Abstract
Introduction
Patients undergoing on-pump cardiac surgery are at an increased risk of acute kidney injury. QPI-1002, a small interfering ribonucleic acid, is under clinical development for the prevention of acute kidney injury. The safety, tolerability, and pharmacokinetics of QPI-1002 was evaluated in this first-in-man, Phase 1 study of a small, interfering ribonucleic acid in patients at risk of acute kidney injury after on-pump cardiac surgery.
Methods
In this phase 1 randomized, placebo-controlled dose-escalation study, a single i.v. dose of QPI-1002 was administered in subjects undergoing on-pump cardiac surgery. Subjects received placebo (n = 4), or QPI-1002 in increasing doses of 0.5 mg/kg (n = 3), 1.5 mg/kg (n = 3), 5 mg/kg (n = 3), and 10 mg/kg (n = 3).
Results
A total of 16 subjects were enrolled in the study. The average maximum concentration and area under the curve from the time of dosing to the last measurable concentration of QPI-1002 were generally dose proportional, indicating that exposure increased with increasing dose. The average mean residence time (mean residence time to the last measurable concentration) was 10 to 13 minutes in all 4 drug-dosing cohorts. Adverse events occurred at a similar rate in all study groups. Of the total 109 reported adverse events, the events were distributed as 26 in the placebo group and 21, 19, 24, and 19 in the QPI-1002 0.5, 1.5, 5.0, and 10.0 mg/kg groups, respectively. Eight of the 16 subjects experienced at least 1 serious adverse event: 4 (100%) in the placebo group and 4 (33.3%) in the combined QPI-1002 cohorts.
Discussion
QPI-1002 was rapidly eliminated from plasma. QPI-1002 was safe and well tolerated across all dose groups. Overall, no dose-limiting toxicities or safety signals were observed in the study. Further development of QPI-1002 for prophylaxis of acute kidney injury is warranted.
Keywords: acute kidney injury, cardiopulmonary bypass, clinical trial, oligonucleotide, pharmacokinetics, siRNA
Acute kidney injury (AKI) is a clinical syndrome characterized by deterioration of renal function that rapidly develops over hours to days. AKI complicates a significant proportion of hospital admissions and is associated with an increased risk of chronic kidney disease, major adverse cardiovascular events, increased length of hospital stay, and additional health care costs and mortality.1, 2, 3 Despite recent advances in surgical techniques, the incidence of AKI remains high, ranging from 33% to 55%.3
Current attempts to treat AKI remain largely supportive, including the adjustment of medications, appropriate nutritional support, correcting volume status, electrolyte imbalance, and acidosis,4 with or without renal replacement therapy. At present, there are no approved therapeutic agents available for the prevention or treatment of AKI.
QPI-1002 (active ingredient: I5NP), a small, interfering ribonucleic acid, is a synthetic, nuclease-resistant, double-stranded RNA oligonucleotide designed to temporarily inhibit the expression of the proapoptotic gene p53 via activation of the RNA interference pathway. The rat analogue of QPI-1002, QM5, was shown to temporarily (<24 hours) suppress p53 protein-mediated apoptosis resulting from ischemia-reperfusion injury (IRI).5 Inhibition of p53 in several preclinical models of ischemia-reperfusion injury demonstrated improved renal function postinjury and improved histology.5 Small, interfering RNA inhibition of p53 may work by providing additional time during which nonlethal injury to renal tubular epithelial cells can be repaired before initiation of apoptosis.6
The objective of this study was to evaluate the safety, tolerability, and pharmacokinetics of QPI-1002 in this first in human study in patients at risk of the development of AKI while undergoing on-pump cardiac surgery.
Materials and Methods
QRK-002 was a phase 1, double-blind randomized, placebo-controlled, dose-escalation study to evaluate the safety and tolerability of a single i.v. bolus injection of QPI-1002 to subjects scheduled to undergo nonemergent, on-pump cardiac surgery at 6 centers globally. Patients were eligible if they were 21 to 85 years of age and up-to-date on screening negative for cancer. Patients were excluded if their postoperative Cleveland Clinic Foundation AKI cumulative risk score was >8, they had received an organ transplant, they were receiving immunosuppressive therapy, or they were women of child-bearing potential.
