INTRODUCTION
Malaria causes substantial morbidity and mortality in many of the most resource-limited areas of the world. In addition, malaria is a threat to travelers to endemic areas and should be considered in the evaluation of any traveler returning from a malaria-endemic region presenting with fever. Malaria infection can rapidly develop into severe disease that can be fatal. Prompt, effective treatment is critical to limiting these complications. Understanding the species-specific epidemiology and drug-resistance patterns in the geographic area where infection was acquired guides treatment. This review contains an overview of the epidemiology and pathogenesis of malaria with a focus on components relevant to treating malaria in nonendemic areas. Guidance for treatment and management of malaria in returned travelers is provided.
CAUSE AND PATHOGENESIS
Malaria is caused by infection with Plasmodium parasites. Five species cause disease in humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi. Infection is spread by the bite of a female Anopheles mosquito and has obligatory human and mosquito stages of the life cycle. The species of Anopheles mosquitoes responsible for Plasmodium transmission has a broad geographic distribution. Typically, Anopheles bite from dusk to dawn. However, exact biting patterns vary based on specific species.
The life cycle of the 5 Plasmodium species is similar, apart from the dormant stages of P vivax and P ovale:
Sporozoites are inoculated into humans by an Anopheles mosquito and immediately invade hepatocytes.
Asexual replication takes place initially in the liver, leading to the release of thousands of merozoites per infected hepatocyte into the blood. This release occurs 1 to 2 weeks after the bite of the infectious mosquito.
Blood stage infection causes clinical disease.
Merozoites invade erythrocytes, undergo asexual reproduction, and then rupture out of the erythrocyte, allowing the daughter merozoites to continue the cycle of invasion and replication.
Some blood stage parasites develop into male and female gametocytes, the stage that is responsible for transmission to the mosquito.
For the infection to be transmitted, a female Anopheles mosquito must ingest erythrocytes containing male and female gametocytes.
Sexual reproduction takes place in the mosquito midgut where the gametocytes mature into gametes, merge to form a zygote, and then develop into an ookinete.
Ookinetes invade the mosquito stomach wall and develop into oocysts, which rupture and release sporozoites.
Sporozoites migrate to the mosquito salivary gland and may infect another human during the mosquito’s next blood meal.
Of note, in P vivax and P ovale, dormant stages, called hypnozoites, may remain quiescent in the liver of the infected human for weeks to years from the initial infection, leading to onset of clinical symptoms or relapses of infections much later. Treatment specifically targeting these dormant stages is required to completely clear infections with P vivax and P ovale.
Malarial disease results from multiple complex parasite-host interactions during the asexual, blood stage of infection. Clinical manifestations of disease are related to parasite modification of the erythrocyte and parasite-induced inflammation.
Plasmodium pathogenesis can be divided into inflammation, anemia, and end-organ damage. Inflammation is caused by the downstream effects of parasite metabolism and erythrocyte rupture, and, in P falciparum, parasite sequestration. Splenic macrophages and monocytes release large amounts of proinflammatory cytokines in response to phagocytosis of hemozoin, a toxic metabolite from the parasite digestion of heme, and other erythrocyte remnants. Proinflammatory cytokines in turn give rise to (1) the systemic inflammatory response syndrome, (2) edema and inflammation in perivascular tissues in end organs due to disruption of endothelial basal lamina and extravasation,1 and (3) increased expression of adhesion molecules and increased sequestration of parasitized erythrocytes.
The anemia caused by Plasmodium infection is multifactorial. Asexual reproduction in infected erythrocytes leads directly to hemolysis. Moreover, intraerythrocytic parasites decrease erythrocyte deformability, leading to increased hemolysis and splenic clearance, compounded by splenic sequestration in P falciparum infection. Hematopoiesis, which would normally compensate for hemolysis, is suppressed by tumor necrosis factor-alpha released during infection.
End organ damage due to P falciparum infection is mediated by cytoadherence of infected erythrocytes, also referred to as sequestration. Intraerythrocytic parasites produce proteins that are expressed on the surface of infected erythrocytes and lead to binding to a variety of cell types. Binding of parasitized erythrocytes in the microvasculature along with uninfected erythrocytes, inflammatory cells, and platelets leads to partial blood flow obstruction, breakdown of the endothelium, and inflammation that causes end organ damage. Sequestered erythrocytes can be found in any organ. Sequestration in the brain leads to the clinical syndrome of cerebral malaria described in later discussion. Sequestration in the placenta leads to the adverse birth outcomes associated with malaria during pregnancy. Sequestration also removes parasites from the circulation, preventing splenic clearance during one phase of parasite replication and permitting on-going infection.
