 10.1371/journal.ppat.1003625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petraitis V, Petraitiene R, Antachopoulos C, Hughes JE, Cotton MP, Kasai M, Harrington S, Gamaletsou MN, Bacher JD, Kontoyiannis DP, et al. Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med Mycol. 2013;51:72–82. doi: 10.3109/13693786.2012.690107. [DOI] [PubMed] [Google Scholar]
- 25.Li CH, Cervantes M, Springer DJ, Boekhout T, Ruiz-Vazquez RM, Torres- Martinez SR, Heitman J, Lee SC. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011;7:e1002086. doi: 10.1371/journal.ppat.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheldon WH, Bauer H. Activation of quiescent mucormycotic granulomata in rabbits by induction of acute alloxan diabetes. J Exp Med. 1958;108:171–178. doi: 10.1084/jem.108.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheldon WH, Bauer H. The development of the acute inflammatory response to experimental cutaneous mucormycosis in normal and diabetic rabbits. J Exp Med. 1959;110:845–852. doi: 10.1084/jem.110.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldorf AR, Diamond RD. Cerebral mucormycosis in diabetic mice after intrasinus challenge. Infect Immun. 1984;44:194–195. doi: 10.1128/iai.44.1.194-195.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbel MJ, Eades SM. Observations on the localization of Absidia corymbifera in vivo. Sabouraudia. 1978;16:125–132. [PubMed] [Google Scholar]
- 30.Voelz K, Gratacap RL, Wheeler RT. A zebrafish larval model reveals early tissue-specific innate immune responses to Mucor circinelloides. Dis Model Mech. 2015;8:1375–1388. doi: 10.1242/dmm.019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamilos G, Lewis RE, Hu J, Xiao L, Zal T, Gilliet M, Halder G, Kontoyiannis DP. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A. 2008;105:9367–9372. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SC, Billmyre RB, Li A, Carson S, Sykes SM, Huh EY, Mieczkowski P, Ko DC, Cuomo CA, Heitman J. Analysis of a Food-Borne Fungal Pathogen Outbreak: Virulence and Genome of a Mucor circinelloides Isolate from Yogurt. MBio. 2014;5 doi: 10.1128/mBio.01390-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gil-Lamaignere C, Simitsopoulou M, Roilides E, Maloukou A, Winn RM, Walsh TJ. Interferon- gamma and granulocyte-macrophage colony-stimulating factor augment the activity of polymorphonuclear leukocytes against medically important zygomycetes. J Infect Dis. 2005;191:1180–1187. doi: 10.1086/428503. [DOI] [PubMed] [Google Scholar]
- 34.Liles WC, Huang JE, van Burik JA, Bowden RA, Dale DC. Granulocyte colony- stimulating factor administered in vivo augments neutrophil-mediated activity against opportunistic fungal pathogens. J Infect Dis. 1997;175:1012–1015. doi: 10.1086/513961. [DOI] [PubMed] [Google Scholar]
- 35.Waldorf AR, Peter L, Polak A. Mucormycotic infection in mice following prolonged incubation of spores in vivo and the role of spore agglutinating antibodies on spore germination. Sabouraudia. 1984;22:101–108. doi: 10.1080/00362178485380171. [DOI] [PubMed] [Google Scholar]
- 36.Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74:150–160. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorens PG, Boelaert JR, Halloy V, Zamora R, Schneider YJ, Herman AG. Human and rat macrophages mediate fungistatic activity against Rhizopus species differently: in vitro and ex vivo studies. Infect Immun. 1995;63:4489–4494. doi: 10.1128/iai.63.11.4489-4494.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeaman MR. The Role of Platelets in Antimicrobial Host Defense. Clinical Infectious Diseases. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 39.Perkhofer S, Kainzner B, Kehrel BE, Dierich MP, Nussbaumer W, Lass-Florl C. Potential antifungal effects of human platelets against zygomycetes in vitro. J Infect Dis. 2009;200:1176–1179. doi: 10.1086/605607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira J, Varon A, Galhardo MC, Santos F, Lyra M, Castro R, Oliveira R, Lamas CC. The burden of mucormycosis in HIV-infected patients: A systematic review. Journal of Infection. 2016;73:181–188. doi: 10.1016/j.jinf.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Corbel MJ, Eades SM. Factors determining the susceptibility of mice to experimental phycomycosis. J Med Microbiol. 1975;8:551–564. doi: 10.1099/00222615-8-4-551. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt S, Tramsen L, Perkhofer S, Lass-Florl C, Roger F, Schubert R, Lehrnbecher T. Characterization of the cellular immune responses to Rhizopus oryzae with potential impact on immunotherapeutic strategies in hematopoietic stem cell transplantation. J Infect Dis. 2012;206:135–139. doi: 10.1093/infdis/jis308. [DOI] [PubMed] [Google Scholar]
