Supplemental Digital Content is available in the text.
Key Words: synovial sarcoma, NY-ESO-1, LV305, lentiviral Vector, ZVex, immunotherapy
Abstract
Effective induction of antitumor T cells is a pivotal goal of cancer immunotherapy. To this end, lentiviral vectors (LV) are uniquely poised to directly prime CD8 T-cell responses via transduction of dendritic cells in vivo and have shown promise as active cancer therapeutics in preclinical tumor models. However, until now, significant barriers related to production and regulation have prevented their widespread use in the clinic. We developed LV305, a dendritic cell-targeting, integration-deficient, replication incompetent LV from the ZVex platform, encoding the full-length cancer-testis antigen NY-ESO-1. LV305 is currently being evaluated in phase 1 and 2 trials in metastatic recurrent cancer patients with NY-ESO-1 positive solid tumors as a single agent and in combination with anti-PD-L1. Here we report on the first patient treated with LV305, a young woman with metastatic, recurrent, therapy-refractive NY-ESO-1+ synovial sarcoma. The patient developed a robust NY-ESO-1-specific CD4+ and CD8+ T-cell response after 3 intradermal injections with LV305, and subsequently over 85% disease regression that is continuing for >2.5 years posttherapy. No adverse events >grade 2 occurred. This case demonstrates that LV305 can be safely administered and has the potential to induce a significant clinical benefit and immunologic response in a patient with advanced stage cancer.
CASE REPORT
The patient is now a 44-year-old woman with synovial sarcoma (SS) originally diagnosed with a tumor originating in the lower lobe of her lung in 2004 when she was 32. She underwent a wedge resection and received 6 cycles of adjuvant doxorubicin and ifosfamide with mesna. In 2007, she developed a regional recurrence and underwent a thoracotomy. She had a mediastinal recurrence in 2009 and received 4 additional cycles of ifosfamide followed by another resection and irradiation with 6480 cGy in 36 fractions.
In 2012, she developed a multifocal recurrence throughout her left lung. She was started on trabectedin (Yondelis) but required dose reductions as a result of thrombocytopenia persisting from her prior ifosfamide treatment. She underwent a left-sided pneumonectomy in January 2013, but her disease quickly recurred again in the left thoracic cavity and in May she began pazopanib (Votrient) at 800 mg daily by mouth. Although this therapy temporarily stabilized her disease, it was stopped in December 2013 due to thrombocytopenia.
The patient had no significant past medical history other than her cancer and Eastern Cooperative Oncology Group performance status of 1. She was a nonsmoker, nondrinker who worked as a teacher. Her disease continued to progress during the months after discontinuation of pazopanib. In May 2014, she was found to have 99% NY-ESO-1 expression in her tumor based on Immunohistochemistry on tissue from her prior thoracotomy. She was enrolled on a phase I dose-escalation trial evaluating the LV305-based immunotherapy (NCT02122861).
Clinical Response After LV305 Therapy
The patient received 3 doses of 5×108 vector genomes (quantified by real time polymerase chain reaction) administered in 8 injections intradermally (2 to each deltoid and at quadriceps muscles) given on days 1, 21, and 42. The patient maintained an excellent quality of life while on study (in contrast to her going on disability while on cytotoxic systemic therapy). She had no grade 3 or higher adverse events. Low-grade toxicities potentially attributable to LV305 included pain and stinging at the injection site, mild fatigue the day after injections, an episode of subjective palpitations, subjective fevers and myalgias in the days after vaccination, each of which resolved within 24 hours. At the time of preparation of this manuscript, she had returned to work full time.
On her first posttreatment scan, 8 weeks after starting therapy and 2 weeks after her last injections, tumor growth had stabilized and by her second posttreatment scan 16 weeks after starting therapy, her tumor had shrunk by 10%. The size of her disease stayed stable until 5 months post-LV305 initiation, when it shrunk to 24.7% below baseline. By month 24 post-LV305, tumor mass had shrunk to 84.8% below baseline. At the time of this report the patient is now over 30 months post-LV305 initiation without evidence of recurrence (Figs. 1A–C).
FIGURE 1.
