Abstract
Black patients with acute myeloid leukemia (AML) experience higher mortality than White patients. We compared induction mortality, acuity of illness prior to chemotherapy, and insurance type between Black and White patients to assess whether acuity of presentation mediates the disparity. Within a retrospective cohort of 1,122 children with AML treated with two courses of standard induction chemotherapy between 2004 and 2014 in the Pediatric Health Information System (PHIS) database, the association between race (Black versus White) and inpatient mortality during induction was examined. Intensive Care Unit (ICU)-level resource utilization during the first 72 hours following admission for initial AML chemotherapy was evaluated as a potential mediator. The total effect of race on mortality during Induction I revealed a strong association (unadjusted HR 2.75, CI: 1.18, 6.41). Black patients had a significantly higher unadjusted risk of requiring ICU-level resources within the first 72 hours after initial presentation (17% versus 11%; RR 1.52, CI: 1.04, 2.24). Mediation analyses revealed the indirect effect of race through acuity accounted for 61% of the relative excess mortality during Induction I. Publicly insured patients experienced greater induction mortality than privately insured patients regardless of race. Black patients with AML have significantly greater risk of induction mortality and are at increased risk for requiring ICU-level resources soon after presentation. Higher acuity amongst Black patients accounts for a substantial portion of the relative excess mortality during Induction I. Targeting factors affecting acuity of illness at presentation may lessen racial disparities in AML induction mortality.
1 | INTRODUCTION
Racial disparities in outcomes are well documented in many pediatric1–3 and adult cancers4 with Black patients consistently experiencing lower overall survival. Access to care and insurance status have been linked to higher mortality in many cancers.5 Studies of adults with acute myeloid leukemia (AML) demonstrate lower rates of survival among Black patients compared with White patients6 and pediatric studies have described higher mortality during the induction phase of therapy and lower overall survival among Black patients.7–9
Although differences in pediatric AML outcomes by race have been documented, the pathways leading to these differences remain largely unexplored. Bhatia10 postulated that advanced stage at diagnosis, disease biology, poor treatment response, nonadherence to therapy, health behaviors, lower socioeconomic status (SES), and differences in health insurance contribute to racial disparities in pediatric cancer survival. A recent study demonstrated that indeed lack of health insurance and lower SES are both associated with worse outcomes in children, adolescents, and young adults with AML in California.11
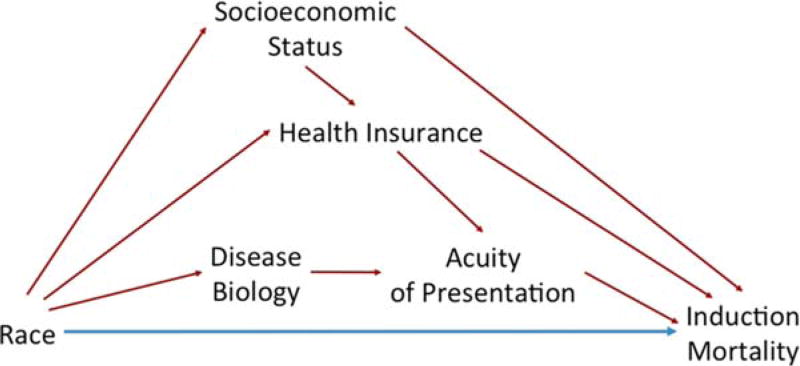
Mediation analyses can elucidate causal intermediates and thus enable planning of targeted interventions to reduce disparities.12,13 We developed a mediation model to delineate the role of insurance type, SES, and acuity prior to chemotherapy initiation in racial disparities in AML induction mortality (Figure 1). Given the complexity of simultaneously modeling multiple associated mediators, we modeled acuity prior to chemotherapy as the primary mediator of mortality differences in Induction I by race while adjusting for SES and insurance type.
FIGURE 1.
Conceptual model: Direct (blue) and potential indirect (red) effects of race on Induction mortality. [Color figure can be viewed at wileyonlinelibrary.com]
We tested this mediation model in a cohort of pediatric AML patients in the Pediatric Health Information Systems (PHIS) database. PHIS includes detailed resource utilization data that allows for assessment of clinical acuity at presentation, as defined by the need for Intensive Care Unit (ICU)-level resources within 72 hours of admission.14–16 We hypothesized that Black children would have higher mortality than White children during the first 50 days following admission and that this difference would be attenuated during Induction II (or the first 100 days following admission). We further hypothesized that access to care, as measured by acuity of presentation prior to chemotherapy, mediates much of the mortality difference in Induction I. Finally, we posited that Black children would have higher resource utilization during Induction I than White children.
