Abstract
Introduction
Calcium oxalate supersaturation is regularly exceeded in the plasma of patients with end-stage renal disease (ESRD). Previous reports have indicated that hemodialfiltration (HDF) lowers elevated plasma oxalate (POx) concentrations more effectively compared with hemodialysis (HD). We reevaluate the therapeutic strategy for optimized POx reduction with advanced dialysis equipment and provide data on the effect of extended treatment time on dialytic oxalate kinetics.
Methods
Fourteen patients with ESRD who underwent HDF 3 times a week for 4 to 4.5 hours (regular HDF; n = 8) or 7 to 7.5 hours (extended HDF; n = 6) were changed to HD for 2 weeks and then back to HDF for another 2 weeks. POx was measured at baseline, pre-, mid-, and postdialysis, and 2 hours after completion of the treatment session.
Results
Baseline POx for all patients averaged 28.0 ± 7.0 μmol/l. Intradialytic POx reduction was approximately 90% and was not significantly different between groups or treatment modes [F(1) = 0.63; P = 0.44]. Mean postdialysis POx concentrations were 3.3 ± 1.8 μmol/l. A rebound of 2.1 ± 1.9 μmol/l was observed within 2 hours after dialysis. After receiving 2 weeks of the respective treatment, predialysis POx concentrations on HD did not differ significantly from those on HDF [F(1) = 0.21; P = 0.66]. Extended treatment time did not provide any added benefit [F(1) = 0.76; P = 0.40].
Discussion
In contrast to earlier observations, our data did not support a benefit of HDF over HD for POx reduction. With new technologies evolving, our results emphasized the need to carefully reevaluate and update traditional therapeutic regimens for optimized uremic toxin removal, including those used for oxalate.
Keywords: clearance kinetics, end-stage renal disease (ESRD), hemodialysis (HD), hemodialfiltration (HDF), oxalate
Oxalate is a terminal metabolite whose homeostasis results from the balance of endogenous synthesis, dietary absorption, and urinary excretion, as recently reviewed.1, 2 With declining kidney function, renal oxalate clearance decreases and oxalate accumulates.3, 4, 5, 6, 7 In patients with end-stage renal disease (ESRD), plasma oxalate (POx) concentrations frequently exceed the oxalosis cutoff of 30 μmol/l.8, 9, 10 Elevated POx can lead to oxalate deposition in various tissues and can cause organ damage.11, 12, 13 Both experimental and clinical evidence indicates a possible role of POx concentrations in the development of cardiovascular complications in patients with ESRD. For example, elevated POx concentrations have been associated with reactive cardiac fibrosis, increased vascular calcification, low low-density lipoprotein levels, and elevated hemodynamic parameters (e.g., pulsewave velocity and central aortic blood pressures).12, 14, 15, 16 Moreover, unrecognized or insufficiently treated hyperoxalemia pre- and post-transplantation can have severe implications for renal allograft survival.8, 17, 18, 19
Because the main physiological excretory route for oxalate removal is the kidneys and this route is not available in patients with ESRD, renal replacement therapy (RRT) is the only option to significantly lower POx concentrations. Therefore, the efficiency of RRT is critical for lowering POx concentrations in patients with ESRD. Hemodialfiltration (HDF) is under extensive investigation for possibly improving clinical outcomes, especially cardiovascular mortality.20 Research is mainly centered in Europe; only 5 of 40 studies in the latest Cochrane meta-analysis on this topic were conducted in the United States, Canada, or Asia.20 This may reflect the increasing implementation of HDF in European RRT units, despite continued controversy about its cost-effectiveness.21, 22, 23, 24, 25, 26 Previous reports have indicated that an oxalate elimination of 6 to 10 mmol/1.73 m2 per week on standard hemodialysis (HD) is inadequate to lower POx; on this regimen, predialysis POx values averaged approximately 45 μmol/l.10, 27, 28, 29 In search of an optimized therapeutic regimen to eliminate POx, 3 studies in patients with secondary hyperoxalemia29, 30, 31 reported higher oxalate clearances with HDF compared with standard HD treatment (Table 1). By performing HDF, higher intradialytic POx reduction and total oxalate extraction, as well as lower postdialysis POx concentrations compared with HD, have been described.29, 30, 31, 32 A POx rebound of 9.6% of the postdialysis concentration within 30 minutes was observed.29 However, dialyzer oxalate clearances in these studies did not exceed 160 ml/min; the maximum intradialytic POx reduction was approximately 60%, and dialyzers had smaller surfaces and lower ultrafiltration coefficients compared with dialyzers currently used for patients undergoing maintenance RRT.29, 30, 31, 32 The positive interaction between diffusive and convective flux on HDF has been suggested to facilitate a higher oxalate extraction rate than the one possible with HD.29, 32 However, in previous studies, patients remained hyperoxalemic at all times.29, 30, 31, 33 Advances in dialyzer filter characteristics and machine technologies have improved oxalate clearances on HDF to 220 ml/min, thereby reducing POx by up to 92% with 1 RRT session.34 Another study recently reported a 71% reduction of a mean pre-HD POx concentration of 22.3 μmol/l during a single high-flux HD session.33 These developments warrant a reevaluation of oxalate kinetics under current regular and extended HD and HDF regimens. Therefore, the present pilot crossover trial was designed to provide an up-to-date reference for clinicians about the benefit of highly efficient RRT on POx reduction in patients with ESRD.
Table 1.
