Abstract
Remote patient management (RPM) offers renal health care providers and patients with end-stage kidney disease opportunities to embrace home dialysis therapies with greater confidence and the potential to obtain better clinical outcomes. Barriers and evidence required to increase adoption of RPM by the nephrology community need to be clearly defined. Ten health care providers from specialties including nephrology, cardiology, pediatrics, epidemiology, nursing, and health informatics with experience in home dialysis and the use of RPM systems gathered in Vienna, Austria to discuss opportunities for, barriers to, and system requirements of RPM as it applies to the home dialysis patient. Although improved outcomes and cost-effectiveness of RPM have been demonstrated in patients with diabetes mellitus and heart disease, only observational data on RPM have been gathered in patients on dialysis. The current review focused on RPM systems currently in use, on how RPM should be integrated into future care, and on the evidence needed for optimized implementation to improve clinical and economic outcomes. Randomized controlled trials and/or large observational studies could inform the most effective and economical use of RPM in home dialysis. These studies are needed to establish the value of existing and/or future RPM models among patients, policy makers, and health care providers.
Keywords: end-stage kidney disease, home dialysis, patient monitoring, peritoneal dialysis, remote, telehealth
Home dialysis (including peritoneal dialysis and home hemodialysis) offers a variety of benefits over in-center hemodialysis. Studies show evidence of benefits for both peritoneal dialysis (PD) and home hemodialysis (HHD) patients related to survival, quality of life, transportation costs, increased patient autonomy, and clinical benefits including enhanced blood pressure and phosphorus control.1, 2, 3, 4, 5, 6 Home dialysis is particularly advantageous in the pediatric population, given the greater schedule flexibility for school and play, the importance of psychosocial aspects, and the limited geographic distribution of pediatric dialysis centers. Furthermore, the cost of delivery of care of home modalities in most countries is less than that of in-center hemodialysis.7, 8, 9
Despite these major advantages, patients on home dialysis represent a small percentage of the total end-stage kidney disease population worldwide, with only a few exceptions.8, 10 Dialysis reimbursement policy seems to be responsible for low uptake in many parts of the world.11, 12, 13 Other notable barriers include patient concerns regarding their ability to learn how to perform home dialysis; a perception that they may receive substandard care and/or have poor outcomes; a feeling of providing self-care in isolation without adequate medical oversight; socioeconomic status; and the fear that home dialysis will burden their family.14, 15 The burden of daily medical responsibility in home dialysis lies with the patients/caregivers, putting the onus on them to know when is the “right” time to contact their health care providers. Physicians may underutilize home dialysis due to a concern that patients may not know when to, or simply will not, contact the health care provider when difficulties do arise. Furthermore, physicians may fear the inability to determine patient adherence with dialysis. Overcoming these barriers could be a large step toward increasing patient uptake of home modalities.
Remote patient management (RPM) may provide a means to overcome some of the aforementioned barriers. RPM is a framework for monitoring patients at home by digital wireless technology and extends the interactive contact of conventional clinical settings to include the patient’s home. The hope is that these technologies would improve clinical outcomes through earlier recognition and correction of problems.15, 16, 17, 18, 19 Although few studies on telehealth in the dialysis population exist, studies do support its technical feasibility, that patient acceptance of this technology is very high, and that RPM may be able to improve outcomes in other comorbid states shared by the end-stage kidney disease population.20, 21 As examples, meta-analyses have shown the benefits of structured telephone support, RPM, and the use of implantable electronic devices in the care of patients with cardiac disease.22, 23 Furthermore, diabetes-related telehealth programs with the ability to change medications remotely have been shown to lower hemoglobin A1c as compared to standard of care.24 However, there is still uncertainty about the role of RPM for home dialysis. Furthermore, nephrology has been slow to accept telehealth technology into its practice, in part due to regulations surrounding telehealth implementation, including information security considerations and reimbursement policies.
What are the current and future applications of RPM in home dialysis, and how can it be integrated into standard of care? How can RPM best be used to improve home dialysis outcomes and to expand the opportunity of home dialysis to patients who otherwise would not have had this option? What evidence is needed to establish the value of existing and/or future RPM models for policy makers, payers, providers, and patients? These critical questions need to be addressed to allow wider adoption of RPM technology for patients with kidney disease. Due to some of the differences in HHD and PD regarding types of monitoring and regulatory considerations, the remainder of this manuscript will focus on RPM as it pertains to PD.
