Abstract
In October 2016, the Advisory Committee on Immunization Practices (ACIP) updated the human papillomavirus (HPV) vaccination recommendation to include a 2-dose schedule for U.S. adolescents initiating the vaccine series before their 15th birthday. We analyzed records for >4 million persons aged 9–17 years receiving any HPV vaccine by the end of each quarter during January 1, 2014–September 30, 2016 from six Immunization Information Systems Sentinel Sites, and reclassified HPV vaccination up-to-date coverage according to the updated recommendations. Compared with HPV vaccination up-to-date coverage by the 3-dose schedule only, including criteria for either a 2-dose or 3-dose schedule increased up-to-date coverage in 11–12, 13–14, and 15–17 year-olds by 4.5–8.5 percentage points. The difference between 3-dose up-to-date coverage and 2- or 3-dose up-to-date coverage was greatest in late 2016. These data provide baseline HPV vaccination coverage using current ACIP recommendations.
1. Introduction
In the United States, the Advisory Committee on Immunization Practices (ACIP) has recommended human papillomavirus (HPV) vaccination for women since 2006 and for men since 2011 [1, 2]. HPV Vaccine is recommended for routine vaccination at age 11–12 years, and can be given starting at age 9 years. Vaccination is also recommended for women through age 26 years, and for men through age 21 years. HPV vaccines were first recommended as a 3-dose schedule, with the second dose 1–2 months after the first dose and the third dose 6 months after the first dose. In October 2016, ACIP recommended a 2-dose schedule for persons initiating the HPV vaccine series before their 15th birthday; this recommendation was adopted by CDC and formalized in December 2016 [3]. In the 2-dose schedule, the second dose is recommended 6–12 months after the first dose1. Persons initiating the series on or after their 15th birthday and those with certain immunocompromising conditions should follow the 3-dose schedule.
Almost all HPV vaccines are delivered through primary care providers in the United States, in clinic-based settings [4]. Routinely recommended vaccines are available at no cost to vaccinees through public or private sectors [5]. National HPV vaccination coverage among adolescents has been increasing, with 34.9% of adolescents aged 13–17 years receiving ≥3 doses in 2015, but still lags behind coverage for other adolescent vaccines [6]. With the revised ACIP recommendations, HPV vaccination up-to-date (UTD) coverage in the United States will be determined by including those who have completed a 2-dose schedule at the appropriate interval and initiation age. Our objective was to assess HPV vaccination UTD coverage according to either a 2-dose or 3-dose schedule among persons aged 9–17 years during each quarter from January 2014 through September 2016.
2. Methods
Immunization information systems (IIS) are confidential, population-based databases of immunization doses administered by participating vaccine providers [7]. The Centers for Disease Control and Prevention funded six IIS Sentinel Sites that had achieved high data quality standards, with ≥85% of persons aged <19 years and ≥85% of provider sites participating in the IIS, to evaluate vaccination coverage: Michigan, Minnesota, New York City, North Dakota, Oregon (six counties), and Wisconsin, constituting approximately 10% of the pediatric population nationwide.
We analyzed records for >4 million persons aged 9–17 years receiving any HPV vaccine by the end of each quarter during January 1, 2014–September 30, 2016. For each quarter, persons were included if they were in any of the following age groups throughout the quarter: 9–10, 11–12, 13–14, 15–17, or 13–17 years (period of time assessment method, without aging in or aging out [8]). Persons known to be deceased or not residing in the jurisdiction were excluded. For age groups including 15–17-year-olds, the analytic period was quarter 3, 2015–quarter 3, 2016 due to birth cohort limitations; persons aged 15–17 years in quarters prior to quarter 3, 2015 may be ≥19 years old as of September 30, 2016 and their immunization records are not available in the dataset.
We considered an HPV vaccine dose valid if it met criteria for ACIP’s recommended age and interval for a 3-dose or 2-dose schedule [3]. We allowed a 4-day grace period for these criteria. We defined UTD status in two ways: (1) all persons aged ≥9 years who received ≥3 valid doses of HPV vaccine per ACIP’s 3-dose schedule; or (2) all persons aged ≥9 years who received ≥2 valid doses of HPV vaccine per ACIP’s 2-dose schedule [3]. The U.S. Census estimates for total population, age group, and gender for each Sentinel Site were used as denominators. We used SAS® 9.4 (SAS Institute, Cary, NC) and Microsoft® Excel® 2010 (Microsoft, Redmond, WA) for all analyses.
3. Results
We found 1,438,161 persons aged 9–17 years (44.3% of corresponding Census population) from the six Sentinel Sites who received at least one HPV vaccination. Among them, 26.0% received only 1 dose, 20.9% received exactly 2 doses, and 53.1% received at least 3 doses of HPV vaccine. Overall, 18.7% of these 1,438,161 persons initiated the HPV vaccine series before age 15 and received exactly 2 doses. Among them, 61.4% received the doses ≥5 months apart, and the other 38.6% received them <5 months apart.