EPIDEMIOLOGY
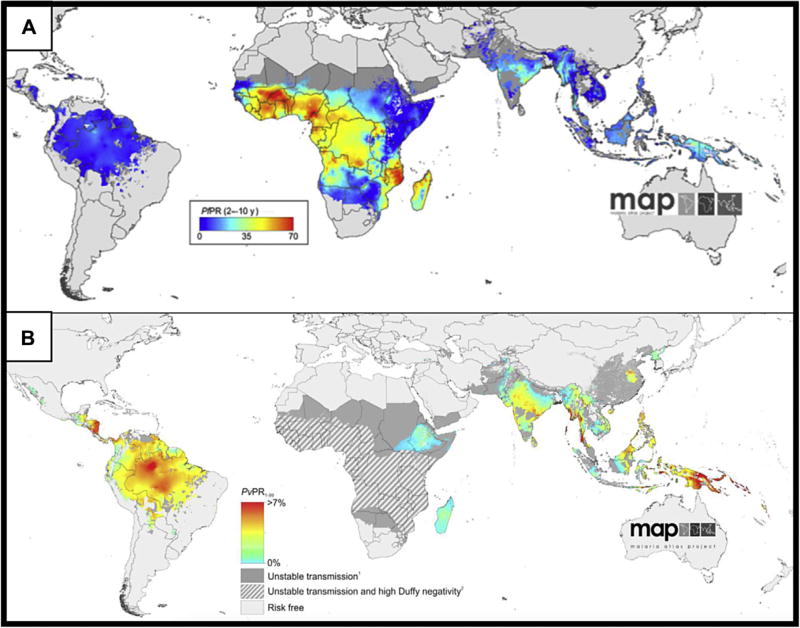
Although rarely encountered in the United States, malaria causes approximately 45% of the world’s population to be at risk of infection.2 P falciparum and P vivax are the most common causes of human malaria and have distinct geographic distributions, as in Fig. 1. P malariae is found in a similar distribution as P falciparum; P ovale is primarily found in West Africa, but cases have been reported in other sub-Saharan African countries. The limited cases of P knowlesi, a primarily nonhuman primate parasite, are reported in Southeast Asia.
Fig. 1.
Spatial distribution of P falciparum (A) and P vivax (B) endemicity in 2010. Prevalence rates are presented in different populations and different scales based on species. The P falciparum map (A) shows the prevalence rate in 2 to 10 year olds (PfPR) and ranges from 0% to 70%; see color scale on map. The P vivax map (B) shows the prevalence rate in 1 to 99 year olds (PvPR) and ranges from 0 to greater than 7%. Shaded areas have unstable transmission (<0.1%), and hatched areas have greater than 90% prevalence of Duffy antigen negativity. Duffy antigen is required for the invasion of P vivax into the erythrocyte and is absent in some African populations. These maps are open source and made available by the Malaria Atlas Project (http://www.map.ox.ac.uk/map/) under the Creative Commons Attribution 3.0 Unported License. (Reproduced from [A] Gething PW, Patil AP, Smith DL, et al. A new world malaria map: plasmodium falciparum endemicity in 2010. Malar J 2011;10:378; and [B] Gething PW, Elyazar IRF, Moyes CL, et al. A long neglected world malaria map: plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 2012;6(9):e1814.)
Worldwide over the last 15 years, there has been a 60% decrease in the malaria death rate due to increased availability of preventive measures, such as bed nets, and effective new diagnostics and treatments. Since 2007 when the World Health Organization (WHO) endorsed a global commitment to eradicate malaria, 5 countries have been declared malaria free (United Arab Emirates, Morocco, Turkmenistan, Armenia, and Sri Lanka), and 26 more are poised for elimination by 2020.3 Despite this progress, in 2015, it is estimated that there were still 214 million new cases of malaria and 438,000 deaths. The vast majority of morbidity and mortality occur in sub-Saharan Africa, where the heaviest burden of disease is shouldered by children less than 5 years of age. Further progress is threatened by the spread of drug and insecticide resistance, the need for new tools for malaria control in areas that have not reduced transmission with current interventions, and the continued demand for a global financial commitment to the goal of eradication.
