Abstract
Purpose: To investigate the efficacy and safety of dexamethasone sodium phosphate administered through Visulex system (DSP-Visulex) in treating experimental uveitis.
Methods: Uveitis was induced in rabbits by subcutaneous injections of complete Freund's adjuvant and an intravitreal injection of H37RA antigen. After induction, the animals of the control group received no treatment and the others received various treatment regimens of DSP-Visulex. Each regimen was different in DSP strength (4%, 8%, and 15%), application time, or treatment frequency. Efficacy and safety of DSP-Visulex were evaluated by ophthalmic observations and histopathological examinations for ocular inflammations and pathology.
Results: The control group exhibited panuveitis with significant inflammation in the vitreous, choroid, and retina, but less in the conjunctiva, cornea, and anterior chamber. The uveitis occurred within 24 h after induction and persisted throughout the study in the control group. All treatments showed some reduction in inflammation in the vitreous, choroid, and retina. The higher dose regimens generally showed more rapid and higher degree of resolution than the lower dose regimens. The posterior eye tissues of the 15% and 8% DSP-Visulex appeared normal with minimal or no inflammation, whereas the untreated eye and the 4% DSP-Visulex eyes showed minimal response.
Conclusions: All DSP-Visulex regimens suppressed the signs of inflammation and were well tolerated over the course of a 29-day study. The 8% and 15% DSP-Visulex treatment regimens were safe and efficacious for anterior, intermediate, and posterior uveitis. On the other hand, the 4% DSP-Visulex regimen may only be considered for anterior and intermediate uveitis.
Keywords: : noninvasive, ocular drug delivery, experimental uveitis, dexamethasone, topical treatment
Introduction
Uveitis is a leading cause of blindness in the United States with 10%–15% of all cases.1–4 Dexamethasone is a potent corticosteroid widely prescribed in ophthalmology for more than 60 years, including the treatment of uveitis.5 Primary treatment options for anterior uveitis entail frequent dosing of eye drops,6 which often leads to poor response due to poor patient compliance.7,8 On the other hand, primary available treatment options for intermediate and posterior uveitis are restricted to either oral medications with significant systemic side effects or local invasive methods, such as periocular injections, intravitreal (IVT) injections, or implantations of a sustained release drug delivery device (eg, Ozurdex®, Retisert®, and Iluvien®) into the eye.5,6 Although local invasive methods are effective, they do pose significant risks9–12 including retinal detachment, endophthalmitis, increased intraocular pressure, and cataractogenesis, and high costs associated with administration. There is an unmet need for a new drug delivery system that addresses these challenges.
Treating back of the eye diseases using topical administration is feasible.13–18 Recent publications suggest that, for topically administered drugs, the transscleral pathway can be a route for a drug molecule to reach posterior eye tissues.19–25 The recent fluorophotometry study in live rabbits suggests that once drug is placed intrasclerally, there is an active, convective flow carrying drug molecules through the suprachoroidal space to the retina–choroid region at the back of the eye.26 To administer drug through this pathway effectively, a high drug concentration on the sclera is an ideal prerequisite. This is because drug diffuses across eye tissues by concentration gradients as described by Fick's first law of diffusion: Flux = PA(C1-C2). Flux is the amount of drug that passes through a membrane per period of time (mg/s). P is the permeability coefficient of the permeant (cm/s), A is the surface area (cm2) over which diffusion is taking place, and (C1-C2) is the difference in concentration (mg/mL) of the permeant across the membrane for the direction of flow from C1 to C2. Thus, a high concentration in the applicator (C1) may significantly increase flux. However, the main challenge of the topical ophthalmic products affecting drug permeation through the sclera is the retention time at the site of application. Particularly, the volume of topically applied solutions is limited to only 30 μL and the amount of solution substantially lost within a fraction of a minute, primarily through drainage and tearing.27,28 Moreover, drug toxicity limits the drug concentrations used in the topical ophthalmic products, including eye drops and ointments.
Visulex-P (referred as Visulex in this publication) developed by Aciont Inc. is a noninvasive drug delivery system that administers drug by passive diffusion through the limbal sclera into the interior of the eye, utilizing the transscleral pathway. While topical ophthalmic products allow drug to spread all over the cornea and leak directly into the nasolacrimal duct leading to inefficient local ocular delivery and systemic exposure, Visulex significantly enhances ocular drug delivery and may reduce systemic exposure through 2 mechanisms. First is the placement of the applicator adjacent to the sclera, which closes off much of the surface clearance (ie, tearing and blinking), permitting optimal drug diffusion to the deeper section of the eye during treatment. Second is the high drug concentration in the applicator creating a main driving force to facilitate the effective passive diffusion through the eye tissues accessing the transscleral pathway. This allows Visulex to be a suitable candidate for a topical treatment using a high concentration of drug. To accomplish the high concentration driving force of dexamethasone, dexamethasone sodium phosphate (DSP), which is a highly water soluble form of dexamethasone, was selected as a candidate for the Visulex drug delivery system. A combination of high-concentration DSP solution and Visulex, called DSP-Visulex, was investigated in this study.
Experimental uveitis, also known as experimental autoimmune uveitis (EAU), has been a method for evaluation of various therapeutic agents as well as new drug delivery systems for intermediate and posterior uveitis.29–35 By preimmunization and challenge of Mycobacterium tuberculosis H37Ra antigen, this induction causes a severe panuveitis in rabbit that lasts for more than 4 weeks.29–36 Endotoxin-induced uveitis (EIU) is a severe panuveitis model used in the early development of DSP-Visulex; the process of uveitis induction is fast and simple.37 However, the severe uveitis condition of the EIU only lasts for a few days.37–39 It was deemed not suitable for longer term studies, including the study in which the weekly frequency of dosing is being considered.
The objective of this study was to assess the efficacy and safety of DSP-Visulex with various dosing regimens in treating the experimental uveitis rabbit. We hypothesized that DSP-Visulex could be a safe and efficacious treatment for panuveitis. The test parameters for the DSP-Visulex treatment regimens included the DSP concentration, application time, and treatment frequency. These data in combination with evaluation for toxicity and pharmacokinetics would facilitate the identification of optimal starting dosing regimen(s) for the first clinical study of DSP-Visulex.