Characteristics of previous studies comparing oxalate kinetics on HD and HDF in patients with secondary hyperoxalemia
| Publication | Predialysis POx, μmol/l |
Postdialysis POx, μmol/l |
POx reduction, % |
POx clearance, ml/min |
||||
|---|---|---|---|---|---|---|---|---|
| HD | HDF | HD | HDF | HD | HDF | HD | HDF | |
| Mydlik et al.30 | 40,3 ± 9,8 | 43,3 ± 6,8 | 18.7 ± 4.5 | 15,9 ± 3,7 | 53.6 | 63.3 | 133.2 ± 17.1 | 158.7 ± 16.7 |
| Marangella et al.29 | 54,4 ± 15,1 | 46,7 ± 13,6 | 23.0 ± 11,7 | 16,2 ± 7,4 | 57.7 | 65.3 | 82.0 ± 25 | 113.0 ± 45 |
| Chimienti et al.31 | 50.0 ± 17.0 | 47.7 ± 10.2 | 34.1 ± 10.2 | 25.0 ± 4.5 | 31.8 | 47.6 | 98.8 ± 10.3 | 143.0 ± 20.5 |
Data are expressed as mean ± SD.
Conversion factor for oxalate in milligram per liter to micromole per liter, ×11.1.
HD, hemodialysis; HDF, hemodialfiltration; POx, plasma oxalate.
Materials and Methods
Study Population
Patients eligible for this study (i) provided written informed consent, (ii) were aged 18 years or older, (iii) had been on HDF treatment for at least 3 months, (iv) had been medically stable with no infections or hospitalizations in the preceding 3 months, (v) had a urinary output of <300 ml/24 h, (vi) an access blood flow of ≥250 ml/min, (vii) a dialysate flow of ≥500 ml/min, (viii) a substitute volume of ≥20 L on HDF, (ix) underwent dialysis for ≥4 or ≥7 hours per session, and (x) received RRT 3 times per week.
The study excluded patients who (i) experienced an acute illness or had been hospitalized in the preceding 3 months, (ii) had a recirculation of >15% (online measurement), or (iii) received single needle dialysis or had a single lumen catheter.
Study Design
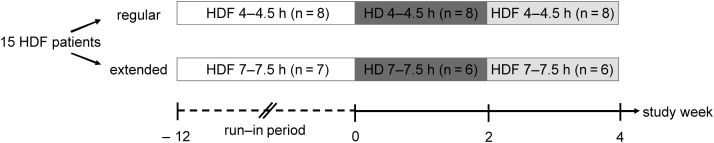
A pilot crossover study comparing POx concentrations during HD and HDF as primary outcome was performed in 2016. Corresponding with their previous prescriptions, patients were stratified to RRT with a regular treatment time (4−4.5 hours per session, regular RRT) during 3 days per week or extended treatment time (7−7.5 hours per session, extended RRT) during 3 nights per week. RRT is used as an umbrella term when mentioning dialysis in general (i.e., both HD and HDF) throughout this article. As shown in Figure 1, both groups received 2 weeks of standard HD followed by 2 weeks of HDF. All blood samples were collected at the first treatment after the long dialytic interval. Immediately before starting the first study period on HD, a predialysis plasma sample was obtained to reflect the baseline POx concentration on the routine HDF regimen of the patients. At the last treatment session during each study period, the patients were sampled at different time points with respect to the RRT session to assess intradialytic POx reduction kinetics with the different RRT modes. Samples on regular RRT were obtained predialysis, at 2 to 2.5 hours after the start of RRT, postdialysis, and 2 hours after the end of RRT. Samples on extended RRT were obtained predialysis, at 4 to 4.5 hours after the start of RRT, postdialysis, and 2 hours after the end of RRT.
Figure 1.
Study design. After a minimum of 12 weeks on the same renal replacement therapy (RRT) modality (hemodialfiltration [HDF]), 8 patients on regular RRT and 6 patients on extended RRT were switched to hemodialysis (HD) for 2 weeks and then back to HDF for another 2 weeks.
To ensure that comparable treatment regimens were administered to all groups, the use of the prescribed RRT equipment, dialysate flow, blood flow, and treatment length were monitored and kept at the target values defined in the study criteria section throughout the study. Dietary intake was restricted as clinically indicated in ESRD, but without special emphasis on dietary oxalate content. Patients were maintained nil p.o. during the 2-hour rebound waiting period. The study protocol was approved by the institutional review board of Friedrich-Alexander-Universität Erlangen-Nürnberg (No. 109_15B) and listed on clinicaltrials.gov (NCT02684656). The study was conducted in compliance with the principles of the Declaration of Helsinki.
RRT Equipment
A 5008 CorDiax dialyzer with a FX100 filter (Fresenius Medical Care; Bad Homburg, Germany) was used for all RRT sessions during the screening period and study. The FX100 uses an advanced Fresenius Polysulfone-based Helixone membrane with an effective surface of 2.2 m2 and an ultrafiltration coefficient of 73 ml/h × mm Hg. The RRT modes applied were standard high-flux HD and online high-flux HDF postdilution.
Sample Handling
Plasma samples were collected from the arterial line or via a venous blood draw into S-Monovette EDTA K3 plasma tubes (Sarstedt; Nuembrecht, Germany), immediately put on ice, centrifuged in a precooled Thermo Scientific Heraeus Megafuge 16R (Thermo Fisher Scientific; Waltham, MA) at 3400 rpm for 10 minutes, aliquoted and stored at −80°C within 2 hours. After a maximum storage of 2 days, plasma samples were thawed, filtered through Vivaspin 500 30,000 MWCO PES filter units (Sartorius; Goettingen, Germany), acidified with 20 μl 1N hydrochloric acid per 500 μl of plasma, and refrozen under the same conditions.