In the United States, there has been a consistent increase the number of cases of malaria reported to the US Centers for Disease Control and Prevention (CDC) since 1973. In 2013, 1727 cases were reported. All infections in which origin was determined (99.6%) were acquired abroad. Most occurred in US residents, and 17% occurred in children (age <18 years). Severe malaria, infection associated with end organ damage, was more common in children less than 5 year old compared with older children and adults. However, none of the 10 deaths caused by malaria in the United States were among children.4
SUSCEPTIBILITY TO INFECTION
In nonendemic and low transmission areas, all individuals are at risk of infection. In highly endemic settings, primarily some areas in sub-Saharan Africa, multiple malaria infections lead to the development of partial immunity. As children in these areas have repeated exposure to malaria infection, they become less likely to experience clinical disease. By adulthood, individuals in high transmission settings may still become infected, but the parasite load is lower and there is very low risk of clinical disease. Sterilizing immunity, complete prevention of infection, does not occur. Moreover, the duration of acquired immunity is not life-long: if individuals from endemic areas are no longer exposed to infection for as little as a year, they are at risk of disease upon repeat exposure. Many cases of malaria in the United States occur when individuals from endemic countries return home to visit friends and relatives. These individuals may not realize that their immunity has decayed, making them again susceptible to high-density infection and disease.
Hemoglobinopathies alter susceptibility to malaria infection and disease. Sickle cell trait (HbAS) is estimated to afford 90% protection from severe disease, 75% protection from hospitalization with malaria, but no protection from asymptomatic infection.5,6 This protection contributes to the persistence of HbS in African populations given the decreased life expectancy of homozygotes. Protection is also seen with hemoglobin C and alpha-thalassemia and beta-thalassemia. However, it is important to recognize that individuals with any of these hemoglobinopathies can still get malaria and have severe manifestations.
HISTORY AND PHYSICAL
During medical evaluation of patients with fever, taking a travel history and considering malaria are critical. Malaria should be suspected in any case of documented or history of fever and residence in or travel to malaria-endemic areas. Key malaria-related questions to ask on history include the following:
Has the patient traveled to a malaria-endemic area? Which species are present in that region? The geographic region determines the possibility of malaria infection and the most likely species, risk of severe disease, and treatment choice based on geographic patterns of antimalarial drug resistance. See the CDC Travelers’ Health Web site (http://wwwnc.cdc.gov/travel) for details on malaria epidemiology by country.
When did exposure occur? The time from the bite of an infected mosquito until presentation with clinical illness of P faliciparum is typically 10 to 14 days but may be as short as 7 days and as long as 30 days. Presentation with clinical illness may be delayed in those with partial immunity or who were taking incomplete or ineffective prophylaxis. P malariae may persist at low levels for long periods of time, up to years. Because of their dormant stages, P vivax and P ovale may present months to years after initial infections. Three to 6 weeks after the initial infectious bite is the most common period for relapse of P vivax infection obtained in tropical areas, and most relapses have occurred by 6 months. Relapse may occur 6 to 12 months after P vivax infection obtained in subtropical or temperate climates.7
Has the patient used antimalarials in the last 1 to 2 months? Drugs recently used for treatment or prophylaxis should not be used for treatment of clinical illness. Among individuals living in or emigrating from endemic areas, it is important to know if they were treated for malaria in the last 1 to 2 months, what drug was used, and if treatment was completed. Among travelers, it is important to know if they were taking malaria prophylaxis, what drug was used, and what their adherence was. Note that taking prophylaxis, even as recommended, does not definitively exclude malaria diagnosis.
Other groups in which Plasmodium infection should be considered in health care include asymptomatic immigrants (refugees, international adoptees, and others) from endemic areas. See the section on “Screening and Treatment of immigrants from malaria endemic areas” in later discussion.
The physical examination and initial laboratory evaluation should be used to determine the likelihood of malaria, evaluate other conditions on the differential, and determine the disease severity if malaria is likely (see Box 2 and the section, “Assessing severity”). General physical examination should be performed with specific attention to the following organ systems:
Ophthalmologic: Check for conjunctival pallor indicative of anemia. If seizures, altered consciousness, or other concern for cerebral malaria, consider dilated funduscopic examination by an ophthalmologist to evaluate for retinal hemorrhages, areas of retinal opacification, papilledema, cotton wool spots, or decolorization of retinal vessels.8
Pulmonary: Note tachypnea, which may be related to pulmonary complications (manifested by crepitation) or to metabolic acidosis (manifested by the characteristic acidotic breathing pattern).
