Abstract
Introduction
H.P. ACTHar gel is a preparation of melanocortin peptides that has been used to treat resistant forms of nephrotic syndrome. To determine whether combination therapy with ACTHar gel and tacrolimus reduces proteinuria and stabilizes renal function, we conducted a prospective, open-label trial in patients with treatment-resistant membranous glomerulopathy (MGN) and focal segmental glomerulosclerosis (FSGS).
Methods
Nine patients with treatment-resistant MGN and 13 with treatment-resistant FSGS received subcutaneous ACTHar gel for 6 months. Patients with no response or a partial response to ACTHar gel alone received an additional 6 months of therapy with combination ACTHar gel and oral tacrolimus. The study endpoint was the percentage of patients achieving a complete or partial remission after 6 months of combination therapy.
Results
Among patients with MGN, treatment with ACTHar gel alone achieved a partial remission in 44% and no response in 56% of patients. No patient achieved a complete response with ACTHar gel therapy alone. An additional 6 months of combination therapy with ACTHar gel and tacrolimus resulted in partial and complete response rates of 25% and 75%, respectively. Among patients with FSGS, ACTHar gel therapy alone resulted in complete and partial response rate of 7.7% and 62.0%. Combination therapy increased complete response rates to 17% and partial responses to 66%. Proteinuria (urinary protein-to-creatinine ratio) was significantly reduced in both patients with MGN and those with FSGS after 6 months of ACTHar gel alone and was further reduced among the patients with MGN with the addition of tacrolimus. There were no significant changes in estimated glomerular filtration rate during the treatment phase or long-term follow-up.
Discussion
Combination therapy with ACTHar gel and tacrolimus was well tolerated by patients with treatment-resistant MGN and FSGS and significantly reduced proteinuria and improved clinical response rates compared with ACTHar gel alone.
Keywords: ACTHar gel, focal segmental glomerulosclerosis, membranous glomerulopathy, proteinuria
The management of primary glomerulopathies and nephrotic syndrome that have failed to respond to conventional therapies remains a persistent problem for clinicians. For patients with treatment-resistant nephrotic syndrome, the lack of consensus therapies is partly due to the wide array of pathogenic mechanisms that contribute to these disorders, as well as a lack of adequately powered, controlled trials. Although glucocorticoids remain the primary therapy for many forms of proteinuric glomerular diseases, prolonged use is associated with significant morbidities including steroid-induced diabetes, metabolic bone disease, and excessive weight gain.1, 2
Although multiple “second-line” agents have been tried in the treatment of patients with nephrotic syndrome that is resistant to conventional therapies, there is no consensus on what constitutes optimal therapy. Previous studies have examined the efficacy of combining i.v. cyclophosphamide with corticosteroids and have shown complete and partial response rates between 40% and 60%.1 More recently, calcineurin inhibitors (CNIs) have become an accepted alternative therapy with similar response rates (between 50% and 70%).3, 4 However, both treatment regimens are limited by the risk of infection, as well as the development of CNI-induced nephrotoxicity and progressive renal disease.5, 6 ACTHar gel is a porcine pituitary preparation composed of adrenocorticotropic hormone (ACTH) and other proopiomelanocortin peptides and is currently approved for treatment of idiopathic nephrotic syndrome.7 Previous studies, as well as more current work, have shown that ACTHar gel can effectively reduce proteinuria in idiopathic membranous glomerulonephritis (MGN) and other forms of glomerulopathy including focal segmental glomerulosclerosis (FSGS) and advanced diabetic nephropathy.8 Although the mechanism is unknown, previous studies have shown that melanocortin 1 receptors are expressed in glomerular podocytes and that activation of this receptor pathway may be involved in the protein-lowering effects of ACTHar gel.9 To investigate the possibility that combination therapy with ACTHar gel and the CNI tacrolimus may be effective in reducing proteinuria and stabilizing renal function, we conducted a prospective, open-label, observational study of 9 patients with treatment-resistant MGN and 13 patients with resistant FSGS.
Methods
This is an institutional review board–approved, prospective, observational study of the safety and efficacy of combination ACTHar gel and tacrolimus therapy in patients with treatment-resistant MGN or FSGS. Between February 2012 and January 2016, 22 patients who were being followed up at the University Tennessee College of Medicine and the Ohio State University were treated with ACTHar gel alone or in combination with oral tacrolimus. All 22 patients had biopsy-proven idiopathic MGN or primary FSGS and were negative for antinuclear, anti-ds-DNA, RPR, C-ANCA, P-ANCA, hepatitis B and C antibodies and had no evidence of monoclonal gammopathies. Patients with dilated cardiomyopathies, glycosylated hemoglobin levels greater than 10%, an estimated glomerular filtration rate (eGFR) of less than 20 ml/min or positive serologies for HIV were not included in the study. Of the 22 patients, 5 had concurrent insulin-dependent or noninsulin-dependent diabetes; in none of these patients was diabetic nephropathy the primary glomerular pathology. All patients were resistant to therapy and had been treated with 2 or more immunosuppressive agents.