Methods
Ten health care providers from around the globe were selected for their expertise in RPM as evidenced by peer-reviewed publications, research, and/or well-established clinical proficiency. Specialties represented included nephrology, cardiology, pediatrics, epidemiology, nursing, and health informatics. The objectives of the meeting were as follows: (i) to understand what forms of RPM tools are already in current use, how they are integrated into care, and what is seen and expected of RPM in terms of better clinical−economic outcomes; (ii) to understand future required and/or desired RPM applications to improve clinical−economic outcomes and to expand access to home dialysis care; and (iii) to understand the data required to achieve greater adoption of RPM and what is required to establish its value among policymakers, payers, providers, and patients. The meeting was supported by the Renal Division, Medical Affairs, Baxter Healthcare Corporation. Consensus recommendations were arrived at by directed group discussion, followed by breakout sessions.
Opportunities and Challenges of RPM
Opportunities of RPM
At present, several small observational studies on RPM in dialysis patients suggest the technical feasibility and numerous potential benefits of RPM.18, 25, 26, 27, 28 However, many potential opportunities identified by the consensus panel have yet to be explored (Figure 1).
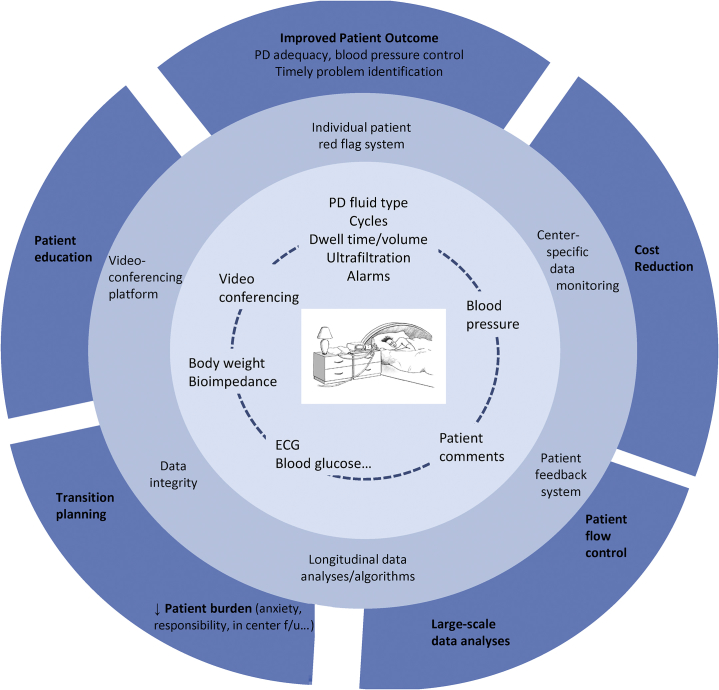
Figure 1.
Opportunities for remote patient management. The inner circle represents examples of individual patient monitoring variables. Moving outward, data can then be used to drive patient- and center-specific clinical care. Finally, in the outermost circle are the predicted health care−related outcome improvements. ECG, electrocardiography; f/u, follow-up; PD, peritoneal dialysis.
RPM provides an opportunity to increase the uptake and technique survival of the home modalities by improving patient satisfaction, patient outcomes, and cost savings. PD patients already spend a significant amount of time engaged in self-care tasks. Facilitating engagement with health care providers has the potential not only to reduce the burden of these activities but also to improve patient satisfaction and quality of life. For example, replacing the face-to-face encounter with a telehealth encounter in the patient’s home would reduce patient driving time, time spent in waiting rooms, and the travel costs associated with these visits. This is important because PD patients on average drive a farther distance to get to their home dialysis unit than patients travel to in-center hemodialysis units.29 Also, it has been demonstrated that frequent and regular home visits can help reduce attrition from home dialysis modalities.30 However, home visits are costly and time consuming, and many centers cannot perform them regularly. Using virtual home visits via RPM may be a preferable substitute to an in-person home visit.
PD patients also spend time collecting and recording detailed data pertaining to their dialysis treatments. Technology (such as Bluetooth connectivity) already exists that would eliminate the need to manually record data pertaining to weight, vitals, and dialysis treatment parameters. This would reduce not only time recording data, but, if entry is automated, would increase both the accuracy of the data collected and the frequency with which that information is sent and reviewed. This would not only increase medical provider oversight, but would potentially improve both physician and patient comfort levels, allaying fears of self-mismanagement. In addition, nurses currently spend a significant amount of time manually collecting data to address patient queries and problems. Having the data already sent remotely would reduce this time, improving efficiency of care and potentially lowering the cost to dialysis units.