Cardiac: Note tachycardia, which could be related to fever, increased cardiac output demand due to anemia, or shock. Assess capillary refill and extremity temperature variation.
Gastrointestinal: Palpate for splenomegaly.
Neurologic: Calculate Glasgow Coma Score if altered mental status and monitor for deterioration. Monitor for seizures. Assess for nuchal rigidity and photophobia, which would suggest meningitis rather than cerebral malaria.
Box 2. Manifestations of severe malaria adapted from World Health Organization and US Centers for Disease Control and Prevention.
|
DIFFERENTIAL DIAGNOSIS
Because the symptoms of malaria are nonspecific, the differential diagnosis is broad. Specific alternative diagnoses are listed in Box 1.
Box 1. Differential diagnoses of malaria.
| Sepsis due to bacteremia |
| Encephalitis (rickettsial or viral) |
| Meningitis (bacterial or viral) |
| Pneumonia (bacterial, viral or fungal) |
| Typhoid fever |
| Dengue fever |
| Chikungunya |
| Leptospirosis |
| Brucellosis |
| Rickettsial infections |
| Acute schistosomiasis (Katayama fever) |
| Amebic liver abscess |
| Acute HIV |
DIAGNOSIS
Malaria has the potential to be fatal. In cases where the index of suspicion is high, treatment can be started before testing results are available or even before they are performed, so that there is no delay in therapy. If presumptive treatment is initiated, diagnostic specimens should still be obtained.
Blood smear and detection by microscopy are considered the gold standard for laboratory confirmation of malaria. Thick and thin smears should be read. The CDC provides details on preparation and interpretation.9 Briefly, smears are stained with either Wright’s or ideally Giemsa stain. The thick smear is the most sensitive measure to detect low-density infection because it allows the microscopist to review a large volume of blood and is read for detection of infection. The thin smear, which allows greater resolution of the red blood cell morphology and the parasite, is used for determining the Plasmodium species and quantifying the specific parasite density. The later features are important for treatment and monitoring decisions. If the initial blood smears are negative but Plasmodium infection remains on the differential, 2 additional smears should be obtained at 12- to 24-hour intervals. Blood smears and trends in parasite quantification are also useful in following response to treatment (see later discussion).
Antigen-detecting rapid diagnostics tests (RDTs) are increasingly available for diagnosis of malaria in both resource-limited and nonendemic settings. The tests are generally cassette- or card-based lateral flow immunochromatographic assays that appear much like a pregnancy test. Labeled antibodies detect 1 of 3 Plasmodium antigens that may or may not be species specific depending on the test. Up-to-date information on the tests available, their mechanisms, and performance characteristics can be found on the WHO Foundation for Innovative New Diagnostics Web site.10
Only one RDT is approved for use in commercial and hospital laboratories in the United States. The BinaxNOW test (Alere Inc, Waltham, MA, USA) detects one antigen that is specific for P falciparum and another that is found in all human Plasmodium species. The sensitivity of detecting P falciparum and non-P falciparum infection in US or Canadian hospitals has ranged from 72% to 100% depending on the study.11–14 This RDT is specifically less sensitive for the detection of P malariae and low-density infections (<200 parasites per microliter). Sensitivity for detection of P knowlesi is low (29%).15 The current recommendations are that RDTs should be used in conjunction with blood smears. RDTs may significantly reduce the time required for preliminary diagnosis and, thus, are useful tools for initial diagnosis.14 Positive RDTs should be considered significant support of Plasmodium infection, but blood smears are required for confirmation, definitive species identification, and quantification. Negative RDTs should not eliminate consideration of malaria, especially if there has been recent treatment or infection is due to low-density or non-falciparum infections. Blood smears should be performed to exclude the diagnosis.
All cases of laboratory confirmed malaria should be reported to the state health department and to the CDC (www.cdc.gov/malaria/report.html).
