Abstract
Introduction
Previous reviews have indicated the effectiveness of exercise in people undergoing hemodialysis. However, these analyses did not take into account whether the subjects were elderly. We performed a systematic review of the effects of exercise training in elderly people undergoing hemodialysis and updated the evidence of exercise for people undergoing hemodialysis by adding recent research data.
Methods
We searched 8 electronic databases up to June 2016. Inclusion criteria were as follows: randomized controlled trial, English publication, subjects aged 18 and older undergoing hemodialysis, evaluation of physical function as an outcome of exercise intervention. We defined elderly as age 60 years and older. The main outcomes were exercise tolerance (peak/maximum oxygen consumption) and walking ability (6-minute walk distance). Secondary outcomes were lower extremity muscle strength and quality of life.
Results
After screening of 10,923 references, 30 comparisons were entered into the analysis. However, because we found only 1 study in which elderly subjects were treated, we could not perform a meta-analysis for these people. For the general population undergoing hemodialysis, supervised exercise training was shown to significantly increase peak/maximum oxygen consumption (standard mean difference, 0.62; 95% confidence interval 0.38–0.87; P < 0.001), 6-minute walk distance (standard mean difference, 0.58; 95% confidence interval 0.24–0.93; P < 0.001), lower extremity muscle strength (standard mean difference, 0.94; 95% confidence interval 0.67–1.21; P < 0.001), and quality of life (standard mean difference, 0.53; 95% confidence interval 0.52–0.82; P < 0.001).
Discussion
Our analysis on the effectiveness of exercise training in elderly people undergoing hemodialysis as compared with nonelderly people was somewhat inconclusive. Future studies should be carried out for elderly people to identify the most favorable exercise program for this population.
Keywords: dialysis, elderly, exercise, meta-analysis, renal replacement therapy
An aging population and the increasing prevalence of lifestyle-related diseases, such as diabetes, hypertension, and cardiovascular disease, have led to a worldwide increase in the rate of chronic kidney disease requiring renal replacement therapy, including hemodialysis.1 The mean age of people undergoing dialysis has been on the rise because of improved survival in this patient population, as well as the reduced availability of transplants for elderly patients. Significant increases in age of people undergoing dialysis were observed in almost all 12 nations included in the Dialysis Outcomes and Practice Patterns Study, an international cohort study.2 Other studies from the United States, Europe, and Japan also report a significant proportion of elderly patients undergoing dialysis.3, 4, 5 In particular, the mean age in the Japanese dialysis population was 66.9 years in 2012, showing an 11.6-year increase since the end of 1991. Furthermore, the proportions of people aged 60 years and older were 78.1% of patients who started undergoing dialysis in 2012 and 75.4% of the entire dialysis population.5
Elderly people undergoing hemodialysis have a high prevalence (70%) of physical frailty, characterized by lower levels of physical functioning.6 However, physical frailty, a well-known indicator of disability and poor prognosis among the elderly,7, 8, 9, 10 could be prevented, postponed, or even reversed with specific interventions and health strategies. Physical exercise has been shown to have positive effects on physical function among frail older adults11 and is recommended for those with kidney disease.12 Previous meta-analyses indicated the effectiveness of exercise interventions on exercise tolerance, physical function, and quality of life (QoL) for people undergoing hemodialysis13, 14; however, these analyses did not take into consideration whether subjects were elderly. Elderly patients face an array of barriers to exercise such as self-efficacy, discomfort, disability, fear of injury, habits, environmental factors, cognitive decline, and fatigue.15 Hence, the concept of exercise intervention for young to middle-aged people undergoing hemodialysis is not entirely applicable to elderly people, and whether exercise training improves physical function, exercise tolerance, or QoL in elderly people undergoing hemodialysis remains unclear. Moreover, how best to manage this patient population is still poorly understood in the field of nephrology. Therefore effectiveness of exercise interventions on patient outcomes needs to be evaluated with patient age in mind, and conclusions regarding the effectiveness of exercise training must be updated with the latest data from new trials targeting elderly people undergoing hemodialysis.16, 17
The main goals of this systematic review and meta-analysis were (i) to compare the benefits of supervised exercise training programs on exercise tolerance (peak/maximum oxygen consumption [VO2]), walking ability (6-minute walk distance), lower extremity muscle strength, and health-related QoL (short-form health survey [SF-36]) between nonelderly and elderly people undergoing hemodialysis, especially those aged 60 years and older and (ii) to update the evidence base for recommendation of supervised exercise interventions for hemodialysis populations by adding data from recent research studies.
