Abstract
Introduction
We hypothesized that at least half of incident hemodialysis (HD) patients on 3-times weekly dialysis could safely start on an incremental, 2-times weekly HD schedule if residual kidney function (RKF) had been considered.
Methods
RKF is assessed in all our HD patients. This single-center, retrospective cohort study of incident adult HD patients, who survived ≥6 months on a 3-times weekly HD regimen and had a timed urine collection within 3 months of starting HD, assessed each patient’s theoretical ability to achieve adequate urea clearance, ultrafiltration rate, and hemodynamic stability if on 2-times weekly HD.
Results
Of the 410 patients in the cohort, we found that 112 (27%) could have optimally and 107 (26%) could have been appropriately considered for 2-times weekly incremental HD. In general, diuretics were underutilized in >50% of subjects who had adequate RKF urea clearance. The optimal 2-times weekly patients had better potassium and phosphorus control. The correlation coefficient of calculated residual kidney urea clearance with 24-hour urine volume and with kinetic model residual kidney clearance was 0.68 and 0.99, respectively.
Discussion
More than 50% of incident HD patients with RKF have adequate kidney urea clearance to be considered for 2-times weekly HD. When additionally ultrafiltration volume and blood pressure stability are taken into account, more than one-fourth of the total cohort could optimally start HD in an incremental fashion.
Keywords: hemodialysis, incremental hemodialysis, residual renal function
In the United States, most individuals who begin long-term hemodialysis (HD) do so on a 3-times weekly prescription. About 40% of incident patients begin HD with an estimated glomerular filtration rate (GFR) of ≥ 10 ml/min,1 and this possibly suggests that many patients have substantial residual renal function (RKF). Indeed, a good proportion of incident patients have meaningful RKF at the time of HD initiation when it is measured.2 In addition to native kidney urea clearance (KRU), RKF also augments weekly volume removal, improves electrolyte balance, lowers erythropoietin-stimulating agent requirement, and provides clearance of protein-bound solutes and middle molecules.3, 4 Most importantly, there is a large positive and robust relationship between RKF and HD patient survival.5, 6, 7
Although guidelines on HD adequacy briefly mention the possibility of adjusting the HD prescription to take into consideration KRU, adjusting the frequency of HD is either not directly addressed or is mentioned only in brief.8, 9 If we applied the concept of incremental HD, the tailoring of the prescription to complement the amount of RKF, perhaps some patients could do well on a less than 3-times weekly HD schedule. It is not known how many incident HD patients in the United States could start HD incrementally, because few incident HD patients ever perform a timed urine collection; in some cohorts, <5% of HD patients ever have a formal evaluation of RKF.10
It is our standard practice to obtain 24-hour urine collections in all HD patients. Timed urine collections are performed within the first 3 months of starting dialysis and then every 3 months thereafter, until either the patient reports no significant urine production or a 24-hour urine collection reveals <100 ml of volume. We have used the KRU information primarily to adjust the duration of dialysis; most patients are on 3-times weekly HD. With extensive RKF data, we are uniquely positioned to determine the theoretical feasibility of incremental start HD across incident HD patients.
Our specific aim was to estimate how many incident HD patients could have started HD on a 2-times weekly instead of a 3-times weekly basis. In this theoretical assessment, we determined that an optimal 2-times weekly HD patient would need to meet the following criteria: (i) adequate weekly total (kidney plus dialyzer) urea clearance; (ii) ultrafiltration (UF) rate ≤ 13 ml/kg/h per dialysis; (iii) stable blood pressure on HD; and (iv) minimal side effects such as nausea and vomiting while on dialysis. In addition, we used a 4-hour per HD treatment time limit in the calculations. Even with these conservative constraints, we hypothesized that the majority of clinically stable incident patients who continue to make urine could have started very safely on HD in an incremental 2-times weekly fashion.
Materials and Methods
Study Subjects
In this single-center, retrospective cohort study, we analyzed all incident adult patients on 3-times weekly HD, who were admitted to 4 dialysis clinics over 14 years, and who survived ≥6 months and had KRU measured within ≤3 months of starting HD. Incidence was defined as receiving a first ever in-center HD treatment within ≤3 months of the first dialysis treatment date listed. Patients were excluded if they had prior renal transplantation, were discharged from the clinic for any reason, received <75% of the expected in-center HD treatments, died <6 months after their first HD treatment, were not initially prescribed a treatment frequency of 3-times weekly, or did not have a 24-hour urine collection within the first 3 months. Data were queried from the dialysis unit database. This study was approved by the Institutional Review Board of the University of California, Davis, as well as by the administrative review board of the dialysis provider (Dialysis Clinic, Inc.).
Patient Data
Demographic information included age, race, ethnicity, gender, and cause of renal failure. For each patient, HD parameters within the first 3 months included treatment times, pre- and post-HD blood pressures, blood flow rates, dialysate flow rates, type of vascular access (the one used for the majority of the time), pre- and post-HD weights, medications given on HD, and adverse symptoms while on dialysis (nausea, vomiting, cramps, or hypotension).
Urine collection data were obtained within 3 months of the in-center HD start date, and included volume, day of the week of collection, and urea concentration. Urea clearance calculation was based on the blood tests done on the next day. All other laboratory information was averaged from the first 3 months. Kinetic modeling data, for the first 3 months, included volume of distribution of urea (V), modeled dialyzer clearance (Kd), urea generation rate (G), and single pool per treatment clearance (spKt/V). Medications were noted for angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, and diuretics (furosemide, torsemide, bumetanide, hydrochlorothiazide, chlorthalidone, metolazone, spironolactone, and eplerenone).