Methods
Materials and animals
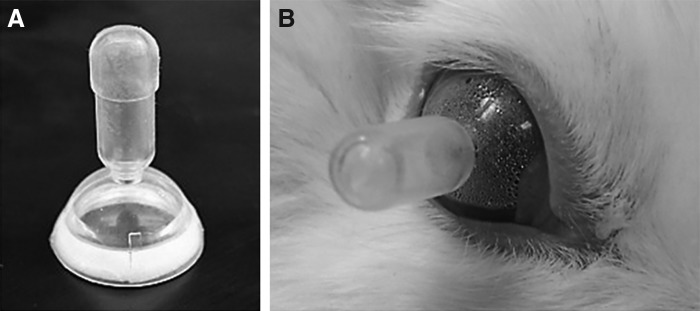
DSP USP grade was obtained from Letco Medical (Decatur, AL). The concentrations of DSP solution were 4.0%, 8.0%, and 15.0% w/v. All DSP formulations containing 0.01% w/v of EDTA (Sigma-Aldrich, St. Louis, MO) with pH adjusted to 7.0 with 1 M hydrochloric acid (LabChem, Zelienople, PA) were freshly prepared in doubly deionized water on the day of dosing using an aseptic technique. The Visulex™ applicator for use in rabbit studies was manufactured by Medical Murray (North Barrington, IL) and fabricated from medical grade silicone rubber, which incorporates a customized sponge material (3–5 mm wide) (Fig. 1). Young adult New Zealand White rabbits (both male and female), each weighing 3–4 kg, were obtained from Western Oregon Rabbit Co. (Philomath, OR). This study complied with the ARVO Statement for the use of Animals in Ophthalmic and Vision Research and was approved by The University of Utah Institutional Animal Care and Use Committee (Salt Lake City, UT). All animals were acclimated and observed for health issues for at least 2 weeks before being used in the study. Freund's complete adjuvant (FCA) and Mycobacterium tuberculosis H37Ra antigen were purchased from Difco Laboratories, Inc. (Detroit, MI), ketamine hydrochloride injectable USP (100 mg/mL) and sodium chloride 0.9% USP were from Hospira, Inc. (Lake Forest, IL), proparacaine hydrochloride ophthalmic solution and gentamicin sulfate ophthalmic solution were from Bausch & Lomb (Tampa, FL), and cyclopentolate hydrochloride ophthalmic solution was from Alcon Laboratories (Fort Worth, TX). The binocular indirect ophthalmoscope used was the Keeler All Pupil II from Keeler Instruments (Broomall, PA) and it was complemented with the double aspheric lens 20D/50 mm for posterior chamber examination from Volk Optical, Inc. (Mentor, OH).
FIG. 1.
(A) Visulex applicator: The Visulex applicator consists of silicone polymer shell incorporated with an annular white sponge located inside the rim of the applicator. Drug solution (eg, DSP) is loaded into the sponge before application. Only the sponge is in contact with the eye on the sclera and the effective surface area of sponge is ∼200 mm2. (B) DSP-Visulex on a rabbit eye: Visulex loaded with DSP solution is temporarily placed onto the eye to deliver DSP to the eye tissue for uveitis treatment. DSP, dexamethasone sodium phosphate.
Study design
Twenty-three animals were randomly assigned into 6 groups after uveitis induction of the right eye according to Table 1. Left eyes were not induced with uveitis to provide some vision in the animals throughout the study. The DSP treatment was on the affected eye (right eye). The first dose occurred ∼30 min after the uveitis induction on Day 1. Ocular examinations and clinical observation were performed during the weekday before and after each dosing. Following the final observations on Day 29, animals were anesthetized with a 2.5 mL intramuscular injection containing 5 mg ketamine and 30 mg xylazine per mL. Depth of anesthesia was confirmed by absence of corneal blink reflex or toe pinch response to ensure humane euthanasia. The animal was then sacrificed by an intracardiac injection of 2 mL of saturated KCl with a 3 mL syringe and 18GA × 1 needle. The eyes were collected and processed for histological evaluation. Please note that this was an exploratory study to understand various DSP-Visulex treatment regimens. The severity of the uveitic conditions limited the number of rabbits per group to 3 in the first part of the study. With the successful experience of the first part of the study, the same number of animals per group was kept for the rest of the study. The study was conducted in 3 parts, and each time a control group was conducted with the treatment group(s). Then, the results were pooled for analysis.
Table 1.
Study Design
| Group | Number of animals | DSP concentration (%) | Application time (minute) | Day of dosing |
|---|---|---|---|---|
| 1 | 8 | No treatment | — | — |
| 2 | 3 | 15 | 15 | 1, 8, 15, 22 |
| 3 | 3 | 15 | 10 | 1 |
| 4 | 3 | 8 | 10 | 1 |
| 5 | 3 | 8 | 5 | 1, 8, 15, 22 |
| 6 | 3 | 4 | 10 | 1, 8 |
DSP, dexamethasone sodium phosphate.
Uveitis induction
The uveitis induction was slightly modified from a rabbit model described by Cheng et al.29 The model provides a rationale to make a qualitative comparison of our DSP-Visulex system with an established dexamethasone implant technology. Rabbits were preimmunized by subcutaneous injections of 0.5 mL FCA H37Ra, a suspension of Mycobacterium tuberculosis H37Ra antigen in FCA. The Freund's Complete Adjuvant H37Ra containing 20 mg/mL of antigen was prepared by mixing dried M. tuberculosis H37 Ra antigen with the FCA. The preimmunized injections of FCA H37Ra were administered in the dorsal area of the animal's neck at 19 and 12 days before induction of uveitis. Then uveitis was induced on Day 1 by 100 μL IVT injection of a suspension containing 33 μg of the M. tuberculosis H37 Ra antigen in sterile balanced salt solution on the right eye using Hamilton syringe with a 30 Ga X ½ needle. No uveitis induction was performed on the left eye. Although a second IVT induction was planned on Day 15, it was not given due to the severity of inflammation in the control group eyes (Group 1). Rabbits were anesthetized with a 2.5 mL intramuscular injection containing 5 mg ketamine and 30 mg xylazine per mL. One drop each of proparacaine and gentamicin was administered to the eye before the IVT injection. The IVT injection entered through the limbus in the superior portion of the sclera and administered approximately in the middle of the vitreous.