The ability of clinicians and their home dialysis patients to have visibility and track daily weights, ultrafiltration (UF), and blood pressures against goals should contribute to more timely intervention and better volume control. Bioimpedance monitoring has thus far shown mixed results in improving outcomes, but with close monitoring and an applied intervention algorithm may augment the above, facilitating improved volume management.31, 32 Oximetry may also be an important parameter to follow, as nocturnal hypoxemia has been associated with increased cardiovascular complications in the hemodialysis population.33 Patients’ care may also be benefited by the answering of daily questions posed through a patient−caregiver interface about perceived medical issues, which can then be relayed to physicians and nursing staff. Questions regarding shortness of breath, appetite, the appearance of their exit site, and PD fluid may serve to help distinguish triage patients with no issues from those who require a call and possible intervention.
Data from the dialysis treatment itself can be of great importance to anticipate dialysis-related complications and treatment adherence. The ability to monitor treatment adherence is of the utmost importance with respect to patient outcomes, as it has been shown to be an indicator for the risk of developing peritonitis, hospitalization, hospital days, technique failure, and death.34, 35 Bernardini et al. demonstrated a significant relationship between nonadherence of PD patients to prescription (defined as performance of less than 90% of prescribed exchanges), as determined by home supply inventory, and technique failure, peritonitis, hospitalization, hospital days, and death. The authors concluded that “identification of noncompliant patients and awareness of risk factors should reduce noncompliance and improve patient outcomes.”34, 35 It can also serve as an early indicator of patient fatigue and a predictor of technique failure. Data such as initial and total drain volumes, UF values, adherence to and duration of therapy, lost dwells, and so forth can all be collected and used to monitor and intervene on behalf of the PD patient. The use of automated data collection reduces the possibility of incorrect or fictitious data entry, thus improving medical oversight. Automated data collection can also improve dialysis unit efficiency but must be able to integrate into existing electronic health records so as not to lead to needless duplication of data entry. On the other hand, excessive data collection may become burdensome to providers and patients, leading to poor data entry, review, and response times. Thus, care models that use data analytics to convert information to a more useable form and provide algorithms to flag concerning values or trends are needed.
RPM also provides an opportunity to increase the efficiency of care delivery. PD patients must order their supplies. Internal data from Baxter Healthcare Corporation from 2015 demonstrate that late orders occur in approximately 10% of home PD patients per month, which can lead to significant costs for expedited deliveries or missed treatments. Delayed supply ordering can affect patient safety and increase cost due to emergency deliveries. Alternatively, nursing staff must ensure that these orders are placed, thus limiting nursing efficiency. Monitoring inventory supplies and automating ordering could reduce such problems. RPM may improve the efficiency of clinics by designing patient flows that decrease patient wait times and increase the patient capacity of a clinic’s existing infrastructure. As a hypothetical example of a use of telehealth to improve efficiency, patients who demonstrate normal laboratory assessments, minimal triggers through remote therapy monitoring and remote vital sign assessments, and were remotely asked a series of questions pertaining to their health indicating no issues may not need to be seen by the physician that month. This would allow more physician time for patients with issues that need to be addressed by a physician. Approaches such as these would increase the utility of physician visits for both patient and physician.