2 | METHODS
2.1 | Data source
PHIS data included inpatient, emergency department, and observation unit information from over 40 not-for-profit, tertiary care pediatric hospitals.17 Data included demographics, dates of service, discharge disposition, and daily inpatient billing data for medications, laboratory tests, imaging procedures, clinical services/procedures, and supplies. Data were de-identified at the time of submission and were exempt from IRB approval. Data were subjected to reliability and validity checks through a joint effort between the Children’s Hospital Association, Truven Health Analytics, and participating hospitals.
2.2 | Study population
The study population of pediatric AML patients in PHIS was assembled using a validated process.14 Patients receiving standard induction chemotherapy (cytarabine, daunorubicin, etoposide - ADE) from January 2004 through June 2014 were identified. Patients with a diagnosis for an alternative malignancy or evidence of bone marrow transplantation within 60 days following the first admission with chemotherapy were excluded. Patients who did not receive ADE or received gemtuzumab ozogamicin were excluded to ensure chemotherapy uniformity.
Patients were followed for two standard courses of induction chemotherapy. Follow-up for our primary analysis began at the start of Induction I chemotherapy until the start of Induction II or 50 days after commencement of Induction I, whichever occurred first. Separate (secondary) analyses also examined outcomes from the start of induction chemotherapy and continued until the start of Intensification I chemotherapy or 100 days after commencement of induction chemotherapy, whichever occurred first.
2.3 | Race
The race of a patient, dichotomized as either Black or White, was identified by contributing hospitals and considered the primary ‘exposure’ variable. Other racial groups were excluded because of modest sample sizes. Ethnicity was not evaluated as this information was missing in a substantial fraction of patients.
2.4 | Outcomes
The primary outcome of interest was inpatient mortality during Induction I. Inpatient deaths were identified based on discharge status for each hospitalization. Inpatient death during Induction II and ICU-level resource usage were secondary outcomes. ICU-level resources were defined by specific ICD-9-CM procedure codes or clinical resource utilization considered a priori as markers of ICU care rather than by physical location.15 ICU-level resource requirements were evaluated by organ system as represented by vasopressor support, mechanical ventilation, renal replacement therapy, and leukapheresis, as well as the need for any of these therapies (versus none).
Daily utilization rates of antibiotic, antifungal, antiviral and vasopressor medications, parenteral nutrition, blood product replacement, and supplemental oxygen were determined from billing data. Binary indicators for each resource designated exposure on each inpatient day were generated and summed to obtain the total number of days exposed. Resource utilization rates were reported as days of use per 100 inpatient days.
2.5 | Proposed mediator
ICU-level resource utilization during the first 72 hours following admission for initial AML chemotherapy was examined as the potential mediator. This timeframe was chosen a priori to evaluate clinical acuity at presentation rather than acuity resulting from chemotherapy toxicity. Acuity of presentation was dichotomized (<2 systems and ≥2 systems) based on the number of organ systems requiring ICU-level resources within the first 72 hours of the initial AML admission in which chemotherapy was billed.15
2.6 | Patient characteristics
Patient characteristics including age,18,19 sex,20 and insurance type (private, public, or other)5,6 were covariates. PHIS zip code was used to determine median household income from 2010 U.S. Census data and was categorized in quartiles.21–23
2.7 | Statistical analyses
Distributions of patient characteristics were compared using chi-squared tests. To evaluate potential confounding, each covariate was added independently to the crude models, and changes in the measure of association were assessed. A change greater than 10% was considered evidence of meaningful confounding; variables that produced such an effect size change were retained in final models.
Cox proportional hazards regression was used to estimate unadjusted and adjusted hazard ratios of induction mortality during Induction I and during Induction I and II. Generalized estimating equations with an exchangeable correlation matrix were used to obtain robust variance estimates to account for clustering by hospital. Log-binomial regression was used to estimate the risk ratios (RR) and 95% CIs comparing occurrence of ICU-level resource utilization.
Mediation analysis was performed to decompose the association of race on induction mortality into direct and indirect components.24 The following are required for acuity of presentation to be considered a potential mediator: statistically significant associations between (1) race and acuity of presentation and (2) acuity of presentation and induction mortality, and (3) a non-null relationship between race and induction mortality.24 First, the requisite component associations were assessed. Then, parameter estimates from the models for induction mortality conditional on acuity of presentation and race and for acuity of presentation conditional on race were combined to decompose the total effect of race on induction mortality into its indirect effect as mediated by acuity of presentation and its direct effect through other undefined pathways.24 The proportion of the total effect mediated through acuity of presentation was computed on the difference scale from the estimates of the direct and indirect effects as HRdirect × (HRindirect − 1)/(HRdirect × HRindirect − 1).24,25
Kaplan-Meier curves and Cox regressions were used to explore the interaction on the additive scale between race and insurance type. White patients with private health insurance were used as the reference group. Robust sandwich estimates of the variance-covariance matrix were used to account for clustering by hospital. The relative excess risk due to interaction was calculated to quantify interaction on the additive scale.26 Only unadjusted results of the exploratory interaction assessment are presented due to small event numbers and concerns regarding overfitting.
