Abstract
Acute kidney injury (AKI) is a common disease with a complex pathophysiology. The old paradigm of identifying renal injury based on location—prerenal, intrarenal, and postrenal—is now being supplanted with a new paradigm based on observable kidney injury patterns. The pathophysiology of AKI on a molecular and microanatomical level includes inflammation, immune dysregulation, oxidative injury, and impaired microcirculation. Treatment has traditionally been supportive, including the avoidance of nephrotoxins, judicious volume and blood pressure management, hemodynamic monitoring, and renal replacement therapy. Fluid overload and chloride-rich fluids are now implicated in the development of AKI, and resuscitation with a balanced, buffered solution at a conservative rate will mitigate risk. Novel therapies, which address specific observable kidney injury patterns include direct oxygen-free radical scavengers such as α-lipoic acid, curcumin, sodium-2-mercaptoethane sulphonate, propofol, and selenium. In addition, angiotensin II and adenosine receptor antagonists hope to ameliorate kidney injury via manipulation of renal hemodynamics and tubulo-glomerular feedback. Alkaline phosphatase, sphingosine 1 phosphate analogues, and dipeptidylpeptidase-4 inhibitors counteract kidney injury via manipulation of inflammatory pathways. Finally, genetic modifiers such as 5INP may mitigate AKI via transcriptive processes.
Keywords: acute kidney injury, angiotensin II, inflammation, intravenous fluids, oxidative stress
Renal injury has traditionally been understood in the context of anatomical location (prerenal, intra-renal, and postrenal). The supportive nature of current therapeutic options stems from a relatively nascent understanding of the complex pathophysiology of acute kidney injury (AKI). Traditionally, therapy for AKI has revolved around maintaining adequate macrovascular renal perfusion through volume and hemodynamic management, as well as the avoidance of nephrotoxins. Renal replacement therapy can be implemented while awaiting signs of recovery. Recently, the amount and type of resuscitative fluids in AKI have come into question as more and more evidence suggests the harmful effects of over-resuscitation.1, 2 Moreover, as we improve our understanding of AKI, we can now point to a complex cascade of microvascular dysregulation and cellular injury that occurs via inflammation, immune dysregulation, and oxidative injury. An array of novel therapies that target specific enzymes or molecules involved in these pathways are in various stages of development.3 This review provides a summary of the emerging understanding of renal injury based on molecular and microvascular injury patterns, before delving into the most recent thinking regarding optimal fluid management for AKI. It will then discuss novel treatment options that target the molecular pathways now implicated in AKI. Tables 1 and 2 summarize the findings.
Table 1.
Novel therapeutic agents for acute kidney injury
| Agent | Mechanism of action | Potential indication(s) |
|---|---|---|
| Renal blood flow modifiers | ||
| Angiotensin | Constricts efferent arterioles to a greater degree than afferent arterioles Regulates release of aldosterone and vasopressin |
Sepsis |
| Adenosine antagonists | Reduces GFR in response to hypoxia Constricts afferent arterioles → increase NaCl levels in proximal tubules |
CIN IRI Cardiorenal syndrome |
| Antioxidants | ||
| Alpha-lipoic acid | Reduced form eliminates free radicals Improves glomerular function Reduces renal inflammation |
IRI CIN |
| Selenium | Cofactor that reduces free radicals | Cisplatin injury ECSL |
| MESNA | Scavenges for free radical oxygen species | CIN |
| Propofol | Converts free oxygen radicals into a phenoxyl form | IRI |
| Curcumin | Scavenges for free oxygen radicals Stimulates activity of antioxidant molecules such as superoxide dismutase, catalase, and glutathione peroxidase |
IRI Diabetic nephropathy Lupus nephritis |
| Anti-inflammatory mediators | ||
| Alkaline phosphatase | Dephosphorylates lipopolysaccharide Dephosphorylates ATP |
Gram-negative sepsis |
| Dipeptidylpeptidase-4 Inhibitors | Extends half-life of glucagon-like peptide-1 | Diabetic nephropathy Cisplatin injury |
| Sphingosine 1 phosphate (S1P) analogues | Mitigates endothelial damage Decreases recruitment of inflammatory mediators to the renal tubules |
None to date |
| Genetic modifiers | ||
| I5NP | Inhibits p53 gene | IRI |
ATP, adenosine triphosphate; CIN, contrast induced nephropathy; ECSL, extracorporeal shockwave lithotripsy; GFR, glomerular filtration rate; IRI, ischemia reperfusion injury; MESNA, sodium 2-mercaptoethane sulfonate; NaCl, sodium chloride.
Table 2.
Therapeutics by disease state with evidence
| Treatment | Study authors/registration name and number on clinicaltrials.gov | Results of study |
|---|---|---|
| Sepsis | ||
| Alkaline phosphatase | Heemskerk et al.4 | Improvement in serum creatinine compared with placebo in gram-negative sepsis |
| Alkaline phosphatase | Pickkers et al.5 | Lower serum creatinine and inflammatory markers in patients with sepsis |
| Alkaline phosphatase | Safety, Tolerability, Efficacy and QoL Study of Human recAP in the Treatment of Patients With SA-AKI (STOP-AKI) (NCT02182440) in progress | Evaluating safety, efficacy, and optimum dosage of ALP in patients with AKI from sepsis |
| Hypoperfusion/shock | ||
| Angiotensin | Angiotensin in Septic Kidney Injury Trial (ASK-IT) (NCT00711789) in progress | Evaluating effect of angiotensin on hemodynamics and urine output in septic shock |
| Angiotensin | Khanna et al.64 | Evaluated angiotensin as a vasopressor for catecholamine-resistant hypotension; AKI as exploratory endpoint |
| Contrast-induced nephropathy | ||
| Adenosine antagonists | Bagshaw and Ghali6 | Theophylline reduced risk of CIN |
| Adenosine antagonists | Dai et al.7 | Theophylline reduced risk of CIN |
| Adenosine antagonists | NCT01469624 in progress | Evaluating effect of pentoxifylline on CIN |
| Alpha-lipoic acid | Jo et al.8 | Older patients (age >70 yr), higher contrast load, “high-risk group” had decreased incidence of AKI |
| MENSA | Ludwig et al.9 | Pretreatment with MESNA decreased risk of CIN |
| Renal transplantation | ||
| Alpha-lipoic acid | Ambrosi et al.10 | Fewer inflammatory markers for kidney-pancreas transplant recipients if given to both donors and recipients |
| Propofol | NCT01132157 in progress | Evaluating incidence of ischemic−reperfusion kidney injury using desflurane versus propofol in renal transplant patients |
| Propofol | NCT01870011 in progress | Evaluating incidence of ischemic−reperfusion kidney injury using desflurane versus propofol in renal transplant patients |
| I5NP | NCT00802347 in progress | Evaluating safety, maximum-tolerated dose, and amelioration of delayed graft function of I5NP in renal transplant patients |
| Drug-induced AIN | ||
| Selenium | Ghorbani et al.11 | Decreased incidence of AKI for cancer patients treated with cisplatin |
| Dipeptidylpeptidase-4 Inhibitors | NCT02250872 in progress | Evaluating effect of DPP-4 inhibitors on AKI due to cisplatin |
| Postoperative (nonrenal transplant) | ||
| Selenium | SodiUm SeleniTe Adminstration IN Cardiac Surgery (SUSTAIN CSX-Trial; SUSTAINCSX) (NCT02002247) in progress | Evaluating effects of selenium on organ dysfunction and mortality in patients undergoing high-risk cardiac surgery |
| Propofol | Yoo et al.12 | Lower serum renal biomarkers and lower hospital length of stay compared with sevoflurane for patients receiving valvular surgery |
| Propofol | Bang et al.13 | Decreased incidence of AKI and shorter ICU stay compared with sevoflurane in colorectal surgery patients |
| Propofol | Ammar et al.14 | Decreased incidence of AKI and serum renal biomarkers compared to sevoflurane in AAA repair patients |
| Propofol | NCT02009280 in progress | Evaluating the incidence of AKI when using propofol in lung transplant patients on ECMO |
| Propofol | NCT01384643 in progress | Evaluating ability of propofol to reduce ischemic−reperfusion kidney injury in valvular surgery patients |
| Circumin | NCT01225094 in progress | Evaluating prevention of AKI in AAA repair patients |
| I5NP | NCT00554359 in progress | Evaluating safety and pharmacokinetics of I5NP in patients undergoing cardiovascular surgery who are at high risk of AKI |
| Diabetic nephropathy | ||
| Circumin | Yang et al.110 | Decreased microalbuminuria and serum inflammatory markers in patients with type 2 diabetes |
| DPP-4 inhibitors | Shih et al.15 | Patients with type 2 diabetes who were hospitalized for AKI were more likely to be on DPP-4 inhibitors |
| DPP-4 inhibitors | Kawasaki et al.16 | Sitagliptin decreased eGFR in type 2 diabetes patients |
| DPP-4 inhibitors | Scirica et al.17 | Saxagliptin decreased eGFR compared with placebo in patients with type 2 diabetes |
| DPP-4 inhibitors | Pendergrass et al.18 | No association between sitagliptin and renal failure |
| DPP-4 inhibitors | Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) | No association between sitagliptin and renal failure |
| Miscellaneous | ||
| Adenosine antagonists | Pharmacology of Aminophylline for Acute Kidney Injury in Neonates (PAANS) trial (NCT02276170) in progress | Evaluating aminophylline as treatment for AKI in neonates (excluding congenital defects) |
| Propofol | Leite et al.19 | Decreased need for renal replacement therapy and mortality compared with midazolam in critical care patients |
| Circumin | Khajehdehi et al.20 | Decreased microalbuminuria and serum inflammatory markers in patients with SLE |
| Propofol | Feng et al.21 | Decreased rates of apoptosis and increased proliferation in renal tubule epithelial cells exposed to anoxia |
AAA, abdominal aortic aneurysm; AIN, acute interstitial nephritis; ALP, alkaline phosphatase; AKI, acute kidney injury; CIN, contrast-induced nephropathy; DPP, dipeptidylpeptidase; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate MESNA, sodium 2-mercaptoethane sulfonate.