Measurement of Plasma Oxalate
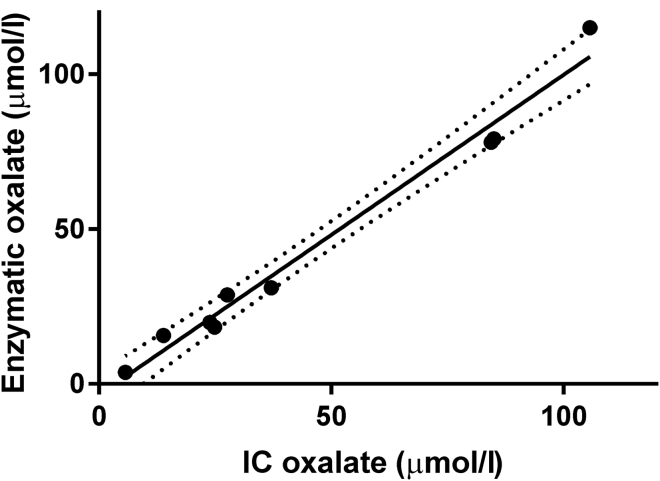
Acidified and purified plasma samples were thawed from storage at −80°C and treated with 20 μl of 5 mM NaNO2 solution per 100 μl of plasma. Oxalate was measured enzymatically using oxalate oxidase (Trinity Biotech; Bray, Co. Wicklow, Ireland), as previously described.35 The standard procedure was adapted by Litholink Corp. (Chicago, Illinois). The lower limit of detection was 2 μmol/l. The samples were assayed using a Beckman DU 650 Spectrophotometer (Beckman Instruments Inc.; Fullerton, CA) at 590 nm wavelength (no background wavelength). A 0.5 mM oxalate standard (Trinity Biotech) was used to create a new standard curve for every set of measurements, and an independent 10 μM sodium oxalate solution was used as a quality control standard. The coefficient of variation was 4.2% for within-run variation and 12.2% for between-run variation. Randomly selected samples were spot-checked with ion chromatography using a Dionex ICS 2000 system (Dionex; Sunnyvale, CA), as previously described.36 As shown in Figure 2, a good correlation between the 2 methods (r = 0.99; P < 0.001) was found.
Figure 2.
Correlation of plasma oxalate concentrations measured with ion chromatography (IC) and enzymatically (Y = 1.033 X – 3.565; r = 0.99; P < 0.001).
Individual Plasma Oxalate Variability Assessment
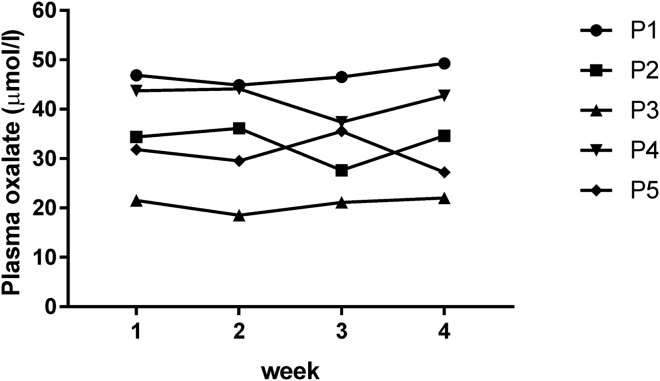
To evaluate the validity of one-time sampling during the different study periods, baseline variability of POx concentrations was determined in 5 patients on regular HDF treatment (4−4.5 hours per session). The same inclusion criteria as for the main study were applied. Pre-HDF blood draws were performed immediately before starting the first treatment session after the long dialytic interval in 4 consecutive weeks during October and November 2015. As shown in Figure 3, all patients had elevated POx concentrations at the indicated sampling time points, which were an average of 34.8 ± 9.7 μmol/l. The coefficient of variation for 4 times repeated pre-HDF POx concentrations in 1-week intervals ranged between 3.4% and 9.9% for different individuals (Table 2), which indicated minimal intraindividual variability of predialysis POx concentrations on maintenance RRT over time.
Figure 3.
Intraindividual variability of predialysis plasma oxalate. Four consecutive weekly plasma oxalate concentrations from 5 individual patients (P) were measured.
Table 2.
Means, SD, and CV for 4 consecutive weekly predialysis plasma oxalate concentrations in 5 individual patients
| Parameters | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Mean POx, μmol/l | 46.9 | 33.2 | 20.8 | 42.0 | 31.0 |
| SD, μmol/l | 1.6 | 3.3 | 1.4 | 2.7 | 3.1 |
| CV, % | 3.4 | 9.9 | 6.5 | 6.4 | 9.9 |
Conversion factor for oxalate in milligram per liter to micromole per liter, ×11.1.
CV, coefficient of variation; POx, plasma oxalate.
Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics, version 22.0 (IBM Corp; Armonk, New York). Descriptive statistics were performed, followed by a repeated measures analysis of variance, and paired and unpaired 2-tailed Student’s t-tests for comparisons of absolute values and differences within and between subjects, respectively. “F” and “t” stand for “F-statistic” and “T-score.” They represent the test statistics used to calculate an analysis of variance and a t-test, respectively. Test statistics provide a measure for how far a sample estimate is from the expected value if the null hypothesis was true. F and t are used to calculate a P value. The higher the test statistic, the more likely a statistically significant P value will result, and the null hypothesis will be rejected. The number in parentheses [e.g., F(1) or t(13)] indicates the degrees of freedom in the calculation and equals the number of observations allowed to vary minus 1. Correlations between parameters were calculated with the parametric Pearson test. A P value of <0.05 was considered statistically significant. Data are expressed as means ± SD.