Study Protocol
All patients were in stable condition while receiving an angiotensin converting enzyme, or an angiotensin receptor blocker, or an angiotensin converting enzyme and mineralocorticoid receptor antagonist, or an angiotensin receptor blocker and mineralocorticoid receptor antagonist therapy for 4 weeks before treatment and had consistent blood pressure measurements below 150/90 mm Hg. All treated patients had urine protein-to-creatinine ratios (UP/Cr) greater that 2500 mg/g and eGFR calculated by Modification of Diet in Renal Disease formula ≥ 20 ml/min per 1.73 m2. Patients with eGFR < 20 ml/min were excluded.
Patients with treatment-resistant MGN and FSGS were treated with subcutaneous ACTHar gel, (40–80 units) 2 to 3 times per week for 6 months. Patients with insulin-dependent or noninsulin-dependent diabetes received 16 units of subcutaneous ACTHar gel daily to reduce glucose fluctuations. A physical examination was performed, and UP/Cr ratio, glycosylated hemoglobin, and routine laboratory data were evaluated every 3 months. Patients who had a glycosylated hemoglobin level > 7.0% were offered the option to discontinue ACTHar treatment or undergo modification of diabetic medications. After 6 months of ACTHar gel therapy, patients who achieved complete remission were weaned off therapy over a period of 3 to 12 months. Patients exhibiting no response or partial remission continued to receive existing ACTHar gel doses and began receiving oral tacrolimus (0.03–0.06 mg/kg/d), which was titrated to a trough level of 5–10 ng/ml for an additional 6 months. A physical examination including measurement of blood pressure and weight and a formal assessment of lower extremity edema was performed every 3 months. Laboratory studies were performed at each follow-up visit and included electrolytes, hemoglobin, hematocrit, white blood cell count, platelets, and glycosylated hemoglobin levels.
Primary/Secondary and Safety Endpoints
The primary endpoint of the study was to determine whether the addition of tacrolimus to treatment with ACTHar gel for 6 months increased the percentage of patients achieving a complete or partial remission. A complete remission was defined as a UP/Cr ratio less than 300 mg/g Cr, and a partial response was defined as a 50% reduction in the pre-ACTH treated UP/Cr ratio to a value less than 3500 mg/g.
Safety Endpoints
Patients were monitored for development of heart failure, worsening lower extremity edema, insomnia, and hyperglycemia. Glycosylated hemoglobin levels were monitored every 3 months during active treatment with ACTHar gel. For patients with diabetes, the dose of insulin or oral hypoglycemic agents was modified to achieve a glycosylated hemoglobin value of 9% or less. For patients with worsening edema, individual site investigators could modify current diuretic therapy or add thiazide diuretics. For patients with diuretic-unresponsive edema, the dose of ACTHar gel could be reduced or discontinued at the discretion of the investigator.
Statistical Analysis
Descriptive statistics were calculated for all variables of interest. Changes in serial levels of proteinuria were calculated for each patient and analyzed using a paired t test. All urinary protein data are presented as mean ± SEM. Changes in complete or partial response rates were calculated by comparison with baseline and prestudy values. The Fisher exact test was used to compare proportions. A P value < 0.05 was deemed statistically significant. All analyses were performed using Graph Pad Instat-3, Version 3.1 (GraphPad Software, Inc., La Jolla, CA).
Results
Patient Outcomes: MGN
Baseline demographics, clinical data, and previous treatments for patients with MGN and FSGS are listed in Table 1. All participating patients were older than 18 years and had biopsy-proven MGN or FSGS. The majority of patients in the MGN group were male (66%) with a mean age of 59 ± 2 years. Six of the 8 patients with MGN (75%) had proteinuria in the nephrotic range at the time of study entry with a mean UP/Cr ratio of 9.47 ± 1.9 g/g before ACTHar gel therapy. Among the patients with MGN, the baseline level of interstitial fibrosis was 20% ± 6%, with an additional 37% of patient having fibrosis of more than 30%. All patients had failed to respond to 2 or more courses of immunosuppressive therapy; 88% of the patients did not respond to treatment with CNIs. The mean time from initial diagnosis of MGN to treatment with ACTHar gel was 25 ± 7 months.
Table 1.