Materials and Methods
This review is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidance18 (Supplementary Appendix S1) and is one of a series of systematic reviews regarding the effectiveness of exercise training in elderly patients undergoing hemodialysis. The protocol used for the systematic review and meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: PROSPERO 2015: CRD42015020701), and our protocol has already been published (http://bmjopen.bmj.com/content/6/5/e010990.long).19
No ethical approval was required because this study did not include confidential personal data and did not involve patient intervention.
Study Selection and Data Management
An electronic database search was performed in MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, CINAHL, Web of Science, PsycINFO, and PEDro. The search was performed with the following terms: dialysis, renal replacement therapy, exercise, physical fitness, cycling, walk, physical therapy. The full strategy is described in Supplementary Appendix S2. To identify any articles missed by the initial search, we also evaluated the reference lists of previously reported systematic reviews.
We used EndNote X7 for Windows (Thompson Reuters, Philadelphia, Pennsylvania) to manage literature records and data. Reviewers screened all titles, abstracts, and the full texts. When required data were not available, the study authors were contacted by e-mail.
Inclusion and Exclusion Criteria
We included only randomized controlled trials (RCTs) published in English that evaluated the effects of supervised exercise training on at least 1 of the outcome measures included for this review and were a measure of physical function. Supervised exercise included resistance training, aerobic exercise, and combined exercise. Only RCTs that included subjects at least 18 years of age who were undergoing hemodialysis were included in this meta-analysis. Patients affected by acute kidney failure were excluded. In the present study, we defined elderly as age 60 years and older. The main outcomes of the study were exercise tolerance (peak/maximum VO2) and walking ability (6-minute walk distance). Secondary outcomes were lower extremity muscle strength measured by using a dynamometer and health-related QoL (short-form health survey: physical component summary score and mental component summary score).
Risk of Bias
The methodological quality of trials included in the review was evaluated independently by using the Cochrane Collaboration tool20 for assessment of risk of bias by 2 reviewers. Studies were graded as having a “low risk,” “high risk,” or “unclear risk” of bias across the following 7 specified domains: random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other sources of bias. Furthermore, we assessed the risk of bias of references using the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX),21 which consists of 15 different items and has been shown to be a reliable tool for performing a comprehensive review of exercise training trials.
Data Analysis and Statistical Methods
Our statistical analysis strategy involved finding the average absolute change in the included patient measures from baseline to endpoint (including SD) in the intervention and control groups. We evaluated the standardized mean difference for exercise training. An analysis was performed according to whether study subjects were elderly (defined as ≥ 60 years old) or nonelderly. The effect consistency across studies was assessed using the I2 statistic,22 with I2 > 25% and 50% considered to indicate moderate and substantial heterogeneity, respectively. We used the random-effects model as the default method of analysis because of the expected clinical heterogeneity between studies, since the alternative fixed-effects model assumes that the true treatment effect of each trial is the same and that any observed differences are caused by chance. We assessed publication bias by plotting the inverse of the SE of the effect estimates using funnel plots to explore symmetry, which was assessed visually and using Egger’s regression test in analyses including 10 or more studies. The analysis was performed using Review Manager Software (RevMan V.5.3; Cochrane Collaboration, Oxford, UK) and R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
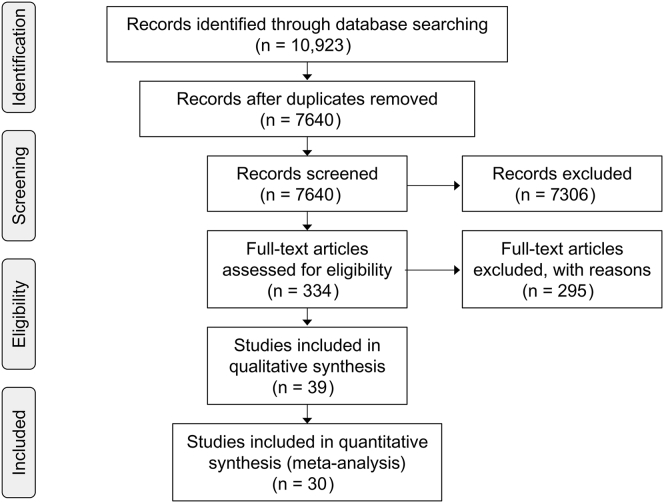
Of the total of 10,923 references that were initially screened, 7640 had no duplicates and 7306 were rejected at the title and abstract stage. We analyzed 334 studies that were identified for potential inclusion and full-text review, and 30 comparisons were entered into the analysis16, 17, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 (Figure 1). Of the 30 comparisons, only 1 study targeted elderly people undergoing hemodialysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing selection of randomized controlled trials.