Expanding the type of patients that use PD may increase patient uptake. Many physicians may not select patients with multiple comorbidities for home modalities. RPM may provide improved patient and physician comfort to use home modalities in patients previously considered “marginal” home candidates, many with multiple comorbid conditions. Online transmission of the previous night PD treatment, alarms, UF rates, body weight, and blood pressure should furthermore allow detection and intervention of impending problems, declining dialysis efficiency, or the need for timely modality switch, thus far associated with worse patient outcomes.36, 37
RPM not only provides an opportunity to improve patient outcomes but may, at the same time, reduce treatment-related costs as well. These cost savings may be realized with the increased uptake of patients to PD and reduced PD technique failure rates, as PD is less expensive to provide than in-center hemodialysis in most countries.8 By reviewing treatment data such as patient adherence to prescription, cycler alarms, UF values, vital signs, and weights on a more frequent basis, unnecessary hospitalizations may be avoided as has been shown in the dialysis population.30, 38, 39 A randomized controlled trial of 49 high-risk hemodialysis patients treated in a remote care nurse setting demonstrated a significant reduction in hospital and emergency visits and reduced costs with RPM.40 Five of the 24 patients on RPM, however, dropped out or withdrew from the study. Although the results warrant further studies, the findings are nonetheless compelling. Well-designed monitoring programs coupled with timely interventions may prevent readmissions. Patients living far from their unit may be able to see physicians more frequently if RPM, is used.41 The ability to provide 2-way communications, including imaging transmission or video-conferencing from the patient home, may allow early identification and intervention in medical problems such as exit site infections and volume overload.18 Finally, patient education may also be achieved through e-learning or nurse-to-patient, using education modules that are standardized to improve patient knowledge regarding self-care. Education could also be done via 2-way communications and allow clinicians to directly visualize PD exchanges and provide real-time feedback to improve technique.18
Significant cost savings may also be realized by reducing transportation costs to in-center facilities by enabling patients in nursing facilities or with significant disabilities to perform PD who would otherwise not have that option.42 RPM may provide a way for these patients to safely dialyze at home without the added need of transportation. Finally, in many areas of the world, there are limited health care providers to care for large populations of patients. RPM has the ability to better distribute the health care provider workforce without the need for transportation of the health care provider team.
Challenges to Implementation and Adoption of RPM
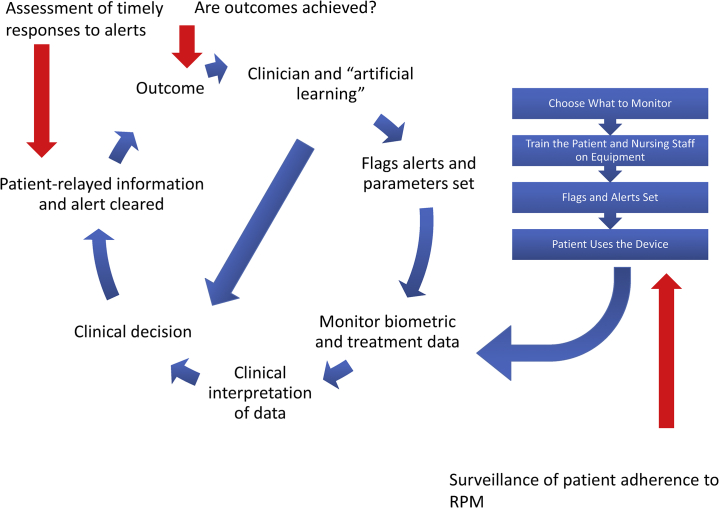
Despite the opportunities that RPM can provide, barriers and questions surrounding the implementation of RPM exist. What factors should be monitored, and what parameters for each factor set to optimize outcomes but not overload caregivers and patients? The number of variables in patient care that can be monitored is immense and can lead to provider fatigue. For the PD patient, factors may include weights, blood pressures, and PD-related parameters. However, numerous additional clinical factors can be measured, leaving clinicians to determine which are most important to improve patient care, and which are superfluous, potentially serving only to contribute to provider and patient burnout. Potential solutions to this include active monitoring of predefined high-risk patients only and computer-based algorithms to identify trends in parameters and to flag abnormal values may be 1 method of addressing these issues and decreasing the burden of sifting through large amounts of data (Figure 2).
Figure 2.
Schematic representation of remote patient monitoring (RPM) program implementation. A remote patient monitoring program begins with the selection of the parameters to be monitored (right-hand side of the diagram). After training of the patient and nurses on remote patient monitoring tools, data are collected and clinical decisions are rendered on these data to effect outcomes. Maintenance of the RPM program is of the utmost importance and requires, at a minimum, continued surveillance of response times, patient adherence, and outcomes (indicated by red arrows) in order to be successful.