Results
Patient and RRT Characteristics
Fifteen patients on regular (n = 8) or extended RRT (n = 7) were recruited for this study. After the baseline blood sampling, 1 patient from the extended RRT group was excluded for noncompliance with his minimum treatment time of 7 hours per session. As shown in Table 3, clinical characteristics at the time of study start differed between groups. However, apart from the differing treatment times of 259 ± 27 minutes on regular and 445 ± 12 minutes on extended RRT, RRT characteristics were largely similar. All patients had arteriovenous fistulas for vascular access.
Table 3.
Patient and RRT characteristics at the time of study start
| Parameters | Regular RRT |
Extended RRT |
P value |
|---|---|---|---|
| Patients, n | 8 | 6 | — |
| Women/men, n | 6/2 | 0/6 | — |
| Age, yr | 72 ± 15 | 48 ± 12 | 0.01 |
| Weight, kg | 64.8 ± 13.8 | 87.7 ± 15.4 | 0.02 |
| Duration of RRT, mo | 154 ± 83 | 155 ± 91 | 0.9 |
| Length of RRT, min | 259 ± 27 | 445 ± 12 | <0.001 |
| Blood flow, ml/min | 299 ± 2 | 294 ± 13 | 0.27 |
| Dialysate flow, ml/min | 513 ± 35 | 497 ± 7 | 0.32 |
| Recirculation on dialysis, % | 11 ± 2 | 6 ± 1 | <0.001 |
| Single pool, Kt/V | 2 ± 0.3 | 2.5 ± 0.5 | 0.05 |
| Hemoglobin, g/dl | 9.9 ± 1 | 11.6 ± 1.5 | 0.04 |
| Substitution volume, l | 24.2 ± 1.2 | 39.5 ± 4.5 | <0.001 |
| Baseline POx, μmol/l | 30.9 ± 7.1 | 24.7 ± 5.5 | 0.08 |
Data are expressed as mean ± SD.
Conversion factor for oxalate in milligram per liter to micromole per liter, ×11.1.
POx, plasma oxalate; RRT, renal replacement therapy.
Baseline Plasma Oxalate Concentrations
At baseline, POx concentrations averaged 28.0 ± 7.0 μmol/l, namely, 30.9 ± 7.1 μmol/l (95% confidence interval [CI]: 25.0−36.9]) in the regular group and 24.7 ± 5.5 μmol/l (95% CI: 19.6−29.8) in the extended group. The interindividual coefficient of variation was 24.9%, and the difference between the groups was not significant [t(13) = 1.89, P = 0.08, 95% CI: −0.9 to 13.4]. Baseline predialysis POx concentrations were independent of sex, age, weight, years on dialysis, hemoglobin, or ultrafiltration. A trend toward a significant correlation with Kt/V (r = 0.50, P = 0.07) and recirculation (r = −0.53, P = 0.05) was observed. Kt/V, the fractional urea clearance, is a measure of dialysis dose and is defined as the ratio of the dialyzer's urea clearance (=K; [ml/min]) multiplied by the treatment time (=t; [min]) to the urea distribution volume in the patient's body (=V; [ml]).
Kinetics of Intradialytic Plasma Oxalate Reduction
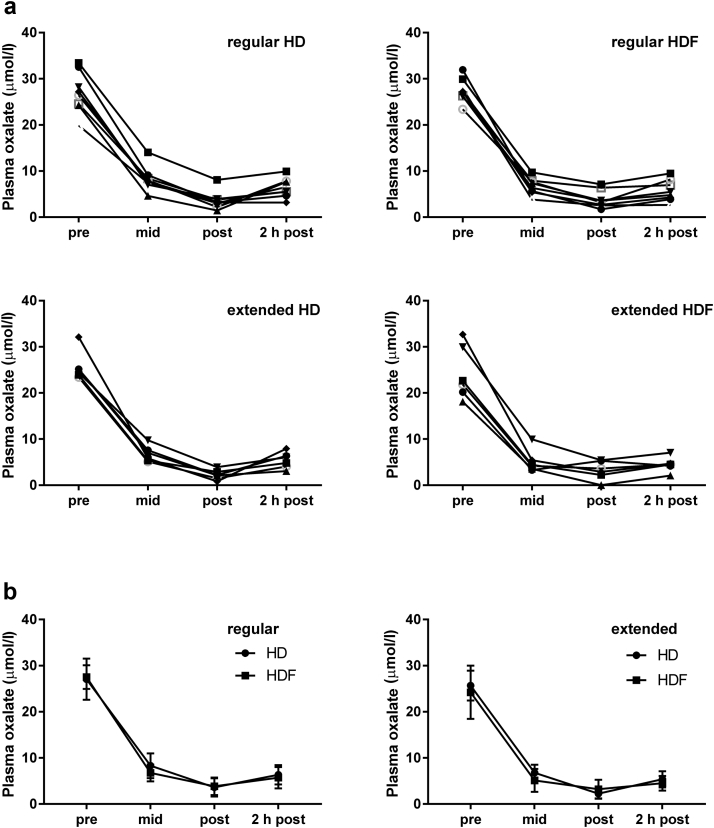
Individual POx reduction curves during RRT grouped by treatment mode are displayed in Figure 4a and summarized in Figure 4b; the corresponding Table 4 lists the mean POx concentrations for the different treatment combinations at the different sampling time points. For most patients, postdialysis POx came close to the normal range of 1 to 3 μM observed in healthy subjects, an average of 3.6 ± 2.0 μmol/l for those who underwent regular HD, 3.9 ± 1.9 μmol/l for those who underwent regular HDF, 2.2 ± 1.1 μmol/l for subjects who underwent extended HD, and 3.2 ± 2.0 μmol/l for those who underwent extended HDF. The major decrease of POx was noted in the first half of the dialysis session in both the regular and extended groups (i.e., between 0 and 2–2.5 hours and between 0 and 4–4.5 hours, respectively). From beginning to the end of 1 dialysis session, POx was lowered by a total of 86.9 ± 5.6% on regular HD and 85.9 ± 6.7% on regular HDF, and by 91.0 ± 4.7% on extended HD and 86.8 ± 9.0% on extended HDF. Intradialytic POx reduction was not significantly different across treatment modes [F(1) = 0.63; P = 0.44], but was correlated strongly with predialysis POx (r = 0.89; P < 0.001).
