Abstract
Introduction
The magnitude of chronic kidney disease (CKD) in Panama has yet to be described. We investigated the association between sociodemographic and cardiovascular exposures with CKD in 2 Panamanian provinces. Further, we analyzed national trends of CKD mortality from 2001 to 2014.
Methods
Data were derived from Prevalencia de Factores de Riesgo de Enfermedad Cardiovascular (PREFREC [Survey on Risk Factors Associated With Cardiovascular Disease]), a cross-sectional study designed to analyze the prevalence of risk factors associated with cardiovascular disease. Biomarkers of kidney function were measured in 3590 participants. CKD was defined as an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2 and/or albuminuria ≥30 mg/g creatinine. Odds ratios (ORs) with 95% confidence intervals (CIs) for CKD were calculated using logistic regression. We calculated age-standardized CKD mortality rates in the country using the National Mortality Register. Annual percentage change and 95% CIs were estimated to evaluate the trends over time.
Results
The prevalence of CKD was 12% (reduced eGFR: 3.3%; albuminuria; 9.9%). CKD was associated with hypertension (OR: 1.8; 95% CI: 1.2−2.7), age 60 years or older (OR: 1.9; 95% CI: 1.2−2.9), and previous myocardial infarction (OR: 2.4; 95% CI: 1.0−5.7), whereas monthly family income was inversely associated with CKD (OR: 0.4; 95% CI: 0.1−0.9) (adjusted). A sustained increase in the trend of CKD mortality was observed from 2001 to 2006, followed by a decreasing trend in subsequent years. Coclé province had the highest adjusted mortality rate.
Discussion
CKD poses a significant health problem for Panama. Health inequalities and an increase of cardiometabolic risk factors warrant robust epidemiological surveillance, improved diagnosis, and treatment. Further national studies aimed to address geographical disparities are necessary.
Keywords: chronic kidney disease, mortality, Panama
In Latin America, the prevalence of end-stage renal disease (ESRD) has been growing by 6.8% annually, and renal replacement therapy is increasing steadily.1, 2 Furthermore, a recent study on global mortality reported that chronic kidney disease (CKD) decreased life expectancy, most notably in central Latin America.3 In addition to the traditional risk factors, emerging evidence also suggested a nonconventional CKD etiology in the region, termed “Mesoamerican nephropathy.”4
Panama is a country of >3.9 million inhabitants located at the southernmost end of Central America. It is divided into 10 provinces and 5 “Comarcas,” which are geographically defined areas populated by various Amerindian groups. As reported by the World Bank, Panama has been one of the fastest growing economies over the past decade; yet, poverty remains pervasive in rural areas, particularly in those inhabited by the Amerindians.5 Moreover, its Gini coefficient is 51.9, ranking it among the uppermost unequal countries.6
In 2013, CKD escalated to the seventh leading cause of years of life lost due to premature mortality compared with 1990, when it ranked in the 17th position.7 By 2009, Panama had one of the highest age-standardized mortality rates (ASMRs) of CKD in the region (12.3 per 100,000 population).8 Despite these data, limited research has been undertaken to assess the epidemiology of this disease. An additional etiology that has contributed to mortality from CKD is the renal sequelae that followed recovery from episodes of acute diethylene glycol poisoning in 2006.9, 10, 11
In 2011, a population-based study with a small sample size (n = 393) evaluated the prevalence of risk factors associated with CKD among 3 communities in the province of Coclé, which has the highest prevalence of patients treated with hemodialysis. The study reported an increasing north-to-south gradient of biomarkers of kidney dysfunction.12
CKD portends great public health challenges for Panama; therefore, a better understanding of CKD epidemiology is urgently needed to generate evidence-based health policies. The aim of this study was 2-fold: (i) to investigate the association between sociodemographic and cardiovascular risk factors with CKD in a population-based study in 2 Panamanian provinces, and (ii) to analyze the mortality trends due to CKD during a 13-year period, using the National Mortality Register.
Material and Methods
PREFREC Study
The data were derived from the cross-sectional descriptive Prevalencia de Factores de Riesgo de Enfermedad Cardiovascular (PREFREC [Survey on Risk Factors Associated With Cardiovascular Disease]) study, which analyzed the risk factors associated with cardiovascular disease (CVD) in Panama, but included only the provinces of Panama and Colon. The study base included citizens older than 18 years of age who resided in private housing between October 2010 and January 2011. These 2 provinces, located in the transisthmian zone where 60.4% of all Panamanians 18 years or older reside, also houses 60% of the total population in the country. Housing was sampled using a probabilistic and randomized approach with a multivariate stratification stage. As a first stage, census segments (according to the national census for the 2000) were used as selection strata, and samples were calculated separately for urban, rural, and indigenous land use areas. In the second stage, primary sampling units (which consisted of 8−30 private occupied homes) were randomly selected and were stratified according to the Administrative Political Code of the Republic and then by population size. The third stage stratified these units according to the education level. A total of 3505 completed interviews were expected by the end of the survey, which would guarantee the maximum relative error for the average estimates of the variables. Fifteen days before the survey started, the population segments were visited (prescreen procedure) to guarantee an adequate response rate, and that participants would be fasting. Up to a maximum of 2 members per household were invited to participate, so that the potentially nonresponding participant could be replaced by another participant who belonged to the household at the time of the visit. A total of 3590 participants agreed to be enrolled, which included 1074 men (29.9%) and 2516 women (70.1%). The PREFREC study was approved by the National Bioethics Committee of Panama. All participants signed an informed consent to be enrolled in the study. A detailed description of the study has been reported elsewhere.13, 14
Briefly, the study participants were invited to answer a questionnaire on sociodemographic information, lifestyle, diet, medical history, and to participate in a health screening in which anthropometric measurements were recorded. Blood and urine samples were obtained after overnight fasting and analyzed by the Central Laboratory of the Gorgas Memorial Institute for Health Studies.