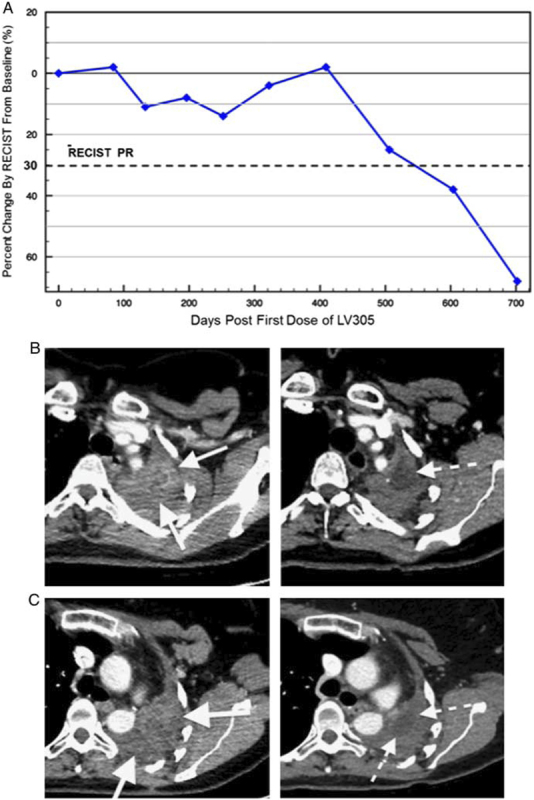
Clinical response to LV305 therapy: percentage change in tumor size. PR according to RECIST criteria (A). CT images before (left) and 2 years after (right) vaccination (B and C). PR indicates partial response.
Immunologic Response to Treatment with LV305
The patient was tested for HLA-A and found to express HLA-A*02:01. We performed flow cytometric analysis on 4 large aliquots (>5 million cells each) of both pretreatment and posttreatment leukapheresis samples stained for CD8 and tetramer for the NY-ESO-1 157-165 peptide. Before treatment, 0.065% of CD8+ cells stained with NY-ESO-1 tetramer, posttreatment this increased to 0.12% (P=0.012) (Fig. 2A). It is interesting to note that, PD-1 expression increased on the posttreatment tet+ cells suggesting activation (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/JIT/A476).
FIGURE 2.
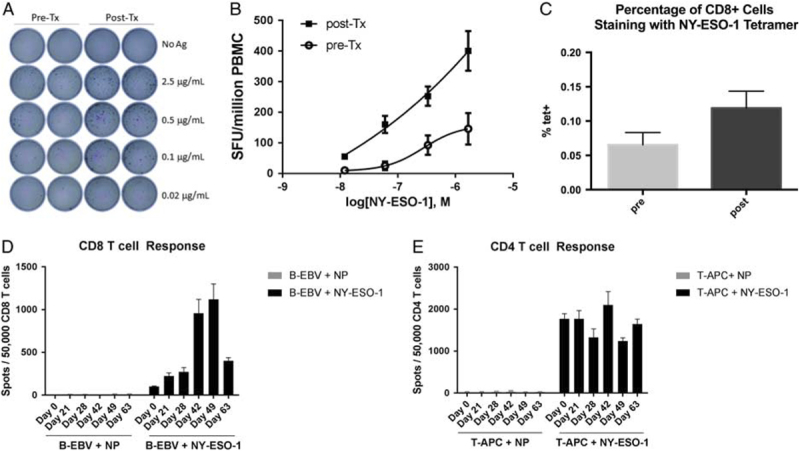
Preexisting CD8 T-cell response to NY-ESO-1 is enhanced by LV305 therapy. A, Tetramer staining of CD8 T cells recognizing NY-ESO-1 p157 peptide pre-LV305 and post-LV305 therapy. B, Ex vivo IFNγ ELISPOT of PBMC collected pre-LV305 and post-LV305 therapy using NY-ESO-1 overlapping peptide pool. C, IFNγ ELISPOT of PBMC collected pre-LV305 and post-LV305 using different concentrations of a NY-ESO-1 overlapping peptide pool. D, Time course of CD8 T-cell response by IFNγ ELISPOT after CD8 T-cell isolation and in vitro stimulation. E, Time course of CD4 T-cell response postvaccination after CD4 T-cell isolation and in vitro stimulation. APC indicates allophycocyanin; IFN, interferon; PBMC, peripheral blood mononuclear cell.
Ex vivo ELISPOT demonstrated a preexisting T-cell response to NY-ESO-1 in peripheral blood mononuclear cell (PBMC) from this patient, which was significantly enhanced in the posttreatment PBMC analysis. Treatment with NY-ESO-1 peptide pool (0.02, 0.1, 0.5, and 2.5 μg/mL) induced Interferonγ production in a concentration-dependent manner (Figs. 2B, C). Posttreatment stimulated PBMC produced higher number of spots and responded to lower concentration of the peptides, indicating higher avidity of the polyclonal T-cells population (pretreatment and posttreatment epitope mapping is shown in Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/JIT/A476). We also measured CD4+ and CD8+ T-cell responses separately by running ELISPOT using isolated CD4+ and CD8+ T cells and in both cases a response was observed, which was highly time dependent for the CD8+ cells (Figs. 2D, E).