Urea Clearance Criterion
We chose to utilize the standard weekly clearance of urea (stdKt/V), which is based on the concept of a stable mean predialysis blood urea nitrogen (BUN) concentration, to add KRU to the dialyzer clearance.11 Standard weekly clearance is a well-established method of assessing dialysis adequacy and allows the addition of clearances obtained from various methods.8, 12 Using explicit equations, the per-session dialyzer single pool clearance (spKt/V) was converted into a continuous weekly equivalent clearance, stdKt/V(dialysis). We used a modified Tattersall equation with a time constant of 30.7 minutes13 to convert spKt/V into an equilibrated value (eKt/V), where “t” is the dialysis duration in minutes:
| (1) |
The next step utilized the simplified equation for standard weekly clearance:
| (2) |
where “F” is number of HD treatments per week and “t” is treatment time. Equation 2 is based on a single-pool, fixed-volume model with no volume removal. Although both spKt/V and eKt/V do theoretically account for convective clearance of urea during ultrafiltration, the effect is not complete when converting the per-session clearance to a standard weekly clearance. We used a correction that was recently published in order to take into account the additional convective urea clearance obtained by dialysis volume removal.13 Therefore, the final standard weekly clearance obtained by the dialyzer is:
| (3) |
where “S” is the result equation 2, “F” is again the number of HD treatments per week, “UFw” is the weekly volume removed by HD expressed in liters, and “V” is the volume of distribution of urea expressed in liters. In our study, we used the patient’s kinetically modeled V. Weekly fluid gains were assumed to remain the same, regardless of 2- or 3-times weekly HD, and were calculated based on the sum of extrapolated weekly urine collection volume and the total HD volume removal on 3-times weekly HD. The UFw for theoretical 2-times weekly HD was based on the difference between this weekly fluid gain and the estimated weekly urine volume if on 2-times weekly HD (discussed below).
We determined KRU based on the patient’s 24-hour urine collection for urea. To determine the correct plasma urea (Purea) to use in the denominator, we multiplied the predialysis Purea that was collected on the same day as the termination of the urine collection by a factor of 0.92 or 0.98 based on whether the 24-hour urine was collected on the last day of the short or long interdialytic period, respectively, to obtain the adjusted Purea.12, 14 Urine collection volume (Vol) was expressed in milliliters, and urine urea concentration (Uurea) and BUN levels were expressed in milligrams per deciliter. In order to convert KRU into the familiar units of milliliters per minute, a factor of 1440 is used:
| (4) |
Because of the intermittent nature of traditional HD, there are daily variations in urine flow and kidney clearance. Work by Daugirdas,15 analyzing clinical data of urine flow, and inulin clearance in HD patients published by van Olden et al.,16 showed that weekly KRU and urine volume can be predicted even with this daily variation. The KRU was adjusted based on whether the 24-hour urine was collected on the last day of the short (2-day) or long (3-day) interdialytic period. We used 0.99 and 0.81 conversion factors for collections in the short and long interdialytic periods, respectively. Conversion of KRU to a standard weekly renal clearance involved the following:
| (5) |
In the above equation, “V” is the individual’s volume of distribution of urea expressed in milliliters, and KRU is expressed in milliliters per minute. With both the dialysis and native kidney clearances in standard weekly form, we can add equations 3 and 5 to obtain the total weekly clearance:
| (6) |
A prior study had suggested that stdKt/V(renal) should be reduced by approximately 30% before adding to dialyzer clearance.13 However, recent discussion suggests that this may be inappropriate and that KRU should be added “full strength” to stdKt/V(dialysis), as is suggested in the latest updated National Kidney Foundation 2015 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines on HD adequacy.8 A fixed-target stdKt/V(total) of 2.3 volumes per week is a recommended prescription target and was the value for which we aimed in this study.8 Combining the above equations, we arrived at the following:
| (7) |
We had each patient’s V, UFw, and KRU. We used the kinetic modeled in vivo dialyzer clearance (K) that was obtained from each patient’s adequacy report while on that individual’s actual 3-times weekly HD schedule. We set F to “2” for the theoretical 2-times weekly schedule. All variables now known, we manipulated and solved for time “t” for each patient, and this determined how long a dialysis treatment would need to be on a 2-times weekly schedule to achieve adequate total urea clearance.
To perform the calculation of “t”, we used a computer to perform an iterative procedure until the “t” that satisfied the equation was determined, if mathematically possible. If the solved value for treatment time was ≤4 hours (our self-imposed maximal time limit), we then concluded that it was possible to attain a total weekly standard clearance of 2.3 with just 2-times weekly HD for that patient. The per-session spKt/V that would be needed to achieve this desired total weekly clearance was then easily calculated.
Volume Removal Criterion
We assumed that patient weekly fluid gains or their weekly fluid burden would be the same between 2 and 3-times weekly HD regimens. Due to the impact of intermittent dialysis, there is variation in daily urine volume, with an expected drop in urine flow immediately after HD and a subsequent increase over the interdialytic period until the next HD. Similar to the adjustment for the daily variation of KRU, we made these adjustments for weekly urine volume (UVolw). Weekly urine output on 3-times weekly HD was based on whether the 24-hour urine was collected on the last day of the short (2-day) or long (3-day) interdialytic period. We calculated UVolw by multiplying the 24-hour urine volume by a correction factor based on when it was collected in the week: if on the last day of the short interdialytic period, UVolw = 24-hour volume × 0.98 × 7; if on the last day of the long interdialytic period, UVolw = 24-hour volume × 0.73 × 7. Total weekly volume was assumed to be UVolw + average UFw on HD. With less frequent HD, daily urine production may, in theory, continue to increase over a 4- or 5-day interdialytic period. We assumed this to be true and estimated the UVolw on 2-times weekly HD to be 1.17 times that of UVolw on 3-times weekly HD. Assuming weekly fluid intake to remain constant, we estimated UFw by subtracting the estimated UVolw for 2-times weekly HD from the weekly fluid intake. To determine highest UF rate, each patient’s theoretical HD UF goal based on a Monday to Thursday HD schedule was divided by the time of dialysis “t” obtained from the determination of urea clearance adequacy (described above) and then divided by the average post-HD weight. We used a weight-based cut-off of ≤13 ml/kg/h as an upper-limit acceptable UF rate. If the estimated value was >13 ml/kg/h using the “t” for urea clearance, we then determined whether extending to a 4-hour treatment, the theoretical maximum HD duration, would allow for a UF rate below our upper-limit (urea clearance adequacy would be more than adequate in this case). We also calculated a UF rate scaled to body surface area. The presence or absence of prescribed diuretics was also noted.