ASSESSMENT OF SEVERITY
Clinical disease is classified as uncomplicated or severe malaria. Uncomplicated malaria is the presence of symptoms and/or signs of malaria and a positive parasitologic test in the absence of evidence of end organ damage. Fever, the quintessential symptom of malaria, is often greater than 40°C and associated with severe rigors and chills and profuse diaphoresis as fever resolves. Although rarely observed, fever is classically periodic with the interval between fevers determined by Plasmodium species causing the infection. Fever as well as other common initial symptoms, including malaise, fatigue, headache, cough, abdominal pain, anorexia, nausea, vomiting, diarrhea, myalgias, and back pain, is nonspecific, requiring a high index of suspicion of malaria in anyone with possible exposure. Signs that may be associated with uncomplicated malaria include mild jaundice due to hemolysis and splenic enlargement. Laboratory abnormalities may include mild anemia, thrombocytopenia, mild coagulopathy, increased blood urea nitrogen, and elevated creatinine not meeting criteria for acute kidney injury. Uncomplicated malaria may be caused by any of the Plasmodium species infecting humans.
Severe malaria is most often caused by cytoadherence of P falciparum–infected erythrocytes in capillary beds of a wide range of organs. The presence of one or more of the features listed in Box 2 and a positive malaria diagnostic test in the absence of an alternative cause are defined as severe malaria. These manifestations may occur alone or in combination. Specific clinical syndromes that can occur in endemic settings as well as in nonimmune travelers include but are not limited to severe anemia and cerebral malaria. Severe anemia can lead to metabolic acidosis, renal impairment, and noncardiogenic pulmonary edema. Cerebral malaria can present with seizures and/or decreased consciousness, including coma, and can lead to cerebral edema, increased intracranial pressure, herniation, and death.
Laboratory tests and potential findings to use in valuation of potential complications include the following:
Complete blood count: anemia, thrombocytopenia, leukopenia, or leukocytosis
Chemistry panel: acidosis, acute kidney injury, and hypoglycemia
Urinalysis: hemoglobinuria and proteinuria
Lumbar puncture (if altered consciousness or other indication): findings may be normal or have elevated opening pressure, mildly elevated protein, or mild pleocytosis
Blood cultures: concomitant bacteremia
Blood gas: metabolic acidosis with or without respiratory compensation
Type and cross-match
TREATMENT AND MANAGEMENT
Uncomplicated Malaria
In cases of malaria infection without signs of severe disease, oral treatment should be initiated as promptly as possible (Table 1). Hospitalization should be considered for individuals from nonendemic regions, young children, and those with high parasite density (>4%). Choice of antimalarial drug is determined by the species of Plasmodium causing the infection, the drug resistance patterns in the region where infection was likely acquired, and whether the patient has taken any other antimalaria drugs in the last 1 to 2 months for treatment or prevention. If the Plasmodium species causing the infection is not certain, then the infection should be treated as though it were P falciparum. In all areas, drugs that were recently used in the last 1 to 2 months for treatment or prophylaxis should not be used for treatment. Specific dosing recommendations and new updates can be found on the CDC Web site,16 (also listed in Key Resources).
Table 1.
General malaria pediatric treatment recommendations and additional information
| Syndrome and Species | Drug Resistance in the Region Acquired |
Drug Recommendations () = See Notes | Notes |
|---|---|---|---|
| Uncomplicated malaria, P falciparum or species unknown | Chloroquine resistant or resistance unknown |
|
|
| Chloroquine sensitive | Chloroquine phosphate or hydroxychloroquine |
|
|
|
| |||
| Uncomplicated malaria, P malariae or P knowlesi | All regions | Chloroquine phosphate or hydroxychloroquine | |
|
| |||
| Uncomplicated malaria, P ovale and P vivax (chloroquine sensitive) | All regions (except where P vivax chloroquine resistance is common, see below) | Chloroquine phosphate plus primaquine or hydroxychloroquine plus primaquine |
|
|
| |||
| Uncomplicated malaria, P vivax (chloroquine resistant) | Chloroquine resistant |
|
|
|
| |||
| Severe malaria | All regions | Quinidine gluconate plus doxycycline, tetracycline, or clindamycin Investigational new drug (contact CDC): artesunate followed by atovaquoneproguanil, doxycycline, or mefloquine |
|
| Investigational new drug (contact CDC): artesunate followed by atovaquoneproguanil, doxycycline, or mefloquine | |||
Adapted from CDC. Guidelines for the treatment of malaria in the US Centers for Disease Control and Prevention. Malaria diagnosis & treatment in the United States. Available at: https://www.cdc.gov/malaria/diagnosis_treatment/index.html. Accessed November 26, 2016.