T-cell receptor (TCR) sequencing was performed on a pretreatment tumor biopsy as well as on pretreatment and posttreatment leukapheresis samples (PBMC). TCR sequences found in tumor-infiltrating lymphocytes (TIL) increased 49% in posttreatment PBMC samples (2.51% overlap of TCR clones between TIL and PBMC), compared with pretreatment samples (1.68% overlap of TCR clones between TIL and PBMC) (Fig. 3A). At the same time, the overall TCR clonality increased by 61.1% from 0.11 to 0.18 We also evaluated the TCR repertoire at long-term follow-up (LTFU), ∼2 years after initial vaccination. The majority of the top 20 clones from pre-LV305 TILs expanded in post-LV305 PBMC as compared with pre-Tx PBMC (Fig. 3B). The top clones remained elevated in the LTFU sample. Both NY-ESO-1-specific CD4+ and CD8+ T cells identified using in vitro stimulation stimulation were detectable in the LTFU sample (Fig. 3C). Antibody responses measured by enzyme-linked immunosorbent assay showed that the patient had high titers (1/30,000) of preexisting anti-NY-ESO-1 antibodies which did not change during the course of the therapy (data not shown).
FIGURE 3.

Tumor-specific T-cell repertoire is enriched in post-Tx PBMC and persists long-term posttherapy. A, Frequency of identical TCR clones detected in pre-Tx PBMC and post-Tx PBMC. TCR sequences that were also detected in a FFPE tumor biopsy pretherapy are shown as red dots, other sequences are shows as blue dots. B, Frequency of top 20 TIL-TCR sequences in pre-LV305 and post-LV305 PBMC. C, NY-ESO-1-specific CD4 and CD8 T-cells responses measured by intracellular cytokine staining in LTFU PBMC. FFPE indicates Formalin-fixed, Paraffin-embedded; ICS, intracellular staining; LTFU, long-term follow-up; PBMC, peripheral blood mononuclear cell; TCR, T-cell receptor; TIL, tumor-infiltrating lymphocytes.
CONCLUSIONS
Despite the impressive clinical successes that have been achieved with immune checkpoint inhibitors in patients with certain solid cancers, many patients currently do not benefit from these agents, likely because they lack a robust preexisting, tumor-specific T-cell response. Several immunotherapies are addressing this with various levels of success, such as adoptive T-cell therapies, ex vivo dendritic cell (DC)-based therapies, and cancer vaccines. The later can be broadly divided into adjuvanted protein or peptide vaccines, inducing mainly antibodies and CD4+ T-cells, and vector-based vaccines, which can induce antibody, CD4+ and CD8+ T-cell responses.1
The priming of naïve CD8 T-cells requires their interaction with activated DCs that present antigens via the major histocompatibility complex (MHC)-I pathway. Most current vaccines (recombinant or vector expressed), achieve this through so-called cross-presentation of protein destined for MHC-II presentation. In contrast, direct presentation of antigen via MHC-I requires its translation in the cytoplasm of the antigen-presenting cell, and this is believed to be significantly more potent for induction of CD8+ T-cell responses than approaches relying on cross-presentation.2
To selectively deliver RNA tumor antigens to DC in vivo, the ZVex platform was created. It is an integration-deficient third-generation lentiviral vector (LV) that selectively targets DC-SIGN (CD209) on the surface of immature human DCs via its envelope glycoprotein derived from the alpha virus, Sindbis.3 In preclinical animal models, vectors derived from the ZVex platform have been shown to target murine DC, induce strong, polyfunctional, and long-lasting CD8+ T-cell responses, and confer robust short-term and long-term immunity to tumor challenge in different tumor models.4 LV305 is the first clinical candidate from the ZVex platform and encodes the tumor-specific cancer testis antigen, NY-ESO-1.5
Because of their pivotal role in initiating the immune response, DCs are an attractive target for cancer immunotherapy. Murine studies suggest that DC-based immunotherapy can efficiently induce strong protective cellular immune responses against tumor targets and LV have been shown to activate myeloid derived DC in vitro and in vivo via toll-like receptor 3 and 7 engagement.4,6 After more than a decade since the concept of targeting DCs with LV to induce antitumor CD8+ T-cell responses was raised,7 we report here the first-in-human treatment with a new class of vector delivery of tumor antigens selectively to DCs in vivo. NY-ESO-1 is an ideal target for cytotoxic T cells because it is not expressed in healthy tissues (with the exception of the immune privileged testis and trophoblast) and is highly expressed in certain tumor types, notably myxoid round cell liposarcoma and SS.8 In this patient, LV305 induced a robust NY-ESO-1-specific CD4+ and CD8+ T-cell response, as demonstrated using multiple methodologies, which has been sustained for over 2 years, allowing this patient to improve clinically while maintaining an excellent quality of life.