Initial costs and maintenance costs of the technology may also be a barrier to implementation of RPM programs. There is a labor cost associated with implementation of an RPM program. Specifically, if not incorporated into the routine care of the patient, and if not used effectively, RPM can require a significant amount of nursing time to review data. Depending on the number of variables and frequency of monitoring, nursing time expenditures and cost can add up. Furthermore, reimbursement for remote monitoring may vary widely by nation and by payer. Providers may invest in RPM technology without compensation, trusting that efficiencies in care and/or reduction in health care cost will ensue. As the cost of dialysis is already high, however, third-party payers may question the return on investment. Although studies in other disease states have shown cost-effectiveness of telehealth interventions, studies evaluating the impact of RPM in the dialysis population are needed to justify its widespread use.43, 44
Patient and physician acceptance of technology may also pose a barrier to the uptake of the RPM in telehealth, although a study has shown that patient acceptance of telehealth is high.21 Patients may fear that their privacy is being invaded in longer-term interventions. Physicians may also not accept telehealth. Physicians already trained to provide care in a traditional fashion may not be willing, or may not have the time or resources to learn another mode of delivery of care. Studies have shown that patients have higher satisfaction with RPM than do physicians.45 Furthermore, the hurdles required to deliver care via telehealth may overwhelm even the most interested of physicians in providing care in this manner. One of these hurdles includes the potential liability surrounding remote patient monitoring. With remote monitoring, critical vital signs such as extremely low or high blood pressures will be captured. If critical values are not addressed in a timely fashion, as an example due to provider fatigue, health care providers may be held accountable. Consent forms for remote monitoring delineating the expectations of the remote monitoring program for the patient are necessary to avoid some of these issues. Some items that may be helpful to include are language to ensure patient understanding that remote monitoring is not expected to be telemetry with coverage for 24 hours a day, 7 days a week, and furthermore, that it should not replace a telephone call when concerning values are obtained with remote monitoring equipment. Continued vigilance to ensure appropriate response times to remote monitoring data is necessary to reduce liability and to ensure the utility and quality of the program. Other hurdles include overcoming information security considerations, establishing the RPM network endpoints be it the patient’s home or another medical facility, lack of reimbursement, and technophobia.
Moving Forward: Recommendations From the Workgroup
In an effort to promote the use of RPM to maximize outcomes in PD patients, the workgroup developed several key recommendations that focus on what we believe are critical issues in the field.
-
1.
Well-defined evaluation and intervention algorithms are needed based on individual patient target ranges for each monitored parameter. This should include a system designed to easily identify when parameters fall outside of prespecified ranges and notify providers at these specified times. The timing of data analysis by nurses and physicians, for examples, once daily during business days, should be communicated to patients and caregivers, as well as the fact that RPM does not address emergency issues (Figure 2).
-
2.
Parameters that would be useful to monitor in a PD patient include total and initial drain values, cycler-based alarms, therapy compliance, duration of therapy, blood pressures, and patient weight. Monitoring of PD-specific parameters may be combined with additional parameters based on patient specific needs, such as blood glucose concentrations in diabetic patients.
-
3.
Real-time collection and review of data for PD therapies is not feasible and is unlikely to substantially improve outcomes.46 Data collection could be transmitted in real time (or as frequently as possible when an Internet/cellular data connection is not readily available), but only viewed when needed in response to a flag indicating values outside accepted parameters or a problem expressed by a patient. Adherence, treatment efficacy, and values of treatment parameter could also be assessed at defined intervals.
-
4.
Patients should have access to their own data in easily accessible format allowing longitudinal data presentation to demonstrate recent and long-term trends and thus performance and compliance. Access could be through an online website or through the device itself. The option for the patient to withhold provider access to that patient’s treatment information through RPM should also be a feature.
-
5.
RPM systems should work with all forms of PD (automated peritoneal dialysis, continuous ambulatory peritoneal dialysis, or assisted PD). Ideally, data should be portable between different RPM platforms. Although complete interoperability between RPM platforms is unlikely, minimum standards as to the format of data storage and collection should be adopted to improve portability.
-
6.
Data should be stored in a fashion that is compatible with local/national laws and regulations. However, this should not be an impediment to access, analysis, or effective use. Data should be available to health care providers and administration to help facilitate quality improvement. Researchers may also benefit from the platform for data capture, analysis, and assessment.
-
7.
Supply ordering and inventory management should be built into the platform, simplifying the process of supply ordering and hopefully reducing the need for emergent deliveries.
-
8.
Attention to the process of remote monitoring as a whole, as opposed to the technology alone, is paramount to the success of an RPM program. This includes continued surveillance as to the effect of the program on targeted outcomes, timeliness and appropriateness of clinical decisions when alarm values are triggered, and patient adherence to RPM.