If P vivax or P ovale is identified or suspected, primaquine should be administered to prevent relapse due to dormant liver stages. However, primaquine can cause hemolytic anemia and is contraindicated in G6PD-deficient persons. G6PD screening should be done before primaquine treatment. Alternate regimens many be considered in consultation with an infectious disease or tropical medicine expert for persons with borderline or true G6PD-deficiency.
During treatment, parasite density should be monitored daily until negative.17
Severe Malaria
When signs or symptoms of severe diseases are present, parenteral treatment should be initiated promptly because death can occur within hours of presentation. Artesunate or quinine/quinidine should be used for treatment. Given the level of monitoring and the frequency of clinical and laboratory assessment required to manage patients with severe malaria, admission to an intensive care unit is recommended. Initial evaluation is described above. Parasite quantification every 12 hours is recommended for the first 2 to 3 days to document response to treatment and to a decrease in parasite quantification. If there is not significant decrease in parasite density by 48 to 72 hours, consider expert consultation.
Supportive care measures, such as fluid resuscitation, cardiac and respiratory monitoring, oxygen, and supportive ventilation, should be provided as clinically indicated. In addition to antimalarial treatment, management of complications may require anticonvulsants, antibiotics, antipyretics, and blood transfusions. Exchange transfusions are no longer recommended.18,19 Clinical assessments should be repeated every 2 to 4 hours. If there is a decline in mental status after initiation of treatment, clinicians should consider new onset of seizures, hypoglycemia, or worsening anemia. Hypoglycemia is a common complication and may mimic cerebral malaria. Laboratory evaluation, including hemoglobin/hematocrit, glucose, and lactate, should be repeated every 6 hours, and supportive care should be administered based on these results.
Quinidine and artesunate are available in the United States for treatment of severe malaria. Treatment should be initiated with intravenous quinidine if it is available. Quinidine can cause arrhythmias, so intravenous administration of the drug requires close cardiac monitoring.20 Hypotension is common and should be treated with volume expansion. Baseline electrocardiogram should be performed, and changes in the width of the QRS and length of the QTc should be monitored hourly. The infusion should be held if the QTc prolongs by more than 50% of the baseline length, but may be restarted when the QTc is no more than 25% longer than its original length. Additional side effects and toxicities include hypoglycemia, tinnitus, reversible hearing loss, dizziness, vision changes, nausea, and vomiting.
Artesunate is available as an investigational new drug through the CDC. Criteria for access to artesunate are confirmed cases of malaria requiring parenteral therapy because of severe malaria, parasitemia greater than 5%, or inability to tolerate oral therapy. In addition, one of the following must be true: artesunate is more readily available than quinidine; quinidine is contraindicated; or there was intolerance to or failure of quinidine.21 A retrospective case series of participants in this experimental protocol showed that artesunate is safe and clinically beneficial.22 Clinical trials in endemic settings and a systematic review show significantly lower rates of mortality using artesunate compared with quinine.23–25 The CDC Malaria Hotline (contact information in “When to Refer” section) should be called for access to and use of artesunate.
After parenteral therapy for at least 24 hours, parasite density is less than 1%, and the patient can tolerate oral medications, treatment may be transitioned to an oral regimen (see Table 1). Complete courses of atovaquone-proguanil or lumefantrine-artemether may be used and may be preferred given they are better tolerated than oral quinine.
WHEN TO REFER
All suspected malaria cases should be seen in consultation with an infectious disease expert. Cases with any manifestations of severe disease may require management in an intensive care setting. The CDC staffs a malaria hotline and can provide additional consultation (Monday-Friday 9 AM to 5 PM EST, call either (770) 488–7788 or (855) 856–4713; after hours, weekends, and holidays call (770) 488–7100 and ask for the malaria clinician on call).
SCREENING AND TREATMENT OF IMMIGRANTS FROM MALARIA-ENDEMIC AREAS
In medical evaluations of asymptomatic immigrants (refugees, international adoptees, and others) from endemic areas, any history of malaria infection and treatment should be noted and signs of chronic infection evaluated, such as anemia and splenomegaly. If these signs of chronic infection are detected, then malaria diagnostic tests should be performed and any detected infections should be treated with oral medication, as described above.
In the absence of evidence of acute or chronic infection, the CDC provides guidelines for the treatment of these populations, which are summarized here.26 More details may be found on the Web site listed in Box 3.
