Introduction
Acute kidney injury (AKI) is common in both critically ill and noncritically ill patients.1, 2 AKI is associated with poorer outcomes including increased mortality, prolonged length of hospital stay, and extended duration of mechanical ventilation in critically ill patients. Patients who survive AKI have an increased risk of progression to chronic kidney disease.1, 3, 4, 5 Current stratification of AKI severity by the Kidney Disease Improving Global Outcomes Work Group is based on increases in serum creatinine (SCr) concentration or decreases in urine output with escalating AKI severity,6 These criteria are diagnostic and not intended to be prognostic, meaning the strata are unable to predict AKI outcome. Because SCr concentration can be influenced by many extrarenal factors, including fluid overload, gender, and muscle composition and because it is particularly variable in children, a significant clinical and translational research effort has been expended toward the discovery and validation of novel AKI biomarkers to detect structural kidney injury.7, 8
One of the novel AKI biomarkers studied is neutrophil gelatinase–associated lipocalin (NGAL). NGAL, a 25-kDa protein expressed in the distal nephron epithelium and secreted into the tubular lumen very early after AKI, is coded for by a transcript demonstrating dramatic upregulation in animal models of AKI.8, 9 Urinary NGAL (uNGAL) has the following characteristics of an ideal biomarker: (i) it is protease resistant and can be rapidly analyzed, (ii) it is highly sensitive to early injury, (iii) it is specific to tubular damage, and (iv) it demonstrates quantitative increases proportional to the presumed degree of damage.8 In clinical studies, uNGAL predicts AKI in patients after cardiac surgery, after radiocontrast administration, in the emergency department, in the intensive care unit (ICU), and after kidney transplantation.8, 10, 11 In these groups of patients with AKI, uNGAL concentration increases can be detected before increases in SCr concentration. Diagnosis of AKI by means of SCr concentration is not possible until the kidney has already had a significant decrease in glomerular filtration rate. Additionally, the diagnosis of AKI based on a change in SCr concentration does not distinguish functional from structural AKI—a distinction now recommended to increase the precision of the AKI phenotype.12 Functional AKI, previously understood as “prerenal” AKI, is driven by insufficient glomerular perfusion pressure and not direct tubular damage; therefore a tubular damage marker such as NGAL will remain normal in the early stages. NGAL rises when there is tubular damage and is a part of damage-associated AKI (previously termed intrinsic AKI).13 Ultimately, because of the different pathophysiology, the management of these AKI phenotypes may be distinct and may be facilitated with the use of appropriate diagnostics.
Despite significant effort and investment in AKI biomarker research, translation of results and incorporation into routine clinical practice has yet to occur. The assay for uNGAL testing was made available on the Cincinnati Children’s Hospital clinical laboratory platform for analysis and reporting within 1 hour in January 2016 (BioPorto Diagnostics A/S, Hellerup, Denmark). The assay requires 1 ml of urine with no special handling for specimens sent directly to the laboratory. The clinical laboratory established cut-off values for uNGAL to determine AKI risk (Table 1), and the assay has now been in routine clinical use at our institution. The purpose of this paper is to demonstrate the utility of an AKI biomarker in clinical practice. In the timeframe between January 1, 2017, and May 1, 2017, a total of 418 individual uNGAL concentrations were assessed for 214 patients. In this series of case reports, we have selected 5 individual cases to demonstrate how assessment of changes in uNGAL concentration, facilitated by access to real-time uNGAL testing, may directly affect and assist in patient management. In these cases, the change in uNGAL over time is labeled “dynamic uNGAL” or “uNGALDYN.” A positive or negative uNGALDYN is indicative of ≥25% in the respective direction.
Table 1.
Urinary neutrophil gelatinase cut-off values and acute kidney injury risk
| uNGAL level | Interpretation |
|---|---|
| < 50 ng/ml | AKI risk: Low Does not exclude subsequent development of AKI Repeat if clinically indicated |
| 50–149 ng/ml | AKI risk: equivocal Repeat measurement is indicated if clinically justified |
| 150–300 ng/ml | AKI risk: moderate High sensitivity/moderate specificity |
| >300 ng/ml | AKI risk: high High specificity |
AKI, acute kidney injury; uNGAL, urinary neutrophil gelatinase–associated lipocalin.