Equipment Requirements
Telehealth equipment for home dialysis should include the capacity for safe bidirectional communication between patient and provider. This will bring, whenever needed, the health care provider and the patient into close contact. This will be useful in more remote areas and for patients with limited transportation or significant disability. The equipment should have the ability to connect even in locations that may not have access to cable-based Internet providers. Methods including either satellite Internet or mobile technologies should be incorporated in these cases. Bidirectional communication will assist in the replacement of clinic visits, allowing early identification of therapy-related complications and enabling remote intervention. Bidirectionality will also allow for remote education sessions and will directly permit observation of therapies to help troubleshoot problems. On the device itself, easy access to local education resources (e.g., training manuals/videos, dietary guidelines, step-by-step procedures) could improve patient performance of dialysis.
Regular communication might include some of the following items: text (messages require little Internet capacity), video (depending on broadband or 4G Internet), speech (same as text or simple phone connectivity), high-resolution pictures (broadband, 3G, 4G), graphics and data of measurements such as weight, blood pressure, and heart rate (require little Internet capacity).
Ideally, data collection should be automated (i.e., Bluetooth capable) to prevent patient fatigue with manual entry of data and to ensure the most accurate data possible. RPM equipment should have the ability to connect to the Internet in a variety of locations, as remote management may be most useful where access to broadband Internet is least available. Preferably, equipment should be portable to allow for travel. Equipment and software should be customizable to ensure that the RPM equipment can be integrated into any clinic’s workflow or electronic medical record.
Future Studies
Future studies aimed at answering questions about RPM and its home dialysis applications are needed to guide its use. Increased surveillance of patients without appropriate interventions may not be beneficial for patients and has the potential to increase cost. As an example, augmented patient surveillance may increase hospitalizations, as opposed to decreasing them, if a proper intervention strategy is not in place. Pragmatic randomized controlled trials as well as large observational studies are needed to determine the potential benefits and harms of these new technologies, as well as to inform best RPM practice. Studies should focus on the impact of RPM on technique failure, PD-related infections, mortality, hospitalizations, PD access complications, and patient-reported outcomes/quality of life. Other measured outcomes variables should be achievement of target weight and blood pressure control that may be facilitated in an environment in which communication between patients and clinicians is optimized and appropriate changes in PD prescription can be implemented as soon as the need arises. Furthermore, studies will be needed to determine the appropriate approach to the handling of large amounts of patient data such that patient benefit is maximized without overwhelming health care providers. Patient-reported outcomes should evaluate the impact of RPM on both the patient and the patient’s family members. Furthermore, economic analyses are needed to determine the cost of providing remote care balanced against total resources expended, including hospitalizations/infections and attrition.
Conclusions
Remote patient management has exciting potential to improve home dialysis patient care and home modalities uptake, to improve quality of life, and to reduce cost. However, up to now, only a few observational studies and 1 small RCT have been accomplished in dialysis patients supporting the role of RPM in this setting. Further RCTs and large registry-based studies that focus on how and what to monitor are needed to guide the most efficacious use of telehealth as it applies to the dialysis patient and provider.
Disclosure
ELW has received consulting fees, honoraria, and grant support from Baxter Healthcare Corporation. MHR has received consulting fees from Baxter Healthcare Corporation and Kadence Ventures. KSR has received honoraria with Baxter Healthcare Corporation and consults for Tytocare. MRM, JAS, CF, and TK are employed by Baxter Healthcare Corporation. All the other authors declared no conflict of interest.