Dialysis Hemodynamic Criterion
The 3-month average of pre- and post-HD blood pressures were used to determine hemodynamic stability. Pre- and post-HD mean arterial pressures were calculated, and a patient with a decrease in post-HD mean arterial pressure of >10 mm Hg or a mean post-HD systolic blood pressure of <90 mm Hg was deemed to be not ideal for 2-times weekly HD.
Dialysis Side Effects Criterion
Reported or observed patient side effects during the HD on actual 3-times weekly HD included nausea, vomiting, cramping or hypotension. Treatment side effects were totaled per month. If more than half of HD treatments in a month included 1 or more side effects, the patient was deemed to be not ideal for 2-times weekly HD.
Theoretically Feasible for Optimal 2-Times Weekly Incremental HD
If a patient was able to achieve adequate urea clearance with 2-times weekly HD, to require a UF rate of ≤ 13 ml/kg/h per HD of ≤4 hours per treatment, to demonstrate stable blood pressure on that individual’s actual 3-times weekly regimen, and to have minimal dialysis-related side effects, the patient was deemed to be an optimal candidate for a 2-times weekly incremental HD regimen. In patients who could theoretically achieve adequate small-molecule clearance on 2-times weekly HD of ≤ 4 hours per HD session, we deemed them to be appropriate to consider for incremental HD. Those who could not realistically obtain adequate weekly urea clearance on such a regimen we labeled as nonideal for such a strategy.
Statistics and Analyses
Continuous values were expressed as mean (± SD) or median (interquartile range). Comparisons of continuous variables used the Student t test. The χ2 test was used for analysis of proportions between groups. Linear regression was used to look at the correlation between urine volume and measured urea clearance as well as between measured urea clearance and kinetic modeled urea clearance (IBM SPSS version 24; IBM Corporation, Armonk, NY). To compute the dialysis time needed to achieve the theoretical stdKt/V(dialysis) assuming 2-times weekly HD frequency (from equations 1, 2, 3, and 7), we used the solver add-in function in Excel (Microsoft Excel 2010; Microsoft Corporation, Redmond, WA) and applied the generalized reduced gradient nonlinear method with constraint precision set at < 1.0 × 10−6 to our model, which varied time “t” to achieve the desired stdKt/V(dialysis).
Results
Patient Characteristics
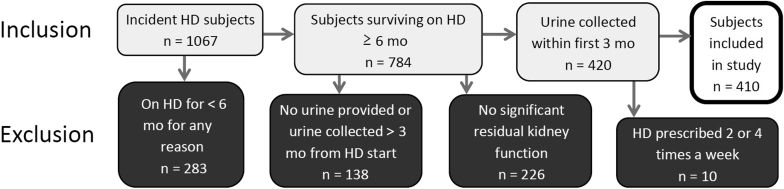
There were 1067 incident adult HD patients accepted into 4 dialysis clinics between January 2000 and December 2014. Patients were excluded for the following reasons: (i) discharged from the dialysis unit in <6 months due to death, transplantation, transfer out, or loss to follow up (n = 283, 26.5%); (ii) no available urine collection, or urine collection completed >3 months from first HD in the clinic (n = 138, 12.9%); (iii) no significant urine output based on patient report (n = 226, 21.2%); and (iv) HD other than 3-times weekly (n = 10, 0.9%) (Figure 1). The remaining 410 patients (38.4%) comprised the basis for the study.
Figure 1.
Inclusion and exclusion of incident hemodialysis (HD) patients.
Incremental 2-Times Weekly HD
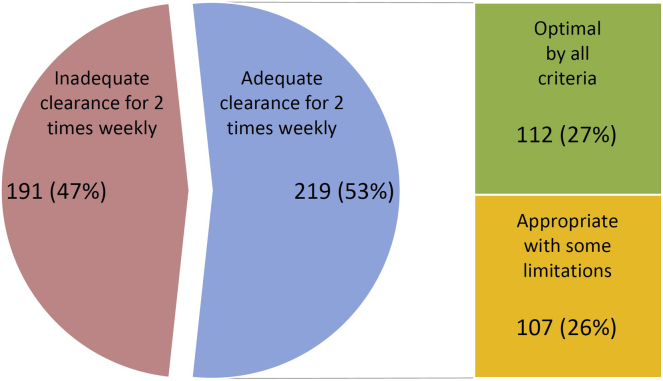
We required an incremental HD start patient to be theoretically able to achieve a stdKt/V(total) of at least 2.3 volumes. We found that 219 subjects (53%) would be able to achieve an adequate urea clearance on 2-times weekly HD within a maximum allowable treatment time of 4 hours per HD session, and the median duration of HD needed to satisfy urea clearance needs was only around 2.4 hours per HD session.