Poisson regression models with inpatient days as offset were used to estimate adjusted rate ratios and 95% CI comparing resource utilization rates by race; a Pearson scale adjustment was used to correct for possible overdispersion. All analyses were performed using SAS (version 9.2, SAS Institute, Inc., Cary, NC).
3 | RESULTS
3.1 | Patient characteristics
The cohort included 1,695 patients diagnosed with AML who received induction chemotherapy at 42 PHIS-contributing institutions between January 1, 2004 and June 30, 2014. Eighty-one percent (n = 1,374) of patients were either Black or White and 82% (n = 1,122) received ADE in Induction I.
Table 1 lists patient characteristics for the study cohort. Sixteen percent of the study population was Black, 52% was male, and the median age was 9 years (interquartile range: 1–14 years). Distributions of sex and age did not differ by race. Black patients were significantly more likely than White patients to be publicly insured (55% versus 38%, P < 0.0001) and to be in the lowest income quartile (39% versus 22%, P < 0.0001). The distribution of chemotherapy regimens administered in the second induction course did not differ by race (P = 0.81). Pre-existing comorbidities as measured by ICD-9-CM codes in the year before the index AML admission could not be assessed because only 11% of the cohort was admitted to a PHIS hospital within that timeframe.
TABLE 1.
Characteristics of AML patient population
| Overall | Black | White | P-valuea | |
|---|---|---|---|---|
| N (%) | 1122 (100%) | 183 (16%) | 939 (84%) | |
|
| ||||
| Sex | ||||
| Male | 584 (52.1) | 102 (54.6%) | 493 (51.6%) | 0.4562 |
| Female | 538 (47.9) | 85 (45.4%) | 463 (48.4%) | |
|
| ||||
| Age at index AML admission, years | ||||
| < 1 | 139 (12.4) | 23 (12.3%) | 117 (12.2%) | 0.8576 |
| 1 to <5 | 284 (25.3) | 46 (24.6%) | 245 (25.6%) | |
| 5 to <10 | 164 (14.6) | 25 (13.4%) | 143 (15.0%) | |
| 10 to <15 | 277 (24.7) | 52 (27.8%) | 230 (24.1%) | |
| 15+ | 258 (23.0) | 41 (21.9%) | 221 (23.1%) | |
|
| ||||
| Insurance Type at AML presentation | ||||
| Private | 480 (42.8) | 46 (24.6%) | 440 (46.0%) | <0.0001 |
| Public | 454 (40.5) | 102 (54.5%) | 361 (37.8%) | |
| Self-pay | 14 (1.3) | 2 (1.1%) | 13 (1.4%) | |
|
| ||||
| Otherb | 174 (15.5) | 37 (19.8%) | 142 (14.9%) | |
|
| ||||
| Median Household Income Quartile (by Zip Code)c | ||||
| < $33,532 | 260 (25.0%) | 63 (38.9%) | 197 (22.4%) | <0.0001 |
| $33,532 to <$42,874 | 260 (25.0%) | 41 (25.3%) | 219 (24.9%) | |
| $42,874 to <$55,145 | 260 (25.0%) | 38 (23.5%) | 222 (25.3%) | |
| > $55,145 | 261 (25.0%) | 20 (12.4%) | 241 (27.4%) | |
|
| ||||
| Induction II Chemotherapy Regimen | ||||
| ADE ± gemtuzumab | 860 (76.6%) | 137 (74.9%) | 723 (77.0%) | 0.8069 |
| MA | 90 (8.0%) | 17 (9.3%) | 73 (7.8%) | |
| AE | 49 (4.4%) | 5 (2.7%) | 44 (4.7) | |
| Other | 82 (7.3%) | 11 (6.0%) | 71 (7.6%) | |
| No Course 2 | 68 (5.7%) | 10 (5.5%) | 54 (5.8%) | |
P-value for the comparison of distributions by race.
Other category includes charity care, admissions without charges, and other as specified by the sites.
A subset of zip codes were invalid and thus could not be matched to U.S. Census Bureau Data, thus 93% (1041 patients) were included.
3.2 | Early and overall induction mortality
Mortality during Induction I (or 50 days from initiation of chemotherapy) was significantly higher among Black patients (4.9%) than White patients (1.9%) (unadjusted HR 2.58, CI: 1.03, 6.46, P = 0.04) with no evidence of confounding by sex or age. Inpatient induction mortality over the first two courses of chemotherapy was also higher among Black patients (4.9%) than White patients (2.6%), but the difference was not statistically significant (unadjusted HR 1.93, CI: 0.90, 4.15, P = 0.09). Six patients (18%) died during Induction II, all of whom were White children. Half of deaths occurred within 9 days of chemotherapy initiation, regardless of race.