Case 1: Prediction of Tubular Injury and Anuria (Prediction)
Background
The patient was a 4-month-old girl who weighed 4 kg and had a history of hypoplastic left heart syndrome (status post Norwood procedure/Sano shunt modification) with respiratory failure requiring mechanical ventilation.
Case
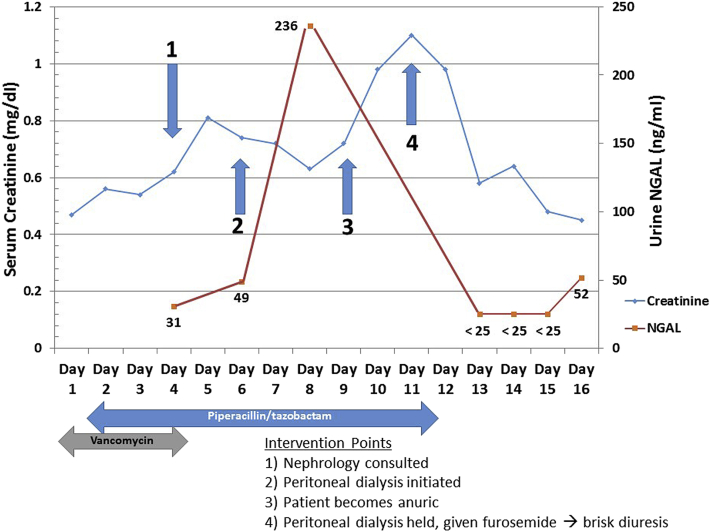
The Nephrology Service was consulted by the cardiac ICU because of a rising SCr concentration with fluid overload (Figure 1). The cardiac ICU team optimized cardiac output using continuous infusions of milrinone, epinephrine, and nicardipine. At the time of the consult, the patient had received vancomycin for the previous 3 days and piperacillin/tazobactam for the previous 2 days. Urinary NGAL on the day of consult (cardiac ICU day 4) was 31 ng/ml, suggestive of no significant tubular damage. Creatinine elevation was therefore assumed to be secondary to poor glomerular perfusion. The cardiac ICU discontinued vancomycin but continued piperacillin/tazobactam. A peritoneal dialysis catheter was placed because of the indication of fluid overload on cardiac ICU day 6. Serial uNGAL concentrations were monitored and found to acutely rise to 236 ng/ml on day 8, suggestive of tubular injury. Concurrent creatinine levels were decreasing. On the following day (day 9), the patient had anuria, and creatinine levels increased 2 days later (day 11). Because of the anuria, uNGAL could not be measured on days 9 to 11.
Figure 1.
Changes in urinary neutrophil gelatinase–associated lipocalin (uNGAL) assist with prediction (Case 1). The graph depicts changes in serum creatinine (blue) concurrent with changes in uNGAL (red) over 16 days for this patient in the cardiac intensive care unit. Intervention points and intervention are depicted along with the time of antibiotic therapy with vancomycin and piperacillin/tazobactam. A spike in uNGAL concentration preceded oliguria and a spike in serum creatinine.
Resolution
Between days 6 and 11, peritoneal dialysis was initiated to address fluid accumulation and resulted in a decrease in edema. On day 11, a decision was made to provide a diagnostic fluid challenge to the patient. Peritoneal dialysis was withheld, and the patient began receiving furosemide, responding with a brisk diuresis. Urine on day 13 demonstrated an undetectable uNGAL value (<25 ng/ml, which is the lower threshold limit) suggesting renal recovery. The SCr concentration continued to decrease in the subsequent days.
Case 2: Prediction of Tubular Recovery and Urine Production (Prognosis)
Background
The patient was an 8-year-old girl with a history of chronic kidney disease (stage 2–3) secondary to autosomal recessive polycystic kidney disease, hepatic fibrosis, and portal hypertension. She was admitted to the general pediatric ICU with septic shock secondary to infection with group B Streptococcus (isolated from blood).