For the present study, those women who reported to be pregnant (n = 47) were excluded, leaving 3543 study participants. Creatinine levels (serum and urinary) and albuminuria were measured using Synchrom Beckman Cx7. Creatinine measurement was calibrated traceable to an isotope dilution mass spectrometry reference method.
Definition of CKD
The CKD-Epidemiology Collaboration group (CKD-EPI) equation was used to estimate the glomerular filtration rate (eGFR).15 Reduced renal function was defined as an eGFR of <60 ml/min/1.73 m2. Urinary albumin-to-creatinine ratio (ACR; milligrams per gram creatinine) was calculated. Study participants with an ACR >30 mg/g were considered to have albuminuria.
CKD was defined as an eGFR of <60 ml/min/1.73 m2 and/or albuminuria (ACR) ≥30 mg/g creatinine, according to the Kidney Disease Improving Global Outcomes guideline.16 A wide range of sociodemographic characteristics, established risk factors for CKD, and biomarker variables were evaluated as exposure of interest. Hypertension was defined as treatment with antihypertensive drugs and/or systolic blood pressure ≥140 mm Hg and a diastolic blood pressure of ≥90 mm Hg, and/or self-reported diagnosis. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dl, self-reported diagnosis, use of antidiabetic medication, and/or a glycosylated hemoglobin of ≥6.5. Smoking was categorized as current versus former or never. Body mass index was calculated as weight in kilograms divided by height in meters squared, and obesity was defined as a body mass index ≥30 kg/m2. Other variables evaluated were hypercholesterolemia (total cholesterol ≥ 200 mg/dl or receiving any lipid-lowering medication), physical inactivity (individuals who reported <150 min/week of physical activity, which was defined as any activity that required energy expenditure under aerobic conditions, such as sports and physical exercises), and family history of ESRD (yes/no). Previous myocardial infarction (MI) was defined as having had a MI or angina diagnosed by a physician. Sociodemographic variables, such as monthly family income (MFI), were assessed according to the following categories in US dollars (USD) (≤$250, $250−$300, $301−$600, $601−$999, $1000−$1200, and >$1201). Years of education were assessed as a continuous variable, whereas geographic area was coded as urban, rural, and areas populated by indigenous groups. Ethnicity (Mestizo, Afro-American, Amerindian, White-Caucasian, Asian, and others) was assessed according to self-reported data.
Statistical Analysis
Prevalence estimates and comparisons were weighted to represent the total adult population of Panama and Colon. Data from the Panamanian Population Sampling Census in 2010 were used as the standard population. Categorical variables are reported as proportions, whereas continuous variables are presented as means ± SD. Age-adjusted prevalence for the indicators of kidney damage was calculated in relation to the total population of Panama and Colon in 2010.
We analyzed the association between indicators of kidney damage and relevant covariates using unconditional logistic regression models. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Single adjustments for covariates were performed, as well as adjustments for combination of covariates, to evaluate their impact on the results. We presented crude and adjusted ORs (adjusted by sociodemographic factors, and traditional CVD and CKD risk factors).
National Mortality Registry
We conducted a registry-based study of mortality due to CKD in Panama, from the years 2001 to 2014. Data were obtained from the Department of Vital Statistics of the National Institute of Statistic and Census, the authority responsible for the compilation and codification of deaths.
The National Mortality Registry lists all deaths reported either from the Civil Registry or the Institute of Legal Medicine (deaths due to external causes). Recently, a global study that assessed the performance of civil registration and vital statistics systems reported the quality of Panamanian data as high.17
The definition of the underlying cause of death was recorded according to the International Classification of Disease-10th Revision (ICD-10). For the present study, a combined endpoint of ICD-10 codes N18 (chronic kidney disease) and N19 (unspecified kidney failures) was used. In addition, we performed an analysis in which acute kidney injury (AKI) (ICD-10 code N17) was evaluated as part of the outcome. Our hypothesis was that deaths due to intoxication that occurred in 2006 might have been reported as “acute renal failure syndrome.” Moreover, AKI is a major risk factor for CKD and vice versa, and has been identified as a long-term risk factor for ESRD and mortality.18
To calculate age-adjusted standardized mortality, the most recent World Health Organization world standard population was used as reference.19 Anonymous secondary data were used; therefore, no ethics approval was required.
Statistical Analysis
For the trend analysis, we computed ASMRs by sex. ASMRs were calculated as the number of deaths per 100,000, using the mortality data and the estimates and projections of the total population of Panama to July 1st for each year (based on the last census conducted in 2010). These specific mortality rates (MRs) were standardized for 5-year age groups using the direct method. Joinpoint regression analyses were performed to evaluate trends of MRs in women and in men.20 The program starts with the minimum number of joinpoints and tests whether more joinpoints are statistically significant and must be added to the model (up to the maximum number). The default value for the maximum number of joinpoints depends on the number of data points; for the present study it was set as 2.21 Therefore, a maximum of 2 joinpoints were calculated, depending on the best fit of the data.
Annual percentage change (APC) and 95% CIs were estimated to describe and test the statistical significance of the trends. The tests of significance used a Monte Carlo Permutation method.20 In the present study, a significance level of 0.05 was used for the permutation test, with 4499 of randomly permuted data sets. The null hypothesis in this analysis was that the trend in MRs was the same over time.20
Analyses were performed using Stata software (version 14; Stata Inc., College Station, TXs) and Joinpoint Regression Program (version 4.3.1.0; National Institutes of Health, Bethesda, MD).
Results
PREFEC Study
Table 1 shows the distribution of sociodemographic, metabolic, and lifestyle characteristics present in the study participants according to indicators of kidney dysfunction. Overall, 12% of the study participants had indicators of CKD (age-adjusted prevalence of 11.1%).
Table 1.