Patient demographics
| Histology | Idiopathic membranous (n = 9) | Primary FSGS (n = 13) |
|---|---|---|
| Age (yr) | 57.8 ± 2 | 56.5 ± 5 |
| Gender (M) | 66.0% | 85.0% |
| Baseline Cr level (mg/dl) | 1.7 ± 0.3 | 1.8 ± 0.2 |
| Baseline eGFR | 59 ± 13.1 | 47 ± 6.8 |
| Baseline UP/Cr ratio (g/g) | 7.7 ± 1.6 | 6.4 ± 1.2 |
| Fibrosis > 20% | 44.0% | 62.0% |
| Prior immunosuppression | ||
| Steroids | 90.0% | 92.3% |
| CNI | 80.0% | 76.9% |
| CTX | 30.0% | 7.7% |
| Rituximab | 0.00% | 15.4% |
| Rapamycin | 20.0% | 7.7% |
| MMF | 20.0% | 0.00% |
| Abatacept | 0.00% | 7.7% |
| Diagnosis to ACTH therapy (mo) | 25 ± 6.4 | 19 ± 6.1 |
| Time to remission (mo) | 5.6 ± 1.2 | 5.4 ± 0.7 |
| Mean ACTHar | 196 ± 18 | 176 ± 17 |
| Duration of ACTHar (mo) | 9.6 ± 2.1 | 7.3 ± 1.0 |
| Duration of ACTHar + tacrolimus (mo) | 7.1 ± 0.9 | 6.3 ± 0.1 |
CNI, calcineurin inhibitor; Cr, creatinine; CTX, cyclophosphamide; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; MMF, mycophenolate mofetil; UP/Cr, urine protein-to-creatinine.
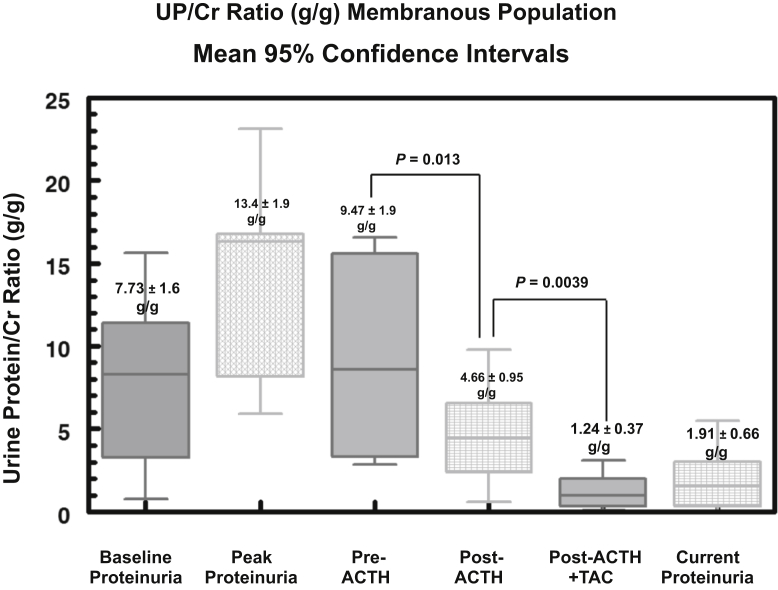
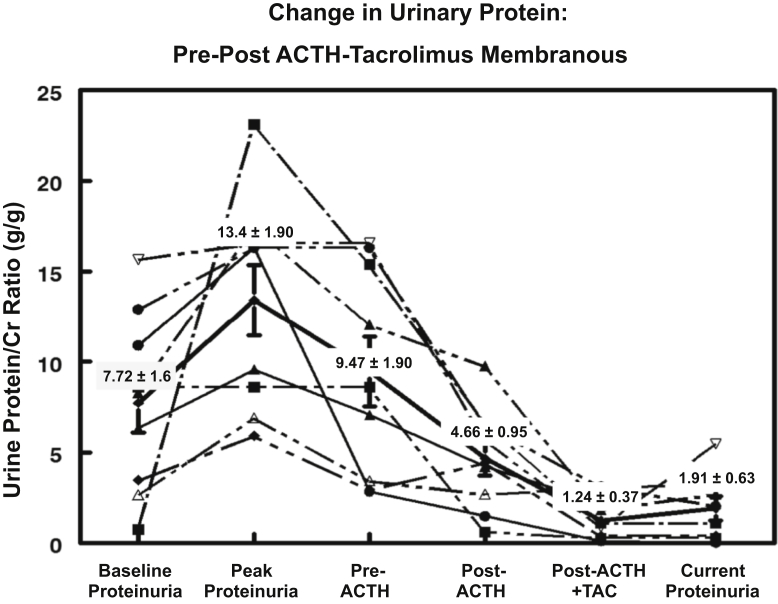
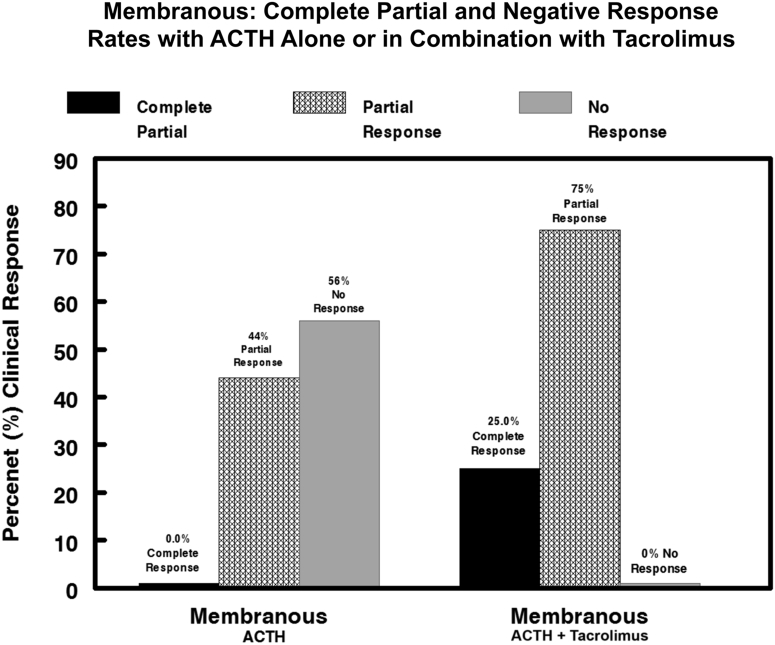
Among the 9 patients in the MGN group, the UP/Cr ratio at baseline presentation was 7.73 ± 1.6 g/g, rising to a peak value of 13.4 ± 1.9 g/g (Figure 1). The mean UP/Cr ratio immediately before treatment with ACTHar gel was 9.47 ± 1.9 g/g, falling to 4.66 ± 0.95 g/g after 6 months of therapy (P = 0.013) (Figure 1). One patient with MGN who achieved a partial response after 6 months chose not to receive combination therapy with tacrolimus. Of the 8 patients with MGN receiving combination therapy, the addition of tacrolimus led to a further reduction in the UP/Cr ratio from a pre-tacrolimus mean of 4.66 ± 0.95 g/g to 1.24 ± 0.37 g/g (P = 0.0039) (Figure 1). The individual