When we applied the restriction of a per-HD UF rate of ≤13 ml/kg/h and no issues with hypotension or adverse symptoms on HD, 112 of the 219 patients fulfilled these rather strict criteria, and we considered this group our “optimal” incremental HD group. Of the remaining 107 patients who met small-solute clearance requirements, whom we deemed “appropriate” to consider for 2-times weekly HD, the majority failed because of higher UF rates. Figure 2 illustrates how a few of these patients were not “optimal” for incremental HD due to other issues such as a drop in blood pressure and/or excessive side effects on their actual 3-times weekly HD. In the group considered “appropriate” for incremental HD the estimated weekly fluid gains were well above the 1.5 L per day intake restriction commonly recommended by renal dieticians. In addition, only about 36% of the “appropriate” group had a diuretic on their active medication log.
Figure 2.
Numbers and percentages of incident patients with 24-hour urine collections, and determination of feasibility of incremental 2-times weekly hemodialysis.
The remaining 191 patients, who could not theoretically achieve minimal weekly clearance of 2.3 volumes, were considered “nonideal” for incremental HD. Table 1 provides demographic, urine collection, and actual and theoretical HD details of the study groups. Notably, patients with a lower body weight were more likely to be able to achieve the adequacy goals with 2-times weekly HD. We also noted that, in the group considered to be nonideal for incremental HD, more than 50% of patients had a collected 24-hour urine volume of > 500 ml, and 11% had a measured kidney urea clearance of > 3 ml/min.
Table 1.
Characteristics of patients who are deemed optimal, appropriate, and not ideal for a 2-times weekly hemodialysis (HD) schedule
| Characteristic | Optimal for 2-times weekly HD (n = 112) |
Appropriate for 2-times weekly HD (n = 107) |
Nonideal for 2-times weekly HD (n = 191) |
|---|---|---|---|
| Demographic | |||
| Age, yr | 58.9 ± 15.0 | 58.0 ± 14.2 | 55.9 ± 16.1 |
| Female gender, % (n)a | 53.6 (60) | 37.4 (40) | 41.7 (71) |
| Race/ethnicity % (n)a | |||
| White/non-Hispanic | 29.5 (33) | 26.2 (28) | 24.6 (47) |
| White/Hispanic | 17.9 (20) | 15.9 (17) | 16.8 (32) |
| Black | 22.3 (25) | 26.2 (28) | 29.8 (122) |
| Asian | 17.9 (20) | 17.8 (19) | 7.3 (14) |
| Other or unknown | 12.5 (14) | 14.0 (15) | 15.2 (29) |
| Cause of ESRD, % (n)a | |||
| Diabetes mellitus | 51.8 (58) | 58.9 (63) | 45.5 (87) |
| Hypertension | 23.2 (26) | 13.1 (14) | 22.5 (43) |
| Glomerular disease | 10.7 (12) | 12.1 (13) | 16.2 (31) |
| Cystic/interstitial disease | 8.0 (9) | 3.7 (4) | 7.3 (14) |
| Other/unknown | 6.3 (7) | 12.1 (13) | 8.4 (16) |
| 24-Hour urine collection | |||
| Time interval between first HD and urine collection, daysa | 14 (7–235) | 29 (15–57) | 26 (12–48) |
| Collected during short (2-day) interdialytic period, % (n) | 36.6 (41) | 38.3 (41) | 42.4 (81) |
| Volume, mla | 1213 (800–1812) | 950 (700–1400) | 550 (400–963) |
| Urine volume > 500, ml, % (n)a | 91.1 (102) | 86.0 (92) | 55.0 (105) |
| Est. weekly urine volume, La,b | 6.9 (4.9–10.4) | 5.1 (4.1–7.8) | 3.4 (2.2–5.6) |
| Urea clearance, ml/mina,b | 4.0 (2.6–5.2) | 3.1 (2.0–4.3) | 1.5 (0.9–2.2) |
| Urea clearance > 3 ml/min, % (n)a | 67.9 (76) | 52.3 (56) | 11.5 (22) |
| stdKt/V (renal)a,b | 1.02 (0.75–1.28) | 0.75 (0.54–0.99) | 0.31 (0.21–0.46) |
| Medications prescribed | |||
| Diuretics, % (n) | 41.1 (46) | 36.4 (39) | 36.1 (69) |
| ACEI or ARB, % (n) | 58.9 (66) | 66.4 (71) | 68.6 (131) |
| EPO units/kg/g Hgb | 51 (29–79) | 63 (38–97) | 53 (33–83) |
| Laboratory data | |||
| Serum albumin, g/dl | 3.6 ± 0.4 | 3.5 ± 0.5 | 3.6 ± 0.4 |
| Hgb, g/dl | 11.2 ± 0.8 | 11.2 ± 0.9 | 10.9 ± 0.8 |
| Serum potassium, mEq/la | 4.6 ± 0.4 | 4.9 ± 0.5 | 4.7 ± 0.5 |
| Serum bicarbonate, mmol/l | 21.1 ± 2.2 | 21.2 ± 2.6 | 21.2 ± 2.3 |
| Serum phosphorus, mg/dla | 5.2 ± 0.9 | 5.5 ± 1.2 | 5.9 ± 1.4 |
