Introduction
Membranoproliferative glomerulonephritis (MPGN) with masked monotypic Ig deposits is a recently described entity.1 Based on recent reports, some patients with this entity were misdiagnosed as C3 GN and subsequent evaluation using paraffin immunofluorescence (IF-P) confirmed the diagnosis. Here we describe the case of MPGN with masked monotypic Ig deposits in a patient who was initially diagnosed with postinfectious GN.
Case Presentation
A 48-year-old Hispanic man with no known past medical history was transferred to our institution from another hospital with a diagnosis of biopsy-proven postinfectious GN and dialysis-dependent renal failure. He was initiated on hemodialysis for uremia and hyperkalemia. He also received 3 doses of methyl prednisone followed by oral prednisone and 1 dose of i.v. cyclophosphamide before the transfer. His presenting symptoms included lower extremity swelling, nausea, and vomiting of 3 weeks’ duration. He denied any history of recent infection, rash, or joint pains. There was no family history of renal disease. On physical examination, he was alert, pale, edematous, and had a blood pressure of 160/100 mm Hg. The rest of the examination was unremarkable. His laboratory evaluation showed the following: Hemoglobin 10.9 g/dl; total leucocyte count: 11,600/mm3; platelets: 163,000/mm3; erythrocyte sedimentation rate: 66 mm/hour; and urine analysis: Protein 3 + protein, red blood cell: 82/high power field, white blood cell: 20 to 25/high power field. There were no cellular casts. Spot urine protein-creatinine ratio was 6100 mg/g, albumin-creatinine ratio 5403 mg/g, blood urea nitrogen 208 mg/dl, and serum creatinine 4.6 mg/dl. Serum protein was 5.8 g/dl, albumin 2.2 g/dl, serum cholesterol 266 mg/dl, and triglyceride 212 mg/dl. His serum complements (C3 and C4) were low. Serology for lupus, antineutrophil cytoplasmic antibody, cryoglobulinemia, antistreptolysin O, hepatitis B, hepatitis C, and human immunodeficiency virus were negative. Serum electrophoresis showed monoclonal gammopathy with an IgG kappa monoclonal protein on immunofixation. Serum-free kappa was elevated at 1246 mg/l and lambda fraction was normal (free kappa-lambda ratio of 95.23). A urine electrophoresis showed M spike of 6 mg/dl in gamma fraction. Sonography showed normal-sized kidney without any obstruction. A repeat kidney biopsy was performed 3 weeks after the first biopsy. Reasons for repeat biopsy included lack of clinical or serologic evidence of preceding or concurrent infection, presence of nephrotic range proteinuria on repeat urine studies, depression of both serum C3 and C4, detection of clinical evidence of monoclonal gammopathy, and lack of electron microscopic studies of the first kidney biopsy. Light microscopy of the repeat biopsy demonstrated marked global endocapillary hypercellularity with numerous intracapillary-infiltrating neutrophils and macrophages (confirmed by a positive immunohistochemical staining for CD68) containing prominent intracytoplasmic silver-negative phagolysosomes. Several glomeruli also showed intracapillary-infiltrating neutrophils with some lymphocytes (Figure 1). Few glomeruli contained large intraluminal silver-negative glassy immune deposits including 1 glomerulus with glassy immune deposits within a hilar arteriole (Figure 2). Some glomeruli show moderate segmental mesangial sclerosis and hypercellularity with lobular accentuation. Immunofluorescence microscopy performed on a frozen section showed trace granular staining for C3 (Figure 3). Staining for IgA, IgG, IgM, C4, C1q, fibrinogen, albumin, kappa, or lambda deposits were all negative. On electron microscopy, glomerular capillaries were globally occluded due to mesangial hypercellularity and numerous intracapillary infiltrating macrophages, many of them contained abundant electron dense phagolysosomes. Large intraluminal electron dense deposits were identified (Figure 4). No deposits with substructure or crystals were seen. In view of histopathological findings and monoclonal gammopathy, immunofluorescence study on pronase-digested, paraffin-embedded tissue (IF-P) was performed that showed bright (2–3+) staining of mesangium, glomerular capillary wall, and phagolysosomes within intracapillary macrophages for IgG and kappa with negative IgA, IgM, and lambda (Figures 5 and 6). There was 1+ granular mesangial and glomerular capillary wall positivity for C1q. The findings were consistent with diffuse endocapillary proliferative GN with masked monoclonal IgG kappa deposits, with histologic features of cryoglobulinemic GN. A subsequent bone marrow aspiration was performed that showed 12.5% plasma cells (by morphology). Immunohistochemistry demonstrated 20% kappa light chain–positive plasma cells. X-ray of the skull showed no evidence of a lytic lesion. He was treated with lenalidomide-bortezomib-dexamethasone regimen for multiple myeloma. He has completed 8 cycles of the treatment so far. Free kappa light chain concentration came down to 33.46 mg/l (free kappa-lambda ratio of 3.27). A repeat bone marrow examination showed 2.6% plasma cells (by morphology) and 5% kappa light chain–positive plasma cells (8 months after kidney biopsy). A repeat urine electrophoresis was negative for monoclonal light chains. He also received hemodialysis support for total of 3 weeks. Subsequently, there was improvement in renal function. The most recent serum creatinine and urine protein-creatinine ratio (3 months after kidney biopsy) were 0.8 mg/dl and 300 mg/g, respectively.