Renal Injury
The traditional anatomically-based classification of kidney injury is being supplanted by a more functional paradigm, in which tissue pathology, regardless of anatomic location, dictates the type of injury. Prerenal disease has traditionally been invoked when the clinical scenario involves the compromise of renal blood flow. Intrarenal disease is traditionally associated with evidence of parenchymal disease in the urine, such as renal tubular casts. Postrenal disease is associated with known or suspected obstruction to urine flow. Although the adequacy of renal blood flow is still important, we have a better understanding, from both microvascular and macrovascular levels, of renal hemodynamics. Insufficient renal perfusion happens both prerenally on a macrovascular scale, such as in states of shock, as well as intra-renally on a microvascular scale, such as with ischemia−reperfusion injury, angiotensin-converting enzyme inhibitor or nonsteroidal anti-inflammatory drug (NSAID) use. Macrovascular renal blood flow may or may not correlate with glomerular perfusion, because changes in glomerular perfusion can occur even in periods of preserved blood pressure via differential effects on afferent and efferent arterioles.22 A significant amount of evidence exists that identifies the appropriate amount of macrovascular renal perfusion pressure required to prevent injury in various states of disease.23, 24, 25 Accordingly, standard of care today includes maintenance of mean arterial pressure at or above approximately 65 mm Hg to preserve renal blood flow. Perfusion at the glomerular level is mediated through the differential dilation and constriction of the afferent and efferent arterioles. In normal human physiology, afferent arteriolar tone is controlled via tubuloglomerular feedback from the juxtaglomerular apparatus, mediated by any of a number of innate molecules, including angiotensin II, thromboxane, catecholamines, nitric oxide, and adenosine.26 Efferent tone is mediated largely by angiotensin II in response to neurohormonal activation of the renin-angiotensin-aldosterone system (RAAS).22 Within physiological blood pressure ranges and absent any external forces affecting afferent or efferent tone, glomerular perfusion pressure is maintained by harmonious autoregulatory mechanisms at a transglomerular pressure gradient of approximately 10 mm Hg.27 Figure 1 illustrates the physiology and pathophysiology of glomerular filtration. Within the past couple of decades, we have begun to understand how afferent and efferent microvascular tone can change in states of disease but also how they are intentionally or unintentionally manipulated. Moreover, we can predict with increasing levels of sophistication the consequences of these effects on glomerular perfusion pressure and ultimately, kidney injury.22, 28, 29
Figure 1.
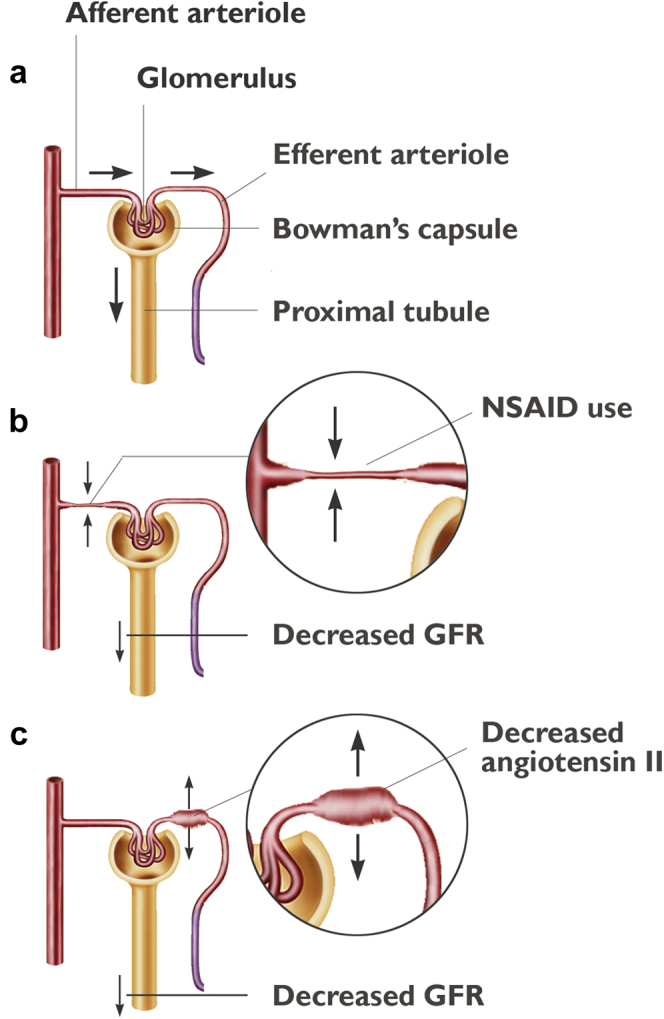
In normal human physiology, glomerular hydrostatic pressure is created via a complex but harmonious manipulation of afferent and efferent arterioles, which maintains a transglomerular pressure gradient that allows for flow of urine across Bowman’s capsule (a). In various states of disease and with nonsteroidal anti-inflammatory drug (NSAID) use, afferent arteriolar vasoconstriction can decrease flow into the glomerulus and across Bowman’s capsule (b). Dilation (or ineffective constriction) of the efferent arteriole, as seen with angiotensin II deficiency in angiotensin-converting enzyme inhibition, can result in the same phenomenon (c). GFR, glomerular filtration rate. Source: Stocktrek Images, Inc./Alamy Stock Photo.
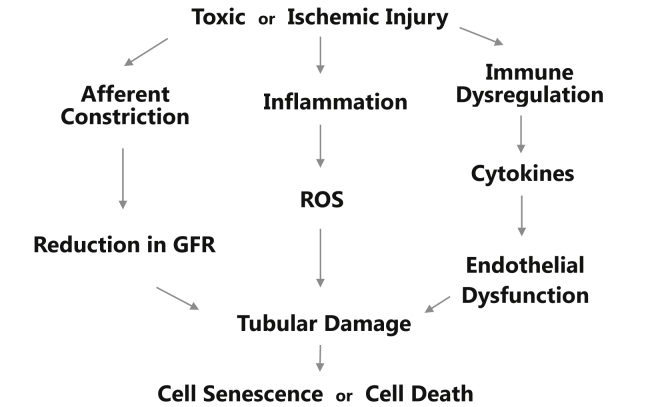
Although renal perfusion is an important factor in understanding the mechanisms of kidney injury, it is by no means the only one, nor does it act in isolation. Injury can occur in the absence of hypotension,22 and glomerular hypoperfusion can lead to tubular damage.30 Tubular damage has also been linked to oxidative stress31, 32 and inflammation.33, 34 Figure 2 illustrates the complex pathway from toxic or ischemic insult to tubular injury in AKI, which can be mediated by microvascular dysfunction, oxidative stress, inflammation, and immune dysregulation. An abundance of inflammatory and immune molecules, including intracellular adhesion molecule-1; tumor necrosis factor-α (TNF-α); interleukin-1 (IL-1), -6, and -8; transforming growth factor-β; and toll-like receptors, have all been identified in AKI.35 AKI may also result from cell death or senescence facilitated by genetic factors, such as cell cycle arrest, which are believed to prevent cell division when DNA may be damaged.36 Anachronistically, oxidative stress, immune dysregulation, and genetically-mediated cell death may all have been considered intrarenal disease, and treated with supportive care, alleviation of precipitating factors, and the tincture of time. However, as our understanding of these mechanisms has grown, so too have potential therapeutic options aimed at addressing the specific pathophysiological mechanisms implicated in renal injury. Current and potential therapeutic options address macrovascular hypoperfusion, microvascular blood flow alteration (glomerular hypoperfusion), tubular and cellular damage from oxidative stress, immune dysregulation and inflammation, and genetic failure, such as cell−cycle arrest.
Figure 2.
A complex relationship exists between toxic or ischemic insult and acute kidney injury. Injury can be mediated via inflammatory and immune mechanisms and microvascular dysfunction, which need not be mutually exclusive. Reactive oxygen species (ROS) and immune mediators (such as interleukins and tumor necrosis factors) can cause mitochondrial failure and trigger apoptosis from cell cycle arrest. Numerous therapies are being developed to counteract the pathophysiological processes involved in acute kidney injury. GFR, glomerular filtration rate.