Dose administration
Each rabbit was placed in a rabbit restrainer to limit movement during the DSP-Visulex administration. One drop of sterile proparacaine hydrochloride ophthalmic solution, a local anesthetic, was given to the right eye of each rabbit ∼5 min before dose administration. DSP solution (250 μL) was loaded into the Visulex applicator using an Eppendorf pipettor. The drug solution saturated the carrier matrix uniformly within a minute. Then, Visulex containing the drug formulation was gently applied to the scleral surface of the right eye of each rabbit. The position of the Visulex system was checked to ensure that the drug matrix was in immediate contact with the white scleral part of the eye, but not the cornea. Digital laboratory timers were used for accurate application times (treatment duration) of 5, 10, or 15 min. After the given treatment duration, the applicator was carefully removed from the eye.
Clinical observation
Body weights of the animal were taken upon arrival, immediately after EAU induction, and before sacrifice. All eyes of the animals (both left and right eyes) were examined by indirect ophthalmoscopy to evaluate respective effects on the cornea, conjunctiva, anterior chamber (AC), vitreous, posterior chamber, and sclera. One to 2 drops each of phenylephrine hydrochloride ophthalmic solution and cyclopentolate hydrochloride ophthalmic solution was used as a mydriatic. Observations pertaining to conjunctival injection, chemosis, discharge, and clarity of anterior and posterior segment of the eye were made, scored, and recorded. An average of all scores over the course of study is calculated for comparison. A modified McDonald-Shadduck scale40 was used for grading inflammation as detailed in Table 2.
Table 2.
A Modified McDonald-Shadduck Scale
| Type | Score | Observation |
|---|---|---|
| Conjunctival discharge | 0 | No discharge or may include a small amount of clear mucoid material normally found in the medial canthus of animal |
| 0.5 | Very small amount of discharge present on the globe | |
| 1 | Discharge is present on the globe of the eye or medial canthus in a significant amount | |
| 2 | Discharge is abundant and has collected on the lids and hairs of the eyelids | |
| 3 | Discharge has been flowing over the eyelids in significant amounts | |
| Chemosis (conjunctival swelling) | 0 | Normal, no swelling observed |
| 0.5 | Mild swelling of the palpebral tissues or very slight swelling of scleral conjunctiva | |
| 1 | Swelling above normal, but without eversion of the lids | |
| 2 | Swelling with misalignment of the normal approximation of the lower and upper eyelids | |
| 3 | Swelling is definite, with partial eversion of the upper and lower eyelids essentially equivalent | |
| 4 | Eversion of the upper eyelid is pronounced with less eversion of the lower eyelid | |
| Conjunctival Injection (hyperemia) | 0 | Normal in appearance for the species, while it may appear blanched to tan or reddish pink, with vessels defined and easily observable mainly at the 12 and 6 o'clock positions |
| 0.5 | Some vessels primarily on upper and lower regions of the globe are more pronounced with more vessels than usual visible | |
| 1 | Flushed reddish color predominantly on the palpebral conjunctiva with some perilimbal injection primarily on the upper and lower regions of the globe. More vessels are clearly visible with some engorged and diffuse looking, including connecting vessels not normally observed | |
| 2 | Palpebral conjunctiva appears bright red with accompanying perilimbal injection covering at least 75% of the perilimbal circumference, while upper and/or lower muscles appear reddish and irritated | |
| 3 | Both bulbar and palpebral conjunctiva exhibit a dark, beefy-red color with pronounced perilimbal injection with petechia often present | |
| Cornea | 0 | No haze observed |
| 0.5 | Slight loss of transparency mainly visible with illuminated scope | |
| 1 | Some loss of transparency with cloudiness readily apparent, but underlying structures visible, perhaps some loss of detail | |
| 2 | More loss of transparency, the affected cornea is homogeneously white in appearance | |
| 3 | Loss of transparency, underlying structures are just barely visible | |
| 4 | Underlying structures cannot be seen | |
| Anterior chamber | 0 | Totally clear |
| 0.5 | Trace Fibrin | |
| 1 | Little fibrin | |
| 2 | Moderate fibrin present | |
| 3 | Aspects of posterior chamber faintly visible | |
| 4 | Totally opacified by fibrin | |
| Vitreous and posterior chamber | 0 | The view through the vitreous is completely clear and all the details of the retina and retinal blood vessels are readily apparent |
| 1 | The view shows a slight degree of uveitis with a small fuzziness of the details of the rabbit retina, but the vessels and optic nerve head are readily seen | |
| 2 | The view shows moderate degree of inflammation where details regarding the optic nerve head and the vessels are still apparent, but are very difficult to appreciate | |
| 3 | The view shows a severe inflammation of the vitreous with only the optic nerve apparent and details of the retinal vessels being lost | |
| 4 | The view shows the inflammation is so severe where the vitreous is so cloudy that no details of the retinal vasculature or optic nerve head are visible | |
| Synechia | + | Present |
| − | Absent | |
| Hypopyon | + | Present |
| ± | Trace | |
| − | Absent |
Histopathology
The enucleated eyes were stored in Davidson's solution (ie, 34.7% deionized water, 11.1% glacial acetic acid, 32.0% ethanol, and 22.2% formalin) for 24 hrs, and then transferred to plastic conical tubes containing 20 mL of 70% ethanol in water. The eyes were sent for histopathological processing and evaluation at Colorado Histo-Prep (Fort Collins, CO). A central cut of the eye globe was taken as well as 2 cuts on either side of the central cut (calottes) at trim. For each eye, the central cut was placed into one cassette and the 2 calottes were placed together into a separate cassette. The tissues were processed, embedded in paraffin wax, sectioned by microtomy, and stained. Histopathology of the tissues was conducted on slides stained with hematoxylin and eosin. The pathologist who evaluated the tissues had no prior knowledge of the specific pharmacologic activity or formulation of the test articles. Standardized toxicologic pathology criteria and nomenclature for the rabbit were used to categorize microscopic tissue changes.36,41 For anterior section, the conjunctiva, cornea, AC, trabecular meshwork, iris, and ciliary body were evaluated and scored from 0 (normal) to 4 (marked) for signs of inflammation, including edema/congestion of the conjunctiva, ciliary body, cornea, inflammatory cell infiltration in the conjunctiva, cornea, AC, trabecular meshwork, iris, ciliary body, and neovascularization on the cornea. Scores from each tissue were combined to give a total inflammatory score of anterior section (maximum score = 40). For posterior section, the vitreous, choroid, and retina were also scored from 0 (normal) to 4 (marked) for signs of inflammatory cell infiltration.