Distribution of selected variables in participants from PREFREC study according to indicators of kidney damage
| No indicator of kidney damage (n = 3113) |
eGFR <60 ml/min/1.73 m2 (n = 109) |
ACR ≥30 mg/g (n = 360) |
CKD (n = 430) |
|
|---|---|---|---|---|
| Weighted prevalencea | 88 | 3.3% | 9.9% | 12 |
| Age-adjusted prevalencea | 88.9% | 1.6% | 7.1% | 11.1% |
| Sociodemographics | ||||
| Sex M/F | 945/2168 | 58/51 | 95/265 | 129/301 |
| Age (yr) | 44.7 ± 15.8 | 68.3 ± 11.7 | 48.8 ± 17.7 | 52.2 ± 18.5 |
| Education (yr) | 10.0 ± 9.7 | 8.4 ± 13.1 | 9.8 ± 10.6 | 9.4 ± 10.8 |
| Areas | ||||
| Urban | 86.4 | 85.5 | 85.5 | 85.2 |
| Rural | 13.4 | 14.3 | 14.3 | 14.6 |
| Indigenous | 0.2 | 0.2 | 0.2 | 0.2 |
| Illiteracy | 4.3 | 4.1 | 3.2 | 3.8 |
| Indigenous ethnicity | 3.2 | 0.8 | 2.4 | 2.2 |
| Afro-Panamanian ethnicity | 18.6 | 27.9 | 22.5 | 23.2 |
| Monthly family income (USD; $) | ||||
| <250 | 27.9 | 27.8 | 28.1 | 29.6 |
| 250−300 | 18.9 | 17.1 | 22.5 | 20.9 |
| 301−600 | 28.0 | 29.3 | 25.8 | 25.4 |
| 601−999 | 11.5 | 5.4 | 12.7 | 11.3 |
| 1000−1200 | 6.8 | 12.7 | 9.6 | 9.9 |
| >1201 | 6.9 | 7.7 | 1.3 | 2.9 |
| Risk factors | ||||
| Hypertension | 34.7 | 80.4 | 46.4 | 53.2 |
| Diabetes | 9.6 | 23.1 | 17.1 | 18.1 |
| Obesity | 28.1 | 20.1 | 37.3 | 34.6 |
| Hypercholesterolemia | 43.8 | 63.9 | 36.7 | 40.8 |
| Family history of ESRD | 11.6 | 11.7 | 13.1 | 12.0 |
| Current smokers | 8.3 | 7.2 | 1.4 | 2.5 |
| Physical inactivity | 8.5 | 20.1 | 10.4 | 12.7 |
| Previous MI | 2.4 | 9.5 | 6.8 | 6.8 |
Data expressed as percentages or mean ± SD.
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MI, myocardial infarction; USD, US dollars.
Age-adjusted prevalence was calculated in relation to the total population of the provinces of Panama and Colon in 2010.
A reduced eGFR was observed in 3.3% of the subjects, whereas 9.9% had albuminuria (age-adjusted prevalence of 1.6% and 7.1%, respectively) (Table 1). Subjects with CKD were more likely to be hypertensive, physically inactive, obese, diabetic, and have a history of MI. Statistically significant differences in sociodemographic and CVD risk factor variables were observed between those with apparently no indicators of kidney dysfunction and those with CKD: age (P < 0.01), hypertension (P < 0.01), diabetes mellitus (P < 0.01), current smokers (P < 0.01), and a history of MI (P < 0.01).
Table 2 shows the crude and adjusted ORs (95% CI) for indicators of CKD, and sociodemographic and CVD risk factors. Age 60 years or older (OR: 1.9; 95% CI: 1.2−2.9), hypertension (OR: 1.8; 95% CI: 1.2−2.7), and a history of MI (OR: 2.4; 95% CI: 1.0−5.7) were independently associated with CKD (adjusted model). Compared with the reference category (MFI of ≤$250 USD), a MFI of >$1201 USD was inversely associated with CKD, with an OR of 0.4 (95% CI: 0.1−0.9).
Table 2.
Association between risk factors and indicators of kidney damage, expressed as odds ratio (OR), 95% confidence intervals (CI) in participants from PREFREC study
| Variables | eGFR <60 ml/min/1.73 m2 |
ACR ≥30 mg/g |
CKD |
|||
|---|---|---|---|---|---|---|
| Crude OR (95% CI) |
Adjusteda OR (95% CI) |
Crude OR (95% CI) |
Adjusteda OR (95% CI) |
Crude OR (95% CI) |
Adjusteda OR (95% CI) |
|
| Sex (female) | 0.3 (0.2−0.6) | 0.4 (0.2−0.9) | 1.1 (0.7−1.6) | 0.9 (0.6−1.4) | 0.9 (0.7−1.4) | 0.9 (0.6−1.4) |
| Age >60 yr | 16.5 (7.7−35.7) | 8.7 (3.6−21.1) | 1.3 (0.8−1.9) | 1.1 (0.6−1.8) | 2.3 (1.6−3.2) | 1.9 (1.2−2.9) |
| Hypertension | 7.5 (3.5−16.0) | 4.0 (1.6−10.2) | 1.5 (1.1−2.2) | 1.5 (0.9−2.4) | 2.1 (1.5−3.0) | 1.8 (1.2−2.7) |
| Diabetes | 2.6 (1.3−5.4) | 1.8 (0.8−4.0) | 1.9 (1.1−3.1) | 1.7 (1.0−2.8) | 2.1 (1.3−3.3) | 1.6 (0.9−2.6) |