LV305 immunotherapy significantly increased the NY-ESO-1-specific CD8+ response, but perhaps more importantly, also changed the quality of the response. The clonality and avidity of the T-cell response changed significantly after therapy, and the frequency of T-cell receptor clones observed in TIL increased in PBMCs posttherapy. These observed changes have persisted for >2 years after therapy and are in line with general observations made of immune responses induced by DC-based therapies using LV based on murine studies. As opposed to immunization with other viral vectors that induce strong inflammatory responses resulting in strong but brisk expansion and rapid contraction of the CD8+ T-cell effector repertoire, such as adeno and poxviral vectors, DC transduction with lentivectors tends to be low-inflammatory, resulting in protracted kinetics of the T-cell response but early induction of memory.9 In mice, LV305 induced vector persistence in lymph nodes draining the dermal injection site for over 28 days and induced long-lasting antitumor effector-memory T-cell responses, suggesting that persisting antigen expression combined with prolonged DC activation may have been responsible for the observed sustained immunologic changes in our patient. Furthermore, human dermal DCs express the C-type lectin DC-SIGN, capture a variety of viral antigens and are potent inducers of CD8+ T-cell immunity. It is important to note that other LV targeting DC through other means, such as through targeting of MHC class II or through use of nanobodies, may also be important for future cancer vaccine studies.10,11
SS is an aggressive cancer-driven through the t(X;18)(p11;q11) translocation. In the metastatic setting, the mean overall survival ranges 12–18 months despite chemotherapy with <10% of patients surviving 5 years. SS tumors naturally have few infiltrating T cells and low expression of PD-L1 suggesting that it may not be ideal target disease for checkpoing inhibitors.12 Despite the historically poor outcomes for patients with metastatic SS, these tumors may be unique targets for NY-ESO-1-directed immunotherapy because of the homogenous expression of NY-ESO-1.8 NY-ESO-1 has been targeted using TCR-modified T cells achieving partial responses in >60% of patients. However, these regimens are HLA-restricted and require intensive lymphodepleting conditioning, are logistically complex to generate and administer and are therefore not applicable to all patients.13
We demonstrate here for the first time that a durable and profound immune response, correlated with a long-term clinical response, can be achieved with an in vivo DC-based immunotherapy directed against NY-ESO-1. Combining this potent generator of antigen specific T cells with checkpoint inhibition warrants further study as it may result in further enhanced T-cell activity and clinical benefit.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
S.M.P. is supported by the Sarcoma Alliance for Research through Collaboration (SARC), the Sarcoma Foundation for America, 1K23CA175167-01, and the Gilman Sarcoma Foundation.
H.L., F.H., and J.t.M. are full-time employees and shareholders of Immune Design. All remaining authors have declared that there are no financial conflicts of interest with regard to this work.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
REFERENCES
- 1.Melero I, Gaudernack G, Gerritsen W, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel RM. On the role of dendritic cells versus other cells in inducing protective CD8+ T cell responses. Front Immunol. 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L, Yang H, Rideout K, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol. 2008;26:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albershardt TC, Campbell DJ, Parsons AJ, et al. LV305, a dendritic cell-targeting integration-deficient ZVex(TM)-based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response. Mol Ther Oncolytics. 2016;3:16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breckpot K, Escors D, Arce F, et al. HIV-1 lentiviral vector immunogenicity is mediated by toll-like receptor 3 (TLR3) and TLR7. J Virol. 2010;84:5627–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esslinger C, Chapatte L, Finke D, et al. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J Clin Invest. 2003;111:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis antigen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer. 2001;94:252–256. [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Tan PH, Beutelspacher SC, Xue SA, et al. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood. 2005;105:3824–3832. [DOI] [PubMed] [Google Scholar]
- 10.Cire S, Da Rocha S, Yao R, et al. Immunization of mice with lentiviral vectors targeted to MHC class II+ cells is due to preferential transduction of dendritic cells in vivo. PLoS One. 2014;9:e101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyvaerts C, Dingemans J, De Groeve K, et al. Targeting of human antigen-presenting cell subsets. J Virol. 2013;87:11304–11308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack SM, He Q, Yearley JH, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer. 2017;123:3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.