Figure 4.
Kinetics of intradialytic plasma oxalate reduction. Plasma oxalate was measured pre-, mid-, immediately post-, and 2 hours postdialysis during the last renal replacement therapy session of the hemodialysis (HD) and hemodialfiltration (HDF) study period, respectively. (a) Individual curves and (b) summary curves for the regular and extended group are shown (mean ± SD).
Table 4.
Plasma oxalate concentrations for different treatment combinations at different treatment time points
| Regular | HD | HDF | P value |
|---|---|---|---|
| Predialysis POx, μmol/l | 27.1 ± 4.5 | 27.5 ± 2.6 | 0.74 |
| Middialysis POx, μmol/l | 8.3 ± 2.7 | 6.7 ± 1.8 | 0.10 |
| Postdialysis POx, μmol/l | 3.6 ± 2.0 | 3.9 ± 1.9 | 0.65 |
| 2 h postdialysis POx, μmol/l | 6.4 ± 2.1 | 5.7 ± 2.3 | 0.36 |
| POx rebound, μmol/l | 2.7 ± 2.2 | 1.9 ± 1.5 | 0.30 |
| POx reduction, % | 86.9 ± 5.6 | 85.9 ± 6.7 | 0.59 |
| Extended | HD | HDF | P value |
|---|---|---|---|
| Predialysis POx, μmol/l | 25.7 ± 3.2 | 24.2 ± 5.8 | 0.46 |
| Middialysis POx, μmol/l | 6.8 ± 1.7 | 5.1 ± 2.5 | 0.08 |
| Postdialysis POx, μmol/l | 2.2 ± 1.1 | 3.2 ± 2.0 | 0.27 |
| 2-h postdialysis POx, μmol/l | 5.4 ± 1.8 | 4.5 ± 1.6 | 0.24 |
| POx rebound, μmol/l | 3.6 ± 2.2 | 1.1 ± 1.3 | 0.16 |
| POx reduction, % | 91.0 ± 4.7 | 86.8 ± 9.0 | 0.29 |
Data are expressed as mean ± SD.
Conversion factor for oxalate in milligram per liter to micromole per liter, ×11.1.
HD, hemodialysis; HDF, hemodialfiltration; POx, plasma oxalate; POx rebound = 2-hour postdialysis POx − postdialysis POx; POx reduction = (predialysis POx – postdialysis POx)/predialysis POx.
Postdialysis Plasma Oxalate Rebound
We observed a postdialysis POx rebound of 2.7 ± 2.2 μmol/l on regular HD and 1.9 ± 1.5 μmol/l on regular HDF; a rebound of 3.6 ± 2.2 μmol/l on extended HD and 1.1 ± 1.3 μmol/l on extended HDF was seen within 2 hours. However, there was no statistically significant difference between treatment modes [F(1) = 0.52; P = 0.48].
Effects of Different Dialysis Modes on Predialysis Plasma Oxalate
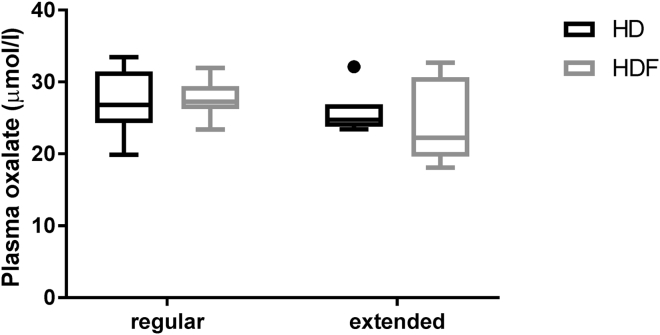
After 2 weeks on HD treatment, predialysis POx concentrations were 27.1 ± 4.5 μmol/l in the regular group and 25.7 ± 3.2 μmol/l in the extended group, and after 2 weeks on HDF, these concentrations were 27.5 ± 2.6 and 24.2 ± 5.8 μmol/l, respectively. The results are displayed in Table 4 and Figure 5. Neither a switch between HD and HDF treatment, nor factoring in extended treatment time, resulted in significantly different predialysis POx concentrations [F(1) = 0.21; P = 0.66 and F(1) = 0.76; P = 0.40, respectively].
Figure 5.
Comparison of predialysis plasma oxalate (POx) concentrations on regular or extended hemodialysis (HD) and hemodialfiltration (HDF). Tukey box plots for predialysis POx after 2 weeks on HD and 2 weeks on HDF in both groups are shown. The box extends from the 25th to the 75th percentiles; the line indicates the median. The upper whisker ends at the 75th percentile + 1.5 times the interquartile range (IQR); the lower whisker ends at the 25th percentile – 1.5 times the IQR. Values that exceed the indicated ranges are plotted as individual points.