Participants and Interventions
Table 1 presents a summary of the trials.16, 17, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 In 21 studies intradialytic exercise was adopted, and interventions ranged from 8 weeks to 12 months in duration, with most lasting for 3 to 6 months. A combination of aerobic exercise and strength exercise training was used in 10 studies, and interventions were performed 3 times per week in most of the studies. There was no trial in which peak/maximum VO2 was reported for elderly participants undergoing hemodialysis, and only 1 trial included reports of 6-minute walk distance, lower extremity muscle strength, and QoL in elderly people.
Table 1.
Characteristics of included studies
| Studies | Location | Mean age (SD), yr | Mean duration of HD (SD), yr | No. of patients | Duration of intervention | Type of intervention | Training program | Intensity of program | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Akiba et al. (1995)50 | Japan | Ex: 38.4 (9.5) Con: 40.6 (10.8) |
Ex: 6.15 (3.9) Con: 5.69 (3.5) |
Ex: 10 Con: 10 |
3 mo | Cycle ergometer before hemodialysis session (+treatment for anemia) | Aerobic for 20 min using cycle ergometer 3 times per wk | No data | Exercise tolerance (VO2 max) |
| Carmack et al. (1995)23 | USA | All: 44.1 | No data | Ex: 23 Con: 25 |
10 wk | Intradialytic | Aerobic exercise for 20–30 min using cycle ergometer 3 times per wk | No data | Exercise tolerance (VO2 peak) Depression |
| Carney et al. (1987)51 | USA | Ex: 36.1 (3.2) Con: 40.7 (5.3) |
Ex: 2.5 (0.7) Con: 3.3 (1.1) |
Ex: 11 Con: 7 |
6 mo | Track walking, bicycle ergometer | Aerobic exercise for 45–60 min using indoor track and bicycle ergometer 3 times per wk | 70%–80% of VO2max | Exercise tolerance (VO2max) Depression |
| Cheema et al. (2007)24 | Australia | All: 62.6 (14.2) | 2.2 | Ex: 24 Con: 25 |
24 wk | Intradialytic | High-intensity progressive resistance training: 2 sets of 8 repetitions of 10 types using weighted ankle cuffs or Thera-Band tubinga 3 times per wk |
Borg scale 15 to 17 (“hard” to “very hard”) |
Lower extremity muscle strength Muscle mass (CT) Walking ability (6MWT) |
| Chen et al. (2010)25 | USA | Ex: 71.1(12.6) Con: 66.9(13.4) |
Ex: 2.6 (2.6) Con: 4.8 (5.2) |
Ex: 25 Con: 25 |
24 wk | Intradialytic | Progressive resistance training 2 sets of 8 repetitions of 8 types using ankle weights 2 times per wk | OMNI scale 6 (somewhat hard) out of 10 (extremely hard), equivalent to 60% of a 1-repetition maximum | ADL level Lower extremity muscle strength Physical performance (SPPB) Physical activity QoL |
| de Lima et al. (2013)52 | Brazil | Ex: 49.6 (9.1) Con: 43.5 (11.1) |
Ex: 5.4 (4.0) Con: 6.5 (4.2) |
Ex: 11 Con: 11 |
8 wk | Intradialytic | Developed peripheral musculature training using anklets consisting of 3 series of 15 repetitions 3 times per wk | 40% of 1RM | QoL |
| de Lima et al. (2013)52 | Brazil | Ex: 43.1 (13.3) Con: 43.5 (11.1) |