changes in UP/Cr ratio for each patient with MGN are shown in Figure 2. No patient achieved a complete response with ACTHar gel alone. Forty-four percent of the patients achieved a partial response after 6 months of therapy, and 57% had no response. As shown in Figure 3, 25% of patients went on to achieve complete remission, and partial response rates increased from 44% with ACTHar alone to 75% with combination therapy.
Figure 1.
Box-whisker plots of urine protein-to-creatinine (UP/Cr) ratios (g/g) for the 9 patients with treatment-resistant membranous glomerulopathy are shown. Data include 5th and 95th percentiles about the median for UP/Cr ratios at baseline, peak levels, pre-ACTH, post-ACTH (6 months), post-ACTH (6 months) and tacrolimus, and current (last recorded data) levels. UP/Cr ratios fell significantly (P < 0.013) after 6 months of ACTHar with a further reduction after 6 months of combination ACTH and tacrolimus therapy (P < 0.0039). TAC, tacrolimus.
Figure 2.
Individual urine protein-to-creatinine (UP/Cr) ratios (g/g) for patients with treatment-resistant membranous glomerulopathy are shown. Average UP/Cr fell significantly from 9.06 ± 1.7 to 4.66 ± 0.9 (P < 0.013) after ACTHar therapy alone and then was further reduced to 1.24 ± 0.4 (P < 0.021) after 6 months of combined ACTHar gel and tacrolimus.
Figure 3.
Combined complete, partial, or no response rates for patients with treatment-resistant membranous glomerulopathy after 6 months of ACTHar alone and 6 months of combined ACTHar and tacrolimus. After 6 months of ACTHar gel therapy alone, 44% of patients achieved a partial response. No patient achieved a complete response with ACTHar alone. After 6 months of combined ACTHar and tacrolimus therapy, the complete response rate increased to 25% and the partial response rate increased to 75%.