Box 3. Key guidelines and sources for malaria diagnosis and treatment.
| World Health Organization |
| Diagnosis and treatment guidelines: http://www.who.int/malaria/areas/treatment/en/ |
| US Centers for Disease Control and Prevention |
| General information: https://www.cdc.gov/malaria/ |
| Diagnosis and treatment guidelines: https://www.cdc.gov/malaria/diagnosis_treatment/index.html |
| Prophylaxis: http://wwwnc.cdc.gov/travel/yellowbook/2016/table-of-contents |
| Immigrant and refugee health: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html |
Refugees from malaria-endemic areas in sub-Saharan Africa without evidence of acute or chronic infection: Refugees should have received presumptive malaria treatment before departure for the United States. If documentation of predeparture treatment is not available, then postarrival presumptive treatment should be considered. Children weighing less than 5 kg and pregnant women may have been screened for infection and only treated if positive. Presumptive treatment or screening and treatment are not recommended for refugees from other areas.
International adoptees without evidence of acute or chronic infection: In contrast to refugees, guidelines for international adoptees do not recommend presumptive treatment or screening. However, a recent study of asymptomatic Plasmodium infection in international adoptees from Ethiopia found 14% had infection, leading the investigators to suggest that screening with polymerase chain reaction be recommended.27
PROPHYLAXIS
Prophylaxis against malaria is recommended for all travelers to malaria-endemic areas. Specific recommendations are updated every 2 years and can be found in the CDC Yellow Book (see link in Box 3). The 5 drugs currently available in the United States for malaria prophylaxis are listed in Table 2. Choice of drug is determined primarily by the species distribution and drug-resistance patterns in the destination and patient characteristics.
Table 2.
Drugs for malaria prophylaxis currently available in the United States
| Pros | Cons | Regimen | |
|---|---|---|---|
| Atovaquone-proguanil |
|
|
Pretravel: 1–2 d During travel: Daily Posttravel: 7 d |
|
| |||
| Doxycycline |
|
|
Pretravel: 1–2 d During travel: Daily Posttravel: 4 wk |
|
| |||
| Mefloquine |
|
|
Pretravel: ≥2 wk During travel: Weekly Posttravel: 4 wk |
|
| |||
| Chloroquine |
|
|
Pretravel: 1–2 wk During travel: Weekly Posttravel: 4 wk |
|
| |||
| Primaquine |
|
|
Primary prophylaxis Pretravel: 1–2 d During travel: Daily Posttravel: 7 d |
| Terminal prophylaxis following use of other regimen for primary prevention Posttravel: 14 d | |||
SUMMARY
Globally, there has been a significant decrease in Plasmodium infection and reduction of malaria-related morbidity and mortality in the last 15 years.
The number of cases of malaria imported to the United States continues to increase likely due to increased global travel.
Prompt provision of effective treatment is critical to limiting the complications of malaria.
Understanding Plasmodium species variation and the epidemiology and drug resistance patterns in the geographic area where infection was acquired is important for determining the most appropriate treatment regimens.
Easy-to-use and up-to-date guidelines for the treatment and management of malaria in endemic areas and in the United States are accessible online from key reference organizations.
KEY POINTS.
Malaria is a significant cause of morbidity and mortality in endemic areas.
Travelers to endemic areas are at risk of malaria.
Identifying patients who may have malaria and providing prompt evaluation and treatment are critical to limit disease and its complications.
Malaria has the potential to be fatal. In cases where the index of suspicion is high, treatment can be started before testing results are available, so that there is no delay in therapy. If presumptive treatment is initiated, diagnostic specimens should still be obtained.
Updated guidelines are available through the US Centers for Disease Control and Prevention and should be consulted whenever a physician is treating patients with suspected or confirmed malaria.
Footnotes
Disclosures: Neither author has any commercial or financial conflicts of interest.