| Pre-HD BUN, mg/dla | 54 ± 20 | 52 ± 18 | 61 ± 22 |
| Post-HD BUN, mg/dla | 18 ± 10 | 18 ± 8 | 23 ± 10 |
| Actual 3-times weekly HD prescription and adequacy parameters | |||
| Dialysis duration per HD, min | 180 ± 15 | 186 ± 14 | 187 ± 17 |
| spKt/Va | 1.30 ± 0.44 | 1.28 ± 0.29 | 1.14 ± 0.29 |
| Total Kt/V (incorporates KUR)a | 1.76 ± 0.36 | 1.66 ± 0.28 | 1.28 ± 0.30 |
| stdKt/V (dialysis)a | 2.08 ± 0.33 | 2.12 ± 0.29 | 1.90 ± 0.33 |
| stdKt/V (total)a | 3.19 (2.91–3.56) | 2.92 (2.75–3.26) | 2.35 (2.12–2.49) |
| UF volume per HD, La | 1.5 ± 0.7 | 2.6 ± 1.0 | 2.2 ± 1.4 |
| Total volume gains per week (est. weekly urine + weekly UF volume), La | 12.1 ± 4.3 | 13.8 ± 4.7 | 10.8 ± 4.4 |
| Dry weight, kga | 75.7 ± 19.1 | 74.0 ± 23.0 | 80.5 ± 21.7 |
| BSA, m2a | 1.83 ± 0.25 | 1.82 ± 0.29 | 1.90 ± 0.27 |
| Pre-HD systolic BP, mm Hga | 145 ± 16 | 151 ± 19 | 149 ± 19 |
| Pre-HD diastolic BP, mm Hg | 76 ± 9 | 78 ± 10 | 79 ± 13 |
| Post-HD systolic BP, mm Hga | 148 ± 20 | 142 ± 17 | 148 ± 19 |
| Post-HD diastolic BP, mm Hg | 78 ± 10 | 76 ± 8 | 79 ± 12 |
| HD access with catheter, % (n) | 69.6 (78) | 66.4 (71) | 64.9 (124) |
| Kinetic modeling data | |||
| Kd (modeled dialyzer clearance), ml/min | 267 ± 40 | 274 ± 39 | 266 ± 39 |
| G (urea generation rate), mg/min | 5.3 (4.0–7.0) | 5.5 (4.4–7.3) | 5.5 (4.2–7.4) |
| Urea distribution volume, La | 38.7 ± 9.6 | 41.1 ± 10.1 | 48.8 ± 13.2 |
| Theoretical 2-times weekly urine output, HD prescription requirement, and adequacy parametersc | |||
|---|---|---|---|
| Est. weekly urine volume (L)a,b | 8.1 (5.7–12.1) | 6.0 (4.8–9.2) | 4.0 (2.5–6.6) |
| stdKt/V (dialysis) needed to achieve 2.3 weekly goala | 1.12 (0.76–1.40) | 1.43 (1.19–1.68) | 1.93 (1.79–2.05) |
| spKt/V per HD, 2 times/wk, to achieve 2.3 stdKt/V goala | 0.87 (0.52–1.25) | 1.20 (0.92–1.66) | 2.47 (1.97–3.06) |
| Hours per HD needed to achieve spKt/Va | 2.0 (1.2–2.9) | 2.9 (1.9–3.6) | 6.4 (4.8–8.1) |
| % Able to reach adequacy goal with 2-times weekly HD ≤ 4-h treatment timea | 100 | 100 | 0 |
| UF volume per HD, La | 2.4 (1.6–3.3) | 3.9 (2.0–4.9) | 3.4 (2.3–4.4) |
| UF rate, ml/kg/ha | 7.9 (5.8–10.2) | 13.9 (10.1–17.9) | 11.0 (7.0–13.9) |
| UF rate, ml/kg/h per m2a | 4.4 (3.1–5.6) | 7.6 (5.3–10.9) | 5.6 (3.6–7.7) |
| UF rate ≤ 13 ml/kg/h, % (n)a | 100 (112) | 44.9 (48) | 72.8 (139) |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; BSA, body surface area; BUN, blood urea nitrogen; EPO, erythropoietin; ESRD, end-stage renal disease; est., estimated; Hgb, hemoglobin; Kd, modeled dialyzer clearance spKt/V, single pool per treatment clearance; stdKt/V, standard weekly clearance of urea; UF, ultrafiltration.
Continuous values expressed as mean ± SD or median (25th−75th percentiles).
Statistically significant among groups by analysis of variance or χ2, P < 0.05, 2-sided.
Weekly urine volume estimation or urea clearance adjusted based on day of actual collection relative to interdialytic period, and adjusted for frequency of HD, either 3-times or 2-times weekly.
Where values were possible; 5 patients had mathematically impossible spKt/V requirements in the “nonideal” group who were not included in the analysis.
Correlation of Measured Urea Clearance to Urine Volume and Modeled Urea Clearance
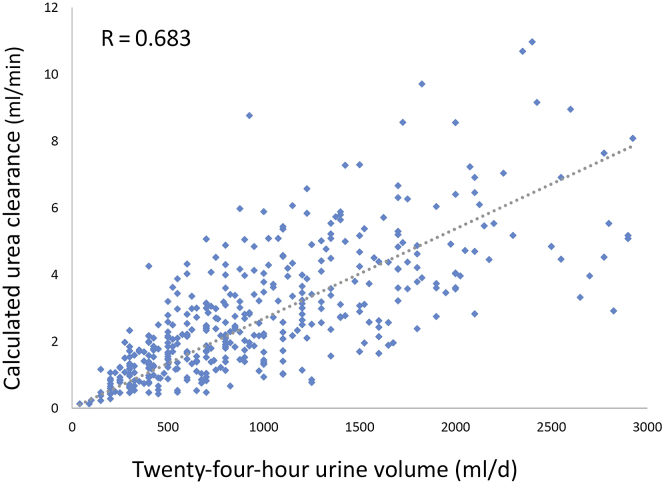
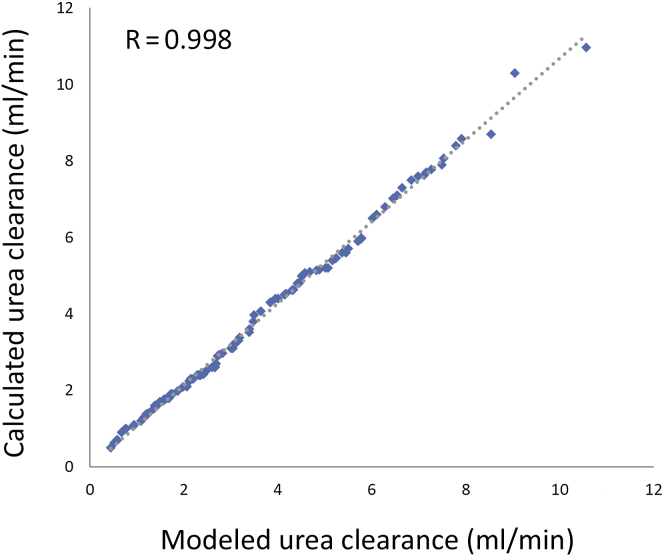
Because many prior studies have used a 24-hour urine volume as surrogate for adequate KRU, we examined the relationship between measured urea clearance and urine volume. We used the calculated urea clearance adjusted for the interdialytic interval and found that the correlation was significant but not ideal (Figure 3). We also explored the relationship between measured urea clearance and kinetically modeled urea clearance in 113 of the 410 subjects whose data were available and who had formal kinetically modeled KRU at the same time as the calculated urea clearance (Figure 4), and the correlation was good.