Discussion
The present pilot study revealed several new findings that challenge the traditional view of oxalate handling in patients with ESRD. First, in contrast to other studies of oxalate kinetics on HD and HDF that reported predialysis POx concentrations between 40 and 50 μmol/l,29, 30, 31 only 3 of our patients exceeded the plasma supersaturation threshold of 30 μmol/l.8, 9, 10 A possible reason for this discrepancy might be different methods for oxalate measurements in earlier studies, which yielded higher POx concentrations due to higher detection limits and thus higher reference ranges.29, 30, 31, 37, 38 In vitro oxalogenesis can cause falsely elevated oxalate measurements. The spectrophotometric assay by Ladwig et al.35 for POx was adapted for our measurements and validated with our established protocol for ion chromatography.36 Another explanation was that RRT is generally more effective today, which leads to lower maintenance POx than in earlier studies. Our patients had an average Kt/V of >2 per treatment while Marangella et al.29 and Mydlík and Derzsiova30 reported means of 1.26 to 1.6. It was conceivable that our patients achieved a higher Kt/V because HDF, and not conventional HD, was administered during the run-in period. It was also noteworthy that baseline POx levels were lower in the extended RRT group. This could suggest that in long-term HDF, extended treatment time might have some benefit over regular treatment time for lowering POx levels. Despite a free diet choice during our trial, intraindividual variability of POx was minimal and interindividual variability was low, which might indicate that dietary oxalate was probably only a minor determinant of already elevated POx concentrations. The low variability could also be a reflection of the uniform and standardized therapy regimen we administered to a homogenous patient population.
Second, independent of the applied treatment mode, we achieved a POx reduction of approximately 90% within 1 RRT session. Moreover, we observed postdialysis POx concentrations that were almost within the range of POx concentrations found in healthy subjects without kidney disease. In previous studies POx was also significantly lowered with both HD and HDF, but patients remained hyperoxalemic at all times during and after the treatment session.29, 30, 31, 33 This implies that both our HD and HDF regimens were more effective than RRT regimens in earlier studies. Although not significant, intradialytic POx reduction was slightly more effective on extended HDF.
Third, we did not detect any benefit of HDF over HD at lowering predialysis POx concentrations. As Mydlik and Derzsiova30 pointed out, it was difficult to compare previous studies that investigated the influence of HD and HDF on oxalate kinetics: their common confounder was the use of 2 different filter materials, a traditional cellulosic membrane for HD and a synthetic membrane for HDF.30, 31, 32 In 2 of these studies,29, 32 HDF duration was shortened because the highly permeable synthetic membrane used for HDF was more efficient than the cellulosic membrane used for HD. Dell’Alquila et al.32 suggested that different solute-membrane interactions may occur dependent on the filter type used. More recently, it was demonstrated that significantly different oxalate clearances can be achieved with different types of filters.34 In our study, the same synthetic FX100 filter was used for both treatment modes, and no benefit of HDF over HD for oxalate reduction was detected. It was previously hypothesized that the positive interaction of diffusive and convective qualities on HDF plays a major role in improving oxalate clearance.32 The uniformity of oxalate kinetics on all treatment modes in our study made it seem more plausible that the membrane was an important determinant of effective and efficient oxalate elimination on current RRT regimens.
It remains possible that a difference in oxalate kinetics using the 2 different RRT modalities would emerge at lower levels of Kt/V than those achieved in our study, closer to the target recommended by the current Kidney Disease Outcomes Quality Initiative Guidelines.39
The 2-hour POx rebound observed in our patients suggested that POx quickly starts to rise again postdialysis. This might explain why placing our patients on HDF rather than HD treatment did not result in lower predialysis POx concentrations. Whether this rise could be solely explained by intercompartmental shifts is questionable. It was more likely that endogenous oxalate synthesis played a role in achieving such elevated predialysis POx concentrations as observed in our patients with ESRD. However, this study was not designed to properly assess oxalate kinetics in the interdialytic period. The lack of benefit of HDF over HD was also consistent with our finding that both HD and HDF effectively reduced POx to low levels by the end of dialysis. Intradialytic POx reduction correlated strongly with predialysis POx. As a consequence, increasing treatment intensity could not reduce the oxalate burden further. However, more frequent sessions could likely provide additional benefit for lowering predialysis POx. This may be of relevance to patients who experience primary hyperoxaluria with higher POx concentrations and who are in need of reducing the complications of systemic oxalosis or in preparation for transplantation.40, 41 The goal of our study was to reevaluate POx reduction by HD and HDF with up-to-date RRT equipment and to provide pilot data on the effect of extended treatment time on dialytic oxalate kinetics. The results from our study could serve as guidance for the design of subsequent larger trials that include more frequent treatment sessions as a promising intervention strategy.
Our study had several limitations. Only a small number of participants could be recruited from 1 university-based outpatient RRT unit in Germany. All patients were Caucasian, and the sex distribution was not even within the groups. Therefore, it was possible that this study did not adequately reflect oxalate kinetics in a broader RRT-dependent population. We balanced these disadvantages by using a crossover study design. Because every patient served as their own control, the influence of confounding covariates was reduced. In addition, the observed differences in POx concentrations with the different treatment modalities were so small that it was questionable whether (i) a higher difference would emerge in a larger study population, and (ii) if so, whether a difference of only a few micromoles per liter would be clinically relevant, as long as predialysis POx remained elevated >10 times normal.
Being in the regular or extended RRT group was a preselection that was fixed before the beginning of the study. The heterogeneity of the groups in their clinical characteristics and the relatively small number of patients did not allow for a direct comparison of regular and extended RRT. Thus, oxalate kinetics in the regular and extended groups represented independent observations. Due to the use of online HDF, measuring oxalate removal directly in the spent dialysate was not possible. The crossover design of our trial enabled us to still include “group” as an independent factor in our repeated measures analysis of variance. Also, (i) there was no significant correlation between predialysis oxalate concentrations and any of the clinical variables, and (ii) the RRT characteristics of the two groups were largely similar (Table 3). It would be of interest to study the influence of extended RRT on oxalate kinetics systematically and in more detail in a larger patient population.