References
- 1.Weinhandl E.D., Foley R.N., Gilbertson D.T. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol. 2010;21:499–506. doi: 10.1681/ASN.2009060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V.A., Sidell M.A., Jones J.P., Vonesh E.F. Survival of propensity matched incident peritoneal and hemodialysis patients in a United States health care system. Kidney Int. 2014;86:1016–1022. doi: 10.1038/ki.2014.224. [DOI] [PubMed] [Google Scholar]
- 3.Yeates K., Zhu N., Vonesh E. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrology Dial Transplant. 2012;27:3568–3575. doi: 10.1093/ndt/gfr674. [DOI] [PubMed] [Google Scholar]
- 4.Nesrallah G.E., Lindsay R.M., Cuerden M.S. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen K.L., Zhang R., Huang Y. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:984–990. doi: 10.1038/ki.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stack A.G., Martin D.R. Association of patient autonomy with increased transplantation and survival among new dialysis patients in the United States. Am J Kidney Dis. 2005;45:730–742. doi: 10.1053/j.ajkd.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2016. 2016 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 8.Robinson B.M., Akizawa T., Jager K.J. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karopadi A.N., Mason G., Rettore E., Ronco C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant. 2013;28:2553–2569. doi: 10.1093/ndt/gft214. [DOI] [PubMed] [Google Scholar]
- 10.Jain A.K., Blake P., Cordy P., Garg A.X. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lameire N., Van Biesen W. Epidemiology of peritoneal dialysis: a story of believers and nonbelievers. Nat Rev Nephrol. 2010;6:75–82. doi: 10.1038/nrneph.2009.210. [DOI] [PubMed] [Google Scholar]
- 12.Hingwala J., Diamond J., Tangri N., Bueti J. Underutilization of peritoneal dialysis: the role of the nephrologist's referral pattern. Nephrol Dial Transplant. 2013;28:732–740. doi: 10.1093/ndt/gfs323. [DOI] [PubMed] [Google Scholar]
- 13.Li P.K.-T., Chow K.-M. Peritoneal dialysis—first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62:993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin K., Manns B., Mortis G., Hons R. Why patients with ESRD do not select self-care dialysis as a treatment option. Am J Kidney Dis. 2003;41:380–385. doi: 10.1053/ajkd.2003.50047. [DOI] [PubMed] [Google Scholar]
- 15.Cafazzo J.A., Leonard K., Easty A.C. Patient-perceived barriers to the adoption of nocturnal home hemodialysis. Clin J Am Soc Nephrol. 2009;4:784–789. doi: 10.2215/CJN.05501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong J., Eakin J., Migram P. Patients' experiences with learning a complex medical device for the self-administration of nocturnal home hemodialysis. Nephrol Nurse J. 2009;36:27–32. [PubMed] [Google Scholar]
- 17.Cafazzo J.A., Leonard K., Easty A.C. Patient perceptions of remote monitoring for nocturnal home hemodialysis. Hemodial Int. 2010;14:471–477. doi: 10.1111/j.1542-4758.2010.00473.x. [DOI] [PubMed] [Google Scholar]
- 18.Gallar P., Vigil A., Rodriguez I. Two-year experience with telemedicine in the follow-up of patients in home peritoneal dialysis. J Telemed Telecare. 2007;13:288–292. doi: 10.1258/135763307781644906. [DOI] [PubMed] [Google Scholar]
- 19.Nayak A., Karopadi A., Antony S. Use of a peritoneal dialysis remote monitoring system in India. Perit Dial Int. 2012;32:200–204. doi: 10.3747/pdi.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong S.W., Jassal S.V., Miller J.A. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11:1054–1062. doi: 10.2215/CJN.10681015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lew S.Q., Sikka N. Are patients prepared to use telemedicine in home peritoneal dialysis programs? Perit Dial Int. 2013;33:714–715. doi: 10.3747/pdi.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klersy C., Boriani G., De Silvestri A. Effect of telemonitoring of cardiac implantable electronic devices on healthcare utilization: a meta-analysis of randomized controlled trials in patients with heart failure. Eur J Heart Fail. 2016;18:195–204. doi: 10.1002/ejhf.470. [DOI] [PubMed] [Google Scholar]
- 23.Inglis S.C., Clark R.A., McAlister F.A. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane Review. Eur J Heart Fail. 2011;13:1028–1040. doi: 10.1093/eurjhf/hfr039. [DOI] [PubMed] [Google Scholar]
- 24.Faruque L.I., Wiebe N., Ehteshami-Afshar A. Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. Can Med Assoc J. 2017;189:E341–E364. doi: 10.1503/cmaj.150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agroyannis B., Fourtounas C., Romagnoli G. Telemedicine technology and applications for home hemodialysis. Int J Artif Organs. 1999;22:679–683. [PubMed] [Google Scholar]