Case
Shortly after initial fluid and hemodynamic resuscitation, the patient was intubated to stabilize oxygen delivery and treat shock (Table 2). Administration of broad-spectrum antibiotics, including piperacillin/tazobactam and vancomycin, was initiated; and continuous infusions of epinephrine and calcium were started to optimize hemodynamics. On day 2 of the ICU course, the SCr concentration increased from 1.06 to 1.37 mg/dl, while uNGALDYN decreased by nearly 50% (6547 to 3567 ng/ml). The patient experienced a progressively greater fluid overload, reaching a maximum of 13% of ICU admission body weight, along with worsening of her baseline ascites. The creatinine and uNGALDYN data, along with some urine output (∼0.5 ml/kg/h), prompted a decision to start a trial of diuresis with a loop diuretic. The patient responded with a brisk diuresis (increased by 2.5 ml/kg/h from baseline). Urinary NGAL on day 4 demonstrated a continued decrease, despite elevated SCr. The SCr concentration decreased between days 5 and 7, suggesting a predictive phenomenon of negative uNGALDYN for renal function recovery.
Table 2.
Urinary neutrophil gelatinase–associated lipocalin aids in determining acute kidney injury prognosis (case 2)
| Timing | Creatinine | uNGAL | UOP (ml/kg/h) | Clinical status |
|---|---|---|---|---|
| Baseline | 0.48 | Stable | Stable | |
| ICU admission (day 0) |
0.78 | n/a | Stable | Unstable Shock |
| Day 1 | 1.06 | 6547 | ∼ 0.3–0.5 | Stable Intubated Vasopressors |
| Day 2 | 1.37 | 3567 | ∼ 0.3 | Stable Intubated Vasopressors |
| Day 3 | 1.42 | 2856 | > 4 | Stable Intubated Weaning from pressors |
| Day 4 | 1.08 | 584 | > 2 | Stable Intubated Pressors discontinued |
| Day 5 | 0.89 | n/a | > 1 | Stable Extubated |
ICU, intensive care unit; uNGAL, urinary neutrophil gelatinase–associated lipocalin; UOP, urine output.
Resolution
After diuresis and continued hemodynamic stability, the patient was extubated on day 5 and left the ICU 36 hours later.
Case 3: Prediction of Response to Therapy (Theragnosis)
Background
The patient was an 8-year-old boy with a history of steroid-resistant nephrotic syndrome admitted for a kidney biopsy and treatment of worsening edema.
Case
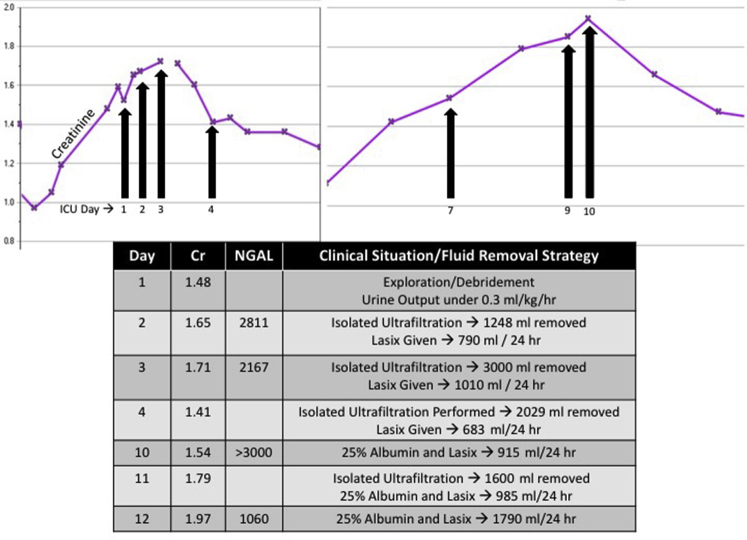
Two days after the biopsy was performed, necrotizing fasciitis developed, and the patient was taken to the operating room for exploration and debridement of the area around the biopsy site (and then began receiving ciprofloxacin, meropenem, and vancomycin) (Figure 2). Postoperatively, the patient was transferred to the pediatric ICU because of worsening edema, a new oxygen requirement, and oliguria. SCr increased over the initial days of his ICU stay. On day 2, a uNGAL concentration of 2811 ng/ml was accompanied by increased urine output in response to diuretics, and the patient had a single isolated ultrafiltration procedure performed (Aquadex Flexflow, Sunshine Heart, Eden Prairie, MN) for fluid removal. On day 3, SCr continued to rise to 1.71 mg/dl; however, the urine NGAL concentration decreased from 2811 to 2167 ng/ml. A furosemide dose was repeated, and this time, it was associated with a brisk urine response. The patient was transferred back to the pediatric medical unit on day 5.