Patient Outcomes: FSGS
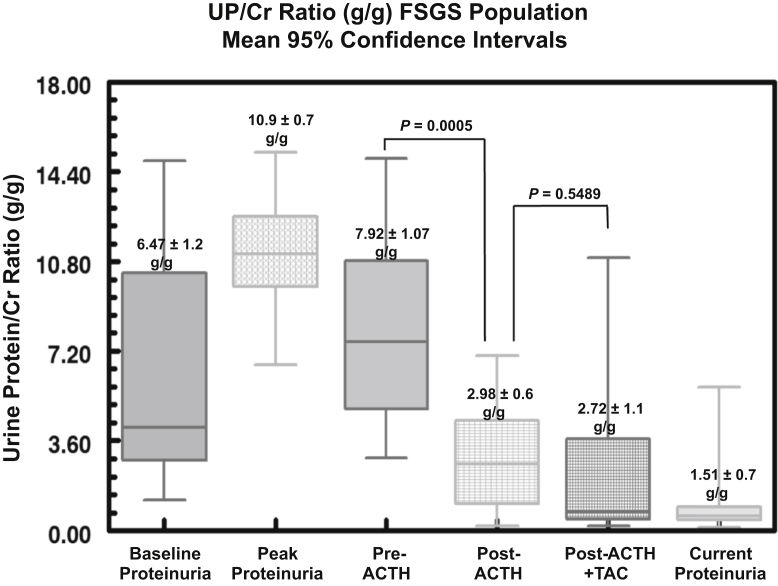
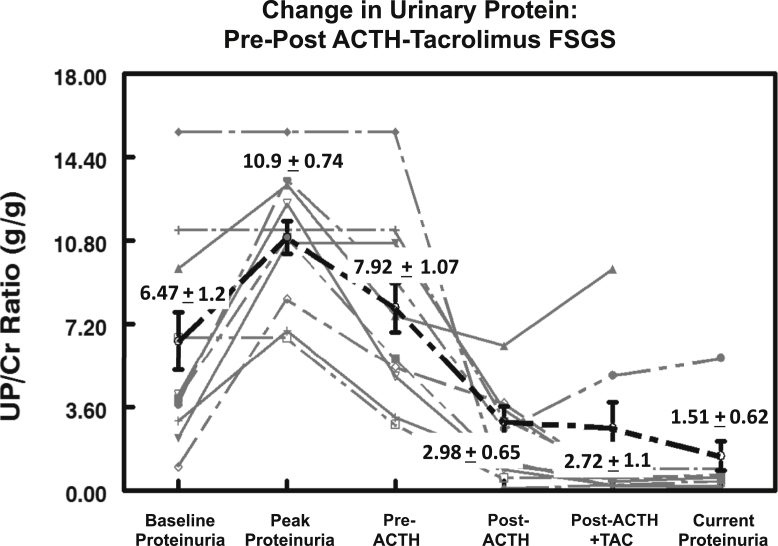
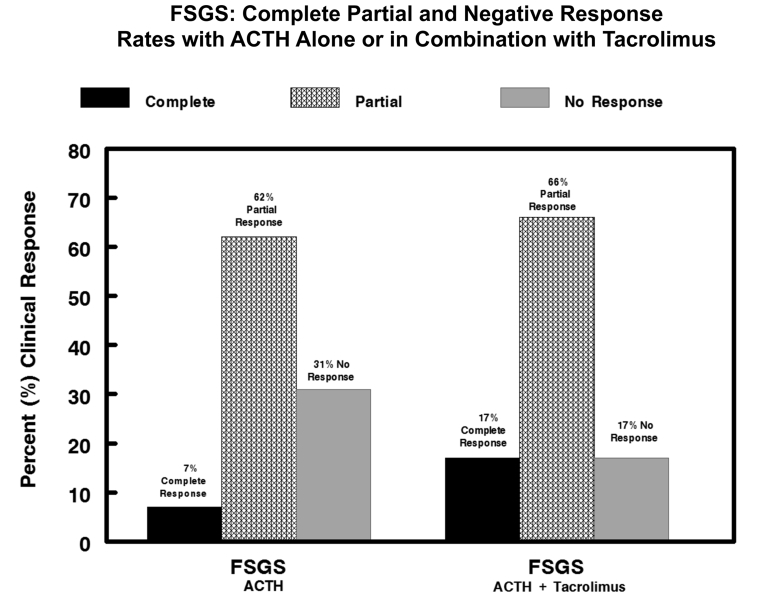
The patients in the FSGS group were also predominately male (85%) with a mean age of 56 ± 5 years. Twelve of the 13 patients (92%) had proteinuria in the nephrotic range at the time of study entry. Of the patients with FSGS, 92% had failed to respond to steroid therapy, with 77% showing no response to CNIs. The mean UP/Cr ratio at the time of diagnosis was 6.47 ± 1.2 g/g, rising to a peak value of 10.9 ± 0.7 g/g (Figure 4). The baseline level of interstitial fibrosis was 25% ± 0.05%, with 46% of patients having fibrosis of more than 30%. The mean time from initial diagnosis of FSGS to treatment with ACTHar gel was 19 ± 6 months. For the 13 patients with FSGS, 6 months of ACTH therapy alone reduced the UP/Cr ratio in aggregate from 7.92 ± 1.1 g/g to 2.98 ± 0.6 g/g (P = 0.0005) (Figure 4). The individual changes in UP/Cr ratios for the patients in the FSGS group are shown in Figure 5. One patient in the FSGS group achieved complete remission after 6 months of treatment with ACTHar gel and did not receive combination therapy with tacrolimus. In contrast to its effect on the patients with MGN, the addition of tacrolimus did not further reduce aggregate UP/Cr ratios. However, complete and partial response rates were improved with combination therapy. As shown in Figure 6, combination therapy with tacrolimus increased the complete response rate from 0% to 25%, and the partial response increased from 40% to 75%.