- 26.Nakamoto H. Telemedicine system for patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2007;27(suppl 2):S21–S26. [PubMed] [Google Scholar]
- 27.Skiadas M., Agroyiannis B., Carson E. Design, implementation and preliminary evaluation of a telemedicine system for home haemodialysis. J Telemed Telecare. 2002;8:157–164. doi: 10.1177/1357633X0200800306. [DOI] [PubMed] [Google Scholar]
- 28.Edefonti A., Boccola S., Picca M. Treatment data during pediatric home peritoneal teledialysis. Pediatr Nephrol. 2003;18:560–564. doi: 10.1007/s00467-003-1147-8. [DOI] [PubMed] [Google Scholar]
- 29.Prakash S., Coffin R., Schold J., Lewis S.A. Travel Distance and Home Dialysis Rates in the United States. Perit Dial Int. 2014;34:24–32. doi: 10.3747/pdi.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martino F., Adıbelli Z., Mason G. Home visit program improves technique survival in peritoneal dialysis. Blood Purif. 2014;37:286–290. doi: 10.1159/000365168. [DOI] [PubMed] [Google Scholar]
- 31.Kurasawa N., Mori T., Naganuma E. Association between home blood pressure and body composition by bioimpedance monitoring in patients undergoing peritoneal dialysis. Adv Perit Dial. 2015;31:38–44. [PubMed] [Google Scholar]
- 32.Tan B.K., Yu Z., Fang W. Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int. 2016;89:487–497. doi: 10.1038/ki.2015.294. [DOI] [PubMed] [Google Scholar]
- 33.Zoccali C., Mallamaci F., Tripepi G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol. 2002;13:729–733. doi: 10.1681/ASN.V133729. [DOI] [PubMed] [Google Scholar]
- 34.Bernardini J., Piraino B. Compliance in CAPD and CCPD patients as measured by supply inventories during home visits. Am J Kidney Dis. 1998;31:101–107. doi: 10.1053/ajkd.1998.v31.pm9428459. [DOI] [PubMed] [Google Scholar]
- 35.Bernardini J., Nagy M., Piraino B. Pattern of noncompliance with dialysis exchanges in peritoneal dialysis patients. Am J Kidney Dis. 2000;35:1104–1110. doi: 10.1016/s0272-6386(00)70047-3. [DOI] [PubMed] [Google Scholar]
- 36.Gill J.S., Rose C., Pereira B.J.G. The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int. 2007;71:442–447. doi: 10.1038/sj.ki.5002072. [DOI] [PubMed] [Google Scholar]
- 37.Boissinot L., Landru I., Cardineau E. Is transition between peritoneal dialysis and hemodialysis really a gradual process? Perit Dial Int. 2013;33:391–397. doi: 10.3747/pdi.2011.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slinin Y., Guo H., Li S. Association of provider-patient visit frequency and patient outcomes on hemodialysis. J Am Soc Nephrol. 2012;23:1560–1567. doi: 10.1681/ASN.2012010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson K.F., Winkelmayer W.C., Chertow G.M. Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol. 2014;25:2079–2087. doi: 10.1681/ASN.2013080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman S.J., Wada C., Minatodani D. Home-based preventative care in high-risk dialysis patients: a pilot study. Telemed J e-Health. 2011;17:283–287. doi: 10.1089/tmj.2010.0169. [DOI] [PubMed] [Google Scholar]
- 41.Sicotte C., Moqadem K., Vasilevsky M. Use of telemedicine for haemodialysis in very remote areas: the Canadian First Nations. J Telemed Telecare. 2011;17:146–149. doi: 10.1258/jtt.2010.100614. [DOI] [PubMed] [Google Scholar]
- 42.Stephens M., Brotherton S., Dunning S.C. High cost of dialysis transportation in the United States: exploring approaches to a more cost-effective delivery system. J Health Econ Outcomes Res. 2013;1:134–150. doi: 10.36469/9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricci R.P., Vicentini A., D'Onofrio A. Economic analysis of remote monitoring of cardiac implantable electronic devices: results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm. 2016;14:50–57. doi: 10.1016/j.hrthm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Dixon P., Hollinghurst S., Edwards L. Cost-effectiveness of telehealth for patients with raised cardiovascular disease risk: evidence from the Healthlines randomised controlled trial. BMJ Open. 2016;6:e012352. doi: 10.1136/bmjopen-2016-012352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krousel-Wood M.A., Re R.N., Abdoh A. Patient and physician satisfaction in a clinical study of telemedicine in a hypertensive patient population. J Telemed Telecare. 2001;7:206–211. doi: 10.1258/1357633011936417. [DOI] [PubMed] [Google Scholar]
- 46.Marshall M.R., Pierratos A., Pauly R.P. Delivering home hemodialysis: is there still a role for real-time treatment monitoring? Semin Dial. 2015;28:176–179. doi: 10.1111/sdi.12327. [DOI] [PubMed] [Google Scholar]