Figure 2.
Changes in urinary neutrophil gelatinase–associated lipocalin (uNGAL) assist with theragnosis (Case 3). The graph depicts changes in serum creatinine (purple) concurrent with changes in urine neutrophil gelatinase associated lipocalin (uNGAL) in the associated table during a stay in the intensive care unit (ICU) for this patient. Clinical context is provided. The change in NGAL demonstrates the prediction of response to diuresis (theragnosis). Cr, creatinine.
Resolution
The patient continued to receive albumin and diuretic treatments for persistent edema. The biopsy results revealed focal segmental glomerulosclerosis. The SCr concentration rose again on days 7 and 8, and on day 10, uNGAL was >3000 ng/ml (standard laboratory upper limit reading before dilution) with marginal response to diuretics, prompting several more isolated ultrafiltration procedures for fluid removal. Although the SCr concentration continued to rise through day 12, uNGAL decreased to 1060 ng/ml, and the Nephrology Service decided to administer furosemide. The creatinine concentration started to decrease on day 13.
Case 4: Renal Function Recovery in the Setting of Extracorporeal Renal Support Therapy (Clarity)
Background
A 14-year old boy with a history of acute myeloid leukemia in remission (10 years) and heart transplantation (4 years) after anthracycline-associated cardiomyopathy presented to the emergency department with decreased oral intake, low-grade fever, dyspnea, and hypoxia (Table 3).
Table 3.
Urinary neutrophil gelatinase–associated lipocalin identifies renal function during renal support therapy
| Date | Creatinine | BUN | uNGAL | Nephrology recommendations |
|---|---|---|---|---|
| Day 1 | 0.88 | 25 | ||
| Day 2 | 1.10 | 32 | 1–1.2 l fluid restriction Avoid negative fluid balance Decrease home diuretics |
|
| Day 3 | 1.92 | 58 | 484 | Isolated ultrafiltration – 1250 ml removed |
| Day 4 | 3.31 | 81 | 1039 | Isolated ultrafiltration – 1500 ml removed |
| Day 5 | 3.67 | 87-18 | 595 | Hemodialysis – 1800 ml |
| Day 6 | 2.40 | 36 | 353 | Isolated ultrafiltration Trial of furosemide and chlorothiazide |
| Day 7 | 2.44 | 38 | Isolated ultrafiltration Continued administration of furosemide and chlorothiazide |
|
| Day 8 | 2.16 | 41 | Changed furosemide to oral form Stopped administration chlorothiazide | |
| Day 9 | 2.13 | 45 | 143 | |
| Day 10 | 2.26 | 53 | ||
| Day 11 | 2.14 | 56 | ||
| Day 12 | 1.68 | 51 | ||
| Day 13 | 0.92 | 44 |
BUN, blood urea nitrogen; uNGAL, urinary neutrophil gelatinase–associated lipocalin.
Case
During a diagnostic cardiac catheterization, the patient experienced an episode of bradycardia requiring a brief round of chest compressions. In addition to a broad cardiac evaluation for rejection, a bronchoscopy demonstrated purulent bronchitis. After catheterization, the patient’s SCr concentration began to rise from baseline (0.7–0.8 mg/dl) to 1.10 mg/dl and then 1.92 mg/dl. Day 2 urinalysis demonstrated a fractional excretion of sodium of 0.05%, indicative of prerenal AKI; however, on day 3, a uNGAL concentration was 484 ng/ml, more indicative of intrinsic AKI. After several days of isolated ultrafiltration, the SCr continued to rise to 3.31 mg/dl, and the uNGAL also increased to 1039 ng/dl. Hemodialysis was initiated to augment clearance, leading to the artificial subsequent decrease in creatinine. Urinary NGAL is not significantly affected by hemodialysis; therefore the associated negative uNGALDYN (595–353 ng/ml) was interpreted as an indicator of renal recovery. Hemodialysis was withheld after day 5.
Resolution
Renal function recovered after day 6, with a demonstrated decrease in SCr and improvement in urine output.