Statistical analysis
All scores are reported as mean ± standard deviation (unless otherwise indicated). The differences in mean score between the control group and each DSP-Visulex treatment group were evaluated by the Wilcoxon rank-sum test. This included vitreous score, AC score, and conjunctiva injection score from clinical observation, and inflammatory score and inflammatory cell infiltration score from histopathological examination. Differences were considered significant at P < 0.05.
Results
Clinical observation
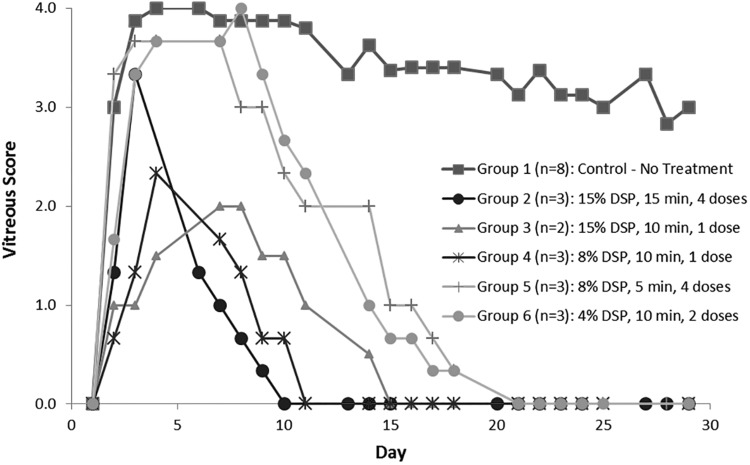
All right eyes showed signs of inflammation within a day after the induction. Left eyes showed no signs of inflammation through the end of the study. One rabbit in Group 3 died due to an unknown cause during the preimmunization period and before the initiation of DSP-Visulex dosing. Inflammation occurred more significantly in the posterior chamber than in the AC. All treatment regimens reduced the signs of uveitis. However, the most prominent finding from ophthalmic examination in assessing the severity of uveitis is the vitreous opacity (Fig. 2). The observations from each section of the eye are the following.
FIG. 2.
Vitreous scores of various treatment groups tested in the experimental uveitis rabbit model.
Vitreous
All animals in the control group (Group 1) reached a severe uveitic state (ie, scores of 3 or 4 for the vitreous), which remained on average above a score of 3 throughout the 28 days of study. Vitreous opacity increased steadily for the first 4 days after initiation of uveitis in all 5 groups. The opacity in Group 1 (control) increased the most. Scores for Group 1 animals decreased slightly around Day 13, but remained on average above a score of 3 throughout the experiment. By Day 4, Groups 2, 3, and 4 had reached the highest scores they would attain and began to decrease steadily thereafter. Group 5 scores began a steady decrease on Day 8, while those for Group 6 began to decrease on Day 10. There were clear decreases in vitreous opacity scores in all treatment groups, while the control group scores remained high. Group 2 animals showed a steady decrease in vitreous opacity scores until reaching zero on Day 10 and remaining at zero throughout the remainder of the study. Group 3 reached zero on Day 15, Group 4 on Day 11, and Groups 5 and 6 reached 0 on Days 21 and 22, respectively. Averaged vitreous scores over the course of study are presented in Table 3. Over the course of study, the average score of vitreous for the control group was 3.3 ± 1.1 and all the DSP-Visulex treatment groups (Group 2–6) were statistically significantly lower than the control.
Table 3.
Inflammation Scores from Clinical Observation Using Indirect Ophthalmoscope
| Inflammation score | |||
|---|---|---|---|
| Treatment regimen | Conjunctival injection | Anterior chamber | Vitreous |
| Group 1 (n = 8): Control - No treatment | 0.9 ± 0.8 | 0.5 ± 0.5 | 3.3 ± 1.1 |
| Group 2 (n = 3): 15% DSP, 15 min, 4 doses | 0.5 ± 0.4a | 0.1 ± 0.2b | 0.4 ± 1.0b |
| Group 3 (n = 2): 15% DSP, 10 min, 1 dose | 0.3 ± 0.4b | 0.1 ± 0.2b | 0.6 ± 1.2b |
| Group 4 (n = 3): 8% DSP, 10 min, 1 dose | 0.5 ± 0.5a | 0.3 ± 0.4a | 0.4 ± 0.8b |
| Group 5 (n = 3): 8% DSP, 5 min, 4 doses | 0.9 ± 0.8 (P = 0.3) | 0.5 ± 0.5 (P = 0.6) | 1.4 ± 1.7b |
| Group 6 (n = 3): 4% DSP, 10 min, 2 doses | 0.5 ± 0.5c | 0.4 ± 0.6 (P = 0.1) | 1.4 ± 1.6b |
Statistical differences in the average scores observed between the control group and each DSP-Visulex treatment group were assessed by the Wilcoxon rank-sum test with aP < 0.01, bP < 0.001, and cP < 0.05.
Anterior chamber
No hypopyon, synechia, or flare was noted in this study. Some fibrin formation in the AC was observed in all groups with slightly different degrees. The signs of inflammation in the AC were not drastic even with the control group. Average AC scores over the course of study was less than 1.0 for all groups (Table 3). The trends of the AC scores were similar for Group 1, Group 5, and Group 6. The average daily score of Group 5 was equal to that of the control group. Group 6 also had fibrin present throughout the study with an average daily score slightly lower than the controls, but not statistically significant. Group 4 displayed a low fibrin score over the course of study with an average of 0.3, which is significantly lower than the average of 0.5 for the control group (Group 1). Group 2 and 3 reached an AC score of 0 within about 1 week after the first treatment. Both groups showed the averaged AC score of 0.1, which is significantly lower than the control group.
Conjunctival injection
Mild to moderate conjunctival injection was present in all animals and was observed throughout the study. The average group scores of conjunctival injection over the course of treatment are presented in Table 3. All treatment groups except Group 5 showed slightly lower average conjunctival injection scores over the course of study than the control group (Group 1). The average conjunctival scores of Group 5 were equal to the control group. There were day to day variations as well as an overall downward trend over the entire experiment in all groups (ie, the average score ranged from 0 to 3 in the first 2 weeks and from 0 to 1 in the last 2 weeks). In Group 1, conjunctival injection scores declined slowly over the course of the experiment, but was still present until the end. Some irritation from placement of the DSP-Visulex was observed in the DSP-Visulex treatment groups. In Groups 2, 5, and 6 (multiple doses), slight increases were observed after each application followed by improvement until the next application. Conjunctival injection scores in Groups 3 and 4 (single dose) declined after Day 3, were minimal after about 10 days, and completely resolved by Day 22.