Ex: 6.4 (4.4) Con: 6.5 (4.2) |
Ex: 10 Con:11 |
8 wk | Intradialytic | Progressive ergometric bicycle exercise 20 min 3 times per wk | Modified Borg scale 2–3 | QoL |
| Deligiannis et al. (1999)26 | USA | Ex: 46.4 (13.9) Con: 50.2 (7.9) |
Ex: 6.5 (5.2) Con: 6.6 (7.2) |
Ex: 16 Con: 12 |
6 mo | Nonintradialytic | Aerobic and low-weight resistance training for 90 min (including 10-min warm- up using cycle ergometer or treadmill, 50-min intermittent aerobic exercise, and 10-min cool-down) 3 times per wk After the first 3 months, the younger patients were playing basketball and football, whereas the older patients were swimming. |
60%–70% of the HRmax | Exercise tolerance (VO2max) |
| DePaul et al. (2002)27 | Canada | Ex: 55 (16) Con: 54 (14) |
Ex: 4.2 (4.8) Con: 4.6 (4.5) |
Ex: 20 Con: 18 |
12 wk | Intradialytic Before and after the dialysis session |
Aerobic training 20 min 3 times per wk, progressive strength training: 1 set of 10 repetitions; number of sets: 1–3 | Borg scale 13 (“somewhat strong”) 5-repetition maximum |
Lower extremity muscle strength Walking ability (6MWT) QoL |
| Dobsak et al. (2002)53 | France | Ex: 58.2 (7.2) Con: 60.1 (8.2) |
Ex: 4.1 (2.1) Con: 4.1 (2.3) |
Ex: 11 Con: 10 |
20 wk | Intradialytic | Progressive ergometric bicycle exercise 20–40 min 3 times per wk | 60% peak workload | Exercise tolerance (peak workload) Walking ability (6MWT) QoL |
| Dong et al. (2010)28 | USA | Ex: 46.5 (12.1) Con: 40.2 (13.5) |
Unknown | Ex: 15 Con: 17 |
6 mo | Intradialytic | 3 sets of 12 repetitions of leg press, under supervision of study personnel, within 30 min 3 times per wk | 70% of the 1-RM | Lower extremity muscle strength Muscle mass (DEXA) |
| Giannaki et al. (2013)29 | Cyprus | Ex: 59.2 (11.8) Con: 58.0 (10.7) |
Ex: 2.0 (1.25) Con: 2.5 (2.2) |
Ex:12 Con:12 |
6 mo | Intradialytic | Ex: progressive aerobic exercise training using a recumbent cycle ergometer for 45 min 3 times per wk | Ex: 60%–65% of the patient's maximal exercise capacity (in Watts) | Depression Lower extremity muscle strength (STS) Sleep quality |
| Giannaki et al. (2013)29 | Greece | Ex: 56.4 (12.5) Con: 55.7 (10.4) |
Ex: 3.9 (1.3) Con: 4.0 (1.7) |
Ex: 15 Con: 7 |
6 mo | Intradialytic | Progressive aerobic exercise training using a recumbent cycle ergometer 3 times per wk | 60%–65% of the patient's maximal exercise capacity (in Watts) | Depression Lower extremity muscle strength (STS) Muscle mass (CT) QoL Walking speed |
| Goldberg et al. (1983)31 | USA | Ex: 38.5 (15.4) Con: 37.1 (12.1) |
Ex: 1.9 (1.4) Con: 3.3 (2.5) |
Ex: 14 Con: 11 |
No data | Indoor training | Progressive treadmill walking or jogging 45–60 min included 5–10 min low-intensity walking | Initial training: 50–60 VO2max By 9 mo: 70–80 VO2max |
Exercise tolerance (VO2max) Depression |
| Goldberg et al. (1986)30 | USA | Ex: 40.0 (14.0) Con: 36.0 (10.0) |
Ex:1.9 (1.5) Con: 3.3 (2.6) |