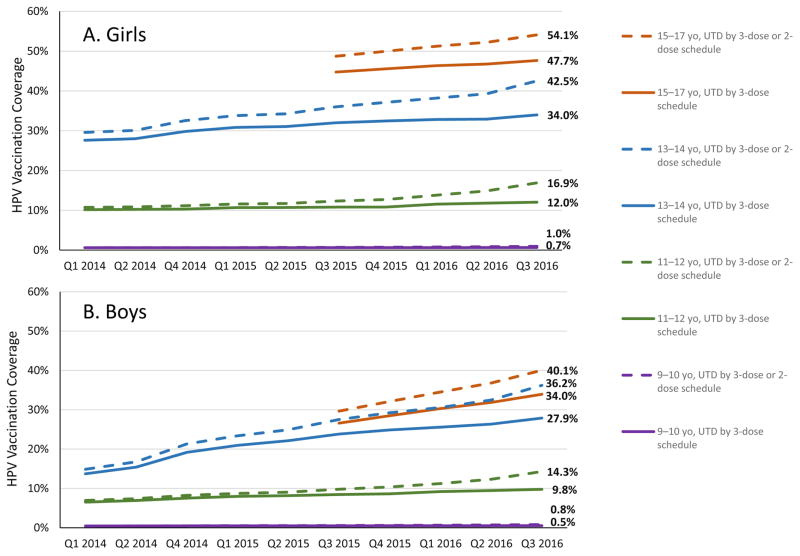
We reclassified HPV vaccination UTD coverage by including a 2-dose schedule (Figure 1). Compared with HPV vaccination UTD coverage according to a 3-dose schedule alone, coverage using 2-dose or 3-dose schedule criteria increased among persons of both genders in all age groups. The difference in UTD coverage increased over the time period of our analysis. The largest difference between UTD coverage using these two sets of criteria occurred in the most recent quarter (quarter 3, 2016) across all age groups, with percentage point increases among girls and boys, respectively, of 0.3 and 0.3 among 9–10-year-olds; 4.9 and 4.5 among 11–12-year-olds; 8.5 and 8.3 among 13–14-year-olds; and 6.4 and 6.1 among 15–17-year-olds. In adolescents aged 13–17 years, the percentage point increase in HPV vaccination UTD coverage from including both schedules was higher in quarter 3, 2016 (7.2; 37.0% vs. 44.2%), compared with quarter 3, 2015 (3.7; 32.6% vs. 36.3%).
Figure 1.
HPV vaccination up-to-date coverage according to 3-dose and 3-dose/2-dose schedules, by age group and gender — six Immunization Information Systems Sentinel Sites, United States, Quarter 1, 2014 – Quarter 3, 2016
yo, year-olds; UTD, up-to-date
4. Discussion
This analysis established baseline HPV vaccination UTD coverage under current ACIP recommendations that can be used for future monitoring. It also assessed how HPV vaccination UTD coverage increased solely due to reclassification based on the revised recommendation. We found that by using 3-dose or 2-dose schedule criteria, the UTD coverage increased 7.2 percentage points among 13–17-year-olds in the most recent quarter, versus using only a 3-dose schedule criterion. We also found that the difference in UTD coverage by including the 2-dose schedule criterion increased over the time period of our analysis. Reasons for this increase are unclear. If this finding reflects intentional delay of the second dose rather than late administration of the second dose in a 3-dose schedule, it may indicate awareness of the 2-dose schedule, which was recommended by the World Health Organization in 2014 [9], and publicly discussed at ACIP meetings throughout 2016. Of note, among persons initiating HPV vaccine series before age 15 years receiving exactly two doses, more than a third received these doses <5 months apart, and therefore would need a third dose to complete the recommended series.
Our results, particularly those from the third quarter of 2015, are consistent with a previous analysis of data from the 2013 National Immunization Survey-Teen (NIS-Teen) [10]. That analysis, conducted using slightly different criteria (6-month interval between doses), found that if 2-dose vaccinees aged 13–17 years who received the second dose ≥6 months after the first dose were reclassified as having completed the HPV vaccination series, overall HPV vaccination UTD coverage in the United States would increase 3.9 percentage points.