3.3 | ICU-level resource utilization and acuity of presentation
Comparisons of ICU-level resource requirements by race are presented in Table 2. Compared with White patients, Black patients experienced a significantly higher risk for any ICU-level resource within the first 72 hours of initial admission in which the patient received chemotherapy (17% versus 11%, adjusted RR 1.52, CI 1.04, 2.24, P = 0.03). In particular, the need for cardiovascular support was markedly higher among Black patients (adjusted RR 2.52, CI 1.31, 4.87, P = 0.006) and, although not statistically significant, the point estimates for all other ICU-level resources also suggested greater requirements among Black patients.
TABLE 2.
Comparison of ICU-level resource requirements during first 72 hours of initial AML admission, over Induction I, and over Inductions I and II by race
| ICU Level Carea,b | Overall (N = 1122) | Black (n = 183) | White (n = 939) | cRR (95% CI) | P-value | aRRc (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| A. First 72 Hours of Index AML Admission | |||||||
|
| |||||||
| ICU Acuity Scored ≥2 | 47 (4.2%) | 18 (9.8%) | 29 (3.1%) | 3.35 (1.84, 6.12) | <0.0001 | ||
|
| |||||||
| Any ICU Level Care, n (%) | 135 (12.0%) | 31 (16.9%) | 104 (11.1%) | 1.52 (1.04, 2.24) | 0.032 | ||
| Cardiovascular, n (%) | 50 (4.5%) | 16 (8.7%) | 34 (3.6%) | 2.52 (1.31, 4.87) | 0.006 | ||
| Respiratory, n (%) | 78 (6.9%) | 20 (10.9%) | 58 (6.2%) | 1.78 (0.96, 3.33) | 0.069 | ||
| Renal, n (%) | 13 (1.2%) | 4 (2.2%) | 9 (1.0%) | 2.30 (0.70, 7.58) | 0.131 | ||
| Leukapheresis, n (%) | 50 (4.5%) | 12 (6.6%) | 38 (4.1%) | 1.56 (0.91, 2.68) | 0.109 | ||
|
| |||||||
| B. Induction I | |||||||
|
| |||||||
| ICU Acuity Scored ≥2 | 88 (7.8%) | 21 (11.5%) | 67 (7.2%) | 1.58 (0.88, 2.86) | 0.045 | 1.00 (0.96, 1.03) | 0.841 |
|
| |||||||
| Any ICU Level Care, n (%) | 205 (18.3%) | 43 (23.5%) | 162 (17.2%) | 1.38 (1.05, 1.81) | 0.046 | 1.09 (0.86, 1.38) | 0.484 |
| Cardiovascular, n (%) | 133 (11.8%) | 30 (16.4%) | 103 (11.0%) | 1.46 (0.92, 2.32) | 0.110 | 1.10 (0.73, 1.66) | 0.645 |
| Respiratory, n (%) | 137 (12.2%) | 29 (15.9%) | 108 (11.5%) | 1.38 (0.88, 2.15) | 0.161 | 0.98 (0.71, 1.34) | 0.897 |
| Renal, n (%) | 26 (2.3%) | 5 (2.7%) | 21 (2.2%) | 1.24 (0.51, 3.04) | 0.633 | 0.80 (0.38, 1.71) | 0.568 |
| Leukapheresis, n (%) | 17 (1.5%) | 3 (1.6%) | 14 (1.5%) | 1.06 (0.36, 3.11) | 0.920 | 0.70 (0.21, 2.26) | 0.695 |
|
| |||||||
| C. Induction I and II | |||||||
|
| |||||||
| ICU Acuity Scored ≥2 | 100 (8.8%) | 22 (12.0%) | 78 (8.3%) | 1.45 (0.85, 2.47) | 0.103 | 0.92 (0.54, 1.55) | 0.780 |
|
| |||||||
| Any ICU Level Care, n (%) | 238 (21.2%) | 48 (26.2%) | 190 (20.2%) | 1.30 (0.99, 1.71) | 0.061 | 1.09 (0.74, 1.61) | 0.597 |
| Cardiovascular, n (%) | 163 (14.4%) | 34 (18.6%) | 129 (13.7%) | 1.36 (0.97, 1.92) | 0.085 | 1.07 (0.63, 1.80) | 0.859 |
| Respiratory, n (%) | 147 (13.1%) | 30 (16.4%) | 117 (12.5%) | 1.33 (0.92, 1.92) | 0.131 | 0.95 (0.59, 1.51) | 0.998 |
| Renal, n (%) | 32 (2.9%) | 6 (3.3%) | 26 (2.8%) | 1.28 (0.53, 3.09) | 0.439 | 1.02 (0.48, 2.17) | 0.824 |
| Leukapheresis, n (%) | 18 (1.6%) | 3 (1.6%) | 15 (1.6%) | 1.10 (0.32, 3.79) | 0.247 | 0.67 (0.20, 2.25) | 0.547 |
Presented as proportion of patients receiving specified ICU level care.