Ex: 13 Con: 12 |
12 mo | Unknown | Endurance training for 45 min (cycling using bicycle ergometer and walking-jogging) | 70%–80% of VO2max | Exercise tolerance (VO2max) Depression |
| Groussard et al. (2015)17 | France | Ex: 66.5 (4.6) Con: 68.4 (3.7) |
Ex: 36.6 (8.2) Con: 41.2 (8.1) |
Ex: 8 Con: 10 |
3 mo | Intradialytic | Aerobic exercise consisting of cycling 3 times per wk (5-min warm-up, 15–30 min at a tolerable pace and 5-min cool-down) | 55%–60% of the peak power output | Exercise tolerance (VO2 peak) Walking ability (6MWT) |
| Guadalupe et al. (2016)54 | Mexico | Ex: 28.5 Con: 29 |
Ex: 1.0 Con: 1.5 |
Ex: 30 Con: 31 |
12 wk | Intradialytic | Resistance training 2 times per wk using ankle weights and bands Four series of 30 repetitions were performed for each of the 4 exercises. |
500-g weight | Grip strength |
| Johansen et al. (2006)32 | USA | Ex: 54.4 (13.6) Con: 56.8 (13.8) |
Ex: 2.8 Con: 2.1 |
Ex: 20 Con: 20 |
12 wk | Intradialytic | Progressive resistance training using ankle weights 2–3 sets of 10 repetitions | 60% of 3RM | Lower extremity muscle strength Lower extremity muscle strength (STS) Muscle mass (MRI) Physical activity QoL Walking speed |
| Kirkman et al. (2014)33 | UK | Ex: 48 (18) Con: 58 (15) |
Ex: 3.8 (4.5) Con: 5.5 (3.9) |
Ex: 12 Con: 11 |
12 wk | Intradialytic | Progressive resistance training: 8 sets of 10 repetitions of 10 types using resistance bands 3 times a wk | 3 sets of 8–10 repetitions at 80% of their predicted 1RM with 2-min rest period between sets | Lower extremity muscle strength Muscle mass (MRI) QoL Walking ability (6MWT) Walking speed |
| Koh et al. (2010)34 | Australia | Ex: 52.3 (10.9) Con: 51.3 (14.4) |
Ex: 2.7 (2.2) Con: 2.2 (1.9) |
Ex: 15 Con: 16 |
6 mo | Intradialytic | Aerobic exercise training for 30–45 min using cycle ergometer 3 times per wk | Borg scale 12–13 | Grip strength QoL Walking ability (6MWT) Walking speed (TUG) |
| Konstantinidou et al. (2002)35 | Greece | Ex: 46.4 (13.9) Con: 50.2 (7.9) |
Ex: 6.5 (5.2) Con: 6.6 (7.2) |
Ex: 16 Con: 12 |
6 mo | Nondialysis days | Aerobic and strengthening training for 60 min 3 times per wk (10 min warm-up, 30 min intermittent aerobic exercise, 10 min stretching, low-weight resistance training and 10 min cool-down) | 60%–70% of the HRmax | Exercise tolerance (VO2 peak) |
| Konstantinidou et al. (2002)35 | Greece | Ex: 48.3 (12.1) Con: 50.2 (7.9) |
Ex: 6.0 (5.5) Con: 6.6 (7.2) |
Ex: 10 Con: 12 |
6 mo | Intradialytic | Aerobic and strength training for 60 min program 3 times per wk (30 min with a bed bicycle ergometer and 30 min for strength and flexibility) | 70% of the HRmax | Exercise tolerance (VO2 peak) |
| Koufaki et al. (2002)36 | UK | Ex: 57.3 (14.3) Con: 50.5 (19) |
Ex: 3.1 (3.8) Con: 4.0 (4.2) |
Ex: 26 Con: 22 |
12 wk | CAPD: in the Renal Rehabilitation Gym HD: Intradialytic |