Fluid Management
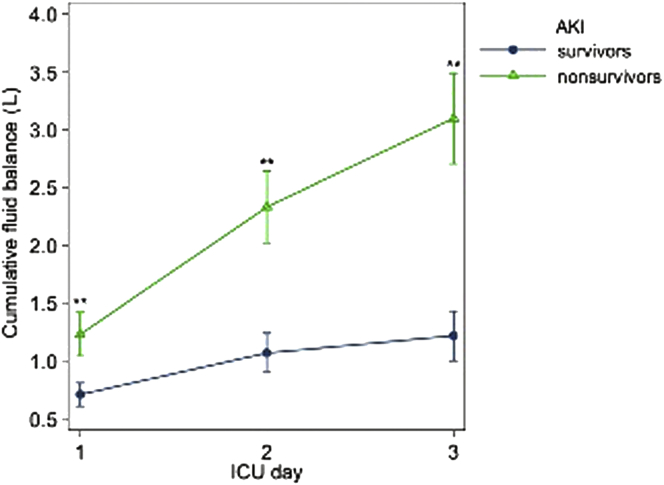
The mainstay of management of AKI from a macrovascular perspective revolves around judicious use of i.v. fluids. To maintain adequate renal macrovascular perfusion, liberal administration of i.v. fluid has historically been standard of care. Numerous studies have focused on the determination of the optimal amount of resuscitation in the mitigation of AKI, which has not necessarily correlated with resuscitation goals for cardiac output or blood volume.37, 38 Recent data from the Sepsis Occurrence in Acutely Ill Patients (SOAP) and Program to Improve Care in Acute Renal Disease (PICARD) studies have suggested that a liberal fluid strategy may actually increase the incidence of AKI.39, 40 Increases in intra-abdominal pressure, interstitial edema, and renal vasculature congestion, which result in disruptions in microanatomy, oxygen transport defects, and small vessel congestion on a cellular level, are believed to be causative.41 The Fluid And Catheter Treatment Trial (FACTT), a multicenter, prospective, randomized, controlled trial that evaluated the effect of a liberal fluid strategy versus conservative fluid strategy on mortality in patients with acute lung injury, found a positive correlation between a liberal fluid strategy and the incidence of AKI.42 Volume overload was also found to independently increase the risk of AKI and death in another prospective, observational, multicenter study—the Beijing Acute Kidney Injury Trial.43 Figure 3 highlights the mortality implications of volume overload.
Figure 3.
Results from the Beijing trial shows the mortality associated with cumulative fluid balance in acute kidney injury (AKI) survivors and nonsurvivors during their first 3 days in the intensive care unit (ICU). (From Wang N, Jiang L, Zhu B, et al. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371.43 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
In addition, evidence has emerged regarding the type of fluid resuscitation used in AKI. Synthetic colloids (starches) are no longer recommended for resuscitation based on accumulating evidence. The Scandinavian Starch for Severe Sepsis/Septic Shock (6S) Trial compared hydroxyethyl starch (HES) with lactated Ringer’s solution in a parallel group, randomized, blinded trial that ultimately found an increased risk of AKI in the HES group.44 HES and normal saline were also compared in the Crystalloid vs Hydroxyethyl Starch Trial (CHEST), which showed no difference in 90-day mortality, but did show a higher incidence of AKI and requirement for renal replacement therapy in the starch group.45 HES was also determined to have an increased risk of AKI and death compared with other crystalloids, albumin, and gelatin in a recent meta-analysis.46
Albumin solutions are believed to increase oncotic pressure and thereby better preserve intravascular volume and renal perfusion pressure than crystalloids.47 Data has been conflicting regarding the use of albumin solutions in resuscitation and prevention of AKI. A 2010 meta-analysis that compared 20% albumin with various isotonic fluids (normal saline, 4%−5% albumin, and lactated Ringer’s) showed that albumin decreased the odds of AKI markedly.48 However, in the Albumin Italian Outcome Sepsis (ALBIOS) trial, 20% albumin and crystalloids were found to be equivalent with regard to mortality at 28 days (primary outcome) and all secondary outcomes, including AKI.49 Studies also do not support the use of isotonic colloids (i.e., 4%−5% albumin) over crystalloid solutions. The Saline versus Albumin Fluid Evaluation (SAFE) trial found that 4% albumin and normal saline were equivalent with regard to all-cause mortality, organ dysfunction, hospital length of stay, ICU length of stay, days requiring mechanical ventilation, and days requiring renal replacement therapy.50
Recent evidence has suggested that chloride-rich solutions may be deleterious to kidney function by inducing renal vasoconstriction and decreasing glomerular filtration rate (GFR).51 Yunos et al. found chlorine-rich fluids to be an independent risk factor for AKI that necessitated renal replacement therapy compared with a balanced solution, such has Hartmann solution, Plasma-Lyte 148, and 20% albumin.52, 53 The authors hypothesized that kidney injury was the result of renal vasoconstriction and changes in tubule-glomerular feedback precipitated by the chloride. In contrast, the 2015 0.9% Saline versus Plasma-Lyte 148 (PL-148) for ICU fluid Therapy (SPLIT) randomized clinical trial compared resuscitation with normal saline versus a balanced solution in critically ill patients, and did not find an increased incidence of AKI.54
In summary, renal perfusion should be monitored at the macrovascular level and maintained via volume and blood pressure adjustment. Kidney injury may be mitigated through the judicious use of fluids to avoid over-resuscitation, avoidance of excessive chloride, and maintenance of mean arterial pressure ≥65 mm Hg. Evidence supporting colloid solutions versus crystalloid solutions is lacking.
Renal Flow Modifiers
Alteration in microvascular renal blood flow at the level of the single nephron has been implicated in AKI. Disease states such as ischemia−reperfusion injury, hypercalcemia, and hepatorenal syndrome, as well as iatrogenic factors, including the use of certain medications (NSAIDs, cyclooxygenase-2 inhibitors, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers) can result in an inadequate transglomerular pressure gradient and a reduction in glomerular filtration.22 The loss of an adequate transglomerular pressure gradient can evolve into tubular damage, because the highly metabolically active tubular epithelial cells are starved of adenosine triphosphate (ATP).30 As such, research has focused on the modification of renal microvascular blood flow to mitigate AKI in the aforementioned clinical conditions. These renal flow modifiers can augment GFR by directly affecting microvascular tone. Within a single nephron, GFR is preserved via sufficient afferent arteriolar vasodilation to allow for adequate blood flow into the glomerulus, but also sufficient efferent arteriolar tone, which results in adequate transglomerular pressure gradient.55 Novel therapeutics such as angiotensin II and adenosine analogues seek to address these microvascular issues.
Angiotensin
The RAAS affects the ability of the kidney to reabsorb water and maintain euvolemia. Increased adrenergic tone and activation of the RAAS occurs as a result of volume depletion to increase renal reabsorption of water. Angiotensin II is an octapeptide with multiple functions.56 In the kidney, angiotensin II participates in the regulation of the release of aldosterone, the maintenance of sodium and water homeostasis, and the release of vasopressin.57 It has been found to cause constriction of efferent arterioles to a greater degree than afferent arterioles, thereby increasing intraglomerular flow and augmenting the transglomerular pressure gradient.58 Figure 1 shows the effect of angiotensin II on the renal microvasculature. In inflammatory or immunoactive states such as sepsis, dysfunction of the RAAS59 and activation of the RAAS leads to a downregulation of angiotensin I type of angiotensin receptors.60 The relative scarcity of angiotensin II may result in inadequately low intraglomerular pressures, which could potentially be mitigated via exogenous angiotensin II.61
In sheep studies, angiotensin II administration was found to increase urine output and creatinine clearance.62 Renal blood flow was noted to be decreased, without inhibition of renal cellular metabolism.63 In contrast, the effect of angiotensin II, norepinephrine, and enalapril on renal plasma flow, prevalence of AKI, and mitochondrial activity was evaluated in a septic pig model, and no difference was noted.61
There are limited data regarding the efficacy of angiotensin II in humans. Older studies evaluated its effect on renal function, but none were randomized, placebo-controlled trials. A pilot study of 20 patients was conducted that assessed the use of angiotensin II as a pressor in high-output shock, but there was inadequate power to determine the effect of the drug on renal function.56 Currently, the Angiotensin in Septic Kidney Injury Trial (ASK-IT) is attempting to evaluate the effect of angiotensin on hemodynamics and urine output in patients with septic shock and acute renal failure (NCT00711789). The recently published Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) phase 3 clinical trial evaluated angiotensin as a vasopressor to treat catecholamine-resistant hypotension, and found a trend toward improved survival at 28 days with no increase in AKI-related adverse events.64 Baseline incidence of AKI in both groups was high, but there was no statistical difference in urine output. Additional research regarding angiotensin II is ongoing.
Adenosine Antagonists
Adenosine has both intra and extrarenal effects that differ depending on the type of receptor. Systemically, it manipulates vascular tone to regulate adequate oxygen delivery to local tissues. In the kidney, adenosine can cause a reduction in GFR in response to hypoxia or an increased level of sodium chloride in the tubule via the constriction afferent arterioles.65 Both A1R and A2R receptors are potential therapeutic targets, and both adenosine agonists of the A2R receptors and adenosine antagonists have been proposed for potentially protective effects.