| Obesity | 0.6 (0.3−1.3) | 0.6 (0.2−1.4) | 1.5 (1.0−2.3) | 1.4 (0.9−2.0) | 1.4 (0.9−1.9) | 1.2 (0.8−1.7) |
| Family history ESRD | 0.9 (0.4−2.3) | 0.9 (0.4−2.4) | 0.9 (0.5−1.5) | 0.9 (0.5−1.5) | 0.9 (0.6−1.6) | 1.0 (0.6−1.7) |
| Illiteracy | 0.9 (0.4−2.4) | 0.5 (0.1−1.5) | 0.7 (0.3−1.6) | 0.6 (0.2−1.5) | 0.9 (0.4−1.7) | 0.6 (0.3−1.3) |
| Physical inactivity | 2.7 (1.3−5.4) | 2.6 (1.1−5.9) | 1.2 (0.7−2.0) | 0.9 (0.5−1.7) | 1.6 (0.9−2.5) | 1.3 (0.8−2.2) |
| Current smokers | 0.9 (0.3−2.6) | 0.8 (0.3−2.5) | 0.7 (0.5−0.9) | 0.7 (0.5−1.1) | 0.3 (0.1−0.7) | 0.7 (0.5−1.1) |
| Previous MI | 3.8 (1.4−10.4) | 1.9 (0.6−5.8) | 2.9 (1.3−6.4) | 3.1 (1.3−7.5) | 3.0 (1.4−6.2) | 2.4 (1.0−5.7) |
| Monthly family income ($) | ||||||
| <250 | Reference | Reference | Reference | Reference | Reference | Reference |
| 250−300 | 0.9 (0.3−2.4) | 1.2 (0.4−3.6) | 1.2 (0.7−2.1) | 1.1 (0.6−2.0) | 1.0 (0.6−1.7) | 1.1 (0.6−1.8) |
| 301−600 | 1.1 (0.4−265) | 1.6 (0.6−4.2) | 0.9 (0.6−1.5) | 0.9 (0.5−1.5) | 0.9 (0.5−1.4) | 0.9 (0.6−1.5) |
| 601−999 | 0.5 (0.1−1.8) | 0.6 (0.1−2.4) | 1.1 (0.6−2.2) | 1.0 (0.5−2.1) | 0.9 (0.5−1.7) | 0.9 (0.5−1.9) |
| 1000−1200 | 1.8 (0.6−6.1) | 1.2 (0.3−5.4) | 1.4 (0.7−2.9) | 1.0 (0.5−2.1) | 1.4 (0.7−2.7) | 1.2 (0.6−2.4) |
| >1201 | 1.2 (0.4−4.1) | 0.9 (0.2−3.7) | 0.2 (0.04−0.8) | 0.2 (0.04−0.7) | 0.4 (0.1−1.0) | 0.4 (0.1−0.9) |
| Indigenous ethnicity | 0.2 (0.04−1.6) | 0.6 (0.07−5.5) | 0.7 (0.1−3.2) | 1.1 (0.2−4.8) | 0.7 (0.2−2.5) | 1.1 (0.3−4.2) |
| Afroamerican | 1.7 (0.8−3.4) | 1.2 (0.5−2.8) | 1.3 (0.8−1.9) | 1.0 (0.6−1.6) | 1.3 (0.9−1.9) | 1.1 (0.8−1.8) |
ACR, albumin-to-creatinine ratio; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease.
Adjusted models: crude + all the socio−demographic, traditional cardiovascular, and chronic kidney disease (CKD) risk variables presented in the table. Missing data: Family history of ESRD (n = 137), illiteracy (n = 8), previous myocardial infarction (MI) (n = 12), monthly family income (n = 152), and ethnicities (n = 3).
When considering an abnormal eGFR as a separate outcome, age 60 years or older (OR: 8.7; 95% CI: 3.6−21.1), hypertension (OR: 4.0; 95% CI: 1.6−10.2), and physical inactivity (OR: 2.6; 95% CI: 1.1−5.9) were associated with an abnormal eGFR (adjusted models). Female sex was inversely associated with an abnormal eGFR (OR: 0.4; 95% CI: 0.2−0.9).
Diabetes mellitus (OR: 1.7; 95% CI: 1.0−2.8) and a history of MI (OR: 3.1; 95% CI: 1.3−7.5) were associated with albuminuria. Similarly, MFI >$1201 USD was associated with a 80% reduced occurrence of albuminuria (OR: 0.2; 95% CI: 0.04−0.7).
Ethnicity, years of education, illiteracy, geographic area, family history of CKD, obesity, and smoking were not associated with the combined outcome, or with reduced eGFR or albuminuria.
National Mortality Registry
During the study period (2001−2014), a total of 4646 deaths due to any kind of chronic or unspecified kidney disease or failure were recorded (ICD-10 code N18: n = 4039 and ICD-10 code N19: n = 607). For the present analysis, all 4 cases that had missing documentation of age were excluded, leaving a sample size of 4642 deaths, of which 57.6% (n = 2675) were men and 42.4% (n = 1967) were women.
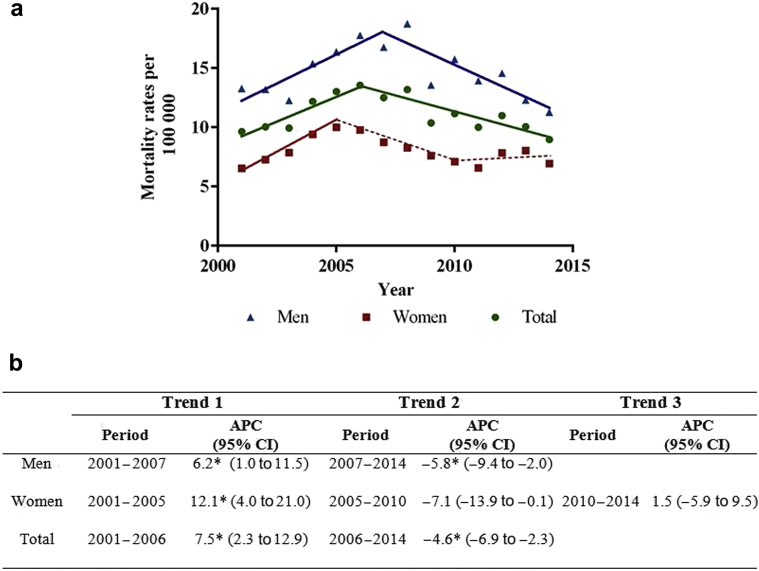
Figure 1a presents the total and sex-specific CKD ASMRs from 2011 to 2014 with their respective APC (95% CIs) during the study period (Figure 1b). Overall, from 2001 to 2006, the ASMRs from CKD showed an increasing trend (APC: 7.5%, 95% CI: 2.3−12.9) (all). From 2006 onward, the CKD mortality rate dropped (APC: −4.6; 95% CI: −6.9 to −2.3).