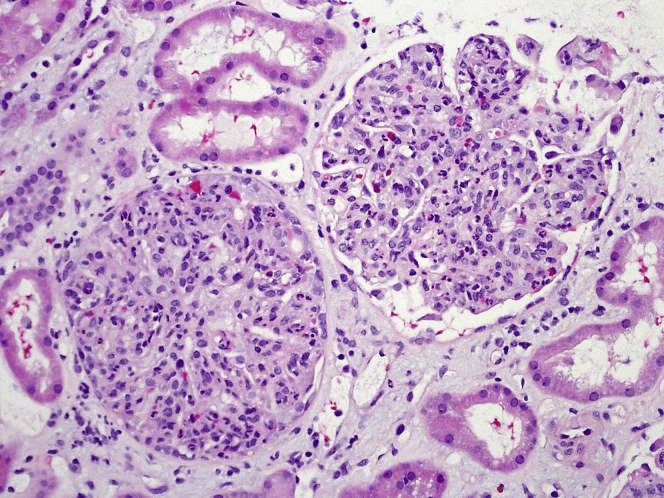
Figure 1.
Light microscopy demonstrating marked global endocapillary hypercellularity with lobular accentuation. Numerous intracapillary infiltrating macrophages and neutrophils are also seen (hematoxylin and eosin, original magnification ×200).
Figure 2.
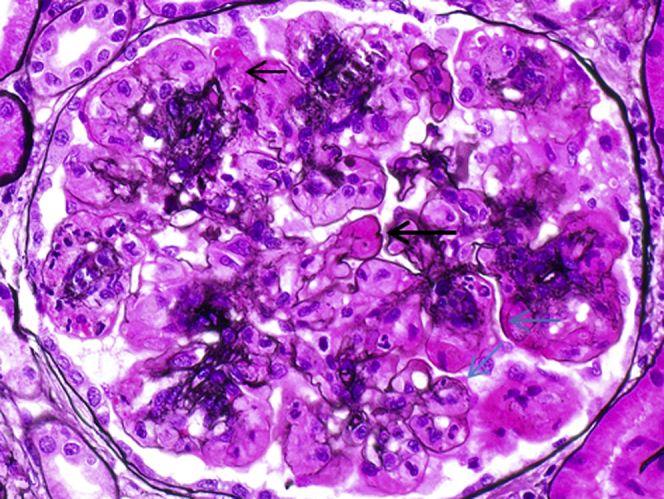
A silver stain showing glomerular large intraluminal silver-negative glassy immune deposits (large black arrow), intracytoplasmic silver-negative phagolysosomes within infiltrating macrophages (small black arrow), and segmental duplication of the glomerular basement membrane (blue arrows) (original magnification ×600).
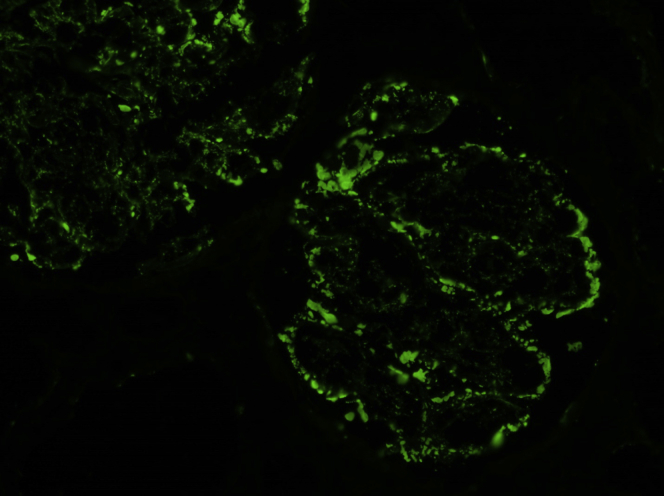
Figure 3.