References
- 1.Prato M, Giribaldi G, Polimeni M, et al. Phagocytosis of hemozoin enhances matrix metalloproteinase-9 activity and TNF-alpha production in human monocytes: role of matrix metalloproteinases in the pathogenesis of falciparum malaria. J Immunol. 2005;175(10):6436–42. doi: 10.4049/jimmunol.175.10.6436. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [Accessed November 25, 2016];World malaria report 2015. 2016 Available at: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/
- 3.Newby G, Bennett A, Larson E, et al. The path to eradication: a progress report on the malaria-eliminating countries. Lancet. 2016;387(10029):1775–84. doi: 10.1016/S0140-6736(16)00230-0. [DOI] [PubMed] [Google Scholar]
- 4.Cullen KA, Mace KE, Arguin PM. Malaria surveillance—United States, 2013. MMWR Surveill Summ. 2016;65(2):1–22. doi: 10.15585/mmwr.ss6502a1. [DOI] [PubMed] [Google Scholar]
- 5.Hill AV, Allsopp CE, Kwiatkowski D, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 6.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192(1):178–86. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewallen S, Bronzan RN, Beare NA, et al. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008;102(11):1089–94. doi: 10.1016/j.trstmh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Centers for Disease Control and Prevention. [Accessed November 25, 2016];DPDx - malaria - diagnostic findings. Available at: http://www.cdc.gov/dpdx/malaria/dx.html.
- 10.World Health Organization. [Accessed November 25, 2016];WHO-FIND malaria RDT evaluation programme. Available at: http://www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/rdt-evaluation-programme/en/
- 11.Farcas GA, Zhong KJY, Lovegrove FE, et al. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am J Trop Med Hyg. 2003;69(6):589–92. [PubMed] [Google Scholar]
- 12.DiMaio MA, Pereira IT, George TI, et al. Performance of BinaxNOW for diagnosis of malaria in a U.S. hospital. J Clin Microbiol. 2012;50(9):2877–80. doi: 10.1128/JCM.01013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobenchik A, Shimizu-Cohen R, Humphries RM. Use of rapid diagnostic tests for diagnosis of malaria in the United States. J Clin Microbiol. 2013;51(1):379. doi: 10.1128/JCM.02509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ota-Sullivan K, Blecker-Shelly DL. Use of the rapid BinaxNOW malaria test in a 24-hour laboratory associated with accurate detection and decreased malaria testing turnaround times in a pediatric setting where malaria is not endemic. J Clin Microbiol. 2013;51(5):1567–9. doi: 10.1128/JCM.00293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster D, Cox-Singh J, Mohamad DSA, et al. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar J. 2014;13:60. doi: 10.1186/1475-2875-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Centers for Disease Control and Prevention. [Accessed November 26, 2016];Malaria diagnosis & treatment in the United States. Available at: https://www.cdc.gov/malaria/diagnosis_treatment/index.html.
- 17.Griffith KS, Lewis LS, Mali S, et al. Treatment of malaria in the United States. JAMA. 2007;297(20):2264. doi: 10.1001/jama.297.20.2264. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Centers for Disease Control and Prevention. [Accessed November 29, 2016];Malaria - exchange transfusion for treatment of severe malaria no longer recommended. Available at: http://www.cdc.gov/malaria/new_info/2013/exchange_transfusion.html.
- 19.Tan KR, Wiegand RE, Arguin PM. Exchange transfusion for severe malaria: evidence base and literature review. Clin Infect Dis. 2013;57(7):923–8. doi: 10.1093/cid/cit429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–58. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Centers for Disease Control and Prevention. [Accessed November 26, 2016];New medication for severe malaria available under an investigational new drug protocol. Available at: http://www.cdc.gov/mmWR/preview/mmwrhtml/mm5630a5.htm.
- 22.Twomey PS, Smith BL, McDermott C, et al. Intravenous artesunate for the treatment of severe and complicated malaria in the United States: clinical use under an investigational new drug protocol. Ann Intern Med. 2015;163(7):498. doi: 10.7326/M15-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dondorp AM, Fanello CI, Hendriksen ICE, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an openlabel, randomised trial. Lancet. 2010;376(9753):1647–57. doi: 10.1016/S0140-6736(10)61924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dondorp A, Nosten F, Stepniewska K, et al. South East Asian QUININE Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717–25. doi: 10.1016/S0140-6736(05)67176-0. [DOI] [PubMed] [Google Scholar]
- 25.Sinclair D, Donegan S, Isba R, et al. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev. 2012;6:CD005967. doi: 10.1002/14651858.CD005967.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Centers for Disease Control and Prevention. [Accessed November 25, 2016];Domestic malaria guidelines immigrant and refugee health. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/malaria-guidelines-domestic.html.
- 27.Adebo SM, Eckerle JK, Andrews ME, et al. Asymptomatic malaria and other infections in children adopted from Ethiopia, United States, 2006–2011. Emerg Infect Dis. 2015;21(7):1227–9. doi: 10.3201/eid2107.141933. [DOI] [PMC free article] [PubMed] [Google Scholar]