- 43.Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Zanetti E, Quadrelli C, Candoni A, Maertens J, Rossi G, et al. Mucorales-specific T cells emerge in the course of invasive mucormycosis and may be used as a surrogate diagnostic marker in high-risk patients. Blood. 2011;118:5416–5419. doi: 10.1182/blood-2011-07-366526. [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE., Jr Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778–783. doi: 10.1128/IAI.73.2.778-783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70:76–83. [PubMed] [Google Scholar]
- 46.Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Jr, Filler SG, Ibrahim AS. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–1924. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebremariam T, Liu MF, Luo GPS, Bruno V, Phan QT, Waring AJ, Edwards JE, Filler SG, Yeaman MR, Ibrahim AS. CotH3 mediates fungal invasion of host cells during mucormycosis. Journal of Clinical Investigation. 2014;124:237–250. doi: 10.1172/JCI71349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chibucos MC, Soliman S, Gebremariam T, Lee H, Daugherty S, Orvis J, Shetty AC, Crabtree J, Hazen TH, Etienne KA, et al. An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nature Communications. 2016;7:12218. doi: 10.1038/ncomms12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebremariam T, Lin L, Liu M, Kontoyiannis DP, French S, Edwards JE, Jr, Filler SG, Ibrahim AS. Bicarbonate correction of ketoacidosis alters host-pathogen interactions and alleviates mucormycosis. J Clin Invest. 2016;126:2280–2294. doi: 10.1172/JCI82744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Jr, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim AS, Gebremariam T, Lin L, Luo G, Husseiny MI, Skory CD, Fu Y, French SW, Edwards JE, Jr, Spellberg B. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol Microbiol. 2010;77:587–604. doi: 10.1111/j.1365-2958.2010.07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu M, Lin L, Gebremariam T, Luo G, Skory C, French SW, Chou TF, Edward JEJ, ibrahim AS. Fob1 and Fob2 Proteins Are Virulence Determinants of Rhizopus oryzae via Facilitating Iron Uptake from Ferrioxamine. PLoS Pathog. 2015;11:e1004842. doi: 10.1371/journal.ppat.1004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma LJ, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, Elias M, Idnurm A, Lang BF, Sone T, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trieu TA, Navarro-Mendoza MI, Perez-Arques C, Sanchis M, Capilla J, Navarro-Rodriguez P, Lopez-Fernandez L, Torres-Martinez S, Garre V, Ruiz-Vazquez RM, et al. RNAi-Based Functional Genomics Identifies New Virulence Determinants in Mucormycosis. PLoS Pathog. 2017;13:e1006150. doi: 10.1371/journal.ppat.1006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez MM, Calvo E, Marine M, Pastor FJ, Fernandez-Ballart J, Guarro J. Efficacy of liposomal amphotericin B combined with gamma interferon or granulocyte-macrophage colony-stimulating factor for treatment of systemic zygomycosis in mice. Antimicrob Agents Chemother. 2009;53:3569–3571. doi: 10.1128/AAC.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tramsen L, Schmidt S, Boenig H, Latge JP, Lass-Florl C, Roeger F, Seifried E, Klingebiel T, Lehrnbecher T. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy. 2013;15:344–351. doi: 10.1016/j.jcyt.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Fu Y, Lee H, Collins M, Tsai HF, Spellberg B, Edwards JE, Jr, Kwon-Chung KJ, Ibrahim AS. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol Lett. 2004;235:169–176. doi: 10.1016/j.femsle.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 58.Lee SC, Li A, Calo S, Inoue M, Tonthat NK, Bain JM, Louw J, Shinohara ML, Erwig LP, Schumacher MA, et al. Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol Microbiol. 2015;97:844–865. doi: 10.1111/mmi.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]