Chemosis
Mild chemosis was found in all groups. Overall chemosis was minor, with no group having an average chemosis score greater than 1 at any point. In Group 1 animals (controls), chemosis decreased slowly, although with variation, throughout the study. Chemosis increased slightly after DSP-Visulex treatment, a trend similar to that seen with conjunctival injection. Groups 2 and 5 showed mild chemosis immediately after each dosing, but resolving to 0 generally within a day. Groups 4 and 6 showed some variations in chemosis scores and reached 0 after Day 11, with Group 6 showing a slight reoccurrence on Days 16 through 18. Neither Group 3 rabbits displayed any significant chemosis.
Conjunctival discharge
Discharge was noted in all groups in a random manner. Discharge never exceeded a score of 1. There was an undistinguishable trend between the treatment regimens and the control.
Cornea
A low grade of cornea cloudiness, mostly with scores of <1, was found in some rabbits in all groups (untreated control group and treatment groups). The corneal haze observed in all rabbits faded with time. Overall, the incidence and severity of corneal haze in treatment groups appeared to be lower than the control group.
Body weight
Group 1 animals (controls) maintained their average body weight throughout the study. Group 2, with the highest dosing of DSP (4 weekly doses of 15% for 15 min), had an average loss of body weight of 0.3 kg, or about 8%. There were no significant weight changes in any of the other treatment groups.
Histopathology of uveitis eyes
The eyes were collected at the end of the study on Day 29 for histopathology evaluation. The average inflammation scores for both anterior and posterior sections of the eyes graded by a veterinarian pathologist are presented in Table 4.
Table 4.
Inflammation Scores and Inflammatory Cell Infiltration Score from Histopathology Examination
| Inflammatory cell infiltration score | |||
|---|---|---|---|
| Treatment regimen | Total inflammatory score of anterior section | Anterior section | Posterior section |
| Group 1 (n = 8): Control - No treatment | 4.4 ± 2.6 | 0.7 ± 1.0 | 2.9 ± 1.2 |
| Group 2 (n = 3): 15% DSP, 15 min, 4 doses | 0.2 ± 0.4a | 0.0 ± 0.2a | 0.1 ± 0.3a |
| Group 3 (n = 2): 15% DSP, 10 min, 1 dose | 1.0 ± 1.1b | 0.2 ± 0.4b | 1.8 ± 1.5b |
| Group 4 (n = 3): 8% DSP, 10 min, 1 dose | 1.8 ± 0.7b | 0.3 ± 0.7b | 1.2 ± 0.9a |
| Group 5 (n = 3): 8% DSP, 5 min, 4 doses | 1.4 ± 1.7b | 0.2 ± 0.8a | 1.9 ± 1.6b |
| Group 6 (n = 3): 4% DSP, 10 min, 2 doses | 1.9 ± 1.1c | 0.3 ± 0.7b | 2.9 ± 1.0 (P = 0.7) |
Statistical differences in the average scores observed between the control group and each DSP-Visulex treatment group were assessed by the Wilcoxon rank-sum test with aP < 0.001, bP < 0.01, and cP < 0.05.
Anterior section
No edema or congestion of conjunctiva, ciliary body, or cornea was observed in all groups. No neovascularization on the cornea was found in this study. In Table 4, the average total inflammatory score of anterior section was 4.4 for the control group, whereas those for the DSP-Visulex treatment groups were significantly lower. The efficacy of DSP-Visulex treatment in the anterior section appears to be related to DSP concentrations. Groups 2 and 3, where the DSP concentration was 15%, the averaged total inflammatory scores were 0.2 and 1.0, respectively; Groups 4 and 5, where the DSP concentration was 8%, the total scores were 1.8 and 1.4, respectively; and Group 6, where the DSP concentration was the lowest at 4%, the total score was the highest among treatment groups at 1.9.
Similarly, the inflammatory cell infiltrations into the anterior section of the eye were less in all DSP-Visulex treatment groups compared to the control. This was reflected by the lower of inflammatory cell infiltration scores of the treatment groups compared to the control group. However, there was no obvious efficacy–concentration relationship among the treatment groups. All animals in Group 1 (untreated) had inflammatory cell infiltrations to the conjunctiva, cornea, AC, trabecular meshwork, iris, and/or ciliary body with the average inflammatory cell infiltration score of 0.7 for the whole anterior section. In contrast, the average inflammatory cell infiltration score of Group 2 was 0.0. No cell infiltrations in the conjunctiva, AC, trabecular meshwork, iris, or ciliary body were found in this group. For Group 3, Group 4, Group 5, and Group 6, few inflammatory cell infiltrations were found in ciliary body, conjunctiva, and/or cornea tissues, but not in the other anterior tissues (ie, AC, trabecular meshwork, and iris) with the average inflammatory cell infiltration scores of 0.2, 0.3, 0.2, and 0.3, respectively.
Posterior section
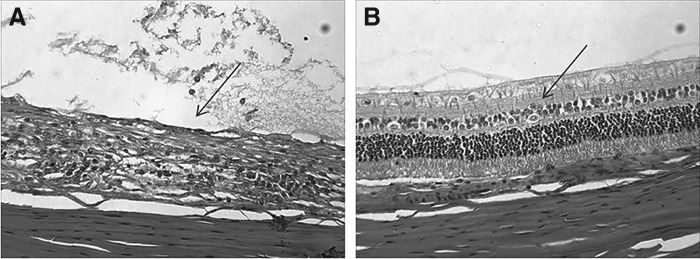
The overall inflammatory cell infiltration scores of the posterior section calculated from the respective individual vitreous, choroid, and retina scores are summarized in Table 4. The results show that all DSP-Visulex treatment groups, except the lowest dosing group (Group 6), were less inflamed in the posterior section than the controls (Group 1). The untreated animals showed moderate to severe inflammation in respective vitreous, choroid, and retina tissues with the average inflammatory cell infiltration score of 2.9. This indicates that intermediate and posterior uveitis were persistent in the control group for 29 days, consistent with the clinical observations. Group 2 animals had almost no pathological signs of uveitis present, with the average inflammatory cell infiltration score of 0.1. This supports that such eyes made a full recovery from induced intermediate and posterior uveitis. The differences in the photoreceptor layer appearance between the untreated eye (Group 1) and the eye from the highest dose regimen (Group 2) can be seen in Fig. 3. The posterior tissues of the treated eye appeared to be healthy with minimal inflammation, where it appeared to be completely impaired in the untreated eye. Histopathology of Group 3, Group 4, and Group 5 showed minimal to mild inflammation with the average infiltration scores of 1.8, 1.2, and 1.9, respectively. All animals in the lowest dosing group (Group 6) had posterior section inflammation nearly identical to the control group.