The HDF and HD regimens were not administered in randomized order. However, it was unlikely that carryover effects played a significant role, because a run-in phase was conducted, and patients were maintained on the respective treatment regimen for 2 weeks before samples for POx measurements were obtained.
In conclusion, our pilot crossover trial provided up-to-date information on oxalate kinetics under regular and extended HD and HDF regimens. POx reduction on current standard HD was highly effective and efficient. Based on our data, there was no indication for HDF treatment to improve oxalate elimination in patients with secondary hyperoxalemia.
Disclosure
FK and K-UE received research grant support from Dicerna Pharmaceuticals. All the authors declared no competing interests.
Acknowledgments
The authors thank all participating patients and the physicians and nurses taking care of them. We thank M. Arend and M. Goppelt-Strübe for their support in setting up the enzymatic oxalate assay and D. Rosenhauer for extensive administrative assistance.
This study was supported by grants to FK from the Deutsche Forschungsgemeinschaft (DFG, project KN 1148/2-1), the Oxalosis and Hyperoxaluria Foundation, the Ulrich Gessler Stiftung, and a thematic network grant from the Deutscher Akademischer Austauschdienst (TRENAL). Peter S. Aronson was supported by NIH grant DK33793. TE is a recipient of a TRENAL medical student scholarship. This study was performed in fulfillment of her requirements for obtaining the degree “Dr. med.” The study also received support by a grant from the Renal Research Institute, NYC, NY. The funders had no role in study design; collection, analysis and interpretation of data; writing the report, or the decision to submit the report for publication.
References
- 1.Ermer T., Eckardt K.U., Aronson P.S. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens. 2016;25:363–371. doi: 10.1097/MNH.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asplin J. The management of patients with enteric hyperoxaluria. Urolithiasis. 2016;44:33–43. doi: 10.1007/s00240-015-0846-5. [DOI] [PubMed] [Google Scholar]
- 3.Cochat P., Rumsby G. Primary Hyperoxaluria. N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 4.Hoppe B., Kemper M.J., Bokenkamp A. Plasma calcium-oxalate saturation in children with renal insufficiency and in children with primary hyperoxaluria. Kidney Int. 1998;54:921–925. doi: 10.1046/j.1523-1755.1998.00066.x. [DOI] [PubMed] [Google Scholar]
- 5.Prenen J.A.C., Mees E.J.D., Boer P. Plasma oxalate concentration and oxalate distribution volume in patients with normal and decreased renal function. Eur J Clin Invest. 1985;15:45–49. doi: 10.1111/j.1365-2362.1985.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen S.M., Chen T.W., Lee Y.H. Renal excretion of oxalate in patients with chronic renal failure or nephrolithiasis. J Formos Med Assoc. 1990;89:651–656. [PubMed] [Google Scholar]
- 7.Gershman B., Sheth S., Dretler S.P. Relationship between glomerular filtration rate and 24-hour urine composition in patients with nephrolithiasis. Urology. 2012;80:38–42. doi: 10.1016/j.urology.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Elgstoen K.B.P., Johnsen L.F., Woldseth B. Plasma oxalate following kidney transplantation in patients without primary hyperoxaluria. Nephrol Dial Transplant. 2010;25:2341–2345. doi: 10.1093/ndt/gfq065. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe B., Kemper M.J., Bokenkamp A. Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 1999;56:268–274. doi: 10.1046/j.1523-1755.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y., Machida N., Ogawa T. Calcium oxalate saturation in dialysis patients with and without primary hyperoxaluria. Urol Res. 2006;34:12–16. doi: 10.1007/s00240-005-0004-6. [DOI] [PubMed] [Google Scholar]
- 11.Mori S., Beppu T. Secondary renal oxalosis. A statistical analysis of its possible causes. Acta Pathol Jpn. 1983;33:661–669. [PubMed] [Google Scholar]
- 12.Salyer W.R., Keren D. Oxalosis as a complication of chronic renal failure. Kidney Int. 1973;4:61–66. doi: 10.1038/ki.1973.80. [DOI] [PubMed] [Google Scholar]
- 13.Doganavsargil B., Akil I., Sen S. Autopsy findings of a case with oxalosis. Pediatr Dev Pathol. 2009;12:229–232. doi: 10.2350/07-06-0293.1. [DOI] [PubMed] [Google Scholar]
- 14.Mulay S.R., Eberhard J.N., Pfann V. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am J Physiol Renal Physiol. 2016;310:F785–F795. doi: 10.1152/ajprenal.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomson C.R.V., Channon S.M., Ward M.K. Plasma oxalate concentration, oxalate clearance and cardiac function in patients receiving haemodialysis. Nephrol DialTransplant. 1989;4:792–799. [PubMed] [Google Scholar]