Figure 3.
Correlation of calculated urea clearance with urine collection volume in 410 patients.
Figure 4.
Correlation of calculated urea clearance with kinetically modeled urea clearance in 112 patients; urine collection performed 24 hours before blood tests used for kinetic modeling.
Discussion
Incremental start of HD involves the prescription of dialysis to complement what is attained by RKF. In this study, we found that a significant number of incident HD patients who were initiated on a traditional 3-times weekly HD schedule could have started in an incremental 2-times weekly fashion. Our idealized candidate for this strategy would have adequate total weekly urea clearance when taking into account KRU, a per-HD UF rate ≤13 ml/kg/h, and hemodynamic stability with minimal adverse symptoms on HD. With these defined and tight constraints, we found that 27% of stable incident HD patients (those who survived ≥6 months) with measured residual kidney function within the first 3 months could have been optimal patients for a 2-times weekly schedule. Another 107 patients, or 26% of the total cohort, were appropriate to consider for 2-times weekly HD. This group had adequate theoretical urea clearance but were mostly not optimal from the standpoint of a higher ultrafiltration need. However, we noted that the majority of the subjects in this group were not on a diuretic; had they been, it would be likely that HD UF requirements could be lowered and would have allowed some patients to be deemed optimal for 2-times weekly HD.
There are growing data and sentiment that incremental HD can be a safe and perhaps even a desirable approach to initiating HD in the appropriate patients.17, 18, 19, 20, 21, 22 We were uniquely positioned to evaluate the feasibility of incremental HD in incident patients, as it has been our practice to obtain timed 24-hour urine collections in all patients, whereas overall very few HD patients in this country have any formal evaluation of RKF,10 with 3-times weekly HD as the default prescription for the majority of new patients.
Residual kidney function has tremendous benefits in HD patients beyond urea clearance, including improved volume control, better electrolyte balance, and lower erythropoietin-stimulating agent requirements.4 Most importantly, mortality is lower in HD patients with RKF.2, 5, 6, 23 We similarly found lower serum potassium and phosphorus in the group optimally suited for incremental HD, which had the highest level of RKF. In addition, studies in both peritoneal dialysis and HD patients suggest that this survival advantage is due almost exclusively to the level of RKF; increasing dialyzer clearances provides little to no additional benefit.7, 24 Therefore, the preservation of RKF should also be a priority in patients on HD.
The rate of decline of RKF is more rapid in HD than in peritoneal dialysis patients.10, 25 Nonetheless, substantial RKF may last for years in HD patients,26, 27 and our own longitudinal urine collections support this. The frequency of HD may have an effect on the longevity of RKF. In HD schedules >3-times weekly, RKF appears to decline at a faster rate.28 The converse, a slower rate of decline of RKF with <3-times weekly HD treatments, is suggested by a recent retrospective analysis of 2- versus 3-times weekly HD patients.20 However, true cause-and-effect nature of this association remains unknown.
Prior studies looking at the effects of RKF on outcomes have used self-reported urine output volumes, often as a cutoff value of 250 or 500 ml per day, as representative of substantial RKF.5 In the review by Kalantar-Zadeh et al.,29 urine volume >0.5 L per day was 1 of 10 criteria that were proposed to identify candidates for 2-times weekly HD. In our study, although the correlation of a 24-hour urine volume with measured urea clearance was not ideal (the model predicting only 49% of variance between the 2 factors), we acknowledge that daily urine output may vary depending on when the urine is collected within the week. In our estimations of weekly urine output, we took this daily variation into account and adjusted accordingly.15 Since about 55% of our “nonideal” subjects had a measured 24-hour urine volume of >500 ml, it is our opinion that if an incremental HD strategy is to be implemented, it is best done with guidance provided by serial measures of urea clearance in a timed urine collection and not by an assessment of urine volume alone. We were also able to compare formal urea kinetic modeled residual renal urea clearance to the measured residual kidney urea clearance in about one-fourth of the patients. The correlation was good, and we believe that the method for calculating the kidney urea clearance (equation 4 in Materials and Methods) is a reasonable approach in the absence of formal kinetic modeling programs. Until techniques to estimate residual renal function without a timed urine collection are refined and validated,30 a urine-based approach is required.