Figure 1.
(a) Chronic kidney disease (ICD-10 codes N18−N19) age-adjusted mortality rates and trends by sex in Panama, from 2001 to 2014. Statistically significant trends are shown as solid lines. Nonstatistically significant trends are presented as dashed lines. (b) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI). Mortality rates were standardized for 5-years age-groups using the direct method. *P < 0.05.
Compared with women, the ASMRs were higher for men. The sex-specific analysis showed a consistent increasing trend in mortality due to CKD in men (APC: 6.2%; 95% CI: 1.0−11.5) until 2007, followed by a decreasing trend (APC: −5.8%; 95% CI: −9.4 to −2.0). In women, the ASMRs from CKD showed an increasing trend (APC: 12.1%; 95% CI: 4.0−21.0) from 2001 to 2005.
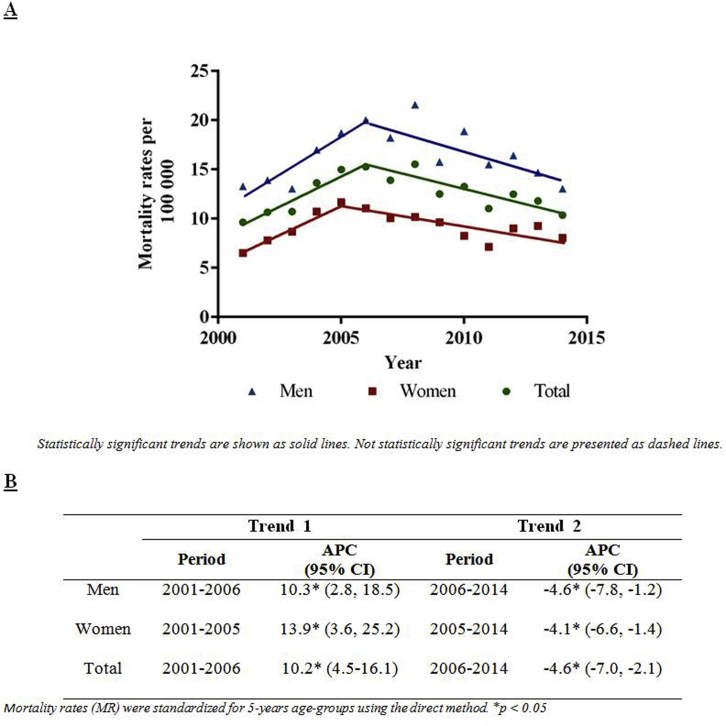
In addition, we examined the mortality trends, including ICD-10 code N17 (n = 646 deaths; n = 372 men and n = 274 women) (Supplementary Figure S1). From 2001 to 2006, the ASMRs from CKD showed an increasing trend (APC: 10.2%; 95% CI: 4.5−16.1) (all). From 2006 onward, the CKD mortality rate dropped (APC: −4.6; 95% CI: −7.0 to −2.1).
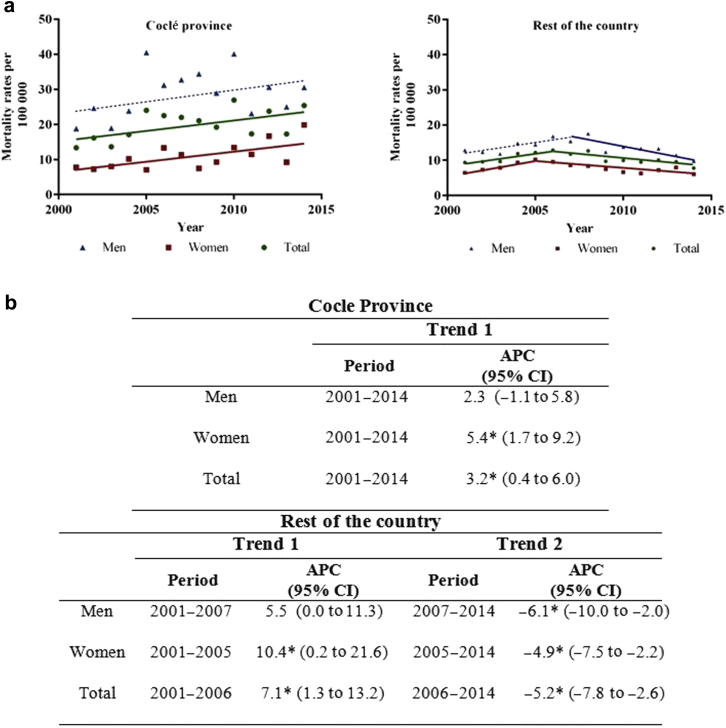
The province of Coclé, previously reported to have the highest hemodialysis rate, had the overall higher age-adjusted CKD mortality rate compared with other national rates (Figure 2). During the study period, the ASMRs from CKD showed a statistically significant increasing trend (APC: 3.2%; 95% CI: 0.4−6.0), a finding that was also confirmed for women (APC: 5.4%; 95% CI: 1.7−9.2).
Figure 2.
(a) Chronic kidney disease (ICD-10 codes N18−N19) age-adjusted mortality rates and trends by sex in the Coclé province in comparison to the rest of the country, from 2001 to 2014. Statistically significant trends are shown as solid lines. Nonstatistically significant trends are presented as dashed lines. (b) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI). Mortality rates were standardized for 5-years age-groups using the direct method. *P < 0.05.