Case 5: Clinical Decision Support (Management)
Background
A 25-year old man presented with a history of Hunter syndrome, morbid obesity (126 kg), critical airway (intubation only possible with fiberoptic laryngoscopy), abdominal hernia (status postrepair with mesh placement) with abdominal infection (Figure 3).
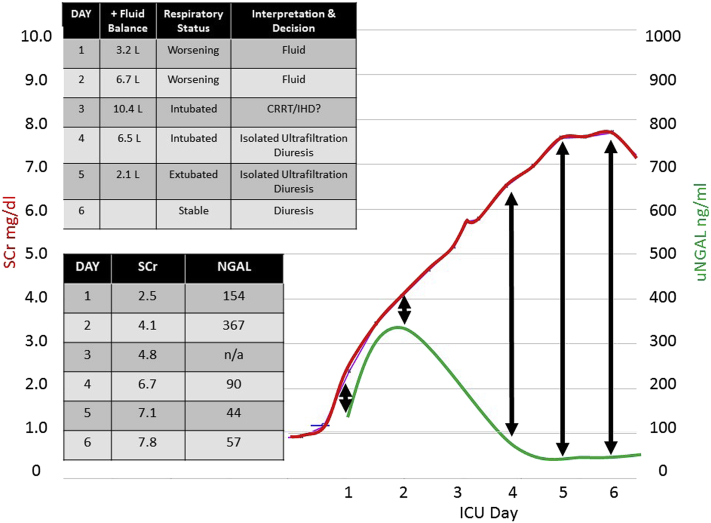
Figure 3.
Changes in urinary neutrophil gelatinase–associated lipocalin (uNGAL) assist with management (Case 5). The graph depicts changes in serum creatinine (SCr) (red) concurrent with changes in urine neutrophil gelatinase associated lipocalin (uNGAL (green) over the first week of admission to the intensive care unit (ICU). This patient had a significant discordance between creatinine and NGAL, starting on day 2. Changes in NGAL influenced the management of this patient, obviating the need for a trip to the operating room, placement of a temporary hemodialysis catheter, and use of continuous renal replacement therapy (CRRT). IHD, intermittent hemodialysis.
Case
The patient underwent port and mesh removal. He was intubated and admitted to the ICU for treatment of Staphylococcus epidermidis bacteremia. Administration of piperacillin/tazobactam and vancomycin was initiated for presumed abdominal sepsis. The patient experienced AKI with an increase in creatinine from 0.7–0.8 mg/dl to a maximum of 7.7 mg/dl over ICU days 1 to 7. Oliguria was present on ICU days 1 to 3, and fluid overload was progressive (net fluid balance: 3.2 l to 10.4 l). The uNGALDYN was initially positive (154–367 ng/ml) but then negative (367 to 90 to 44 ng/ml). These levels were concurrent with a persistent, steady increase in creatinine. The Nephrology Service was consulted for initiation of continuous dialysis on day 3, when the patient had a fluid overload of 10 l. The patient had been prophylactically intubated the day before because of progressive respiratory distress and critical airway. Hemodialysis catheter placement was attempted at the bedside on day 4 but was unsuccessful because of the patient's body habitus, so a 7F central venous catheter was placed instead. In lieu of hemodialysis, isolated ultrafiltration was performed on day 4, resulting in the removal of 3 l of fluid. A planned trip to the operating room for dialysis catheter placement was cancelled on day 5 because of the negative uNGALDYN, and the patient instead began receiving a continuous diuretic infusion (resulting in additional urine output of 3 l).
Resolution
Negative uNGALDYN continued, and despite a steadily increasing SCr concentration, the patient’s urine output remained brisk in response to diuretics. He was rapidly extubated after the fluid removal and was transferred out of the ICU in stable condition on day 9.