Our study had limitations and unknowns. First, it is a small, single-center evaluation. Second, we made some important assumptions about a theoretical 2-times weekly HD, using the actual 3-times weekly dialysis treatment data. Specifically, total weekly fluid volume gains were assumed not to change regardless of the frequency of dialysis. We did, however, attempt to adjust weekly urine volume for the effect that HD has on urine flow. Even with these adjustments, the true volume balance and residual kidney function, given the longer interdialytic periods with 2-times weekly dialysis, are unpredictable and would require careful monitoring to see if “real life” mirrors these theoretical adjustments. Nonetheless. we believe that these assumptions would be a good starting point from a practical perspective in singling out potential patients for 2-times weekly HD. Third, we assumed that removing weekly fluid accumulation in 2 sessions would be tolerated without additional side effects, and assumed a spacing of 3 and 4 days between HD sessions. Also, evaluation of diuretic use was based on the electronic medication list, which is frequently updated but has an inherent level of inaccuracy. Finally, we used a fixed-target total stdKt/V of 2.3, which assumes equivalent clinical value of dialyzer versus residual kidney small-molecule clearance. This target is generally accepted,8 and adjustment of HD prescriptions to take into account KRU>2 ml/min has been suggested as reasonable, although the question of frequency of HD has not been specifically mentioned.31 However, each milliliter per minute of KRU may be worth more than each milliliter per minute of dialyzer clearance. Thus, a variable-target model dependent on the relative contribution of native kidney function to the total clearance, as provocatively proposed by Casino and Basile,32 may be an even more precise approach to prescribing HD in the setting of RKF. At this point, assuming equivalency of KRU to dialyzer clearance errs on the safe side; if anything, it conservatively manages the urea clearance determination.
Our study can be seen as a “thought exercise” in examination of the prospect of incremental HD in an incident contemporary dialysis population. Strengths of our study include the very detailed and formal analysis of theoretical urea clearance adequacy, HD ultrafiltration rate, blood pressure, and dialysis treatment side effects using patient-level data. We believed that these parameters would be important to most clinicians in their decision to prescribe incremental HD, and therefore we placed very conservative constraints on these parameters in our determination of ideal candidacy for 2-times weekly HD. For example, the threshold UF rate of ≤13 ml/kg/h is based on data associating higher dialysis volume removal rates with mortality33; however, the risk of UF rate is probably not a threshold effect,33 and the benefit of targeting a particular UF rate ceiling is unclear.34 Scaling of the UF rate to body surface area rather than to weight has been proposed as an alternative method,35 with 1 recent study showing a more stable effect across body surface area strata. Nonetheless, higher UF rates appear to be associated with higher mortality, regardless of indexing technique.36 Our inclusion of incident patients who were dialyzed for at least 6 months would likely have excluded the most unstable patients as well as those who may have had acute kidney injury with subsequent recovery. Most importantly, our clinics attempt to obtain 24-hour urine collections in all HD patients. This allows us to provide a comprehensive snapshot of the viability of incremental HD start across a contemporary incident patient cohort in the United States.
Incremental HD is not new, and it is sporadically applied by many nephrologists. Nephrologists in our practice have used residual function data mainly to adjust treatment times, but maintain most patients on 3-times weekly HD prescriptions. The lack of good, high-value medical evidence regarding incremental HD in incident patients is the primary reason that incremental 2-times weekly prescriptions are not universally used more often with nephrologists at our center, even when RKF data are available. Our center has used incremental 2-times weekly HD for patients whose transition from chronic kidney disease to ESRD is smooth and without acute clinical events, and for renal transplant patients whose graft function has gradually declined to the point of requiring dialysis. Other groups have also recently outlined details of their experience with incremental HD37 and have reported outcomes similar to those in patients on 3-times weekly HD.38
Incremental start HD can initially be a much more labor-intensive process.39 It requires not only patient understanding and counseling that the HD prescription will change over time, but also the cooperation of the patient and dialysis provider in obtaining frequent urine collections and ensuring that stdKt/V(renal) is reported and reviewed frequently by the care team. We want to emphasize that serial urine monitoring is critical to such a program, as has been recommended by societal guidelines as it pertains to adjusting HD prescriptions when taking into account KRU.8 We also believe that incremental start HD is not contradictory to the notion that longer and perhaps more frequent HD may benefit some patients. In fact, the 2 can be seen as both ends of a continuum of options that best customizes the dialysis regimen for a given patient’s evolving clinical status and needs.
With the growing observational and retrospective information suggesting that it may be safe and feasible, the time seems right for a prospective randomized trial to look at incremental start HD as appropriate for a subset of incident patients.40 Our findings show that 2-times weekly HD may be applicable and appropriate for more than 50% of clinically stable incident dialysis patients with RKF in this country.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors would like to thank Albert I. Chin for assistance is developing the Excel-based generalized reduced gradient algorithm utilized in solving for the duration of the dialysis treatment. We also would like to thank Dialysis Clinic, Inc., for support of investigator-initiated research. Portions of this manuscript were presented at the American Society of Nephrology Kidney Week Meeting, San Diego, California, 4–8 November 2015. The conclusions in this report are the responsibilities of the authors and in no way should be seen or interpreted as official policy of the US Government.