Figure 4.
Box-whisker plots of urine protein-to-creatinine (UP/Cr) ratios (g/g) for 13 patients with treatment-resistant focal segmental glomerulosclerosis (FSGS) are shown. Data include 5th and 95th percentiles about the median for UP/Cr ratios at baseline, peak levels, pre-ACTH, post-ACTH (6 months), post-ACTH (6 months) and tacrolimus, and current (last recorded data) levels. UP/Cr ratios fell significantly (P < 0.0005) after 6 months of ACTHar gel. The addition of tacrolimus did not further reduce urinary protein levels in the population with FSGS (P < 0.550).
Figure 5.
Individual urine protein-to-creatinine (UP/Cr) ratios (g/g) for patients with steroid-resistant focal segmental glomerulosclerosis (FSGS) are shown. Average UP/Cr fell significantly from 7.92 ± 1.1 to 2.98 ± 0.6 (P < 0.0005) but did not decline further after 6 months of therapy with the addition of tacrolimus (P < 0.550).
Figure 6.
Combined complete, partial, or no response rates for patients with steroid-resistant focal segmental glomerulosclerosis (FSGS) after 6 months of ACTHar alone and 6 months of combined ACTHar and tacrolimus. After 6 months of ACTHar alone, 7.7% achieved a complete response, and 62% achieved a partial response. After 6 months of combination therapy with ACTHar and tacrolimus, the complete response rate rose to 17.0% and the partial response rate rose to 66%.
Change in eGFR
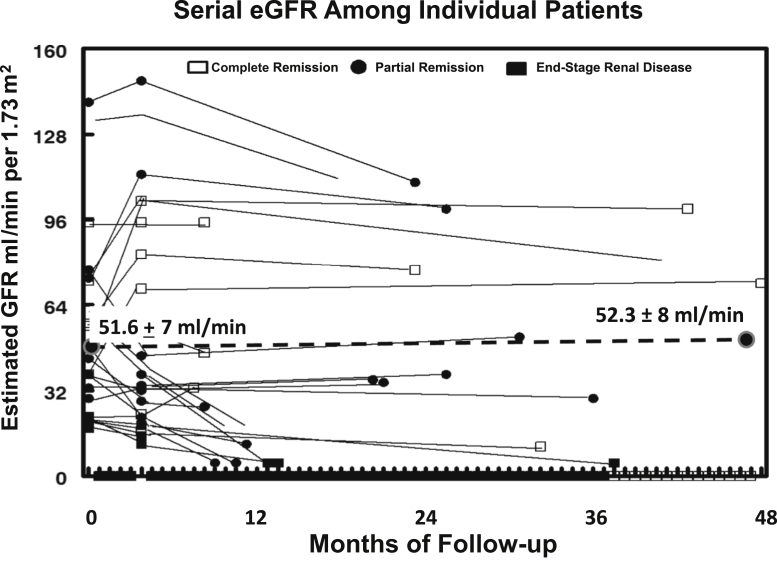
To determine the effects of ACTH or combination therapy on renal function, the eGFR was calculated for each patient. The addition of tacrolimus reduced mean eGFR from 53.6 ± 8.1 to 48.6 ± 8.6 ml/min per 1.73 m2, but this difference did not reach statistical significance (P = 0.112). As shown in Figure 7, the mean eGFR did not change (51.6 ± 7 to 52.3 ± 8 ml/min per 1.73 m2) after 48 months of follow-up. Four patients reached end-stage renal disease (1 partial responder and 3 nonresponders) after an average follow-up of 4 years.
Figure 7.
Individual changes in estimated glomerular filtration rate (eGFR) during treatment and follow-up. Twenty-one patients completed 4 years of follow-up. Mean eGFR did not change over the course of 4 years of follow-up. These data include eGFR values for 4 patients who progressed to end-stage renal disease.
Safety Profile and Side Effects
All patients completed a minimum of 6 months of treatment with ACTH (range, 6–18 months) with a mean duration of therapy of 9.2 ± 0.81 months. Among the 22 patients in the study, hyperglycemia was noted in 22% (5 of 22). Three of the 5 patients had been previously receiving oral agents requiring increased dosing to maintain glucose control. One patient required a reduction in ACTHar dose; no patient required discontinuation of ACTHar. Insomnia was observed in 9% (2 of 22) of patients and treated without complications by using benzodiazepine-based sleep medications. Increased lower extremity edema was observed in 14% (3 of 22) and corrected with modification of diuretic therapy; no patient required discontinuation of ACTHar. One patient experienced dysgeusia, which improved with a reduction in tacrolimus.