FIG. 3.
Comparative histopathologic presentation of the posterior section of the eyes at the end of study (Day 29). (A) Control (untreated eye). The inflammation is severe and the photoreceptor layer is completely damaged (arrow). (B) 15% DSP (15 min, 4 doses). Inflammation is very minimal and the tissue structure is well preserved (arrow).
Discussion
Panuveitis was successfully induced in the experimental uveitis rabbit. In this study, the inflammation in the vitreous was found to be severe and lasted longer than previously reported by other investigators when a single IVT injection of the antigen was given.29–33,35 This longer lasting effect of posterior inflammation might have been caused by the differences in adjuvant composition employed and amounts of mycobacterial cells used for preimmunization. It has been discussed that the compositions of the FCA can have an impact on the production of high-titer, high-affinity, and high-avidity antibodies and respective overall antibody response.42 Most studies29–31,33,35 used 10 mg of M. tuberculosis H37RA as in the present study, but mineral oil was used instead of a mixture of mannide monooleate (15%v/v) and paraffin oil (85% v/v). One study used only 0.5 mg of M. tuberculosis H37RA with TiterMax® Gold adjuvant (ie, a mixture of squalene, sorbitan monooleate 80, a patented block copolymer, and microparticulate silica).32 TiterMax adjuvant was reported to be less effective than FCA for antibody titer production in rabbit.43–45 On the other hand, the inflammation of the anterior section was less severe in our study compared to other studies. While 33 μg of mycobacterial cells was used in our study and in Cheng's study,29 the other studies used 50 μg.30,32,35 The lower response in anterior section might be concentration dependent as reported by Jaffe et al.33
Both clinical observation and histopathological examination support the hypothesis that DSP administration through the Visulex drug delivery system can treat experimental uveitis. Vitreous opacity is the most apparent finding from ophthalmic examination in assessing the severity of uveitis. While the vitreous scores of the untreated group remained high through the end of study, the clear vitreous (scores of 0) indicates that treatment with the DSP-Visulex is efficacious in EAU in rabbit. Histopathology scores of the posterior segment generally agreed well with the vitreous opacity scores in all groups except Group 6, which showed significant presence of inflammatory cells in the posterior section, despite the clearing of the vitreous opacity. The general trend was that the more rapid the resolution of the vitreous opacity, the less inflammation observed by histopathology. The resolution speed of inflammation in the vitreous appears to be related with the strength and frequency of the DSP-Visulex treatment. By Day 10, the highest dosing regimen showed a complete resolution of the vitreous opacity and by Day 22, the vitreous of all the treated animals, including the lowest dose, was completely clear. Although the vitreous in Group 5 (8% DSP, 5 min, 4 weekly doses) had the slow resolution similar to the lowest dose group (4% DSP, 10 min, 2 doses), the histopathology scores support that the higher dose is more efficacious than the lower dose. Overall, all the DSP-Visulex treatments, except the lowest dose group, can reasonably suppress the signs of inflammation in the intermediate and posterior section of the eye.
Clinical signs of inflammation on the anterior section, including AC, conjunctiva, and cornea, were mild, which make it difficult to see significant differences between the control group and the treatment groups using an indirect ophthalmoscope. Most of the efficacy of the DSP-Visulex treatment on the anterior section was supported by the more microscopic and sensitive method of histopathology examinations. In the case of the cornea, the slight haze was observed in the control group and treatment groups alike. The histopathological examinations indicate that the inflammatory cell infiltration of the cornea is higher in the control group compared to the DSP-Visulex treatment groups. This suggests that the cloudiness on the cornea may be caused by the disease and the corneal inflammation was alleviated by the DSP-Visulex treatments. For the AC, the high-dose regimens (Groups 2–4) showed lower inflammation scores compared to the lower dose regimens (Group 5 and 6) in both clinical observation and histopathological examination. The lower dose regimens only show significantly lower inflammation scores in the anterior section under the histopathological examination. This maybe because the clinical sign of AC inflammation was average to begin with even when compared against the control group. Unlike the AC, the histopathology and the clinical observations of conjunctiva did not show a strong correlation with the efficacy of the higher dose regimens.
The successful treatment of the single dose of DSP-Visulex (Groups 3 and 4) on this uveitis model was not anticipated. This is because the DSP was estimated to be cleared from the eye tissues and eventually from the body within 24 h based on the ocular pharmacokinetic study of dexamethasone disodium phosphate46 and the systemic half-life of DSP.47 Although the duration of action for dexamethasone can last up to 72 h,48 it cannot explain the long anti-inflammatory effect of the single dose of DSP-Visulex on this chronic uveitis model. We speculate that the very high dose of DSP can stop the inflammatory process in the uveitic eye without repeating the dose. More studies (eg, a dose-ranging study of DSP ocular injections in experimental uveitis rabbit) need to be done to confirm this hypothesis.
Clinical observation and histopathological examinations strongly indicate that the DSP-Visulex treatment was safe and well tolerated in the rabbit uveitis model. All the rabbits appeared to cooperate with the operator during the application with the use of topical anesthetic eye drop. The experiences of applying the DSP-Visulex on uveitic eyes were not significantly different than healthy rabbit eyes. Although the uveitis model can obscure any potential inflammation caused by the DSP-Visulex application, the data provide no evidence of ocular adverse event other than conjunctiva injection and chemosis in some occasions. The slight loss of body weight was observed only in the highest dose group. This could be an indication of systemic effects of DSP, which may occur with the high-dose regimen on a small animal such as a rabbit. Slight weight loss was also found in our toxicity study of DSP-Visulex with a similar dosing regimen (unpublished data). This is not a surprise because the rabbits are known to be sensitive to the effects of administered glucocorticoids even after a single ocular administration of dexamethasone.49
All 3 factors, including, drug concentration, application time, and administration frequency, appear to contribute to the effectiveness of the DSP-Visulex treatment regimen. With limited treatment regimens tested in this study, the results are inconclusive to show which the most dominant factor is. However, the application time and administration frequency are likely to be major factors for patient compliance. Therefore, the concentration can be a preferred factor from a clinical standpoint; for instance, one dose of 10 min of 15% DSP is preferred over 4 doses of 5 min of 8% DSP.