The randomization was performed manually via a centralized randomization system by unblinded Quark personnel, per the QRK.002 Pharmacy Manual and Patient Enrollment Guidelines, using the enrollment form. In each QPI-1002 dosing cohort, the first subject was dosed as single, followed by double-blind, placebo-controlled enrollment.
For each dose level, patients were allocated 3:1 to receive a single i.v. bolus injection of QPI-1002 in doses of 0.5, 1.5, 5.0, or 10.0 mg/kg or placebo (isotonic saline) at 4 ± 0.5 hours after discontinuation of cardiopulmonary bypass. The study protocol and informed consent were reviewed and approved by institutional review board or an independent ethics committee at each site. The study was conducted in accordance with the International Conference on Harmonization guidance on Good Clinical Practice based on the Declaration of Helsinki.
Pharmacokinetic plasma samples were collected just before administration of the study drug (QPI-1002 or placebo) and at 5, 15, and 30 minutes and 1, 2, 4, 8, and 24 hours post-dose. In addition to drug concentrations, these samples were also analyzed for complement activation products (Bb, C3a, C4a, and C5a) to monitor for the development of this previously described class effect, reported for the related, but structurally modified, phosphorothioate-substituted single-stranded oligodeoxyribonucleotides.7, 8 Baseline samples for complement activation were taken before surgery. Blood samples drawn into commercially available K2-ethylenediamine tetraacetic acid–containing Vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, NJ) and were cold-centrifuged within 30 minutes of phlebotomy, and the plasma was frozen at −70 °C pending shipment on dry ice to a central laboratory (National Jewish Laboratory, Denver CO) for complement activation analyses and to Charles River Laboratories (Senneville, Quebec, Canada) for determination of QPI-1002 (I5NP) concentrations.
Additional blood and urine samples were collected at screening and postoperatively for routine determination of serum chemistry and hematology analytes, indices of coagulation, and measures of renal (blood urea nitrogen, serum creatinine), hepatic (aspartate transaminase, alanine transaminase, alkaline phosphatase, and total bilirubin), and pancreatic (serum amylase and lipase) function.
The QPI-1002 pharmacokinetic profile for each subject was evaluated by noncompartmental analysis of I5NP plasma data using commercially available computer software (WinNonlin Professional, version 5.3, Pharsight Corp., Mountain View, CA). Values below the limit of quantitation were treated as “missing.” The area under the QPI-1002 plasma concentration versus time curve from time zero to the last quantifiable plasma concentration (AUClast) was calculated using the linear trapezoidal method (linear interpolation). Other values determined, where possible, included maximum concentration (Cmax), clearance (Cl), elimination half-life (T1/2), Vss, AUClast extrapolated to infinite time (AUC0-∞), and the mean residence time over the interval from time zero to the last quantifiable time point mean residence time (MRTlast).
Treatment emergent adverse events were defined as any adverse events occurring at any time from study drug administration through day 7, based on the relatively short residence time of QPI-1002 in plasma and proximal tubular epithelium.5 All adverse events and serious adverse events were reported through study day 30. Subjects were contacted by telephone at 6 and 12 months to assess medically significant adverse events, including death, need for renal replacement therapy, and malignancy. Therapeutic efficacy was not assessed in this study.
There were no formal statistical tests of hypotheses. A safety analysis was performed including all subjects who were randomized to a study arm and had received either the study drug or placebo and analyzed according to the treatment that the subject actually received.
Summary statistics including number of subjects (n), mean, SD, median, minimum, and maximum were presented for continuous variables. Categorical variables were summarized using counts and percentages.