Defined as the occurrence of at least one ICD-9-CM procedure code or clinical resource considered a priori as a marker of ICU care, rather than by physical location.
Adjusted for ICU acuity score in the first 72 hours of index admission.
Acuity score represents the number of systems requiring ICU-level resources.
Abbreviations: RR, risk ratio; CI, confidence interval.
Black patients had three times the risk for ICU-level care involving two or more systems within the first 72 hours (unadjusted RR 3.35, CI: 1.84, 6.12, P < 0.0001) compared to White patients. After adjustment for acuity of presentation, there was no significant difference in the overall risk for ICU-level care outside of the first 72 hours for Black patients relative to White patients (adjusted RR 1.09, CI: 0.86, 1.38, P = 0.48) during Induction I. There was a clear association between acuity of presentation and mortality during Induction I (adjusted HR 15.4, CI: 7.09, 33.6, P < 0.0001, Supporting Information eTable Ia).
3.4 | Mediation analysis
All three criteria were met to consider acuity of presentation as a potential mediator of the association between race and mortality during Induction I: (1) a statistically significant association between race and acuity of presentation (adjusted RR 2.94, CI: 1.58, 5.46, P < 0.0001, Supporting Information eTable II), (2) a statistically significant association between acuity of presentation and induction mortality (adjusted HR 15.4, CI: 7.09, 33.6, P < 0.0001, Supporting Information eTable Ia), and (3) a non-null association between race and mortality during Induction I (adjusted HR 2.60, CI: 1.05, 6.45, P = 0.04).
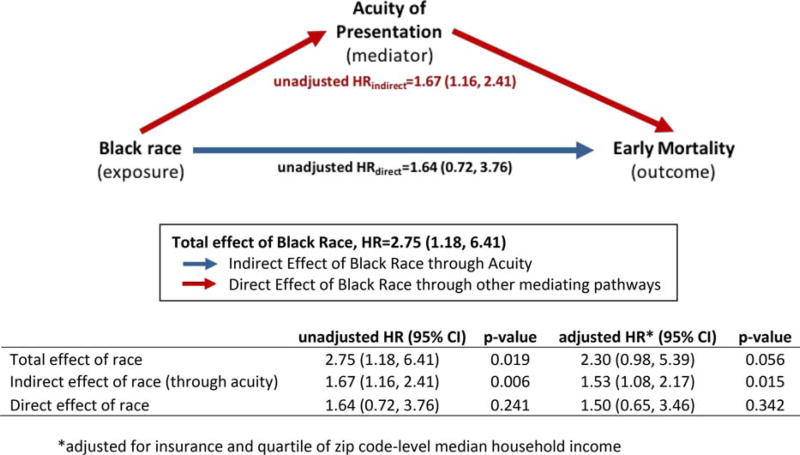
Mediation analysis defined the direct effect of race (unadjusted HR 1.64, CI: 0.72, 3.76, P = 0.24) on mortality during Induction I and the indirect effect mediated by acuity of presentation (unadjusted HR 1.67, CI: 1.16, 2.41, P = 0.006) as shown in Figure 2. When adjusted for insurance type and median household income, both effects were modestly attenuated (indirect effect adjusted HR 1.53, CI: 1.08, 2.17, P = 0.02, direct effect adjusted HR 1.50, CI: 0.65, 3.46, P = 0.34). Thus, acuity of presentation mediates approximately 61% of the absolute effect of Black race on mortality during Induction I.
FIGURE 2.
Mediation Assessment: Estimates of the direct (blue) and indirect (red) effects of Black race on AML mortality during Induction I. [Color figure can be viewed at wileyonlinelibrary.com]
The role of acuity of presentation in mortality over the course of Induction I and II was also examined and the total effect of race is attenuated as are the component effects (Supporting Information eTable III). While there were many fewer deaths during Induction II, all of the deaths were amongst White patients.