In qualitative comparison of the DSP-Visulex treatments with the IVT implants of dexamethasone and subconjunctival implant of prednisolone using a similar experimental uveitis rabbit, the treatment results on the posterior sections are comparable.29–31 This suggests that DSP-Visulex, a topical noninvasive once-a-week treatment from the front of the eye, may be as effective as a sustained drug release intraocular implant for the treatment of intermediate and posterior uveitis. In addition, this intermittent administration of DSP-Visulex may cause fewer incidents of steroid-induced glaucoma and cataract than a corticosteroid implant.
Overall, we think that DSP-Visulex addresses the problems of the existing corticosteroid treatment options for noninfectious uveitis. This includes eliminating the need for frequent dosing of eye drops, reducing the systemic side effects of oral therapy, and avoiding the serious risks associated with IVT and periocular injections, all of which potentially may hinder patient compliance. DSP-Visulex can potentially benefit some other posterior eye diseases such as diabetic macular edema, diabetic retinopathy, and age-related macular degeneration. In the future, many other molecules can be incorporated into the Visulex drug delivery platform for other ophthalmic applications.
Conclusion
The efficacy of the DSP-Visulex in treating experimental uveitis was assessed with 5 different treatment regimens. All DSP strengths (ie, 4%, 8%, or 15% DSP) administered through Visulex were able to suppress the signs of inflammation and were well tolerated over the course of dosing regimens tested for 29 days. The reduction of the dosing regimens by decreasing concentration, frequency of treatment, and application time generally results in a slower resolution of vitreous opacity and essentially reduced efficacy of the uveitis treatment. Based upon the findings, all the 8% and 15% DSP-Visulex treatment regimens in this study are considered safe and efficacious treatment modalities for anterior uveitis, posterior uveitis, and/or panuveitis. On the other hand, the 4% DSP Visulex regimen may only be considered for the treatment of anterior and intermediate uveitis, but not for posterior uveitis, unless more frequent dosing is tested.
Acknowledgments
This study was supported by NEI SBIR Grant R44EY014772. The authors thank Dr. Kevin Li at University of Cincinnati for his help on article preparation, Dr. Albert Vitale at Moran Eye Center for his guidance on uveitis, and Dr. Nick Mamalis at Moran Eye Center for training and protocol for uveitis clinical assessment in rabbit.
Author Disclosure Statements
Kongnara Papangkorn, and John Higuchi are employees of Aciont, Inc. Eri Prendergast is a biostatistic consultant at Aciont, Inc. Balbir Brar is a consultant at Aciont, Inc. William Higuchi is a founder of Aciont, Inc.
References
- 1.Nussenblatt R.B. The natural history of uveitis. Int. Ophthalmol. 14:303–308, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Tomkins-Netzer O., Talat L., Bar A., et al. Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology. 121:2387–2392, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Gritz D.C., Schwaber E.J., and Wong I.G. Complications of Uveitis: The Northern California Epidemiology of Uveitis Study. Ocul. Immunol. Inflamm. 1–11, 2017. [Epub ahead of print]; DOI: 10.1080/09273948.2016.1247174 [DOI] [PubMed] [Google Scholar]
- 4.Gritz D.C., and Wong I.G. Incidence and prevalence of uveitis in Northern California: The Northern California Epidemiology of Uveitis Study. Ophthalmology. 111:491–500, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez Villanueva J., Rodriguez Villanueva L., and Guzman Navarro M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int. J. Pharm. 516:342–351, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Airody A., Heath G., Lightman S., and Gale R. Non-Infectious Uveitis: optimising the Therapeutic Response. Drugs. 76:27–39, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Winfield A.J., Jessiman D., Williams A., and Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br. J. Ophthalmol. 74:477–480, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An J.A., Kasner O., Samek D.A., and Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J. Cataract. Refract. Surg. 40:1857–1861, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Jager R.D., Aiello L.P., Patel S.C., and Cunningham E.T. Risks of Intravitreous Injection: a Comprehensive Review. Retina. 24:676–698, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Sen H.N., Vitale S., Gangaputra S.S., et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 121:2275–2286, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geck U., Pustolla N., Baraki H., Atili A., Feltgen N., and Hoerauf H. Posterior vitreous detachment following intravitreal drug injection. Graefe's Arch. Clin. Exp. Ophthalmol. 251:1691–1695, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowder C., Belfort R., Lightman S., et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch. Ophthalmol. 129:545–553, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Boddu S.H., Gupta H., and Patel S. Drug Delivery to the Back of the Eye Following Topical Administration: an Update on Research and Patenting Activity. Recent. Pat. Drug Deliv. Formul. 8:27–36, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Hughes P.M., Olejnik O., Chang-Lin J.E., and Wilson C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 57:2010–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Koevary S.B. Pharmacokinetics of topical ocular drug delivery: potential uses for the treatment of diseases of the posterior segment and beyond. Curr. Drug Metab. 4:213–222, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Loftsson T., Sigurdsson H.H., Konrádsdóttir F., Gísladóttir S., Jansook P., and Stefánsson E. Topical drug delivery to the posterior segment of the eye: anatomical and physiological considerations. Pharmazie. 63:171–179, 2008 [PubMed] [Google Scholar]