Results
A total of 16 subjects were enrolled in the study (Supplementary Figures S1 and S2; QRK 002 ClinicalTrials.gov number, NCT00554359). The study was initiated in October 2007, and the last subject was enrolled in October 2009. The baseline and demographic characteristics of the randomized subjects are summarized in Table 1. The mean ± SD age was 61.5 ± 10 and 63 ± 20 years for the QPI-1002–treated and placebo-treated patients, respectively. Insulin-dependent diabetes mellitus was present in 1 placebo-treated and 5 QPI-1002–treated subjects (25.0% and 41.7%), respectively. A history of congestive heart failure was present in 4 subjects treated with QPI-1002 and none in the placebo group.
Table 1.
Patient demographics and baseline characteristics
| Patient demographics/baseline characteristics | Treatment groups |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 4) |
QPI-1002 |
All (n = 16) |
|||||
| 0.5 mg/kg (n = 3) |
1.5 mg/kg (n = 3) |
5.0 mg/kg (v = 3) |
10 mg/kg (n = 3) |
(Pooled) (n = 12) |
|||
| Sex, n (%) | |||||||
| Male | 3 (75) | 2 (66.7) | 3 (100) | 2 (66.7) | 3 (100) | 10 (83.3) | 13 (81.3) |
| Race, n (%) | |||||||
| White | 3 (75) | 1 (33.3) | 3 (100) | 3 (100) | 3 (100) | 10 (83.3) | 13 (81.3) |
| Age (yr) | |||||||
| Mean | 63.0 | 65.0 | 54.3 | 72.0 | 54.7 | 61.5 | 61.9 |
| SD | 20.61 | 8.72 | 14.19 | 4.36 | 0.58 | 10.68 | 13.00 |
| BMI (kg/m2) | |||||||
| Mean | 28.2 | 36.0 | 28.4 | 34.7 | 38.7 | 34.4 | 32.9 |
| SD | 4.46 | 8.91 | 2.07 | 5.92 | 7.00 | 6.80 | 6.76 |
| Type of surgery | |||||||
| Valve only | 2 (50) | 1 (33.3) | 3 (100) | 1 (33.3) | 0 (0) | 5 (41.7) | 7 (43.8) |
| CABG and valve | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 1 (33.3) | 2 (16.7) | 2 (12.5) |
| Other cardiac surgery | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (8.3) | 1 (6.3) |
| Preoperative serum creatinine (mg/dl) | |||||||
| <1.2 | 2 (50) | 2 (66.7) | 2 (66.7) | 1 (33.3) | 3 (100) | 8 (66.7) | 10 (62.5) |
| 1.2–2.1 | 2 (50) | 1 (33.3) | 1 (33.3) | 2 (66.7) | 0 (0) | 4 (33.3) | 6 (37.5) |
| CCF score | |||||||
| Mean | 2.8 | 3.0 | 2.3 | 3.7 | 2.3 | 2.8 | 2.8 |
| SD | 0.50 | 2.65 | 2.31 | 3.06 | 2.31 | 2.29 | 1.97 |
BMI, body mass index; CABG, coronary artery bypass graft; CCF, Cleveland Clinic Foundation.
Of the 16 participating subjects, 15 underwent coronary artery bypass graft involving ≥1 coronary arteries (n = 6), valve replacement or repair (n = 7), or both (n = 2). The remaining subject was scheduled for aortic valve replacement, but additional procedures were deemed necessary during surgery including surgical repair of a dilated aortic root and a portion of the ascending aorta. The mean preoperative Cleveland Clinic Foundation risk scores,9 determined to assess the level of risk for the development of postsurgical AKI requiring acute dialysis, was 2.8 (on a scale of 0 to 13) in both placebo- and QPI-1002–treated subjects; among the QPI-1002–treated subjects, mean scores ranged from 2.3 in the 10-mg/kg dose group to 3.7 in the 5-mg/kg dose group (Table 1).