In animal studies, activation of selective adenosine 2A receptors was found to be protective of kidney injury following ischemia in rodents.66, 67 In humans, theophylline, a nonselective adenosine A1R and A2R antagonist, has been compared with N-acetylcysteine in the prevention of contrast-induced nephropathy (CIN). A meta-analysis published in 2005 found a temporary benefit in the prevention of CIN with the use of theophylline, but there was significant heterogeneity within the study.6 Another meta-analysis by Dai et al. noted a reduction in the risk of CIN-related AKI with the use of theophylline (odds ratio 0.48).7 In contrast, the nonselective adenosine antagonist aminophylline showed no beneficial effect on the incidence of kidney injury compared with placebo in children with congenital heart disease.68 A similar result was noted with rolofylline in the PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) study.69 Despite the mixed results, there is still considerable interest in the evaluation of adenosine antagonism in AKI. The Pharmacology of Aminophylline for Acute Kidney Injury in Neonates (PAANS) trial (NCT02276170) is attempting to evaluate aminophylline as a treatment for AKI by measuring changes in urine output, creatinine, and other urine biomarkers. Another study is examining the effect of pentoxifylline on CIN (NCT01469624).
Antioxidants
There is an increasing amount of data describing the role of free radical oxygen species in the development of AKI. Once formed, oxygen radicals enact deleterious effects on the kidney via oxidation of proteins, peroxidation of lipids, damage to DNA, and induction of apoptosis.70 Free radical oxygen species are formed under such disease states as ischemia−reperfusion injury, CIN, sepsis, and malignancy (especially with the use of chemotherapeutic agents). There are some potential therapies that directly target free radicals in the prevention of AKI caused by these clinical scenarios.
Alpha-Lipoic Acid
Alpha-lipoic acid (ALA) eliminates free radicals when it is converted to its reduced form in tissues.71 In animal models, ALA both improves glomerular function and reduces renal inflammation.72, 73 In humans, ALA has been used to treat diabetic neuropathy and retinopathy, and it has been proposed in the treatment of both CIN and ischemia−reperfusion kidney injury. Blood urea nitrogen (BUN), creatinine, cystatin C, and urinary neutrophil gelatinase-associated lipocalin have been found to be elevated in CIN.74, 75, 76 A 2013 study assessed the effect of ALA on the incidence of CIN in diabetic patients using the biomarkers neutrophil gelatinase-associated lipocalin and cystatin C in addition to the conventional biomarkers (BUN and creatinine) and found no beneficial effect.77 Another 2013 study measured the effect of ALA on the incidence of CIN in 200 patients with chronic renal disease (creatinine clearance <60 ml/min) and found no appreciable difference between the control and experimental groups.8 However, patients aged older than 70 years and those in a predefined high-risk group who received a higher contrast load did experience a protective effect with ALA. ALA may also have some anti-inflammatory properties independent of its antioxidant properties. In patients who receive kidney-pancreas transplantations, when ALA was given to both the donor and recipient, markers of inflammation were diminished compared with the untreated group or the recipient-only treated group.10 Currently, no human studies are being conducted using ALA, but in animal studies, ALA continues to show improvement in surrogate and clinical outcomes when used in such disease processes as ischemia−reperfusion injury,78 sepsis,79 toxic injury,72, 80 and obstructive uropathy.81
Selenium
Selenium is a trace element that is involved in the reduction of free radicals during cellular aerobic respiration.82 Deficiency in selenium has been linked to AKI in rat models.83 Selenium supplementation correlated with improved serum creatinine, urea, and histopathological evidence in cisplatin-induced kidney injury in rats.82 Rats with gentamycin-induced AKI exhibited similar histological and functional improvements with selenium.84 When given in combination with erythropoietin in a murine model, selenium seemed to bestow some benefit in ischemia−reperfusion injury.85 In a porcine model, selenium improved the antioxidant profile (byproducts of ischemia−reperfusion injury) on immunohistopathology of transplanted kidneys in pigs.86
The data regarding the renal protective effect of selenium in humans are more ambiguous. Although patients who were pretreated with selenium showed better kidney biomarker profiles (urine N-acetylglucosaminidase, γ-glutamyl transpeptidase, alanine aminopeptidase, leucine aminopeptidase, and alkaline phosphidate) after receiving cisplatin, this did not translate into any clinical benefit.87 A European study of cisplatin-based chemotherapy patients pretreated with a multivitamin cocktail that included selenium showed that creatinine clearance was equivalent in the control group versus the pretreatment group.88 Micronutrient supplementation (including selenium) failed to provide a benefit in patients who underwent extracorporeal shockwave lithotripsy, cardiac surgery, major trauma, or subarachnoid hemorrhages.89, 90 In contrast, a randomized, controlled trial by Ghorbani et al. in 2013 found that cancer patients who received cisplatin therapy had a decreased incidence of AKI when pretreated with selenium.11 At this time, the Sodium Selenite Administration in Cardiac Surgery (SUSTAIN CSX) trial is attempting to compare the effects of selenium versus placebo on organ dysfunction (including renal function) and mortality in 1400 critically ill patients.91
Sodium-2-Mercaptoethane Sulphonate
The sulfhydryl moiety in sodium-2-mercaptoethane sulphonate (MESNA) is a scavenger of free radical oxygen species. It is found in high concentrations in the renal tubular cells and is easily filtered across the glomerulus.92 In a mouse model, MESNA was noted to ameliorate renal injury, both histologically and functionally, after ischemia−reperfusion injury.92 Throughout the early 1990s, MESNA was widely used as a means to protect renal function when paired with chemotherapy.93, 94, 95 In 2011, a study that consisted of 100 patients suggested that MESNA might decrease the risk of CIN if used as pretreatment to contrast administration.9 This is the only randomized controlled trial of MESNA in kidney injury to date.
Propofol
Because of its molecular structure which is similar to that of vitamin E, propofol exhibits antioxidant properties by converting free oxygen radicals into a less toxic phenoxyl form.96 Propofol has been studied in a variety of animal models and has been associated with lower incidences of AKI. As an anesthetic, propofol correlated with lower levels of serum creatinine and other inflammatory markers such as TNF-α, IL-1, and interferon-γ compared with that of sevoflurane in pigs.97 Propofol tempered the degree of renal injury due to ischemia−reperfusion injury in both piglets and rats.98, 99
Propofol has been studied in humans both in vitro and in vivo. Human renal tubular cells exhibited decreased rates of apoptosis and abundant proliferation when treated with propofol.21 In numerous surgical studies, propofol proved superior to sevoflurane for protecting the kidneys. Yoo et al. found that in comparison to the general anesthetic sevoflurane in 112 valvular surgery patients, certain biomarkers such as serum creatinine and cystatin C were lower with propofol, and hospital length of stay was also shorter.12 A retrospective study of 4320 colorectal surgery patients found that anesthesia with propofol resulted in a decreased incidence of AKI and shorter intensive care unit and hospital lengths of stay compared with sevoflurane.13 In addition, patients who were anesthetized with propofol before undergoing cardiopulmonary bypass surgery for elective open abdominal aortic aneurysm repair experienced less kidney injury, as measured in serum biomarkers compared with sevoflurane.14 When propofol was compared with midazolam in a retrospective trial with propensity-matched critically ill patients, a similar trend was noted, as well as a decreased need for renal replacement therapy and mortality.19 Additional research is ongoing in other settings, including extracorporeal mechanical oxygen−assisted lung transplantation (NCT02009280), valvular heart surgery (NCT01384643), and renal transplantation (NCT01132157, NCT01870011).
Curcumin
Curcumin is an herbal supplement related to turmeric, which is typically used as food coloring. However, it is also a scavenger of free oxygen radicals and stimulates the activity of additional antioxidant molecules such as superoxide dismutase, catalase, and glutathione peroxidase.100
Numerous rat models have demonstrated the protective effect of curcumin on the kidneys. Curcumin has been found to mitigate cisplatin, gentamicin, and carbon-tetrachloride−induced kidney injury in pretreated rats, resulting in histological improvement of inflammation, curtailment of inflammatory markers (TNF-α, myeloperoxidase, IL-1 β), reduction in serum creatinine and BUN, and inhibited expression of proapoptotic genes such as p53.100, 101 In a septic rat model, pretreatment with curcumin resulted in a mortality benefit, reduction in TNF-α, and decline of fibrin deposition in the glomerulus.102 Multiple animals subjected to ischemia−reperfusion injury and treated with curcumin exhibited attenuated renal damage.103, 104, 105, 106, 107 In contrast, there appeared to be no benefit from curcumin if renal disease was induced by hypertonic glycerol in rats.108
In humans, curcumin has been shown to diminish the apoptotic and necrotic effects of shiga toxin on renal proximal tubule cells in vitro, but the authors hypothesized this to be the result of activation of heat shock proteins rather than any antioxidant properties.109 Among human trials, most of the benefit has been demonstrated in diabetic nephropathy, where curcumin was noted to reduce proteinuria and inflammatory markers.110, 111, 112 A similar effect was found in an randomized control trial of patients with lupus nephritis.20 Notably, these human studies were conducted on patients with chronic renal disease rather than AKI. Currently, curcumin is being evaluated in the prevention of kidney injury after abdominal aortic aneurysm repair using endpoints, including serum creatinine, urine IL-18, time to dialysis, and mortality (NCT01225094).