References
- 1.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2015. 2015 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 2.Vilar E., Wellsted D., Chandna S.M. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24:2502–2510. doi: 10.1093/ndt/gfp071. [DOI] [PubMed] [Google Scholar]
- 3.Marquez I.O., Tambra S., Luo F.Y. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol. 2011;6:290–296. doi: 10.2215/CJN.06100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penne E.L., van der Weerd N.C., Grooteman M.P. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:281–289. doi: 10.2215/CJN.04480510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shafi T., Jaar B.G., Plantinga L.C. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi: 10.1053/j.ajkd.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shemin D., Bostom A.G., Laliberty P., Dworkin L.D. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 7.Termorshuizen F., Dekker F.W., van Manen J.G. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15:1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Tattersall J., Martin-Malo A., Pedrini L. EBPG guideline on dialysis strategies. Nephrol Dial Transplant. 2007;22(suppl 2):ii5–ii21. doi: 10.1093/ndt/gfm022. [DOI] [PubMed] [Google Scholar]
- 10.Moist L.M., Port F.K., Orzol S.M. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 11.Chin A.I., Depner T.A. Measuring hemodialysis using solute kinetic models. In: Lerma E.V., Weir M.R., editors. Henrich's Principles and Practice of Dialysis. 5 ed. Wolters Kluwer; Philadelphia, PA: 2017. pp. 70–94. [Google Scholar]
- 12.Chin A.I., Depner T.A., Daugirdas J.T. Assessing the adequacy of small solute clearance for various dialysis modalities, with inclusion of residual native kidney function. Semin Dial. 2017;30:235–240. doi: 10.1111/sdi.12584. [DOI] [PubMed] [Google Scholar]
- 13.Daugirdas J.T., Depner T.A., Greene T. Standard Kt/Vurea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77:637–644. doi: 10.1038/ki.2009.525. [DOI] [PubMed] [Google Scholar]
- 14.Daugirdas J.T. Estimating time-averaged serum urea nitrogen concentration during various urine collection periods: a prediction equation for thrice weekly and biweekly dialysis schedules. Semin Dial. 2016;29:507–509. doi: 10.1111/sdi.12554. [DOI] [PubMed] [Google Scholar]
- 15.Daugirdas J.T. Estimating weekly urine flow rate and residual kidney urea clearance: a method to deal with interdialytic variability. Semin Dial. 2016;29:510–514. doi: 10.1111/sdi.12558. [DOI] [PubMed] [Google Scholar]
- 16.van Olden R.W., van Acker B.A., Koomen G.C. Time course of inulin and creatinine clearance in the interval between two haemodialysis treatments. Nephrol Dial Transplant. 1995;10:2274–2280. doi: 10.1093/ndt/10.12.2274. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K., Casino F.G. Let us give twice-weekly hemodialysis a chance: revisiting the taboo. Nephrol Dial Transplant. 2014;29:1618–1620. doi: 10.1093/ndt/gfu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libetta C., Nissani P., Dal Canton A. Progressive hemodialysis: is it the Future? Semin Dial. 2016;29:179–183. doi: 10.1111/sdi.12455. [DOI] [PubMed] [Google Scholar]
- 19.Lin X., Yan Y., Ni Z. Clinical outcome of twice-weekly hemodialysis patients in shanghai. Blood Purif. 2012;33:66–72. doi: 10.1159/000334634. [DOI] [PubMed] [Google Scholar]
- 20.Obi Y., Streja E., Rhee C.M. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68:256–265. doi: 10.1053/j.ajkd.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee C.M., Unruh M., Chen J. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26:720–727. doi: 10.1111/sdi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong J., Vilar E., Davenport A., Farrington K. Incremental haemodialysis. Nephrol Dial Transplant. 2015;30:1639–1648. doi: 10.1093/ndt/gfv231. [DOI] [PubMed] [Google Scholar]
- 23.Lee M.J., Park J.T., Park K.S. Prognostic value of residual urine volume, GFR by 24-hour urine collection, and eGFR in patients receiving dialysis. Clin J Am Soc Nephrol. 2017;12:426–434. doi: 10.2215/CJN.05520516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bargman J.M., Thorpe K.E., Churchill D.N., CANUSA Peritoneal Dialysis Study Group Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001;12:2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 25.Jansen M.A., Hart A.A., Korevaar J.C. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 26.Hung A.M., Young B.S., Chertow G.M. The decline in residual renal function in hemodialysis is slow and age dependent. Hemodial Int. 2003;7:17–22. doi: 10.1046/j.1492-7535.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 27.Patel N., Hu S.L. Preserving residual renal function in dialysis: what we know. Semin Dial. 2015;28:250–258. doi: 10.1111/sdi.12302. [DOI] [PubMed] [Google Scholar]
- 28.Daugirdas J.T., Greene T., Rocco M.V. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K., Unruh M., Zager P.G. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong J., Kaja Kamal R.M., Vilar E., Farrington K. Measuring residual renal function in hemodialysis patients without urine collection. Semin Dial. 2016;30:39–49. doi: 10.1111/sdi.12557. [DOI] [PubMed] [Google Scholar]
- 31.Hemodialysis Adequacy Work Group Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 32.Casino F.G., Basile C. The variable target model: a paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant. 2017;32:182–190. doi: 10.1093/ndt/gfw339. [DOI] [PubMed] [Google Scholar]
- 33.Flythe J.E., Kimmel S.E., Brunelli S.M. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer H., Yee J., Weiner D.E. Ultrafiltration rate thresholds in maintenance hemodialysis: an NKF-KDOQI controversies report. Am J Kidney Dis. 2016;68:522–532. doi: 10.1053/j.ajkd.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Daugirdas J.T., Schneditz D. Hemodialysis ultrafiltration rate targets should be scaled to body surface area rather than to body weight. Semin Dial. 2017;30:15–19. doi: 10.1111/sdi.12563. [DOI] [PubMed] [Google Scholar]
- 36.Assimon M.M., Wenger J.B., Wang L., Flythe J.E. Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2016;68:911–922. doi: 10.1053/j.ajkd.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghahremani-Ghajar M, Rojas-Bautista V, et al. Incremental hemodialysis: the University of California Irvine experience. Semin Dial., in press. [DOI] [PMC free article] [PubMed]
- 38.Mathew A., Obi Y., Rhee C.M. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90:1071–1079. doi: 10.1016/j.kint.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 39.Golper T.A. Incremental hemodialysis: how I do it. Semin Dial. 2016;29:476–480. doi: 10.1111/sdi.12530. [DOI] [PubMed] [Google Scholar]
- 40.Marshall M.R. Observations of twice a week hemodialysis. Kidney Int. 2016;90:936–938. doi: 10.1016/j.kint.2016.06.040. [DOI] [PubMed] [Google Scholar]