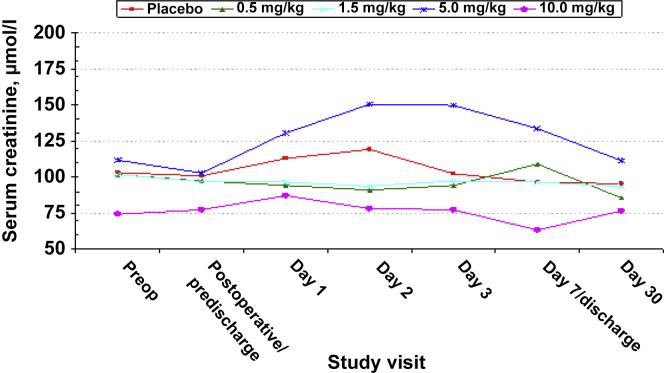
Baseline mean serum creatinine concentrations ranged from 0.84 (10 mg/kg) to 1.26 (5 mg/kg) mg/dl. Post-dosing, mean serum creatinine remained stable through day 7/hospital discharge in all but the 5-mg/kg dose QPI-1002 group, in which the mean serum creatinine increased from 1.16 mg/dl at baseline to peak at 1.70 mg/dl on day 2, and progressively decreased toward baseline (1.26 mg/dl) by day 30. This was deemed attributable to the increased serum creatinine observed in 1 patient with underlying chronic kidney disease. The change in serum creatinine over time by treatment group is presented in Figure 1.
Figure 1.
Change in mean serum creatinine concentration over time by treatment group and study visit.
Overall, no differences were observed for plasma thromboplastin, activated partial thromboplastin time, or international normalized ratio between treatment groups at any time before or during the first 7 days after surgery and study drug administration.
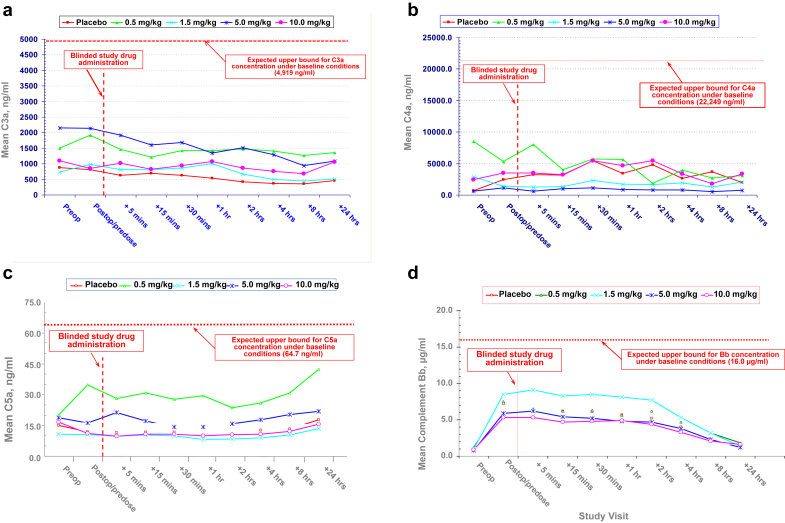
The mean blood concentrations of the activated complement components C3a, C4a, C5a, and Bb are presented for placebo- and QPI-1002–treated patients in Figure 2. The mean blood concentrations reflecting classic complement pathway activation (C’3a, C’4a, and C’5a) remained stable across all QPI-1002 and placebo groups. Unlike the classic pathway factors, mean postoperative, predose levels of alternate pathway activated factor B (Bb) increased by 5.9- to 8.5-fold compared with baseline (screening) levels in all groups, consistent with the previously described impact of on-pump bypass surgery.1 In all patients, Bb levels decreased at variable rates thereafter (Figure 2). No differences in classic or alternate pathway complement activation were observed between placebo- and QPI-1002–treated patients at any dose level.
Figure 2.
(a) Mean C3a levels by treatment group before and after on-pump cardiac surgery. (b) Mean C4a levels by treatment group before and after on-pump cardiac surgery. (c) Mean C5a levels by treatment group before and after on-pump cardiac surgery. (d) Mean Bb levels by treatment group before and after on-pump cardiac surgery.