Inflammatory Mediators
Kidney injury is associated with the release of inflammatory cytokines and chemokines that in turn attract immune cells such as neutrophils, macrophages, and natural killer cells.3 The dysregulation of these molecular and cellular elements results in the functional impairment of the kidney. Inflammation and immune dysregulation occur in such disease states as gram-negative sepsis, ischemia−reperfusion, diabetes (highlighted by chronic inflammation), and cancer (especially with the use of chemotherapeutic gents). As such, the various proteins involved in these complex pathways could serve as targets for innovative therapies.
Alkaline Phosphatase
Alkaline phosphatase (AP) confers renal protection during sepsis via the dephosphorylation of lipopolysaccharide, which activates inflammatory pathways when the lipid-A molecule is phosphorylated.113, 114 The presence of lipopolysaccharide results in oxidative stress and release of inflammatory cytokines such as TNF-α and IL-6, which leads to endothelial damage and regional hypoxia within the kidney.115, 116, 117 In addition, in sepsis, mitochondria release large quantities of adenosine triphosphate in response to inflammatory cytokines and hypoxia.118 AP dephosphorylates adenosine triphosphate, converting it to adenosine. In vitro, adenosine has exhibited some protective effects on proximal renal tubule cells, depending on the receptor activated.119 However, the effect of adenosine, as previously noted, is quite variable, and its manipulation is the subject of alternative therapies.
Recent studies have evaluated the effect of AP on AKI in humans. A 2009 study found AP significantly improved serum creatinine compared with placebo in a small group of patients with gram-negative sepsis. However, the study was underpowered with regard to clinical outcome data.4 In a 2012 study, AP was found to lower creatinine clearance and levels of inflammatory markers in septic patients, although there was no significant difference between AP and placebo with regard to the need for renal replacement therapy.5 The ongoing STOP-AKI trial is designed to evaluate the efficacy of a human recombinant form of AP in reducing serum creatinine and progression to renal replacement therapy.120
Dipeptidylpeptidase-4 Inhibitors
Dipeptidylpeptidase-4 (DPP-4) inhibitors were originally designed to extend the biological half-life of glucagon-like peptide-1 (GLP-1). GLP-1 is an incretin hormone, and is known to play a role in blood glucose regulation via stimulation of insulin secretion while inhibiting glucagon secretion.121 However, evidence suggests that GLP-1 also has anti-inflammatory properties by suppressing the activity of various proinflammatory cytokines such as TNF-α and -γ, IL-1 β, plasminogen activator inhibitor type-1, and intercellular adhesion molecule-1.122, 123, 124 GLP-1 receptors have been found in the glomeruli of animal models, and it has been hypothesized that a deficiency in the receptors is involved in the pathogenesis of diabetic nephropathy.125, 126, 127 DPP-4 inhibitors may have a benefit in renal disease, particularly when associated with diabetes.121
In rats with type 1 diabetes, DPP-4 inhibitors were found to decrease the level of inflammatory markers and oxidative stress, resulting in less albuminuria and glomerular hyperfiltration.128 This result has been replicated in other animal models as well.129, 130, 131 In nondiabetic rat kidneys, DPP-4 inhibitors were found to decrease the levels of inflammatory macrophages,132 and mice exposed to cisplatin had lower serum BUN and creatinine when pretreated with DPP-4 inhibitors.133 When sitagliptin was administered to rats who experience renal ischemia−reperfusion injury, serum creatinine and BUN were lower, whereas urine output was increased at 24 and 72 hours.134
Human studies of DPP-4 inhibitors have been more inconclusive. Shih et al. found retrospectively that DPP-4 inhibition was associated with an increased risk of AKI in a case−control study of 13,000 diabetic patients (one-half of whom were taking DPP-4 inhibitors).15 An observational study found a decrease in the estimated glomerular filtration rate in 247 diabetic patients who received sitagliptin.16 In addition, the large Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which evaluated the effect of saxagliptin versus placebo on cardiovascular events in patients with type 2 diabetes mellitus, found that DPP-4 inhibitor use was associated with decreased GFR compared with placebo.17 Conversely, a retrospective analysis by Pendergrass et al. showed no association between sitagliptin and renal failure.18 The Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) study randomized 14,000 diabetic patients to sitagliptin versus placebo and echoed these findings.135 However, subsequent analysis using the TECOS trial data with primary renal endpoints failed to find any improvement in renal function with sitagliptin.136 The effect of DPP-4 inhibitors on renal dysfunction continues to be evaluated in at least 1 clinical trial (NCT02250872).
Sphingosine 1 Phosphate Analogues
Sphingosine 1 phosphate (S1P) analogues mitigate endothelial damage and decrease recruitment of inflammatory mediators in the renal tubules. In mice subjected to cisplatin, the S1P analogue FTY720 was found to decrease the levels of TNF-α and IL-6.137 This same effect was also seen with ischemia−reperfusion kidney injury in mice with both FTY720 and the S1P analogue SEW2871,138 whereas SEW2871 was found to reduce tubular necrosis, lower serum creatinine, and lessen leukocyte infiltration of glomerular tissue.139 However, when used as an immunosuppressant for renal allografts in mice, FTY720 showed no improvement in graft survival and was associated with worsening kidney function.140 Data on S1P analogues in humans is lacking, and currently, there are no clinical trials evaluating S1P analogues in human AKI.
Genetic Modifiers
In AKI, there is damage to the highly metabolically active cells in the tubular epithelium, which signals quiescent and terminally differentiated cells to enter the cell cycle as part of the repair process.141 The cell cycle arrest and apoptosis during repair may ultimately lead to acute tubular necrosis.36 Cell cycle arrest, apoptosis, or cellular senescence is often mediated by the p53 tumor suppressor protein (resulting from the transcription of the TP53 gene) in response to stress signals, including DNA damage.142 Genetic modification or manipulation of this process may be of benefit in ameliorating AKI.
I5NP
I5NP, a small interfering RNA (siRNA) that can be filtered through the glomerulus and inhibit p53 in the renal tubules, has been found to decrease serum creatinine.143, 144 I5NP interferes with the transcriptive process of p53, which in the tubular epithelium, may result in delay of apoptosis, permitting repair of damaged DNA, and ultimately, cellular function.144 Inhibition of the well-studied pro-apoptotic p53 gene in animal models has shown some benefit after ischemic and toxic injury to the kidney. In models of ischemia−reperfusion kidney injury (cross-clamp) and toxin-mediated kidney injury (cisplatin) in rats, I5NP was found to be associated with lower serum creatinine.145 Administration of an siRNA molecule after ischemia−reperfusion injury resulted in improved tubular injury, less frequent apoptosis, and reduced swelling of mitochondria in cells of the thick ascending limb of Henle at the outer medullary regions in mice.146 A few studies have tried to evaluate the safety and dose escalation of I5NP with regard to AKI in patients undergoing cardiovascular surgery (NCT00683553, NCT00554359) and renal transplantation (NCT00802347), but results were never reported. Genetic manipulation of transcriptive processes through the delivery of I5NP and other siRNA molecules through various mechanisms is an evolving area of research, especially in the arena of renal transplantation medicine.147
Conclusion
AKI is a common disease, but heretofore has been poorly understood. Rudimentary understanding of the pathophysiology of AKI has meant that effective therapeutics to treat this disease are scarce. Although current therapy is supportive, a great deal of data has emerged that calls the conventional practices into question. More conservative resuscitation practices have supplanted traditional liberal volume resuscitation with normal saline. Accumulating evidence suggests chloride-rich fluids worsen renal function, and increasingly, balanced, buffered solutions are being considered for resuscitation. Crystalloids are still the mainstay of therapy, with equivocal data supporting the use of albumin over crystalloid therapy. Based on the most recent evidence, synthetic starches are no longer recommended. Research is also beginning to elucidate the microvascular and molecular pathways that lead to patterns of injury in AKI, which has resulted in a number of new and prospective therapies to prevent this disease. Renal flow modulators such as angiotensin II and adenosine antagonists convey benefit to the kidney through the manipulation of renal microvasculature. Antioxidants such as ALA, selenium, propofol, MESNA, and curcumin mitigate the effects of free radical oxygen species commonly found in AKI. Inflammatory modifiers such as AP, DPP-4 inhibitors, and S1P analogues attenuate inflammatory kidney injury processes via direct effect on immune-active molecules, such as TNFs and Ils. Finally, genetic modifiers seek to derail the genetic processes that lead to cell cycle arrest and apoptosis in kidney failure. Although many of these novel therapies have yet to be studied extensively in humans, their promise in animal models or smaller human studies lays the groundwork for more thorough future investigation.
Disclosure
LWB reports having received consulting fees from La Jolla Pharmaceutical Company. The other author declared no competing interests.