Progressive aerobic training on a cycle ergometer 3 times per wk | 90% of VT | Exercise tolerance (VO2 peak) Lower extremity muscle strength (STS) Physical activity |
| Kouidi et al. (1997)55 | Greece | Ex: 49.6 (12.1) Con: 52.8 (10.2) |
Ex: 5.9 (4.9) Con: 6.2 (5.4) |
Ex: 20 Con: 11 |
6 mo | Nondialysis days | Supervised exercise (stationary cycling, walking or jogging, calisthenics, aerobics, swimming and/or game sports) 90 min 3–4 times per wk | 50%–60% of their VO2max or 60%–70% of their HRmax | Exercise tolerance (VO2max) QoL |
| Kouidi et al. (2009)37 | USA | Ex: 54.6 (8.9) Con: 53.2 (6.1) |
Ex: 6.3 (3.7) Con: 6.2 (3.9) |
Ex: 32 Con: 31 |
10 mo | Intradialytic | Supervised training (40 min: cycling ergometer; 30 min: progressive muscle strengthening 3 sets of 15 repetitions using Thera-Band tubinga and weights to the limbs) 3 times per wk |
Borg scale 13 (somewhat hard) | Exercise tolerance (VO2 peak) |
| Matsufuji et al. (2015)16 | Japan | Ex: 69 (61–78) Con: 69 (64–79) |
Ex: 14 Con: 15 |
Ex: 12 Con: 15 |
12 wk | On dialysis day | 5 sets chair stand exercise as resistance training 3 times per wk |
5 sets of half of the maximum duration for each participant with 4 short breaks | Lower extremity muscle strength QoL Walking ability (6MWT) |
| Matsumoto et al. (2007)56 | Japan | Ex: 60.8 (9.5) Con: 57.2 (8.3) |
Ex: 12.4 (6.8) Con:12.7(7.5) |
Ex: 17 Con: 32 |
12 mo | Endurance training before each hemodialysis treatment | 20 min of continuous cycling 3 times per wk | Borg scale 11–13 (60%– 70% of peak heart rate) | QoL |
| Molsted et al. (2004)38 | Denmark | Ex: 59.0 Con: 48.0 |
Ex: 2.0 Con: 1.4 |
Ex: 22 Con: 11 |
5 mo | Unknown | Strength and aerobic exercises for 1 h twice a wk (10 min of warm-up, 20–30 min of strength and aerobic exercises, 20 min of interval cycling, and 10 min cooling down) | Borg scale 14–17 | Exercise tolerance (VO2max) Lower extremity muscle strength (STS) QoL |
| Ouzouni et al. (2009)39 | Greece | Ex: 47.4 (15.7) Con: 50.5(11.7) |
Ex: 7.7 (7.0) Con: 8.6 (6.0) |
Ex: 19 Con: 14 |
10 mo | Intradialytic | 60–90 min 3 times per wk (cycling: 30 min, strengthening: 30 min, flexibility exercise: 30 min) | Borg scale 13–14 (“somewhat hard”) | Exercise tolerance (VO2 peak) QoL |
| Painter et al. (2002)40 | USA | Ex: 43.5 (10.5) Con: 50.1 (13.8) |
Ex: 5.0 (6.7) Con: 5.7 (4.5) |
Ex: 12 Con: 12 |
5 mo | Intradialytic (+ Normalized hematocrit) | Continuous cycling 30 min 3 times per wk Interval exercise 20 min 3 times per wk |
Borg scale 12–14 (70% of peak heart rate) Borg scale 15–17 |
Exercise tolerance (VO2 peak) QoL |
| Parsons et al. (2004)41 | Canada | Ex: 60.0 (17.0) Con: 49.0(25.0) |
Ex: 2.9 (2.1) Con: 4.1 (2.2) |
Ex: 6 Con: 7 |
8 wk | Intradialytic | Cycle ergometry exercise for 15 min 3 times per wk | 40%–50% maximal work capacity | QoL |
| Pellizzaro et al. (2004)42 | Brazil | Ex: 48.9 (10.1) Con: 51.9 (11.6) |
Ex: 4.5 Con: 4.5 |
Ex: 14 Con: 14 |