Immunofluorescence microscopy performed on a frozen section showed trace granular global mesangial and glomerular capillary wall staining for C3 (original magnification ×200).
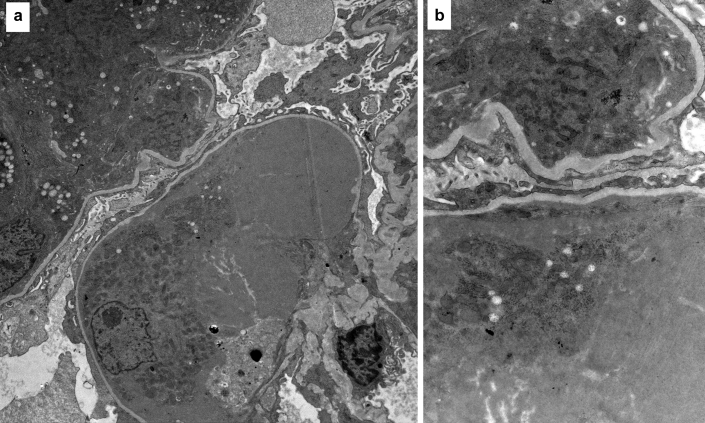
Figure 4.
(a) An electron microscopy image showing large intraluminal electron dense deposits (original magnification ×1100). (b) The deposits on high magnification appear granular without substructure (original magnification ×5650).
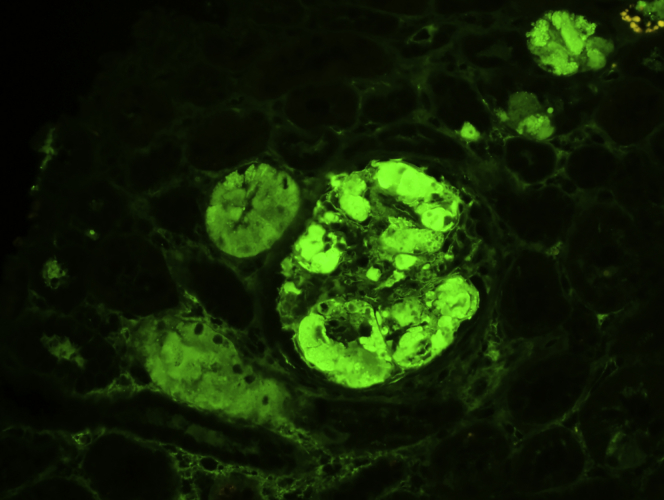
Figure 5.
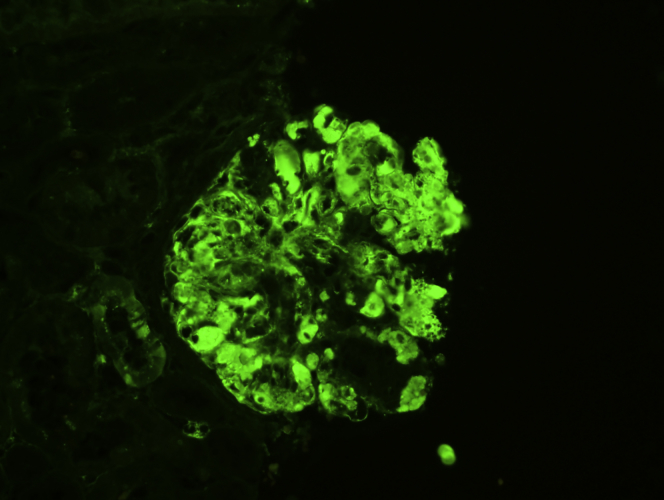
Immunofluorescence study on pronase-digested, paraffin-embedded tissue showing bright (2–3+) staining of IgG (original magnification ×100).
Figure 6.
Immunofluorescence study on pronase-digested, paraffin-embedded tissue showing staining for kappa (original magnification ×200).
Discussion
MPGN with masked monotypic Ig deposits is a recently described disorder characterized by little or no reactivity for Igs on immunofluorescence on frozen tissue (IF-F) but positivity for Ig and 1 of the light chains on IF-P.1 Most patients had monoclonal gammopathy of renal significance while a minority had multiple myeloma or lymphoma.1 This entity also highlights the importance of performing IF-P when the findings by routine IF do not match the clinical scenario, laboratory tests, light microscopy, or electron microscopic findings.