Discussion
PREFEC Study
We evaluated the association of markers of CKD and various sociodemographic variables and CVD risk factors in a representative sample of the inhabitants of 2 Panamanian provinces, where approximately 60% of the total population of the country resides. The estimated crude prevalence of CKD was 12%, with albuminuria being the most common manifestation of renal dysfunction. A higher MFI reduced the likelihood of having CKD.
Prevalence estimates of CKD have been described in other Latin American countries. Peru reported a prevalence of 16.8% in 2 of its regions. Similar to our study, proteinuria, rather than reduced eGFR, was more prominent.22 Population-based studies in Nicaragua documented CKD prevalences between 9.1% and 13.1%, with the higher prevalence noted in rural areas,23, 24 whereas in the urban areas of Mexico, the estimated prevalence was 8.5%.25 It is noteworthy that the outcome definitions, baseline characteristics, confounding factors, and methods used to determine eGFR and albuminuria assays were inconsistent across these studies, limiting their comparisons. We defined CKD as a combined outcome according to epidemiological studies that considered abnormalities, such as albuminuria and low eGFR.22, 26 The CKD-EPI formula was used because it has been validated in a Mexican population.27
We found a strong relation between age older than 60 years and CKD, particularly with eGFR. The decline in eGFR with aging has been widely discussed, and thus, there is concern that some older adult patients with reduced eGFR but without evidence of kidney damage may be misclassified as having CKD.28 Therefore, prevalence estimates might be overestimated because 22% of the PREFREC population was older than 60 years of age. Yet, the prevalence of CKD in the population that was younger than 60 years was 9.7%. (0.8% low eGFR and 9.4% albuminuria), and the overall age-adjusted prevalence was 11.4%. New formulas for risk stratification of CKD in the older population have been described29; however, reduced eGFR and higher levels of ACR are independent predictors of all-cause mortality across a broad age range, including adults aged 80 years or older.30
Traditional risk factors such as hypertension, diabetes mellitus, and physical inactivity were independently associated with our outcomes of interest. It could be expected that in low- and middle-income countries like Panama, the increasing prevalence of cardiometabolic diseases could become a risk multiplier for CKD. With this perspective, special attention should be paid to tailoring successful preventive and management strategies for these risk multipliers. In PREFREC, 32.1% and 19.8% of the hypertensive and diabetic study participants, respectively, were not taking medication. Furthermore, consistent associations were observed between a history of MI with CKD, as well as with albuminuria. It has been shown that a first ischemic cardiac event appears to increase the natural decline in renal function.31 Taken together, a corollary of these observations is the need to identify gaps in the diagnosis, access to medications, and compliance with treatment.
Physical inactivity was more commonly observed in the CKD group, particularly in those with reduced eGFR. CKD has been linked with a poor functional status, and regular physical activity has been associated with a lower risk of adverse outcomes in a CKD population.32 In addition, CKD and lower GFR were associated with physical inactivity in participants from the National Health and Nutrition Examination Survey III study.33 Of note, the PREFREC study did not use a standardized instrument; thus, misclassification of the exposure was likely. Further prospective studies are required to evaluate the relation among these variables in greater detail.
Female sex had a protective effect for reduced eGFR. Our results mirrored the results of other studies, which showed an elevated CKD prevalence among men in El Salvador, Mexico, and Nicaragua,23 but differed from the Chinese population in which women were more likely to have low eGFR or albuminuria.26 Sex differences have been documented in the field of CKD, and women are commonly known to be at lower risk than men,34 although the underlying mechanisms are not fully clear.
Poverty and social deprivation affect CKD. In agreement with our findings, in urban China, the prevalence of low eGFR and albuminuria were low in areas with high economic development.26 This might be related to direct impacts, but might also be indirectly related to access to renal care, dialysis, and transplantation.35 Beyond the potential implications for CKD definition, the present findings underscore the importance of social determinants of health for this disease.
National Mortality Registry
A sustained increase in the mortality trend was observed until 2006, followed by a moderately decreasing trend in the ensuing years. This finding underscores the impact of the massive poisoning with diethylene glycol that resulted from a contaminated cough syrup distributed in Panama, which became public in 2006, and probably contributed to the observed peak (Figure 1).11 Despite the severity and the fatality rate among these patients,10 the total number of cases reported probably was underestimated because of subclinical damages that did not become apparent early during the incident. It is worth mentioning that consistent results were obtained when deaths coded as ICD-10 N17 were included (Supplementary Figure S2). Likewise, it was conceivable that increased CKD mortality noted that year could have been produced by heightened awareness of the disease, which, in turn, might have influenced a greater likelihood of CKD coding. The reduced mortality observed afterward might be related to an increase in testing of renal function in the general population following the diethylene glycol poisonings, and the availability of peritoneal dialysis and hemodialysis after 2006.
Finally, our results confirmed the intriguing finding of higher CKD MRs in the province of Coclé. During many years, clinicians had suspected a higher occurrence of CKD in Coclé, where the hemodialysis rate was reported as high as 220 × 100,000 in a county.12 A study performed in 3 regions of this province (2 located in the south and 1 in the north) showed statistically significant higher levels of creatinine and a family history of CKD in the southern regions of the province compared with the north. Likewise, hypertension and diabetes were more prevalent in the southern regions.
The underlying reason for the high CKD MR in Coclé is still uncertain. Coclé is known for its rice and sugar cane agriculture, salt industry, and livestock production. Similar to our findings, a recent study from Costa Rica reported that excess CKD mortality occurred primarily in areas with hot and dry lowland.36 Moreover, men had higher MRs compared with women. Remarkably, women in Coclé had a higher CKD mortality than women elsewhere in Panama. From the public health standpoint, the previous findings evoke testable hypotheses and suggest the need for epidemiological studies aimed at addressing diverse exposures, including biological interactions between environmental and occupational risk factors.