In 2015, as measured by the NIS-Teen, HPV vaccination coverage (≥3 doses) among U.S. adolescents aged 13–17 years (girls: 41.9%; boys: 28.1%) was below the Healthy People 2020 goals (80% for both boys and girls aged 13–15 years) [6, 11]. HPV vaccination coverage in our analysis was consistent with these national estimates. ACIP recommends a 2-dose schedule for persons starting the series before age 15 years based on immunologic data demonstrating that the 2-dose schedule in this age group is non-inferior to the 3-dose schedule. Although a 2-dose schedule decreases the total number of doses needed to complete the HPV vaccination series, it is not known whether the new recommendations will result in an increase in series initiation, since reasons reported by parents for not vaccinating their children with HPV vaccine have highlighted lack of knowledge, beliefs that vaccine is unnecessary, safety concerns, and lack of recommendation from a healthcare provider [12], rather than number of doses in the series. Future evaluations are warranted to assess impact of the 2-dose schedule recommendation on HPV vaccination coverage.
Strengths of this analysis include good quality and reliability of provider-submitted IIS data. Limitations include the following: first, data from six Sentinel Sites do not necessarily represent the entire U.S. population, although our population-based data contain records for >4 million persons and our HPV vaccination coverage (≥3 valid doses) was within the 2015 NIS-Teen confidence intervals for both girls and boys; second, potentially incomplete vaccination histories in IIS data might underestimate vaccination coverage in the current analysis. However, the underestimation on HPV vaccination coverage is likely small due to high participation of children aged <19 years (≥85%) in the IIS Sentinel Sites, and the consistent HPV vaccination coverage estimates between IIS sentinel site and NIS-Teen data sources; the impact of any such data incompleteness on relative UTD coverage changes according to two sets of criteria is likely even smaller.
HPV vaccination coverage reclassification by applying the 3-dose/2-dose schedule criteria will result in modest increases in UTD coverage in the United States. Coverage remains below that of Healthy People 2020 goals and ongoing collaborative efforts involving state, local, and federal government resources, public health and vaccine programs, healthcare providers, parents, and patients are critical to sustain improvements in national HPV vaccination coverage and to protect U.S. adolescents against vaccine-preventable HPV-associated diseases and cancers.
Acknowledgments
Funding
The sentinel site project is funded by the Centers for Disease Control and Prevention.
We thank the following representatives from the IIS Sentinel Sites for assistance in reviewing study proposals, revising the manuscript, and providing vaccination records: Rachel Potter, DVM, MS2 ; Cristi Bramer, MPH2; Miriam Muscoplat, MPH3 ; Sydney Kuramoto, MPH3; Vikki Papadouka, PhD, MPH4 ; Alexandra Ternier, MPH4; Dominick Ftizsimmons5 ; Mary Woinarowicz, MA5; Andrew Osborn, MBA6 ; Aaron Dunn, MPH6; and Matthew J. Verdon7, Stephanie Schauer, PhD7.
Footnotes
Minimum interval between the first and second doses is 5 months.
Michigan Department of Health and Human Services
Minnesota Department of Health
New York City Department of Health and Mental Hygiene
North Dakota Department of Health
Oregon Health Authority
Wisconsin Department of Health Services
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The following are author contributions: X.L. wrote the article; L.Z. and X.L. did data analysis; all authors contributed to study design, data interpretation, and article editing, and approved of the final manuscript. All authors meet authorship criteria.
Conflict of Interest
All authors have disclosed no financial relationships relevant to this article.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 2.Petrosky E, Bocchini JA, Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 4.Smith PJ, Stokley S, Bednarczyk RA, Orenstein WA, Omer SB. HPV vaccination coverage of teen girls: the influence of health care providers. Vaccine. 2016;34:1604–10. doi: 10.1016/j.vaccine.2016.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. [Accessed August 2017];The Vaccines for Children (VFC) Program. https://www.cdc.gov/features/vfcprogram.
- 6.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Curtis CR, MacNeil J, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:850–8. doi: 10.15585/mmwr.mm6533a4. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Progress in immunization information systems - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:1005–8. [PMC free article] [PubMed] [Google Scholar]
- 8.American Immunization Registry Association. [Accessed August 2017];Analytic guide for assessing vaccination coverage using an IIS. 2015 http://www.immregistries.org/resources/other-aira-resources/Analytic_Guide_for_Assessing_Vaccination_Coverage_Using_an_IIS.pdf.
- 9.Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89:465–91. [PubMed] [Google Scholar]
- 10.Cloessner EA, Stokley S, Yankey D, Markowitz LE. Timing of HPV vaccine intervals among United States teens with consideration to the current ACIP schedule and the WHO 2-dose schedule. Hum Vaccin Immunother. 2016;12:1375–80. doi: 10.1080/21645515.2015.1110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healthy People 2020 Goals. [Accessed August 2017];Increase the vaccination coverage level of 3 doses of human papillomavirus (HPV) vaccine for females by age 13 to 15 years. https://www.healthypeople.gov/2020/topics-objectives/objective/iid-114.
- 12.Stokley S, Jeyarajah J, Yankey D, Cano M, Gee J, Roark J, et al. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014--United States. MMWR Morb Mortal Wkly Rep. 2014;63:620–4. [PMC free article] [PubMed] [Google Scholar]