The cardinal features of bacterial infection–associated GN are diffuse endocapillary proliferative and exudative GN on light microscopy, C3-dominant glomerular deposits on IF, hump-shaped subepithelial electron-dense deposits on electron microscopy, low serum C3 with normal C4, and preceding or concomitant bacterial infection. In the case presented here, the initial diagnosis based on the biopsy findings was consistent with either postinfectious GN or C3 GN. The repeat biopsy showed glomerular staining for C3 only on routine IF-F and numerous intraglomerular infiltrating neutrophils mimicking bacterial infection–associated GN or C3 GN or both. The absence of a definite history of infection, presence of nephrotic range proteinuria, and normal antistreptolysin O titer on repeat testing were the reasons for the initiation of repeat investigation. It is uncommon to see nephrotic range proteinuria in postinfectious GN and in the absence of clinical suspicion, the diagnosis of multiple myeloma could have been missed.2 Since renal involvement might be the initial presentation in some of the patients with plasma cell dyscrasia, it is important to identify such patients early and institute appropriate therapy. Fifty percent of patients with myeloma present with renal failure and renal involvement is considered a poor prognostic indicator. It is the most common cause of death in myeloma after infection.3 It should also be noted that patients with monoclonal gammopathy have a particularly high incidence of MPGN recurrence as compared with patients without monoclonal proteins.4
IF is very critical in the accurate diagnosis of many immunologically mediated glomerular diseases and dysproteinemia that result from abnormal protein deposition in the renal tissue.5 Routine IF-F remains the most widely used technique of immunohistochemistry in renal pathology. It is performed on cryostat sections cut from unfixed frozen tissue.6 Although frozen, unfixed tissue is thought to be superior for detection of the immune deposits in most forms of GN, the process is associated with a number of drawbacks. During the processing of the tissue for routine IF, the proteins of interest may be washed off from the slide due to a charge-charge interaction with the slide.7 Additionally, the Igs deposited in the tissue in such cases are rendered resistant to antibody binding due to the quaternary arrangement of the Ig or a charge-charge interaction between the antibody used and the Ig in the tissue.7 It is also possible that the transport media (e.g., Zeus fixative [ZEUS Scientific, Branchburg, NJ]) of the immunofluorescence tissue before sample freezing could interfere with the Ig reactivity on IF-F. Use of IF-P can circumvent these limitations (Table 1). IF-P first described by Fogazzi et al.,8 and it was initially developed mainly as a salvage technique when frozen tissue for routine IF was inadequate. The portion of the renal tissue allocated for light microscopy can be used for performing the IF using this technique. Formalin-fixation achieves excellent preservation of tissue morphology by cross-linking proteins and prevents their washout.8 IF-P have been shown to be superior to IF-F in the diagnosis of light chain proximal tubulopathy, membranous-like glomerulopathy with masked IgG kappa deposits, and MPGN with masked monotypic Ig deposits as it may unmask monoclonal deposits in the kidney.1, 6, 9 Only a single case series of MPGN with masked monoclonal deposits has been reported so far. In this series from Arkana Laboratories (Little Rock, AR; previously Nephropath) and Mayo Clinic (Rochester, MN), there were 16 patients (9 female, 7 male) with a mean age of 61.6 years at the time of biopsy. Patients presented with hematuria, proteinuria, and renal injury. Serum creatinine was elevated in 88% of the patients. All patients had proteinuria with a mean value of 7.1 g per 24 hours and 13 of 16 patients (80%) met criteria for full nephrotic syndrome. Hematuria was present in 15 of 16 patients. Testing for serum C3 and C4 was performed in 15 patients and 10 (67%) had hypocomplementemia. A positive serum electrophoresis was found in 88% of the patients and a subsequent bone marrow biopsy was positive in 81% of the patients (13 of 17). Of these, 9 of 13 had evidence of plasma cell dyscrasia and 4 of that 9 had multiple myeloma as in our case. On renal biopsy, 10 patients (63%) had findings consistent with C3 GN on the routine IF. But IF-P showed “masked” deposits that were positive for IgG kappa in 12 cases and the rest were positive for IgG lambda, IgM lambda, and IgM kappa. Electron microscopy showed subepithelial (38%), subendothelial (100%), and mesangial deposits (75%), which were granular (nonorganized) in most patients. Classical findings of C3 GN including subepithelial “humps,” intramembranous deposits, or deposits with only mild electron density were not present in any case. In 10 of 16 patients for whom follow-up data was available, most patients were treated with chemotherapy with variable response rate. Proteinuria improved or stabilized in 87% of the patients, whereas creatinine improved or stabilized in 70% of the patients. Six patients had persistent renal dysfunction, and 1 patient progressed to end-stage renal disease. It is not clear how many patients required renal replacement therapy at the time of diagnosis.1 Our patient was dialysis-dependent at time of diagnosis and following treatment there was gradual improvement of renal function and proteinuria and eventually complete recovery. It is important to emphasize that only one-third of patients with monoclonal gammopathy in whom IF-F show glomerular staining for C3 only (i.e., without Igs) have masked monoclonal deposits detectable on IF-P.1 The remaining two-thirds have negative IF-P for Ig and should be labeled as C3 glomerulopathy with monoclonal gammopathy as has been recently reported.10, 11 In these patients, the monoclonal protein causes GN in an indirect mechanism through continuous activation of the alternative pathway of complement, possibly by acting as an inhibitor of 1 of the complement-regulating proteins.10, 11
Table 1.