I5NP (active ingredient) was detected in all patients who received QPI-1002 but was not detected in any patient who received placebo. In Cohort 1, I5NP was quantified in plasma up to 4 hours. In Cohorts 2 through 4, I5NP was quantifiable up to 4 to 8 hours and up to 24 hours in 1 patient in Cohort 4. Due to the lack of plasma concentrations above the assay limit, pharmacokinetic analysis could not be conducted in 1 of 3 patients in each of Cohorts 1 and 2. Therefore, pharmacokinetic parameters were estimated for 10 patients. AUC0-∞ was not reported for the majority of patients due to the unacceptably large percentage of extrapolation from AUClast.
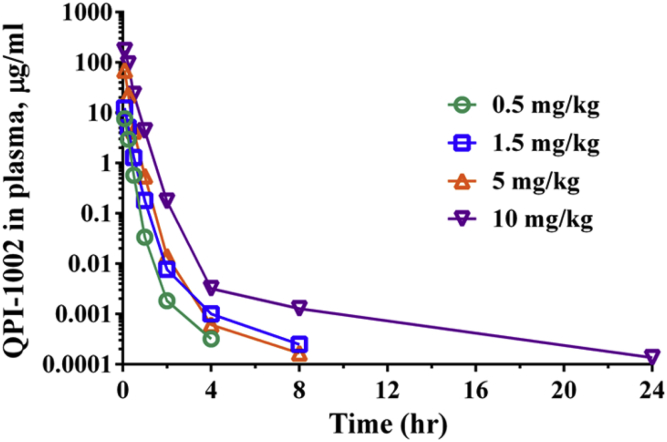
Average QPI-1002 Cmax and AUClast values increased with increasing dose from 7.64 to 261 μg/ml and from 2.33 to 85.6 μg·hr/ml, respectively, across the range of studied doses (Table 2). The increase in both parameters generally increased proportionally with increasing dose, despite high interpatient variability and the small number of observations. The average dose-normalized Cmax values were 14.6, 8.30, 10.8, and 26.0 for Cohorts 1 through 4, respectively. For patients in all 4 cohorts, QPI-1002 was rapidly cleared from plasma with a mean residence time (MRTlast) of 10-13 minutes (Figure 3). This is consistent with the rapid elimination of I5NP and its analogues observed in nonhuman primates, in normal rats and in rats with impaired kidney function (5/6 nephrectomy model).
Table 2.
Mean (min-max) QPI-1002 plasma PK estimates
| PK parameter | QPI-1002 dose (mg/kg) |
|||
|---|---|---|---|---|
| 0.5 | 1.5 | 5.0 | 10 | |
| n | 2 | 2 | 3 | 3 |
| Cmax (μg/ml) | 7.64 (5.7–9.6) | 12.4 (9.80–14.9) | 54.0 (22.8–89.7) | 261 (169–441) |
| AUClast (μg·hr/ml) | 2.33 (1.81–2.85) | 3.82 (3.01–4.62) | 21.1 (15.1–31.4) | 85.6 (63.6–129.4) |
| MRTlast (hr) | 0.166 (0.152–0.180) | 0.172 (0.164–0.179) | 0.174 (0.141–0.210) | 0.216 (0.184–0.244) |
| Tlast (hr) | 4 (4–4) | 6 (4–8) | 8 (8–8) | 13.4 (8–24) |
| Clast (ng/ml) | 0.335 (0.320–0.349) | 0.572 (0.250–0.894) | 0.175 (0.167–0.192) | 0.581 (0.136–1.28) |
AUClast, area under the curve from the time of dosing to the last measurable concentration; Clast, concentration corresponding to Tlast; Cmax, maximum observed plasma concentration; MRTlast, mean residence time to the last measurable concentration; Tlast, time of last measurable concentration.
Figure 3.
Plasma concentrations of QPI-1002 after i.v. administration of 0.5 mg/kg (Cohort 1), 1.5 mg/kg (Cohort 2), 5 mg/kg (Cohort 3), or 10 mg/kg (Cohort 4).