3.5 | Adjustment for socioeconomic status and insurance type
When comparing the extremes of area-based median household income quartile (the highest versus lowest quartilies), they were neither significantly associated with mortality during Induction I (adjusted HR 1.43, CI: 0.61, 3.06, P = 0.35, Supporting Information eTable Ia) nor associated with acuity of presentation (adjusted RR 0.70, CI: 0.26, 1.90, P = 0.48, Supporting Information eTable II). Public insurance was not significantly associated with mortality during Induction I (adjusted RR 1.40, CI: 0.53, 3.70, P = 0.49), but was associated with acuity of presentation (unadjusted RR 2.02, CI: 1.08, 3.78, P = 0.03). Following adjustment for race and area-based median household income quartile, the association between insurance and acuity of presentation was attenuated (adjusted RR 1.80, CI: 0.91, 3.53, P = 0.09, Supporting Information eTable II).
3.6 | Interactions between race and insurance type
The independent and joint effects of Black race and public insurance on mortality during Induction I were examined using White patients with private insurance as the reference group (Supporting Information eFigure 1A). The joint effect of Black race and public insurance (unadjusted HR 3.91, CI: 1.32, 11.6, P = 0.01) was greater than expected based on the independent effects of Black race (unadjusted HR 1.37, CI: 0.17, 10.9, P = 0.77) and public insurance (unadjusted HR 2.01, CI: 0.78, 5.18, P = 0.15). The relative excess risk due to interaction was 1.53 (CI: −2.74, 5.80) with 39% of the joint effect due to interaction. These results suggest that the absolute difference in mortality during Induction I between Black and White patients is larger among publicly insured children than privately insured children.
3.7 | Resource utilization
Overall resource utilization in Induction I did not differ significantly between Black and White children. When stratified by the need for ICU-level resources, only two resources differed significantly as shown in eTable IV. Among patients receiving ICU-level resources, Black patients received more parenteral nutrition (adjusted RR 1.60, CI: 1.07, 2.38, P = 0.02). Among patients not receiving ICU-level resources, Black patients received more antihypertensive medications (adjusted RR = 2.47, CI: 1.22, 5.00, P = 0.01).
4 | DISCUSSION
In a large nationally representative cohort, Black pediatric patients receiving standard AML chemotherapy experienced significantly greater inpatient mortality than White patients and 61% of this difference is explained by acuity of illness at presentation. From a methodological perspective, our approach of using resource utilization data in a mediation analysis enabled the determination of both the direct and indirect effects of race mediated through acuity of presentation while adjusting for area-based SES and insurance type as confounders.
Although the decreased survival of Black patients with AML relative to White patients has been described previously,7–9 our data provide valuable insights into this disparity. Most notably, these data demonstrate an important role for presenting severity of illness and provide evidence that post-diagnostic supportive care does not substantially differ by race. Our findings also demonstrate that racial disparities persist despite reductions in mortality with the transition to ADE induction regimens.27
The outcome gap observed previously within pediatric trials is seen in this cohort of patients unselected by clinical trial participation, which itself varies by race.28–30 Moreover, our finding of increased acuity of presentation among Black patients is consistent with data from studies in pediatric and adult solid tumors showing Black patients present with more advanced disease.1,31–33
The novel finding of higher acuity of presentation among Black patients with AML provides direction for investigating this mortality disparity. Specifically, qualitative assessments of patient experiences leading up to and at time of AML diagnosis may uncover unknown barriers to accessing care and identifying signs and symptoms. Additionally, geocoding analyses may allow more nuanced assessments of socioeconomic variables and distance to care that may modify a patient’s clinical status at presentation.
Our exploratory interaction analysis suggests a synergistic effect between insurance type and race in influencing mortality during Induction I. While publicly insured patients experienced greater mortality than privately insured patients regardless of race, the difference in mortality during Induction I between Black and White patients was substantially greater among publicly insured patients. These results are consistent with data from St. Jude Children’s Research Hospital that demonstrated no difference in outcome between Black and White patients in a health care setting of uniform access to care among those referred.34 In the pediatric population, barriers to accessing care are likely multifaceted and reflect multiple complexities including access to transportation,35 parental financial resources and employment,36–38 childcare for siblings,39,40 and cultural and educational barriers.41–43
Several limitations of our study should be acknowledged. PHIS data do not contain laboratory results, thus limiting the evaluation of biologic risk factors. However, prior investigations of pediatric AML reported no difference in clinical characteristics, FAB subtype, cytogenetics, or prognostic molecular markers between Black and White children in the large St. Jude cohort as well as in cooperative group trials.7,34 Since ethnicity is incompletely reported in PHIS, the Hispanic population is included within the two racial groups examined. Legacy cooperative group data suggest that Hispanic patients have decreased survival compared to White patients. Thus the differential misclassification of Hispanic patients as White would be expected to hinder the detection of a true association between race and mortality. In addition, PHIS resource utilization data do not allow for analyses using medication dose or hour-level timing of administration. Likewise, comorbidities could not be fully evaluated using the inpatient data available. Furthermore, the use of zip code-derived household income provides only an approximation of SES, leading to the possibility of resonant confounding. Our mediation approach is very informative, but as with all mediation analyses, it does not address potential exposure-induced confounding of the acuity-mortality association, such as from SES or insurance type; however, given the small amount of change following adjustment, their role as confounders is likely limited.