Several limitations deserve mentioning. First, temporality could not be precluded as the inherent limitation of a cross-sectional study design, such as that in the PREFREC study. Second, all the indicators of CKD were obtained on the basis of single-time measurements, which might result in misclassification of the outcome. Third, the prevalence estimates presented were not national, and thus, our findings might not be extrapolated to other regions. Nevertheless, by 2010, the distribution of the urban−rural setting, income, and ethnic origin were comparable to the remainder of the population of the country that was not evaluated by this study, according to the National Institute of Statistics and Census.13 However, there was a higher participation among women, which was possibly explained by the type of sampling strategy used (stratified according to education level), the greater acceptance of women participating in population-based studies, and/or the requirement to abstain from alcohol for 24 hours before the survey, which might have prompted a lower participation among men. Likewise, there was higher proportion of participants aged older than 60 years (compared with the nonstudied population). Self-selection bias should be carefully considered when interpreting the results of this study; our findings require validation in the national population, preferably demonstrating the chronicity of kidney dysfunction. Fourth, the Cause of Death register did not record the contributing causes of death, which might affect the quality of data, particularly in older adults who might have several comorbidities. In addition, the reliance on diagnostic code data alone to define CKD as a cause of death might have resulted in underestimation of people with early stages of CKD. Fifth, underreporting of deaths still persists predominantly in the regions populated by Amerindians, but it was likely that the impact of this on our mortality estimates was minor because it might have affected small groups. Lastly, residual or unmeasured confounding (i.e., genetic predisposition, nephrotoxic drug use) needs to be considered while interpreting the results. Nevertheless, incident registries and national representative surveys of CKD are not available for Panama as yet; to the best of our knowledge, this was the first attempt to describe the association between sociodemographic and cardiovascular risk factors with CKD and to investigate the mortality trends due to CKD in Panama.
In conclusion, our results suggested that CKD is an important public health problem in Panama. Health inequalities and the rapid increase of cardiometabolic diseases warrant broad collaborative public health efforts and robust epidemiological surveillance similar to registries of the incidence of non-ESRD. National studies are needed to assess the true magnitude and address geographic disparities, like those seen for the Coclé province, to mitigate the risk of CKD and the development of ESRD.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Maribel Tribaldos Causadias, Julio Toro and the Service of Nephrology at the Social Security Hospital for valuable comments on the article. A special thanks to Jean Paul Carrera, Karen Neira, and Bernardo González for the biostatistics guidance.
This work was supported by an institutional research grant from Panama (9044.053). The funding source had no involvement in the study design, in the collection, analysis, interpretation of data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Figure S1. (A) Chronic kidney disease age-adjusted mortality rates and trends by sex in Panama, from 2001 to 2014. (B) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI).
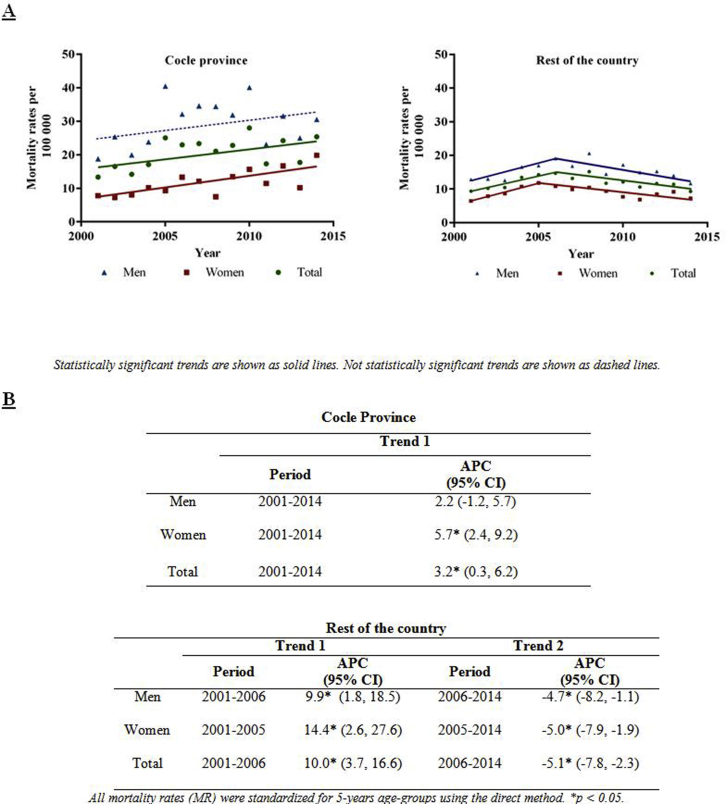
Figure S2. (A) Chronic kidney disease age-adjusted mortality rates and trends by sex in Coclé province in comparison to the rest of the country, from 2001 to 2014. (B) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI).
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Figure S1.
(A) Chronic kidney disease age-adjusted mortality rates and trends by sex in Panama, from 2001 to 2014. (B) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI).
Figure S2.
(A) Chronic kidney disease age-adjusted mortality rates and trends by sex in Coclé province in comparison to the rest of the country, from 2001 to 2014. (B) Trends expressed as annual percentage change (APC) and 95% confidence intervals (CI).
References
- 1.Cusumano A.M., Gonzalez Bedat M.C. Chronic kidney disease in Latin America: time to improve screening and detection. Clin J Am Soc Nephrol. 2008;3:594–600. doi: 10.2215/CJN.03420807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cusumano A.M., Gonzalez Bedat M.C., Garcia-Garcia G. Latin American Dialysis and Renal Transplant Registry: 2008 report (data 2006) Clin Nephrol. 2010;74 Suppl 1:S3–S8. [PubMed] [Google Scholar]
- 3.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson L., Haynes L.K., Turiano L. Chronic kidney disease in Central America: the big picture. Am J Public Health. 2014;104:e9. doi: 10.2105/AJPH.2014.301984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The World Bank. Panama. Available at: http://www.worldbank.org/en/country/panama/overview. Accessed September 1, 2016.