Discussion
Although uNGAL has been studied in the research setting for more than a decade, it has not yet found a place in routine clinical care. Access to near real-time uNGAL measurement has added a layer of information that enriches the AKI phenotype for our practice. In the cases reported, we have demonstrated the clinical impact of monitoring uNGAL serially, showing that the dynamic changes in uNGAL are predictive, prognostic, and theragnostic and may potentially serve as clinical decision support for an aspect of the AKI syndrome—fluid overload. Incorporation of uNGAL measurement into practice in this manner mirrors the care provided for critically ill patients—real-time and dynamic. Urinary NGAL measurement will not replace traditional methods of assessing kidney function during clinical care, nor should we expect a biomarker that will do so to be identified in the immediate future. In fact, a limitation to this select group of cases is that these are observations and the practitioners were not blinded to the value of uNGAL, creatinine, or urine output. It is notable, however, that in this context, the uNGAL information was additive to the entire armory of clinical information available for a given patient. This biomarker, and potentially others, can provide additional information to the care team 1 to 2 days earlier than SCr, which is not an insignificant amount of time, particularly for patients with critical illness. During the timeframe from which these cases were extracted, more than 200 patients had at least 1 uNGAL measurement. It should be noted that we have selected illustrative examples of the benefit of sequential uNGAL measurements, and a true clinical study—in fact a series of studies—is required to confirm our findings. If validated, our findings require extension and analyses of efficacy when matched against the status quo of creatinine measurements and urine output assessment. We believe our cases give this arena of inquiry momentum. Ronco et al. have recently reviewed the current status of NGAL and whether it is ready for clinical use.14 We agree with their findings that clinical judgment, prognosis, and management of the various pathologies that lead to AKI can benefit from its adoption, as illustrated in a small cohort of patients from our institution.
The following key learning points can be gleaned from our selected cases:
-
•
A low uNGAL concentration concurrent with creatinine elevation delineates glomerular versus structural tubular damage.
-
•
The change in uNGAL over time (uNGALDYN) refines understanding of the AKI disease process and assists in the management of AKI-related sequelae, such as fluid accumulation.
-
•
Urinary NGALDYN is informative (prognostic, theragnostic) even in the context of a patient with chronic kidney disease and repeated acute-on-chronic changes in SCr.
-
•
NGAL is not dialyzed, and thus uNGALDYN can be used to track renal recovery or disease progression during renal replacement therapy. (Blood urea nitrogen and creatinine levels are not reliable for tracking renal recovery after hemodialysis.)
-
•
Positive uNGALDYN predicts tubular damage–associated AKI and oliguria at a time when concurrent sequential measurements of creatinine are decreased.
-
•
Negative uNGALDYN is indicative of tubular recovery and responsiveness (with associated urine production) and thus indicates AKI prognosis.
-
•
Negative uNGALDYN correctly identifies stability and recovery of renal function, even during the administration of diuretics.
-
•
Negative uNGALDYN predicts response to therapy in response to loop diuretic administration (theragnosis).
-
•
The uNGALDYN can guide management, serving as clinical decision support. Monitoring the negative uNGALDYN for patient 5 led to the prevention of a high-risk trip to the operating room, a high-risk operative procedure for dialysis line placement, and use of either intermittent hemodialysis or continuous renal replacement therapy for fluid removal. Expeditious fluid removal facilitated earlier extubation and shortened ICU length of stay.
Disclosure
SLG, PD, and RKB disclose consulting agreements with BioPorto Diagnostics (Hellerup, Denmark). The other author declared no competing interests.
Acknowledgments
This work was partially supported by a P50 Center of Excellence Grant (PD): NIH P50 DK096418.
References
- 1.Kaddourah A., Basu R.K., Bagshaw S.M. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste E.A., Bagshaw S.M., Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 3.Devarajan P., Jefferies J.L. Progression of chronic kidney disease after acute kidney injury. Prog Pediatr Cardiol. 2016;41:33–40. doi: 10.1016/j.ppedcard.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla L.S., Eggers P.W., Star R.A. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland S.M., Byrnes J.J., Kothari M. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10:554–561. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group KDIGOKAKIW KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 7.Haase-Fielitz A., Haase M., Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51:335–351. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra J., Ma Q., Prada A. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 10.Parikh C.R., Devarajan P., Zappitelli M. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Geus H.R., Bakker J., Lesaffre E.M. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183:907–914. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- 12.Murray P.T., Mehta R.L., Shaw A. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative Consensus Conference. Kidney Int. 2014;85:513–521. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Endre Z.H., Kellum J.A., Di Somma S. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the Tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 14.Ronco C., Legrand M., Goldstein S.L. Neutrophil gelatinase-associated lipocalin: ready for routine clinical use? An international perspective. Blood Purif. 2014;37:271–285. doi: 10.1159/000360689. [DOI] [PMC free article] [PubMed] [Google Scholar]