Adverse Events
During the first 30 post-operative days, a total of 109 AEs were reported among all 16 study participants. The distribution of AEs was similar across all treatment group: 26, 21, 19, 24, and 19 in the placebo and QPI-1002 0.5, 1.5, 5, and 10 mg/kg cohorts, respectively (Table 3); the majority (90 [83.5%]) were mild or moderate (NCI CTC AE Grades 1 or 2) in severity. The overall distribution of Grade 1 and 2 events was also similar across cohorts: 22 (84.6%), 20 (95.2%), 13 (63.4%), 20 (83.4%) and 18 (85.3%), respectively. The most frequent of these were hypotension, reported in 2 placebo and 5 QPI-1002 recipients overall (50% and 41.7%, respectively), pleural effusion in 1 (25%) and 5 (41.7%), respectively, and constipation in 0 and 4 (33.3%), respectively (Table 4). Overall, only 2 AEs were investigator-assessed as “Possibly Related;” both were for Grade 3 worsening anemia that developed during the perioperative period—one in a placebo- and the other in a QPI-1002 (1.5 mg/kg)-treated patient.
Table 3.
Counts of adverse events (through day 30) by dosing cohort
| Through day 30 | Placebo n = 4 |
QPI-1002 |
Pooled n = 16 |
|||
|---|---|---|---|---|---|---|
| 0.5 mg/kg n = 3 |
1.5 mg/kg n = 3 |
5.0 mg/kg n = 3 |
10.0 mg/kg n = 3 |
|||
| No. of events | 26 (100.0%) | 21 (100.0%) | 19 (100.0%) | 24 (100.0%) | 19 (100.0%) | 109 (100.0%) |
| CTCAE severity grades | ||||||
| 1 | 13 (50.0%) | 12 (57.1%) | 6 (31.6%) | 7 (29.2%) | 15 (78.9%) | 53 (48.6%) |
| 2 | 9 (34.6%) | 8 (38.1%) | 7 (36.8%) | 13 (54.2%) | 3 (15.8%) | 40 (36.7%) |
| 3 | 4 (15.4%) | 1 (4.8%) | 5 (26.3%) | 4 (16.7%) | 1 (5.3%) | 15 (13.8%) |
| 4 | 0 (0.0%) | 0 (0.0%) | 1 (5.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.9%) |
CTCAE, Common Terminology Criteria for Adverse Events.
Table 4.
Most frequently reported adverse events for QPI-1002 versus placebo-treated patients Through day 30
| Event | QPI-1002, n (%) | Placebo, n (%) |
|---|---|---|
| Hypotension | 5 (41.7) | 2 (50.0) |
| Pleural effusion | 5 (41.7) | 1 (25.0) |
| Anemia | 3 (25.0) | 2 (50.0) |
| Constipation | 4 (33.3) | 0 (0.0) |
| Nausea | 3 (25.0) | 0 (0.0) |
| Leukocytosis | 3 (25.0) | 2 (50.0) |
| Elevated AST | 3 (25.0) | 1 (25.0) |
| Pulmonary edema | 3 (25.0) | 0 (0.0) |
| Peripheral edema | 3 (25.0) | 0 (0.0) |
AST, aspartate transaminase.
A total of 17 serious adverse events (SAEs) were reported in 8 (4 placebo-treated [100%] and 4 QPI-1002-treated [33.3%]) subjects through 180 days of follow-up. Through study day 365, an additional 7 SAEs were reported in 4 (Placebo: 1; QPI-1002: 3) subjects, none of which were investigator-assessed as related or possibly related. Of these, only 1 episode of atrial fibrillation in the Placebo group and another 2 episodes of bradycardia in the QPI-1002 group, 1.5- and 10- mg/kg treatment groups, respectively, were reported for >1 patient. A single event of grade 3 prostate cancer was reported as an AE at study day 237 in a 70-year-old subject with a history of benign prostatic hypertrophy treated with 5 mg/kg I5NP in whom a locus of adenocarcinoma was identified in prostate tissue resected for benign prostatic hypertrophy. The event was considered not related and had resolved without sequelae at the time of study completion. No dose-limiting toxicities were identified, either per the prospectively defined criteria specified in the protocol or by the independent Data Safety Monitoring Board for this study. There were no deaths or study discontinuations due to reported AEs.