Teaching points
| 1 | Paraffin IF should be done in all patients with clinical evidence of monoclonal gammopathy and light microscopy and routine IF showing evidence of C3 GN, PIGN, or MPGN with negative IF findings. |
| 2 | Paraffin IF is recommended when the findings by routine IF do not match either the clinical scenario or electron microscopic findings. |
| 3 | It is important to maintain a high index of suspicion for the possibility of masked deposits given the potential of misdiagnosis if they are not detected. |
IF, immunofluorescence; GN, glomerulonephritis; MP, membranoproliferative; PI, postinfectious.
When kidney biopsy shows apparent monoclonal deposits, correlation with full hematologic-oncologic work-up is needed, the results of which will drive treatment decisions.3 A bone marrow biopsy and flow cytometry will aid in confirming the type of plasma cell dyscrasia, and once confirmed, targeting early chemotherapy aimed at controlling the underlying clonal disorder should be adopted to prevent the progression toward end-stage renal disease.3 In patients with non-IgM–type monoclonal gammopathy, treatment regimens used include bortezomib, thalidomide, lenalidomide, cyclophosphamide, and dexamethasone in varying combinations. In our case, we used a combination of lenalidomide (revlimid), bortezomib (velcade), and dexamethasone. In IgM-secreting disorders, rituximab is the preferred agent, and it is used either alone or in combination with other agents (bendamustin, cyclophosphamide, bortezomib, carfilzomib, fludarabine cladribine, and ibrutinib).12 In addition to specific therapy aimed at eradicating the disease, supportive measures for control of hypertension, fluid balance, and proteinuria should also be considered. Disorders associated with cryoglobulin production usually require plasmapheresis. Although the histologic features in our case were compatible with cryoglobulinemic glomerulonephritis type 1, plasmapheresis was not tried due to absence of clinical features of cryoglobulinemia and cryoglobulins in the serum. Renal transplant can be associated with recurrence in the graft unless the monoclonal plasma cell disorder is adequately eradicated before the procedure.
Disclosure
All the authors declared no competing interests.
References
- 1.Larsen C.P., Messias N.C., Walker P.D. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015;88:867–873. doi: 10.1038/ki.2015.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldwin D.S., Gluck M.C., Schacht R.G. The long-term course of poststreptococcal glomerulonephritis. Ann Intern Med. 1974;80:342–358. doi: 10.7326/0003-4819-80-3-342. [DOI] [PubMed] [Google Scholar]
- 3.Katagiri D., Noiri E., Hinoshita F. Multiple myeloma and kidney disease. Sci World J. 2013;2013:487285. doi: 10.1155/2013/487285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S., Zand L., Leung N. Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol. 2010;5:770–782. doi: 10.2215/CJN.06760909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridoux F., Leung N., Hutchison C.A. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 6.Larsen C.P., Ambuzs J.M., Bonsib S.M. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86:154–161. doi: 10.1038/ki.2013.548. [DOI] [PubMed] [Google Scholar]
- 7.Messias N.C., Walker P.D., Larsen C.P. Paraffin immunofluorescence in the renal pathology laboratory: more than a salvage technique. Mod Pathol. 2015;28:854–860. doi: 10.1038/modpathol.2015.1. [DOI] [PubMed] [Google Scholar]
- 8.Fogazzi G.B., Bajetta M., Banfi G. Comparison of immunofluorescent findings in kidney after snap-freezing and formalin fixation. Pathol Res Pract. 1989;185:225–230. doi: 10.1016/S0344-0338(89)80256-0. [DOI] [PubMed] [Google Scholar]
- 9.Stokes M.B., Valeri A.M., Herlitz L. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27:1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zand L., Kattah A., Fervenza F.C. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62:506–514. doi: 10.1053/j.ajkd.2013.02.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridoux F., Desport E., Fremeaux-Bacchi V. Glomerulonephritis with isolated C3 deposits and monoclonal gammopathy: a fortuitous association? Clin J Am Soc Nephrol. 2011;6:2165–2174. doi: 10.2215/CJN.06180710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajkumar S.V., Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]