Discussion
Patients with treatment-resistant forms of FSGS or MGN are at higher risk for progressive disease. Patients who do not achieve complete or partial remission with conventional therapy have a poorer prognosis, including a more rapid loss of renal function and an increased risk for end-stage renal disease.10 For patients with FSGS, initial therapy is often associated with multiple comorbidities, including excessive weight gain, glucocorticoid-induced diabetes, and metabolic bone disease. As a consequence, there has been a broad effort among clinicians to find effective alternatives to steroid therapy or regimens that can reduce total steroid exposure.11 To address these concerns, we conducted a prospective observational study of the safety and efficacy of ACTHar gel in combination with oral tacrolimus in 22 patients with treatment-resistant MGN or FSGS. All patients were referred for study participation after they had failed to respond to a minimum of 2 different forms of immunosuppression. CNI treatment had previously failed for more than 80% of patients.
ACTHar gel is a mixture of porcine derived pituitary hormones that is primarily composed of ACTH, alpha melanocyte stimulating hormone, and other pro-opiomelanocortin peptides.7 The full-length ACTH is a 39–amino acid peptide that is able to activate 5 different G protein–coupled melanocortin receptors, leading to downstream generation of cyclic adenosine monophosphate and subsequent activation of protein kinase A.12 Recent studies have shown that melanocortin receptors are expressed in numerous cell lines and can exhibit a broad range of physiologic functions. For example, Andersen et al. demonstrated that melanocortin 1 receptors are expressed in several lymphoid cells lines including CD4+ T helper cells, CD8+ T suppressor cells, and CD19+ B cells.13 Olsen et al. demonstrated that incubation of freshly isolated CD19+ B cells with ACTH led to the suppression of IgG production and the inhibition of proliferating B-cell clones.14 These observations suggest that activation of the melanocortin pathways can regulate both humeral and cell-mediated immunity through glucocorticoid-independent mechanisms.
The regulatory role of melanocortin receptors in the kidney has been recently demonstrated. Lindskog et al. have shown that melanocortin 1 receptors co-localize with synaptopodin in the glomerular podocyte and can be detected within endocapillary cells and renal tubular cells.9 Using a puromycin model of nephrosis, Elvin et al. demonstrated that melanocortin 1 receptors are upregulated after cell injury and that activation of melanocortin receptors blocks podocyte apoptosis via a cyclic AMP–dependent pathway. These observations suggest that activation of melanocortin pathways may serve to stabilize podocyte function and viability.15 Results of subsequent preclinical studies have suggested that in addition to their effects on cellular viability, melanocortin pathways also regulate cytoskeletal functions within the podocyte. For example, Faul et al. demonstrated that synaptopodin is a substrate of protein kinase A and that serine-threonine phosphorylation reduces its intracellular degradation. The protective effect of protein kinase A was enhanced when cells were co-incubated with cyclosporin A, suggesting that a combination of melanocortin agonists and CNI may reduce urinary protein losses through effects on the cell cytoskeleton,16 which was one of the factors considered in the design of our trial.
A growing body of clinical studies has shown that ACTHar gel has efficacy in the treatment of a variety of types of glomerular disease including diabetic nephropathy.7, 8, 17, 18 Hogan et al. have reported on the use ACTHar gel in the treatment of 24 patients with adult-onset FSGS: after a maximum of 24 weeks of ACTHar therapy (80 units administered subcutaneously twice a week), the overall (complete or partial) response rate was 35%.19 In a smaller retrospective series, Filippone et al. studied the efficacy of ACTHar gel in 10 patients with FSGS that was previously resistant to multiple different immunosuppressive therapies. Using a similar dose regimen (80 units administered subcutaneously twice a week), Filippone et al. achieved a complete or partial response rate of 40%.20 In a prospective open-label study, Hladunewich et al. studied the efficacy of ACTHar gel in the treatment of 20 patients with idiopathic MGN.21 In this study, patients were in stable condition while receiving angiotensin converting enzyme/angiotensin receptor blocker therapy before study entry but had not received prior immunosuppressive therapy. The dose of ACTHar gel varied between 40 and 80 units 2 times per week for a 6-month period. At the end of 6 months of active therapy, 50% of patients had achieved a complete or partial response, which rose to 65% at the end of 1 year of follow-up. As in the study by Hladunewich et al., none of the patients with MGN in our study achieved a complete remission after 6 months of ACTHar gel therapy. However, when these patients with previously resistant MGN were treated with a combination of ACTHar gel and tacrolimus, the complete response increased to 25%, and the partial response rate increased to 75%.
In previous studies investigators have attempted combination therapy for both FSGS and MGN. Gibson and the National Institutes of Health FSGS Investigators compared the efficacy of monotherapy with cyclosporin A with that of combination therapy with dexamethasone and mycophenolate mofetil in 138 patients with steroid-resistant FSGS. After 12 months of therapy, the complete or partial response rate in the dexamethasone-mycophenolate mofetil–treated group was 33% compared with 46% in the cyclosporin A–treated group.22 In our study, patients with FSGS treated with a combination of ACTHar gel and tacrolimus were found to have complete and partial remission rates of 17% and 66%, respectively. It is unclear why combination therapy did not further reduce the UP/Cr ratios, but complete and partial response rates were improved with combination therapy. Our study is limited by small sample size, a lack of power, and a variable dosing regimen. It is also unknown whether the improvement in complete or partial response rates was the result of a true additive effect between tacrolimus and ACTH or simply a reflection of prolonged ACTH therapy. Until appropriately powered, controlled studies have been performed, use of ACTH should be limited to patients with nephrotic syndrome that is refractory to conventional treatment.