- 17.Ying L., Tahara K., and Takeuchi H. Drug delivery to the ocular posterior segment using lipid emulsion via eye drop administration: effect of emulsion formulations and surface modification. Int. J. Pharm. 453:329–335, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Patel A., Cholkar K., Agrahari V., and Mitra A.K. Ocular drug delivery systems: an overview. World J. Pharmacol. 2:47–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sigurdsson H.H., Konrádsdóttir F., Loftsson T., and Stefánsson E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 85:598–602, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Tanito M., Hara K., Takai Y., et al. Topical dexamethasone-cyclodextrin microparticle eye drops for diabetic macular edema. Invest. Ophthalmol. Vis. Sci. 52:7944–7948, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Ohira A., Hara K., Jóhannesson G., et al. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol. 93:610–615, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Shulman S., Johannesson G., Stefansson E., Loewenstein A., Rosenblatt A., and Habot-Wilner Z. Topical dexamethasone-cyclodextrin nanoparticle eye drops for non-infectious Uveitic macular oedema and vitritis - a pilot study. Acta Ophthalmol. 93:411–415, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Shikamura Y., Ohtori A., and Tojo K. Drug penetration of the posterior eye tissues after topical instillation: in vivo and in silico simulation. Chem. Pharm. Bull (Tokyo). 59:1263–1267, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Geroski D.H., and Edelhauser H.F. Transscleral drug delivery for posterior segment disease. Adv. Drug Deliv. Rev. 52:37–48, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Gaudana R., Ananthula H.K., Parenky A., and Mitra A.K. Ocular drug delivery. AAPS J. 12:348–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berezovsky D.E., Patel S.R., McCarey B.E., and Edelhauser H.F. In vivo ocular fluorophotometry: delivery of fluoresceinated dextrans via transscleral diffusion in rabbits. Invest. Ophthalmol. Vis. Sci. 52:7038–7045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishima S., Gasset A., Klyce S.D., Jr., and Baum J.L. Determination of tear volume and tear flow. Invest. Ophthalmol. 5:264–276, 1966 [PubMed] [Google Scholar]
- 28.Davies N.M. Biopharmaceutical considerations in topical ocular drug delivery. Clin. Exp. Pharmacol. Physiol. 27:558–562, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Cheng C.K., Berger A.S., Pearson P.A., Ashton P., and Jaffe G.J. Intravitreal sustained-release dexamethasone device in the treatment of experimental uveitis. Invest. Ophthalmol. Vis. Sci. 36:442–453, 1995 [PubMed] [Google Scholar]
- 30.Ang M., Ng X., Wong C., et al. Evaluation of a prednisolone acetate-loaded subconjunctival implant for the treatment of recurrent uveitis in a rabbit model. PLoS One. 9:e97555, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghosn C.R., Li Y., Orilla W.C., et al. Treatment of experimental anterior and intermediate uveitis by a dexamethasone intravitreal implant. Invest. Ophthalmol. Vis. Sci. 52:2917–2923, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Eperon S., Balaskas K., Vaudaux J., and Guex-Crosier Y. Experimental uveitis can be maintained in rabbits for a period of six weeks after a safe sensitization method. Curr. Eye Res. 38:405–412, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Jaffe G.J., Yang C.S., Wang X.C., Cousins S.W., Gallemore R.P., and Ashton P. Intravitreal sustained-release cyclosporine in the treatment of experimental uveitis. Ophthalmology. 105:46–56, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Sloper C.M., Powell R.J., and Dua H.S. Tacrolimus (FK506) in the treatment of posterior uveitis refractory to cyclosporine. Ophthalmology. 106:723–728, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Sakurai E., Nozaki M., Okabe K., Kunou N., Kimura H., and Ogura Y. Scleral Plug of Biodegradable Polymers Containing Tacrolimus (FK506) for Experimental Uveitis. Invest. Ophthalmol. Vis. Sci. 44:4845–4852, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Jubb Ê.V.F., Kennedy P.C., and Palmer N. Pathology of Domestic Animals: Fourth Edition. Vol 1 San Diego, CA: Academic Press, 2013 [Google Scholar]
- 37.Miller D.J., Li S.K., Tuitupou A.L., et al. Passive and oxymetazoline-enhanced delivery with a lens device: pharmacokinetics and efficacy studies with rabbits. J. Ocul. Pharmacol. Ther. 24:385–391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldblum D., Fausch K., Frueh B.E., Theurillat R., Thormann W., and Zimmerli S. Ocular penetration of caspofungin in a rabbit uveitis model. Graefe's Arch. Clin. Exp. Ophthalmol. 245:825–833, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Rosenbaum J.T., and Angell E. Paradoxical effects of IL-10 in endotoxin-induced uveitis. J. Immunol. 155:4090–4094, 1995 [PubMed] [Google Scholar]
- 40.Hackett R., and McDonald T. Eye irritation. In: Marzulli F., Maibach H., eds. Advances in Modern Toxicology: Dermatoxicology. 4th ed. Washington, DC: Hemisphere Publishing; 1991, p. 749–815 [Google Scholar]
- 41.Banks W.J. Applied Veterinary Histology. 3rd ed. St. Louis: Mosby Year Book; 1993 [Google Scholar]
- 42.Stills H.F. Adjuvants and antibody production: dispelling the myths associated with Freund's complete and other adjuvants. ILAR J. 46:280–293, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Leenaars P.P.A.M., Hendriksen C.F.M., Angulo A.F., Koedam M.A., and Claassen E. Evaluation of several adjuvants as alternatives to the use of Freund's adjuvant in rabbits. Vet. Immunol. Immunopathol. 40:225–241, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Leenaars P.P.A.M., Koedam M.A., Wester P.W., Baumans V., Claassen E., and Hendriksen C.F.M. Assessment of side effects induced by injection of different adjuvant/antigen combinations in rabbits and mice. Lab. Anim. 32:387–406, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Smith D.E., O'Brien M.E., Palmer V.J., and Sadowski J.A. The selection of an adjuvant emulsion for polyclonal antibody production using a low-molecular-weight antigen in rabbits. Lab. Anim. Sci. 42:599–601, 1992 [PubMed] [Google Scholar]
- 46.Hosseini K., Matsushima D., Johnson J., et al. Pharmacokinetic study of dexamethasone disodium phosphate using intravitreal, subconjunctival, and intravenous delivery routes in rabbits. J. Ocul. Pharmacol. Ther. 24:301–308, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Czock D., Keller F., Rasche F.M., and Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44:61–98, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Schimmer B.P., and Parker K.L. Chapter 60. Adrenocorticotropic Hormone; Adrenocortical Steroids and Their Synthetic Analogs; Inhibitors of the Synthesis and Actions of Adrenocortical Hormones. In: Goodman & Gilman's, ed. The Pharmacological Basis of Therapeutics, 10th Edition New York, NY: McGraw Hill, 2001 [Google Scholar]
- 49.Rosenthal K.L. Therapeutic contraindications in exotic pets. Semin. Avian. Exot. Pet. Med. 13:44–48, 2004 [Google Scholar]