References
- 1.Legrand M., Dupuis C., Simon C. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd J.H., Forbes J., Nakada T.A., Walley K.R., Russell J.A. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 3.Bajwa A., Kinsey G.R., Okusa M.D. Immune mechanisms and novel pharmacological therapies of acute kidney injury. Curr Drug Targets. 2009;10:1196–1204. doi: 10.2174/138945009789753174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heemskerk S., Masereeuw R., Moesker O. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37:417–423. doi: 10.1097/CCM.0b013e31819598af. e411. [DOI] [PubMed] [Google Scholar]
- 5.Pickkers P., Heemskerk S., Schouten J. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care. 2012;16:R14. doi: 10.1186/cc11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagshaw S.M., Ghali W.A. Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Medicine. 2005;165:1087–1093. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- 7.Dai B., Liu Y., Fu L., Li Y., Zhang J., Mei C. Effect of theophylline on prevention of contrast-induced acute kidney injury: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;60:360–370. doi: 10.1053/j.ajkd.2012.02.332. [DOI] [PubMed] [Google Scholar]
- 8.Jo S.-H., Kim S.-A., Kim H.-S., Han S.-J., Park W.-J., Choi Y.J. Alpha-lipoic acid for the prevention of contrast-induced nephropathy in patients undergoing coronary angiography: the ALIVE study - a prospective randomized trial. Cardiology. 2013;126:159–166. doi: 10.1159/000353812. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig U., Riedel M.K., Backes M., Imhof A., Muche R., Keller F. MESNA (sodium 2-mercaptoethanesulfonate) for prevention of contrast medium-induced nephrotoxicity - controlled trial. Clin Nephrol. 2011;75:302–308. doi: 10.5414/cn106651. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosi N., Arrosagaray V., Guerrieri D. Alpha-lipoic acid protects against ischemia-reperfusion injury in simultaneous kidney-pancreas transplantation. Transplantation. 2016;100:908–915. doi: 10.1097/TP.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 11.Ghorbani A., Omidvar B., Parsi A. Protective effect of selenium on cisplatin induced nephrotoxicity: a double-blind controlled randomized clinical trial. J Nephropathol. 2013;2:129–134. doi: 10.12860/JNP.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo Y.C., Shim J.K., Song Y., Yang S.Y., Kwak Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014;86:414–422. doi: 10.1038/ki.2013.532. [DOI] [PubMed] [Google Scholar]
- 13.Bang J.Y., Lee J., Oh J., Song J.G., Hwang G.S. The influence of propofol and sevoflurane on acute kidney injury after colorectal surgery: a retrospective cohort study. Anesth Analg. 2016;123:363–370. doi: 10.1213/ANE.0000000000001274. [DOI] [PubMed] [Google Scholar]
- 14.Ammar A.S., Mahmoud K.M. Comparative effect of propofol versus sevoflurane on renal ischemia/reperfusion injury after elective open abdominal aortic aneurysm repair. Saudi J Anaesth. 2016;10:301–307. doi: 10.4103/1658-354X.174907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih C.-J., Lee Y.-J., Lo Y.-H., Kuo S.-C., Ou S.-M., Chen Y.-T. Association between use of dipeptidyl peptidase-4 inhibitors and the risk of acute kidney injury: a nested case-control study. Mayo Clin Proc. 2016;91:867–872. doi: 10.1016/j.mayocp.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki I., Hiura Y., Tamai A. Sitagliptin reduces the urine albumin-to-creatinine ratio in type 2 diabetes through decreasing both blood pressure and estimated glomerular filtration rate. J Diabetes. 2015;7:41–46. doi: 10.1111/1753-0407.12153. [DOI] [PubMed] [Google Scholar]
- 17.Scirica B.M., Bhatt D.L., Braunwald E. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 18.Pendergrass M., Fenton C., Haffner S.M., Chen W. Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes Metab. 2012;14:596–600. doi: 10.1111/j.1463-1326.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 19.Leite T.T., Macedo E., Martins Ida S., Neves F.M., Liborio A.B. Renal outcomes in critically ill patients receiving propofol or midazolam. Clin J Am Soc Nephrol. 2015;10:1937–1945. doi: 10.2215/CJN.02330315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khajehdehi P., Zanjaninejad B., Aflaki E. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: a randomized and placebo-controlled study. J Renal Nutr. 2012;22:50–57. doi: 10.1053/j.jrn.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y., Bai T., Ma H., Wang J.-K. Propofol attenuates human proximal renal tubular epithelial cell injury induced by anoxia-reoxygenation. Lab Med. 2008;39:356–360. [Google Scholar]
- 22.Abuelo J.G. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357:797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 23.Bagshaw S.M., George C., Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun L.Y., Wijeysundera D.N., Tait G.A., Beattie W.S. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 25.Walsh M., Devereaux P.J., Garg A.X. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 26.Schnermann J. Concurrent activation of multiple vasoactive signaling pathways in vasoconstriction caused by tubuloglomerular feedback: a quantitative assessment. Ann Rev Physiol. 2015;77:301–322. doi: 10.1146/annurev-physiol-021014-071829. [DOI] [PubMed] [Google Scholar]
- 27.Prowle J.R., Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol. 2015;35:64–74. doi: 10.1016/j.semnephrol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Whiting P., Morden A., Tomlinson L.A. What are the risks and benefits of temporarily discontinuing medications to prevent acute kidney injury? A systematic review and meta-analysis. BMJ Open. 2017;7:e012674. doi: 10.1136/bmjopen-2016-012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlondorff D. Renal complications of nonsteroidal anti-inflammatory drugs. Kidney Int. 1993;44:643–653. doi: 10.1038/ki.1993.293. [DOI] [PubMed] [Google Scholar]
- 30.Sutton T.A., Fisher C.J., Molitoris B.A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z., Holthoff J.H., Seely K.A. Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol. 2012;180:505–516. doi: 10.1016/j.ajpath.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holthoff J.H., Wang Z., Seely K.A., Gokden N., Mayeux P.R. Resveratrol improves renal microcirculation, protects the tubular epithelium, and prolongs survival in a mouse model of sepsis-induced acute kidney injury. Kidney Int. 2012;81:370–378. doi: 10.1038/ki.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko G.J., Jang H.R., Huang Y. Blocking Fas ligand on leukocytes attenuates kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:732–742. doi: 10.1681/ASN.2010010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenhammar J., Rundgren M., Forestier J., Kalman S., Eriksson S., Frithiof R. Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology. 2011;114:1130–1137. doi: 10.1097/ALN.0b013e31820b8b44. [DOI] [PubMed] [Google Scholar]
- 35.Basile D.P., Anderson M.D., Sutton T.A. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashani K., Al-Khafaji A., Ardiles T. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourgoin A., Leone M., Delmas A., Garnier F., Albanese J., Martin C. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Crit Care Med. 2005;33:780–786. doi: 10.1097/01.ccm.0000157788.20591.23. [DOI] [PubMed] [Google Scholar]
- 38.Poukkanen M., Wilkman E., Vaara S.T. Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: data from the prospective observational FINNAKI study. Crit Care. 2013;17:R295. doi: 10.1186/cc13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Payen D., de Pont A.C., Sakr Y., Spies C., Reinhart K., Vincent J.L. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouchard J., Soroko S.B., Chertow G.M. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 41.Claure-Del Granado R., Mehta R.L. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17:109. doi: 10.1186/s12882-016-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grams M.E., Estrella M.M., Coresh J., Brower R.G., Liu K.D. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–973. doi: 10.2215/CJN.08781010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang N., Jiang L., Zhu B., Wen Y., Xi X.M. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371. doi: 10.1186/s13054-015-1085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perner A., Haase N., Guttormsen A.B. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 45.Myburgh J.A., Finfer S., Bellomo R. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 46.Zarychanski R., Abou-Setta A.M., Turgeon A.F. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 47.Rackow E.C., Falk J.L., Fein I.A. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839–850. [PubMed] [Google Scholar]
- 48.Wiedermann C.J., Dunzendorfer S., Gaioni L.U., Zaraca F., Joannidis M. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care. 2010;14:R191. doi: 10.1186/cc9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caironi P., Tognoni G., Masson S. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 50.Finfer S., Bellomo R., Boyce N., French J., Myburgh J., Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 51.Wilcox C.S. Regulation of renal blood flow by plasma chloride. J Clin Investig. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yunos N.M., Bellomo R., Glassford N., Sutcliffe H., Lam Q., Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intens Care Med. 2015;41:257–264. doi: 10.1007/s00134-014-3593-0. [DOI] [PubMed] [Google Scholar]