- 6.United Nations Development Programme. Human Development Reports. Income Gini coefficient. Available at: http://hdr.undp.org/es/content/income-gini-coefficient. Accessed September 1, 2016.
- 7.Institute of Health Metrics and Evaluation. Global Burden of Disease Profile. Panama. 2013. Available at: http://www.healthdata.org/panama. Accessed April 1, 2017.
- 8.Pan American Health Organization (PAHO). Visualizing renal failure and chronic kidney diseases age-standardized mortality rate in countries of the Americas, 2000-2009. Non-communicable Diseases and Mental Health. Washington, DC. 2014. Available at: http://www.paho.org/hq/index.php?option=com_content&view=article&id=9381:renal-failure-chronic-kidney-disease-ckd&Itemid=2391&lang=es. Accessed June 25, 2016.
- 9.Conklin L., Sejvar J.J., Kieszak S. Long-term renal and neurologic outcomes among survivors of diethylene glycol poisoning. JAMA Intern Med. 2014;174:912–917. doi: 10.1001/jamainternmed.2014.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rentz E.D., Lewis L., Mujica O.J. Outbreak of acute renal failure in Panama in 2006: a case-control study. Bull World Health Organ. 2008;86:749–756. doi: 10.2471/BLT.07.049965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sosa N.R., Rodriguez G.M., Schier J.G., Sejvar J.J. Clinical, laboratory, diagnostic, and histopathologic features of diethylene glycol poisoning–Panama, 2006. Ann Emerg Med. 2014;64:38–47. doi: 10.1016/j.annemergmed.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez F., Cuero C., Delgado E., Camargo I., Tuñon R. Diagnóstico de la Enfermedad Renal Crónica y Factores de Riesgo Asociados en Áreas Seleccionadas de la Provincia de Coclé, Panamá. Revista Médica de Panamá. 2014;34:31–38. [Google Scholar]
- 13.Mc Donald A., Bradshaw R.A., Fontes F. Prevalence of obesity in Panama: some risk factors and associated diseases. BMC Public health. 2015;15:1075. doi: 10.1186/s12889-015-2397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mc Donald P.A., Montenegro G.J., Cruz G.C., Moreno de Rivera A.L., Cumbrera O.A. Prevalence, sociodemographic distribution, treatment and control of diabetes mellitus in Panama. Diabetol Metab Syndr. 2013;5:69. doi: 10.1186/1758-5996-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsen L., Phillips D.E., AbouZahr C. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–1406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 18.Bydash J.R., Ishani A. Acute kidney injury and chronic kidney disease: a work in progress. Clin J Am Soc Nephrol. 2011;6:2555–2557. doi: 10.2215/CJN.09560911. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Surveillance Epidemiology and End Results. World (WHO 2000-2025) Standard. Available at: http://seer.cancer.gov/stdpopulations/world.who.html. Accessed July 15, 2016.
- 20.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Division of Cancer Control & Population Sciences. Surveillance Research Program. Number of joinpoints. Available at: https://surveillance.cancer.gov/help/joinpoint/setting-parameters/advanced-tab/number-of-joinpoints. Accessed April 12, 2017.
- 22.Francis E.R., Kuo C.C., Bernabe-Ortiz A. Burden of chronic kidney disease in resource-limited settings from Peru: a population-based study. BMC Nephrol. 2015;16:114. doi: 10.1186/s12882-015-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebov J.F., Valladares E., Pena R. A population-based study of prevalence and risk factors of chronic kidney disease in Leon, Nicaragua. Can J Kidney Health Dis. 2015;2:6. doi: 10.1186/s40697-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell J.K., Tobey M., Weiner D.E. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–2805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amato D., Alvarez-Aguilar C., Castaneda-Limones R. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int Suppl. 2005:S11–S17. doi: 10.1111/j.1523-1755.2005.09702.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Wang F., Wang L. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 27.Arreola-Guerra J.M., Rincon-Pedrero R., Cruz-Rivera C., Belmont-Perez T., Correa-Rotter R., Nino-Cruz J.A. Performance of MDRD-IDMS and CKD-EPI equations in Mexican individuals with normal renal function. Nefrologia. 2014;34:591–598. doi: 10.3265/Nefrologia.pre2014.Jun.12538. [DOI] [PubMed] [Google Scholar]
- 28.Glassock R.J., Winearls C. CKD–fiction not fact. Nephrol Dial Transplant. 2008;23:2695–2696. doi: 10.1093/ndt/gfn331. author reply 2696−2699. [DOI] [PubMed] [Google Scholar]
- 29.Tarantini L., McAlister F.A., Barbati G. Chronic kidney disease and prognosis in elderly patients with cardiovascular disease: comparison between CKD-EPI and Berlin Initiative Study-1 formulas. Eur J Prev Cardiol. 2016;23:1504–1513. doi: 10.1177/2047487316638454. [DOI] [PubMed] [Google Scholar]
- 30.Muntner P., Bowling C.B., Gao L. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6:2200–2207. doi: 10.2215/CJN.02030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eijkelkamp W.B., de Graeff P.A., van Veldhuisen D.J. Effect of first myocardial ischemic event on renal function. Am J Cardiol. 2007;100:7–12. doi: 10.1016/j.amjcard.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 32.Ricardo A.C., Anderson C.A., Yang W. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beddhu S., Baird B.C., Zitterkoph J., Neilson J., Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iseki K. Gender differences in chronic kidney disease. Kidney Int. 2008;74:415–417. doi: 10.1038/ki.2008.261. [DOI] [PubMed] [Google Scholar]
- 35.Hossain M.P., Goyder E.C., Rigby J.E. El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 36.Wesseling C., van Wendel de Joode B., Crowe J. Mesoamerican nephropathy: geographical distribution and time trends of chronic kidney disease mortality between 1970 and 2012 in Costa Rica. Occup Environ Med. 2015;72:714–721. doi: 10.1136/oemed-2014-102799. [DOI] [PubMed] [Google Scholar]