In summary, we conducted a prospective open-label study of 22 patients with treatment-resistant MGN and FSGS and examined the safety and efficacy of ACTHar gel in combination with oral tacrolimus in reducing urinary protein losses. We found that the addition of tacrolimus to 6 months of ACTHar gel therapy resulted in improved complete and partial response rates for patients with treatment-resistant MGN and FSGS. Although these data will need to be verified by larger randomized trials, these encouraging results suggest that stimulation of melanocortin pathways in combination with calcineurin inhibition may be an effective therapy for patients with refractory nephrotic syndrome.
Disclosure
All the authors worked on this manuscript under the sponsorship of NephroNet, a 501C3 nonprofit clinical trials organization. JT and BR have received research grant funding and consultant fees from Mallinckrodt Pharmaceuticals and are active consultants. All the other authors declared no competing interests.
References
- 1.Korbet S.M. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23:1769–1776. doi: 10.1681/ASN.2012040389. [DOI] [PubMed] [Google Scholar]
- 2.Moghadam-Kia S., Werth V.P. Prevention and treatment of systemic glucocorticoid side effects. Int J Dermatol. 2010;49:239–248. doi: 10.1111/j.1365-4632.2009.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponticelli C., Rizzoni, Edefonti A. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. doi: 10.1038/ki.1993.194. [DOI] [PubMed] [Google Scholar]
- 4.Cattran D.C., Appel G.B., Hebert L.A. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int. 1999;56:2220–2226. doi: 10.1046/j.1523-1755.1999.00778.x. [DOI] [PubMed] [Google Scholar]
- 5.Choudhry S., Bagga A., Hari P. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis. 2009;53:760–769. doi: 10.1053/j.ajkd.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Meyrier A., Noel L.H., Auriche P. Long-term renal tolerance of cyclosporin A treatment in adult idiopathic nephrotic syndrome. Kidney Int. 1994;45:1446–1456. doi: 10.1038/ki.1994.189. [DOI] [PubMed] [Google Scholar]
- 7.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2012;8:122–128. doi: 10.1038/nrneph.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tumlin J.A., Galphin C.M., Rovin B.H. Advanced diabetic nephropathy with nephrotic range proteinuria: a pilot study of the long-term efficacy of subcutaneous ACTH gel on proteinuria, progression of CKD, and urinary levels of VEGF and MCP-1. J Diabetes Res. 2013;6:1–8. doi: 10.1155/2013/489869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindskog A., Ebefors K., Johansson M.E. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekahli D., Liutkus A., Ranchin B. Long term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr Nephrol. 2009;24:1525–1532. doi: 10.1007/s00467-009-1138-5. [DOI] [PubMed] [Google Scholar]
- 11.Abeyagunawardena A.S., Dillon M.J., Rees L. The use of steroid-sparing agents in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2003;18:919–924. doi: 10.1007/s00467-003-1216-z. [DOI] [PubMed] [Google Scholar]
- 12.Catania A., Gatti S., Colombo G. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Andersen G.N., Hägglund M., Nagaeva O. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand J Immunol. 2005;61:279–284. doi: 10.1111/j.1365-3083.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 14.Olsen N.J., Decker D.A., Higgins P. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300–308. doi: 10.1186/s13075-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elvin J., Buvall L., Lindskog Jonsson A. Melanocortin 1 receptor agonist protects podocytes through catalase and RhoA activation. Am J Physiol Renal Physiol. 2016;310:F846–F856. doi: 10.1152/ajprenal.00231.2015. [DOI] [PubMed] [Google Scholar]
- 16.Faul C., Donnelly M., Merscher-Gomez S. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponticelli C., Passerini P., Salvadori M. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2005;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Berg A.L., Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004;19:1305–1307. doi: 10.1093/ndt/gfh110. [DOI] [PubMed] [Google Scholar]
- 19.Hogan J., Bomback A.S., Meht K. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013;8:2072–2081. doi: 10.2215/CJN.02840313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippone E.J., Dopson S.J., Rivers D.M. Adrenocorticotropic hormone analog use for podocytopathies. Int Med Case Rep J. 2016;9:125–133. doi: 10.2147/IMCRJ.S104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hladunewich M.A., Cattran D., Beck L.H. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29:1570–1577. doi: 10.1093/ndt/gfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gipson D.S., Trachtman H., Kaskel F.J. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]