Discussion
We report the i.v. adminstration of QPI-1002 in a phase I safety, tolerability, and pharmacokinetic study in subjects undergoing on-pump cardiac surgery and at risk of the development of AKI. We demonstrated that single i.v. bolus injections of QPI-1002 were safe, well tolerated, and without immediate reaction at any dose in the range of 0.5 to 10.0 mg/kg when administered at 4 hours post–cardiopulmonary bypass. The drug was rapidly eliminated from plasma, with an average mean residence time (MRTlast) of 10 to 13 minutes in all 4 dosing cohorts. In preclinical models, the siRNAs were shown to undergo rapid glomerular filtration and proximal tubular epithelial uptake.5 Thus, bolus i.v. injection is an effective means of delivering a “naked” siRNA to the proximal tubule, which is a key target of IRI.
QPI-1002 is a siRNA targeting p53 mRNA and designed to temporarily downregulate the expression of p53 protein via the RNA interference (RNAi) pathway. P53 plays a major role in ischemia reperfusion–induced AKI and has emerged as the pivotal regulator of apoptosis (i.e., programmed cell death).10, 11, 12, 13, 14 QPI-1002 and other synthetic siRNAs are metabolized by cellular endonucleases in a manner similar to endogenously produced oligoribonucleotides.15 RNA interference via QPI-1002 is expected to mitigate the premature elimination of reversibly damaged proximal tubular epithelial cells via programmed cell death, without exacerbating the postischemic inflammatory response; this is demonstrated by the fact that administration of murine double minute-2 antagonist, which metabolizes p53 protein, suppresses inflammation following AKI in p53 knockout mice in a p53-independent manner.16, 17, 18
Although p53 is also involved in the longer term process of immune surveillance, studies in wild-type and p53 knockout mice have clearly shown that transient p53 suppression, for periods of up to 7 days, was not associated with a long-term increase in the risk of malignant transformation, so long as p53 activity was subsequently restored.19 No unexpected malignancy was reported in any patient in this small and short-duration trial. Overall, QPI-1002 is rapidly eliminated via the kidneys and minimally distributed to other organs/tissues and has a duration of action limited to ∼48 to 72 hours in preclinical studies.5
Subjects treated with QPI-1002 had an AE profile similar to what was observed in the placebo group. Overall, other than worsening anemia, no other AEs were investigator-assessed as “possibly related” in either group. Some structually altered oligodeoxyribonucleotides, those with phosphorothioate-substituted backbones, were previously reported to cause dose-dependent prolongation of coagulation times (activated partial thromboplastin times greater than prothrombin times) and/or exposure-dependent complement activation (C’3a, C’5a, Bb).20 However, no evidence of increased complement activation or coagulopathy was observed with single-dose i.v. bolus administration of QPI-1002 in this study.
In summary, we report results for a phase I, safety, tolerability, and pharmacokinetics study of single-dose, i.v. bolus administration of QPI-1002 in subjects undergoing on-pump cardiac surgery who were at risk of the development of AKI. This was the first study that involved the systemic administration of a siRNA in human subjects. Our study has all the limitations of small-sized randomized studies. We conclude that QPI-1002, when administered as a single i.v. bolus injection at 4 hours after discontinuation of cardiopulmonary bypass, was well tolerated and was not associated with unexpected or unfavorable AEs. Further study of QPI-1002 is needed to assess its clinical efficacy and additional safety for the prevention of AKI and its associated outcomes in patients undergoing cardiovascular surgery.
At present, 2 studies are ongoing to assess the efficacy and additional safety of QPI-1002: prevention of acute kidney injury following cardiac surgery (NCT02610283 phase 2) and prevention and reduction in severity of delayed graft function in recipients of an older donor kidney transplant (NCT02610296 phase 3).