- 53.Yunos N.M., Bellomo R., Hegarty C., Story D., Ho L., Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 54.Young P., Bailey M., Beasley R. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: The SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 55.Prowle J.R., Echeverri J.E., Ligabo E.V., Ronco C., Bellomo R. Fluid balance and acute kidney injury. Nature Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 56.Chawla L.S., Busse L., Brasha-Mitchell E. Intravenous angiotensin II for the treatment of high-output shock (ATHOS trial): a pilot study. Crit Care. 2014;18:534. doi: 10.1186/s13054-014-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Correa T.D., Takala J., Jakob S.M. Angiotensin II in septic shock. Crit Care. 2015;19:98. doi: 10.1186/s13054-015-0802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown N.J., Vaughan D.E. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 59.Rosa R.M., Colucci J.A., Yokota R. Alternative pathways for angiotensin II production as an important determinant of kidney damage in endotoxemia. Am J Physiol Renal Physiol. 2016;311:F496–F504. doi: 10.1152/ajprenal.00121.2014. [DOI] [PubMed] [Google Scholar]
- 60.Bucher M., Ittner K.P., Hobbhahn J., Taeger K., Kurtz A. Downregulation of angiotensin II type 1 receptors during sepsis. Hypertension (Dallas, Tex : 1979) 2001;38:177–182. doi: 10.1161/01.hyp.38.2.177. [DOI] [PubMed] [Google Scholar]
- 61.Correa T.D., Jeger V., Pereira A.J., Takala J., Djafarzadeh S., Jakob S.M. Angiotensin II in septic shock: effects on tissue perfusion, organ function, and mitochondrial respiration in a porcine model of fecal peritonitis. Crit Care Med. 2014;42:e550–e559. doi: 10.1097/CCM.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 62.Wan L., Langenberg C., Bellomo R., May C.N. Angiotensin II in experimental hyperdynamic sepsis. Crit Care. 2009;13:R190. doi: 10.1186/cc8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.May C.N., Ishikawa K., Wan L. Renal bioenergetics during early gram-negative mammalian sepsis and angiotensin II infusion. Intens Care Med. 2012;38:886–893. doi: 10.1007/s00134-012-2487-2. [DOI] [PubMed] [Google Scholar]
- 64.Khanna A., English S.W., Wang X.S. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017 doi: 10.1056/NEJMc1714511. [e-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Vallon V., Muhlbauer B., Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- 66.Day Y.J., Huang L., McDuffie M.J. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okusa M.D., Linden J., Macdonald T., Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277(3 Pt 2):F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- 68.Axelrod D.M., Sutherland S.M., Anglemyer A., Grimm P.C., Roth S.J. A double-blinded, randomized, placebo-controlled clinical trial of aminophylline to prevent acute kidney injury in children following congenital heart surgery with cardiopulmonary bypass. Pediatr Crit Care M. 2016;17:135–143. doi: 10.1097/PCC.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Voors A.A., Dittrich H.C., Massie B.M. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) J Am Coll Cardiol. 2011;57:1899–1907. doi: 10.1016/j.jacc.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 70.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 71.Lee S.R., Jeong M.H., Lim S.Y. The effect of alpha lipoic acid (Thioctacid HR®) on endothelial function in diabetic and hypertensive patients. Korean Circ J. 2006;36:559–564. [Google Scholar]
- 72.Kang K.P., Kim D.H., Jung Y.J. Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury in mice by suppressing renal inflammation. Nephrol Dial Transplant. 2009;24:3012–3020. doi: 10.1093/ndt/gfp242. [DOI] [PubMed] [Google Scholar]
- 73.Melhem M.F., Craven P.A., Derubertis F.R. Effects of dietary supplementation of alpha-lipoic acid on early glomerular injury in diabetes mellitus. J Am Soc Nephrol. 2001;12:124–133. doi: 10.1681/ASN.V121124. [DOI] [PubMed] [Google Scholar]
- 74.Briguori C., Visconti G., Rivera N.V. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 75.Haase M., Bellomo R., Devarajan P., Schlattmann P., Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 76.Malyszko J., Bachorzewska-Gajewska H., Poniatowski B., Malyszko J.S., Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Renal Fail. 2009;31:910–919. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]
- 77.Cicek M., Yıldırır A., Okyay K. Use of alpha-lipoic acid in prevention of contrast-induced nephropathy in diabetic patients. Renal Failure. 2013;35:748–753. doi: 10.3109/0886022X.2013.790298. [DOI] [PubMed] [Google Scholar]
- 78.Takaoka M., Ohkita M., Kobayashi Y., Yuba M., Matsumura Y. Protective effect of alpha-lipoic acid against ischaemic acute renal failure in rats. Clin Exp Pharmacol Physiol. 2002;29:189–194. doi: 10.1046/j.1440-1681.2002.03624.x. [DOI] [PubMed] [Google Scholar]
- 79.Li G., Gao L., Jia J., Gong X., Zang B., Chen W. Alpha-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clin Exp Pharmacol Physiol. 2014;41:459–468. doi: 10.1111/1440-1681.12244. [DOI] [PubMed] [Google Scholar]
- 80.Abdel-Zaher A.O., Abdel-Hady R.H., Mahmoud M.M., Farrag M.M.Y. The potential protective role of alpha-lipoic acid against acetaminophen-induced hepatic and renal damage. Toxicology. 2008;243:261–270. doi: 10.1016/j.tox.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Wongmekiat O., Leelarungrayub D., Thamprasert K. Alpha-lipoic acid attenuates renal injury in rats with obstructive nephropathy. Biomed Res Int. 2013;2013:138719. doi: 10.1155/2013/138719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aksoy A., Karaoglu A., Akpolat N., Naziroglu M., Ozturk T., Karagoz Z.K. Protective role of selenium and high dose vitamin E against cisplatin-induced nephrotoxicty in rats. Asian Pac J Cancer Prev. 2015;16:6877–6882. doi: 10.7314/apjcp.2015.16.16.6877. [DOI] [PubMed] [Google Scholar]
- 83.Iglesias P., Selgas R., Romero S., Diez J.J. Selenium and kidney disease. J Nephrol. 2013;26:266–272. doi: 10.5301/jn.5000213. [DOI] [PubMed] [Google Scholar]
- 84.Randjelovic P., Veljkovic S., Stojiljkovic N. Protective effect of selenium on gentamicin-induced oxidative stress and nephrotoxicity in rats. Drug Chem Toxicol. 2012;35:141–148. doi: 10.3109/01480545.2011.589446. [DOI] [PubMed] [Google Scholar]
- 85.Liu L., Liu C., Hou L. Protection against ischemia/reperfusioninduced renal injury by cotreatment with erythropoietin and sodium selenite. Mol Med Rep. 2015;12:7933–7940. doi: 10.3892/mmr.2015.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Treska V., Kuntscher V., Hasman D. Importance of selenium for the influence of ischemia-reperfusion syndrome after kidney transplantation from a non-heart beating donor in a pig model. Transplant Proc. 2002;34:3057–3059. doi: 10.1016/s0041-1345(02)03694-1. [DOI] [PubMed] [Google Scholar]
- 87.Hu Y.J., Chen Y., Zhang Y.Q. The protective role of selenium on the toxicity of cisplatin-contained chemotherapy regimen in cancer patients. Biol Trace Element Res. 1997;56:331–341. doi: 10.1007/BF02785304. [DOI] [PubMed] [Google Scholar]
- 88.Weijl N.I., Elsendoorn T.J., Lentjes E.G. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer. 2004;40:1713–1723. doi: 10.1016/j.ejca.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 89.El-Nahas A.R., Elsaadany M.M., Taha D.E. A randomised controlled trial evaluating renal protective effects of selenium with vitamins A, C, E, verapamil, and losartan against extracorporeal shockwave lithotripsy-induced renal injury. BJU Int. 2017;119:142–147. doi: 10.1111/bju.13667. [DOI] [PubMed] [Google Scholar]
- 90.Berger M.M., Soguel L., Shenkin A. Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care. 2008;12:R101. doi: 10.1186/cc6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stoppe C., McDonald B., Rex S. SodiUm SeleniTe Adminstration IN Cardiac Surgery (SUSTAIN CSX-trial): study design of an international multicenter randomized double-blinded controlled trial of high dose sodium-selenite administration in high-risk cardiac surgical patients. Trials. 2014;15:339. doi: 10.1186/1745-6215-15-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mashiach E., Sela S., Weinstein T., Cohen H.I., Shasha S.M., Kristal B. Mesna: a novel renoprotective antioxidant in ischaemic acute renal failure. Nephrol Dial Transplant. 2001;16:542–551. doi: 10.1093/ndt/16.3.542. [DOI] [PubMed] [Google Scholar]
- 93.Dechant K.L., Brogden R.N., Pilkington T., Faulds D. Ifosfamide/mesna. A review of its antineoplastic activity, pharmacokinetic properties and therapeutic efficacy in cancer. Drugs. 1991;42:428–467. doi: 10.2165/00003495-199142030-00006. [DOI] [PubMed] [Google Scholar]
- 94.Schoenike S.E., Dana W.J. Ifosfamide and mesna. Clin Pharm. 1990;9:179–191. [PubMed] [Google Scholar]
- 95.Skinner R., Sharkey I.M., Pearson A.D., Craft A.W. Ifosfamide, mesna, and nephrotoxicity in children. J Clin Oncol. 1993;11:173–190. doi: 10.1200/JCO.1993.11.1.173. [DOI] [PubMed] [Google Scholar]
- 96.Murphy P.G., Myers D.S., Davies M.J., Webster N.R., Jones J.G. The antioxidant potential of propofol (2,6-diisopropylphenol) Br J Anaesth. 1992;68:613–618. doi: 10.1093/bja/68.6.613. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez-Lopez J.M., Sanchez-Conde P., Lozano F.S. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth. 2006;53:701–710. doi: 10.1007/BF03021629. [DOI] [PubMed] [Google Scholar]
- 98.Sánchez-Conde P., Rodríguez-López J.M., Nicolás J.L. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106:371–378. doi: 10.1213/ane.0b013e318160580b. [DOI] [PubMed] [Google Scholar]