This study represents a step toward understanding the complex factors underlying racial disparities in AML outcome and employs a methodological approach that is novel in this arena. However, additional studies are needed to extend this approach to outcomes beyond early mortality and to other possible mediators. Such analyses will require integration of different datasets44 and large-scale cooperative studies across international borders to provide the more sophisticated understanding that is necessary for empirical interventions to address the disparities.
Racial disparities in early mortality among pediatric patients undergoing treatment for AML, may be explained in part by differences in acuity of illness prior to initiation of chemotherapy, which exceed the effects of SES and insurance type. Combined with the synergistic role of insurance type, these findings imply that access to care figures prominently in the greater early mortality among Black children than White children. Additional investigation into the events preceding and during presentation will help further elucidate what drives the differences in acuity, enabling the development of more targeted interventions to improve outcomes and eliminate this well-documented racial disparity.
Supplementary Material
Acknowledgments
Funding information
NIH, Grant/Award Number: R01CA133881; Pediatric Hospital Epidemiology Outcomes Research Training Program (T32).
CONFLICT OF INTERESTS
Dr Brian Fisher receives research funding from Pfizer and Merck for projects unrelated to this research.
Footnotes
AUTHOR CONTRIBUTIONS
The authors would like to acknowledge Matt Hall for his provision of the SES data from PHIS. In addition, we would like to thank Dr Marilyn Schapira and Dr Scott Lorch for their critical review of the manuscript.
Designed research, performed cohort assembly, analyses, and interpretation of data, and drafted manuscript: Winestone
Designed research, performed analyses and interpretation of data, and drafted manuscript: Getz
Aided in cohort assembly, interpretation of data, and revision of manuscript: Miller, Wilkes
Aided in analyses, interpretation of data, and revision of manuscript: Li
Performed cohort assembly: Sack, Huang
Aided interpretation of data and revision of manuscript: Seif, Bagatell, Fisher
Aided in study design, interpretation of data, and revision of manuscript: Epstein
Provided study supervision, designed research, performed interpretation of data, and drafted manuscript: Aplenc
Additional Supporting Information may be found in the online version of this article.
References
- 1.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29:76–82. doi: 10.1200/JCO.2010.29.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–1964. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 3.Kadan-Lottick NS, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 4.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 5.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58:9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 6.Bradley CJ, Dahman B, Jin Y, et al. Acute myeloid leukemia: how the uninsured fare. Cancer. 2011;117:4772–4778. doi: 10.1002/cncr.26095. [DOI] [PubMed] [Google Scholar]
- 7.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- 9.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia S. Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. doi: 10.1002/pbc.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrahão R, Keogh RH, Lichtensztajn DY, et al. Predictors of early death and survival among children, adolescents and young adults with acute myeloid leukaemia in California, 1988–2011: a population-based study. Br J Haematol. 2016;173:292–302. doi: 10.1111/bjh.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meghani SH, Kang Y, Chittams J, et al. African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. J Clin Oncol. 2014;32:2773–2779. doi: 10.1200/JCO.2013.54.7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–2e24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 14.Kavcic M, Fisher BT, Torp K, et al. Assembly of a cohort of children treated for acute myeloid leukemia at free-standing children’s hospitals in the United States using an administrative database. Pediatr Blood Cancer. 2013;60:508–511. doi: 10.1002/pbc.24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maude SL, Fitzgerald JC, Fisher BT, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr Crit Care Med. 2014;15:112–120. doi: 10.1097/PCC.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar EG, Li Y, Fisher BT, et al. Supportive care utilization and treatment toxicity in children with Down syndrome and acute lymphoid leukaemia at free-standing paediatric hospitals in the United States. Br J Haematol. 2016;174:591–599. doi: 10.1111/bjh.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Child Health Corporation of America. Child Health Corporation of America-a business alliance of children’s hospitals. [Accessed August 12, 2014]; http://www.chca.com.