Disclosure
GA is a consultant for Abbott Vascular, Medtronic, St. Jude, Edwards, and Atricure. MC has received consulting fees from Quark Pharma. SE, DR, and ECS are employees of Quark Pharmaceuticals. MP was an employee of Quark Pharmaceuticals during the conduct of the study. SK was an employee of Quark Pharmaceuticals during the finalization of manuscript. All the other authors declared no competing interests.
Acknowledgments
Funded by Quark Pharmaceuticals, Inc; QRK 002 ClinicalTrials.gov number, NCT00554359. Quark provided funding for study QRK-002 and was involved in the study design, study execution, collection, analysis, and interpretation of data and in the writing, reviewing, and approval of the report. All authors had access to study results, and the lead author vouches for the accuracy and completeness of the data reported. All authors had the final decision to submit the publication. The study was overseen by a data review committee. Formatting assistance was provided by Risë Ivy, Quark.
Footnotes
Figure S1. CONSORT 2010 checklist of information.
Figure S2. CONSORT 2010 flow diagram.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Author Contributions
GA, DB, MB, SD, MH, SK, DR, SS, and SKS contributed to providing the content and reviewing the article. MC, SE, and ECS contributed to the trial design, to providing the content, and to reviewing the article. MP provided medical monitoring for the study and contributed to the trial design, to providing the content, and to reviewing the article.
Supplementary Material
CONSORT 2010 checklist of information.
CONSORT 2010 flow diagram.
References
- 1.Chertow G.M., Burdick E., Honour M. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Chawla L., Amdur R., Shaw A. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobson C.E., Yavas S., Segal M.S. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 5.Molitoris B.A., Dagher P.C., Sandoval R.M. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komarov P.G., Komarova E.A., Kondratov R.V. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald K., Frank-Kamenetsky M., Shulga-Morskaya S. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomized single-blind placebo-controlled phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly K.J., Plotkin Z., Vulgamott S.L. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14:128–138. doi: 10.1097/01.asn.0000040596.23073.01. [DOI] [PubMed] [Google Scholar]
- 9.Thakar C.V., Arrigain S., Worley S. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 10.Naito A.T., Okada S., Minamino T. Promotion of CHIP-mediated p53 degradation protects the heart from ischemic injury. Circ Res. 2010;106:1692–1702. doi: 10.1161/CIRCRESAHA.109.214346. [DOI] [PubMed] [Google Scholar]
- 11.Nijboer C.H., Heijnen C.J., van der Kooij M.A. Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol. 2011;70:255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- 12.Singaravelu K., Padanilam B.J. p53 target Siva regulates apoptosis in ischemic kidneys. Am J Physiol Renal Physiol. 2011;300:F1130. doi: 10.1152/ajprenal.00591.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino A., Matoba S., Iwai-Kanai E. p53-TIGAR axis attenuates mitophagy to exacerbate cardiac damage after ischemia. J Mol Cell Cardiol. 2012;52:175–184. doi: 10.1016/j.yjmcc.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Vaseva A.V., Marchenko N.D., Ji K. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fire A., Xu S., Montgomery M.K. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 16.Sutton T.A., Hato T., Mai E. p53 is renoprotective after ischemic kidney injury by reducing inflammation. J Am Soc Nephrol. 2012;24:113. doi: 10.1681/ASN.2012050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Q., Dong G., Yang T. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2007;293:F1282–F1291. doi: 10.1152/ajprenal.00230.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulay S.R., Thomasova D., Ryu M. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012;81:1199–1211. doi: 10.1038/ki.2011.482. [DOI] [PubMed] [Google Scholar]
- 19.Christophorou M.A., Ringshausen I., Finch A.J. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 20.Henry S.P., Monteith D., Levin A.A. Effects of intravenous infusion of phosphorothioate oligonucleotides on coagulation, complement activation and hemodynamics. Nucleosides Nucleotides. 1997;16:1673–1676. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT 2010 checklist of information.
CONSORT 2010 flow diagram.