- 99.Wang H.H., Zhou H.Y., Chen C.C., Zhang X.L., Cheng G. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin. 2007;28:1175–1180. doi: 10.1111/j.1745-7254.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 100.Topcu-Tarladacalisir Y., Sapmaz-Metin M., Karaca T. Curcumin counteracts cisplatin-induced nephrotoxicity by preventing renal tubular cell apoptosis. Ren Fail. 2016;38:1741–1748. doi: 10.1080/0886022X.2016.1229996. [DOI] [PubMed] [Google Scholar]
- 101.He L., Peng X., Zhu J. Protective effects of curcumin on acute gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol. 2015;93:275–282. doi: 10.1139/cjpp-2014-0459. [DOI] [PubMed] [Google Scholar]
- 102.Chen H.W., Kuo H.T., Chai C.Y., Ou J.L., Yang R.C. Pretreatment of curcumin attenuates coagulopathy and renal injury in LPS-induced endotoxemia. J Endotoxin Res. 2007;13:15–23. doi: 10.1177/0968051907078605. [DOI] [PubMed] [Google Scholar]
- 103.Aydin M.S., Caliskan A., Kocarslan A. Intraperitoneal curcumin decreased lung, renal and heart injury in abdominal aorta ischemia/reperfusion model in rat. Int J Surg (London) 2014;12:601–605. doi: 10.1016/j.ijsu.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Chen T.H., Yang Y.C., Wang J.C., Wang J.J. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant Proc. 2013;45:3546–3549. doi: 10.1016/j.transproceed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Hammad F.T., Al-Salam S., Lubbad L. Curcumin provides incomplete protection of the kidney in ischemia reperfusion injury. Physiol Res. 2012;61:503–511. doi: 10.33549/physiolres.932376. [DOI] [PubMed] [Google Scholar]
- 106.Awad A.S., El-Sharif A.A. Curcumin immune-mediated and anti-apoptotic mechanisms protect against renal ischemia/reperfusion and distant organ induced injuries. Int Immunopharmacol. 2011;11:992–996. doi: 10.1016/j.intimp.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 107.Bayrak O., Uz E., Bayrak R. Curcumin protects against ischemia/reperfusion injury in rat kidneys. World J Urol. 2008;26:285–291. doi: 10.1007/s00345-008-0253-4. [DOI] [PubMed] [Google Scholar]
- 108.Vlahovic P., Cvetkovic T., Savic V., Stefanovic V. Dietary curcumin does not protect kidney in glycerol-induced acute renal failure. Food Chem Toxicol. 2007;45:1777–1782. doi: 10.1016/j.fct.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 109.Sood A., Mathew R., Trachtman H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem Biophys Res Commun. 2001;283:36–41. doi: 10.1006/bbrc.2001.4749. [DOI] [PubMed] [Google Scholar]
- 110.Yang H., Xu W., Zhou Z. Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes. 2015;123:360–367. doi: 10.1055/s-0035-1545345. [DOI] [PubMed] [Google Scholar]
- 111.Suresh Babu P., Srinivasan K. Amelioration of renal lesions associated with diabetes by dietary curcumin in streptozotocin diabetic rats. Mol Cell Biochem. 1998;181:87–96. doi: 10.1023/a:1006821828706. [DOI] [PubMed] [Google Scholar]
- 112.Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26:269–270. [PubMed] [Google Scholar]
- 113.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 114.Peters E., Heemskerk S., Masereeuw R., Pickkers P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis. 2014;63:1038–1048. doi: 10.1053/j.ajkd.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 115.Kalakeche R., Hato T., Rhodes G. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol. 2011;22:1505–1516. doi: 10.1681/ASN.2011020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zarjou A., Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol. 2011;22:999–1006. doi: 10.1681/ASN.2010050484. [DOI] [PubMed] [Google Scholar]
- 117.Wu L., Tiwari M.M., Messer K.J. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292:F261–F268. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 118.Roberts V., Lu B., Rajakumar S., Cowan P.J., Dwyer K.M. The CD39-adenosinergic axis in the pathogenesis of renal ischemia–reperfusion injury. Puriner Signal. 2013;9:135–143. doi: 10.1007/s11302-012-9342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee H.T., Emala C.W. Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol. 2002;282:F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- 120.Peters E., Mehta R.L., Murray P.T. Study protocol for a multicentre randomised controlled trial: Safety, Tolerability, efficacy and quality of life Of a human recombinant alkaline Phosphatase in patients with sepsis-associated Acute Kidney Injury (STOP-AKI) BMJ Open. 2016;6:e012371. doi: 10.1136/bmjopen-2016-012371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee Y.S., Jun H.S. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Mediat Inflamm. 2016;2016:3094642. doi: 10.1155/2016/3094642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwai T., Ito S., Tanimitsu K., Udagawa S., Oka J.-I. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 123.Blandino-Rosano M., Perez-Arana G., Mellado-Gil J.M., Segundo C., Aguilar-Diosdado M. Anti-proliferative effect of pro-inflammatory cytokines in cultured beta cells is associated with extracellular signal-regulated kinase 1/2 pathway inhibition: protective role of glucagon-like peptide -1. J Mol Endocrinol. 2008;41:35–44. doi: 10.1677/JME-07-0154. [DOI] [PubMed] [Google Scholar]
- 124.Liu H., Dear A.E., Knudsen L.B., Simpson R.W. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201:59–66. doi: 10.1677/JOE-08-0468. [DOI] [PubMed] [Google Scholar]
- 125.Fujita H., Morii T., Fujishima H. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579–589. doi: 10.1038/ki.2013.427. [DOI] [PubMed] [Google Scholar]
- 126.Crajoinas R.O., Oricchio F.T., Pessoa T.D. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol. 2011;301:F355–F363. doi: 10.1152/ajprenal.00729.2010. [DOI] [PubMed] [Google Scholar]
- 127.Schlatter P., Beglinger C., Drewe J., Gutmann H. Glucagon-like peptide 1 receptor expression in primary porcine proximal tubular cells. Regulatory Peptides. 2007;141:120–128. doi: 10.1016/j.regpep.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 128.Kodera R., Shikata K., Takatsuka T. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem Biophys Res Commun. 2014;443:828–833. doi: 10.1016/j.bbrc.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 129.Gangadharan Komala M., Gross S., Zaky A., Pollock C., Panchapakesan U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology (Carlton, Vic) 2016;21:423–431. doi: 10.1111/nep.12618. [DOI] [PubMed] [Google Scholar]
- 130.Nakashima S., Matsui T., Takeuchi M., Yamagishi S.I. Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis. Hormone Metab Res. 2014;46:717–721. doi: 10.1055/s-0034-1371892. [DOI] [PubMed] [Google Scholar]
- 131.Marques C., Mega C., Goncalves A. Sitagliptin prevents inflammation and apoptotic cell death in the kidney of type 2 diabetic animals. Mediators Inflamm. 2014;2014:538737. doi: 10.1155/2014/538737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Higashijima Y., Tanaka T., Yamaguchi J., Tanaka S., Nangaku M. Anti-inflammatory role of DPP-4 inhibitors in a nondiabetic model of glomerular injury. Am J Physiol Renal Physiol. 2015;308:F878–F887. doi: 10.1152/ajprenal.00590.2014. [DOI] [PubMed] [Google Scholar]
- 133.Katagiri D., Hamasaki Y., Doi K. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J Am Soc Nephrol. 2013;24:2034–2043. doi: 10.1681/ASN.2013020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen Y.T., Tsai T.H., Yang C.C. Exendin-4 and sitagliptin protect kidney from ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2013;11:270. doi: 10.1186/1479-5876-11-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Green J.B., Bethel M.A., Armstrong P.W. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 136.Cornel J.H., Bakris G.L., Stevens S.R. Effect of sitagliptin on kidney function and respective cardiovascular outcomes in type 2 diabetes: outcomes from TECOS. Diabetes Care. 2016;39:2304–2310. doi: 10.2337/dc16-1415. [DOI] [PubMed] [Google Scholar]
- 137.Bajwa A., Rosin D.L., Chroscicki P. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J Am Soc Nephrol. 2015;26:908–925. doi: 10.1681/ASN.2013121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Awad A.S., Ye H., Huang L. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 139.Lien Y.H., Yong K.C., Cho C., Igarashi S., Lai L.W. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 140.Budde K., Schutz M., Glander P. FTY720 (fingolimod) in renal transplantation. Clin Transpl. 2006;20 Suppl 17:17–24. doi: 10.1111/j.1399-0012.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 141.Yang Q.H., Liu D.W., Long Y., Liu H.Z., Chai W.Z., Wang X.T. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infection. 2009;58:459–464. doi: 10.1016/j.jinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Rodier F., Campisi J., Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson J.D., Kornbrust D.J., Foy J.W. Toxicological and pharmacokinetic properties of chemically modified siRNAs targeting p53 RNA following intravenous administration. Nucleic Acid Ther. 2012;22:255–264. doi: 10.1089/nat.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Powell JT, Tsapepas DS, Martin ST, Hardy MA, Ratner LE. Managing renal transplant ischemia reperfusion injury: novel therapies in the pipeline. Clin Transpl.27:484–491. [DOI] [PubMed]
- 145.Molitoris B.A., Dagher P.C., Sandoval R.M. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fujino T., Muhib S., Sato N., Hasebe N. Silencing of p53 RNA through transarterial delivery ameliorates renal tubular injury and downregulates GSK-3beta expression after ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2013;305:F1617–F1627. doi: 10.1152/ajprenal.00279.2013. [DOI] [PubMed] [Google Scholar]
- 147.Glebova K., Reznik O.N., Reznik A.O. siRNA technology in kidney transplantation: current status and future potential. BioDrugs. 2014;28:345–361. doi: 10.1007/s40259-014-0087-0. [DOI] [PubMed] [Google Scholar]