- 18.Creutzig U, Buchner T, Sauerland MC, et al. Significance of age in acute myeloid leukemia patients younger than 30 years: a common analysis of the pediatric trials AML-BFM 93/98 and the adult trials AMLCG 92/99 and AMLSG HD93/98A. Cancer. 2008;112:562–571. doi: 10.1002/cncr.23220. [DOI] [PubMed] [Google Scholar]
- 19.Razzouk BI, Estey E, Pounds S, et al. Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 institutions. Cancer. 2006;106:2495–2502. doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- 20.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Oncologist. 2007;12:341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 21.Du XL. Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol. 2005;23:8620–8628. doi: 10.1200/JCO.2005.02.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keating NL. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 23.Yabroff KR, Dowling EC, Guy GP, et al. Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–267. doi: 10.1200/JCO.2015.62.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York: Oxford University Press; 2015. [Google Scholar]
- 25.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposuremediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenland S, Lash TL, Rothman KJ. Concepts of Interaction. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. p. 7183. [Google Scholar]
- 27.Kavcic M, Fisher BT, Li Y, et al. Induction mortality and resource utilization in children treated for acute myeloid leukemia at free-standing pediatric hospitals in the United States. Cancer. 2013;119:1916–1923. doi: 10.1002/cncr.27957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. [DOI] [PubMed] [Google Scholar]
- 29.Lund MJ, Eliason MT, Haight AE, et al. Racial/ethnic diversity in children’s oncology clinical trials: ten years later. Cancer. 2009;115:3808–3816. doi: 10.1002/cncr.24437. [DOI] [PubMed] [Google Scholar]
- 30.Shaya FT, Gbarayor CM, Huiwen Keri Y, et al. A perspective on African American participation in clinical trials. Contemp Clin Trials. 2007;28:213–217. doi: 10.1016/j.cct.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Baker KS, Anderson JR, Lobe TE, et al. Children from ethnic minorities have benefited equally as other children from contemporary therapy for rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 2002;20:4428–4433. doi: 10.1200/JCO.2002.11.131. [DOI] [PubMed] [Google Scholar]
- 32.Metzger ML, Castellino SM, Hudson MM, et al. Effect of race on the outcome of pediatric patients with Hodgkin’s lymphoma. J Clin Oncol. 2008;26:1282–1288. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 33.Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 34.Rubnitz JE, Lensing S, Razzouk BI, et al. Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatr Blood Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 35.Samuels RC, Ward VL, Melvin P, et al. Missed appointments: factors contributing to high no-show rates in an urban pediatrics primary care clinic. Clin Pediatr. 2015;54:976–982. doi: 10.1177/0009922815570613. [DOI] [PubMed] [Google Scholar]
- 36.Son M, Kim J, Oh J, et al. Inequalities in childhood cancer mortality according to parental socioeconomic position: a birth cohort study in South Korea. Soc Sci Amp Med (1982) 2011;72:108–115. doi: 10.1016/j.socscimed.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Buckle GC, Collins JP, Sumba PO, et al. Factors influencing time to diagnosis and initiation of treatment of endemic Burkitt Lymphoma among children in Uganda and western Kenya: a cross-sectional survey. Infect Agent Cancer. 2013;8:36. doi: 10.1186/1750-9378-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bona K, Blonquist TM, Neuberg DS, et al. Impact of socioeconomic status on timing of relapse and overall survival for children treated on Dana-Farber cancer institute ALL Consortium Protocols (2000–2010) Pediatr Blood Amp Cancer. 2016;63:1012–1018. doi: 10.1002/pbc.25928. [DOI] [PubMed] [Google Scholar]
- 39.Erdmann F, Winther JF, Dalton SO, et al. Survival from childhood hematological malignancies in Denmark: is survival related to family characteristics? Pediatr Blood Amp Cancer. 2016;63:1096–1104. doi: 10.1002/pbc.25950. [DOI] [PubMed] [Google Scholar]
- 40.Erdmann F, Kaatsch P, Schüz J. Family circumstances and survival from childhood acute lymphoblastic leukaemia in West Germany. Cancer Epidemiol. 2015;39:209–215. doi: 10.1016/j.canep.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Simony SB, Lund LW, Erdmann F, et al. Effect of socioeconomic position on survival after childhood cancer in Denmark. Acta Oncol (Stockholm, Sweden) 2016;55:742–750. doi: 10.3109/0284186X.2016.1144933. [DOI] [PubMed] [Google Scholar]
- 42.Dang-Tan T, Franco EL. Diagnosis delays in childhood cancer: a review. Cancer. 2007;110:703–713. doi: 10.1002/cncr.22849. [DOI] [PubMed] [Google Scholar]
- 43.Adam M, Rueegg CS, Schmidlin K, et al. Socioeconomic disparities in childhood cancer survival in Switzerland. Int J Cancer. 2016;138:2856–2866. doi: 10.1002/ijc.30029. [DOI] [PubMed] [Google Scholar]
- 44.Aplenc R, Fisher BT, Huang YS, et al. Merging of the National Cancer Institute-funded cooperative oncology group data with an administrative data source to develop a more effective platform for clinical trial analysis and comparative effectiveness research: a report from the Children’s Oncology Group. Pharmacoepidemiol Drug Saf. 2012;21:37–43. doi: 10.1002/pds.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.