- 16.Gulhan B., Turkmen K., Aydin M. The relationship between serum oxalic acid, central hemodynamic parameters and colonization by Oxalobacter formigenes in hemodialysis patients. Cardiorenal Med. 2015;5:164–174. doi: 10.1159/000381219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinheiro H.S., Câmara N.O.S., Osaki K.S. Early presence of calcium oxalate deposition in kidney graft biopsies is associated with poor long-term graft survival. Am J Transplant. 2005;5:323–329. doi: 10.1111/j.1600-6143.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Taheri D., Gheissari A., Shaabani P. Acute oxalate nephropathy following kidney transplantation: report of three cases. J Res Med Sci. 2015;20:818–823. doi: 10.4103/1735-1995.168408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parasuraman R., L. Zhang P., Samarapungavan D. Primary nonfunction of renal allograft secondary to acute oxalate nephropathy. Case Rep Transplant. 2011;2011:876906. doi: 10.1155/2011/876906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nistor I., Palmer S.C., Craig J.C. Haemodiafiltration, haemofiltration and haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev. 2015;5:CD006258. doi: 10.1002/14651858.CD006258.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramponi F., Ronco C., Mason G. Cost-effectiveness analysis of online hemodiafiltration versus high-flux hemodialysis. Clinicoecon Outcomes Res. 2016;8:531–540. doi: 10.2147/CEOR.S109649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque R., Marcelli D., Cardinal H. Cost-effectiveness analysis of high-efficiency hemodiafiltration versus low-flux hemodialysis based on the Canadian Arm of the CONTRAST Study. Appl Health Econ Health Policy. 2015;13:647–659. doi: 10.1007/s40258-015-0179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takura T., Kawanishi H., Minakuchi J. Cost-effectiveness analysis of on-line hemodiafiltration in Japan. Blood Purif. 2013;35 Suppl 1:85–89. doi: 10.1159/000346358. [DOI] [PubMed] [Google Scholar]
- 24.Mazairac A.H.A., Blankestijn P.J., Grooteman M.P.C. The cost-utility of haemodiafiltration versus haemodialysis in the Convective Transport Study. Nephrol Dial Transplant. 2013;28:1865–1873. doi: 10.1093/ndt/gft045. [DOI] [PubMed] [Google Scholar]
- 25.Lebourg L., Amato S., Toledano D. Hémodiafiltration en ligne : y a-t-il réellement un surcoût. Nephrol Ther. 2013;9:209–214. doi: 10.1016/j.nephro.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Oates T., Cross J., Davenport A. Cost comparison of online haemodiafiltration with high-flux haemodialysis. J Nephrol. 2012;25:192–197. doi: 10.5301/jn.5000046. [DOI] [PubMed] [Google Scholar]
- 27.Ogi M., Abe R., Nishitani T. The oxalate level in ultrafiltrate fluid collected from a dialyzer is useful for estimating the plasma oxalate level in hemodialysis patients. Clin Exp Nephrol. 2006;10:118–123. doi: 10.1007/s10157-006-0406-y. [DOI] [PubMed] [Google Scholar]
- 28.Hoppe B., Graf D., Offner G. Oxalate elimination via hemodialysis or peritoneal dialysis in children with chronic renal failure. Pediatr Nephrol. 1996;10:488–492. doi: 10.1007/s004670050145. [DOI] [PubMed] [Google Scholar]
- 29.Marangella M., Petrarulo M., Mandolfo S. Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron. 1992;60:74–80. doi: 10.1159/000186708. [DOI] [PubMed] [Google Scholar]
- 30.Mydlik M., Derzsiova K. Renal replacement therapy and secondary hyperoxalemia in chronic renal failure. Kidney Int Suppl. 2001;78:S304–S307. doi: 10.1046/j.1523-1755.2001.59780304.x. [DOI] [PubMed] [Google Scholar]
- 31.Chimienti S., Mele G., Perrone F. Oxalate depuration during biofiltration with AN69 and in conventional hemodialysis in chronic renal failure (CRF) patients. Int J Artif Organs. 1986;9 Suppl 3:73–74. [PubMed] [Google Scholar]
- 32.Dell'Aquila R., Feriani M., Mascalzoni E. Oxalate removal by differing dialysis techniques. ASAIO J. 1992;38:797–800. [PubMed] [Google Scholar]
- 33.Liu Y., Weisberg L.S., Langman C.B. Plasma oxalate levels in prevalent hemodialysis patients and potential implications for ascorbic acid supplementation. Clin Biochem. 2016;49:1133–1139. doi: 10.1016/j.clinbiochem.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 34.Franssen C.F. Oxalate clearance by haemodialysis–a comparison of seven dialysers. Nephrol Dial Transplant. 2005;20:1916–1921. doi: 10.1093/ndt/gfh971. [DOI] [PubMed] [Google Scholar]
- 35.Ladwig P.M., Liedtke R.R., Larson T.S. Sensitive spectrophotometric assay for plasma oxalate. Clin Chem. 2005;51:2377–2380. doi: 10.1373/clinchem.2005.054353. [DOI] [PubMed] [Google Scholar]
- 36.Knauf F., Asplin J.R., Granja I. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013;84:895–901. doi: 10.1038/ki.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rolton H.A., McConnell K.N., Modi K.S. A simple, rapid assay for plasma oxalate in uraemic patients using oxalate oxidase, which is free from vitamin C interference. Clin Chim Acta. 1989;182:247–254. doi: 10.1016/0009-8981(89)90102-2. [DOI] [PubMed] [Google Scholar]
- 38.Kohlbecker G., Butz M. Direct spectrophotometric determination of serum and urinary oxalate with oxalate oxidase. J Clin Chem Clin Biochem. 1981;19:1103–1106. doi: 10.1515/cclm.1981.19.11.1103. [DOI] [PubMed] [Google Scholar]
- 39.National Kidney F KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Tang X., Voskoboev N.V., Wannarka S.L. Oxalate quantification in hemodialysate to assess dialysis adequacy for primary hyperoxaluria. Am J Nephrol. 2014;39:376–382. doi: 10.1159/000360624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi T., Quillard M., Takahashi S. Oxalate removal by daily dialysis in a patient with primary hyperoxaluria type 1. Nephrol Dial Transplant. 2001;16:2407–2411. doi: 10.1093/ndt/16.12.2407. [DOI] [PubMed] [Google Scholar]