10 wk | Intradialytic | Resistance training using leg weights 3 sets of 15 knee extension repetitions | 50% of 1RM | Walking ability (6MWT) QoL |
| Pellizzaro et al. (2004)42 | Brazil | Ex: 43.0 (13.8) Con: 51.9 (11.6) |
Ex: 5.0 Con: 4.5 |
Ex: 11 Con: 14 |
10 weeks | Intradialytic | Inspiratory muscle training using the Threshold Loader 3 sets of 15 inspirations Resistance training using leg weights: 3 sets of 15 knee extension repetitions |
50% of PImax 50% of 1RM |
Walking ability (6MWT) QoL |
| Petraki et al. (2008)43 | Greece | Ex: 50.05 (13.2) Con: 50.52 (1.4) |
Ex: 6.4 (0.6) Con: 6.1 (0.4) |
Ex: 22 Con: 21 |
7 mo | Intradialytic | Progressive 60 min cycling using specific bed cycles (including 5-min warm -up and terminated 5-min recovery) and 30 min strengthening and flexibility exercises 3 times per wk | Borg scale 13 | Exercise tolerance (VO2 peak) |
| Reboredo et al. (2011)44 | Brazil | Ex: 50.7 (10.7) Con: 42.2 (13.0) |
Ex: 3.3 (3.4) Con: 4.8 (4.4) |
Ex: 12 Con: 12 |
12 wk | Intradialytic | Warmed up for 10 min (lower-limb stretching exercise, low work rate cycling) Aerobic training program for 40 min 3 times per wk |
No data Modified Borg scale 4–6 |
Exercise tolerance (VO2 peak) |
| Reboredo et al. (2015)57 | Brazil | Ex: 50.7 (10.7) Con: 42.2 (13.0) |
Ex: 3.3 (3.4) Con: 4.8 (4.4) |
Ex: 12 Con: 12 |
12 wk | Intradialytic | Aerobic exercise at moderate exertion for 43 min 3 times per wk (10 min warm-up by lower limb stretching, 5 min low-intensity cycling, 35 min moderate intensity cycling, and 3 min cool-down) | Modified Borg scale 4–6 | Exercise tolerance (VO2 peak) |
| Segura-Orti et al. (2009)45 | Spain | Ex: 53.5 (18.0) Con: 60.1(16.9) |
Ex: 3.1 (2.9) Con: 4.5 (3.5) |
Ex: 17 Con: 8 |
24 wk | Intradialytic | Progressive resistance training that targeted major muscle groups of lower extremities, 3 sets of 4 exercises using weights and elastic bands | Borg scale 12–15 | Exercise tolerance (VO2 peak) Lower extremity muscle strength Lower extremity muscle strength (STS) QoL Walking ability (6MWT) |
| Song et al. (2012)46 | Korea | Ex: 52.1 (12.4) Con: 54.6 (10.1) |
Ex: 3.2 (2.2) Con: 3.8 (4.7) |
Ex: 20 Con: 20 |
12 wk | Predialysis resistance training | 5 min warm-up and cool-down; progressive resistance training consisted of upper and lower body exercise using elastic bands and sandbags for 30 min 3 times per wk | Borg Scale 11–15 (moderate to hard) | Grip strength Lower extremity muscle strength Lower extremity muscle strength (STS) Balance function QoL |
| Tsuyuki et al. (2003)47 | Japan | Ex: 40.1 (11.9) Con: 39.7(10.7) |
Ex: 2.1 (2.5) Con: 2.7 (2.6) |
Ex:17 Con: 12 |
20 wk | Nondialysis days | Combination training of bicycle ergometry, walking, and jogging for 30 min 2–3 times per wk | 50%–60% of the peak heart rate | Exercise tolerance (VO2 peak) |
| van Vilsteren et al. (2005)48 | Netherlands | Ex: 52 (15) Con: 58 (16) |
Ex: 3.2 (4.1) Con: 3.9 (4.4) |
Ex: 53 Con: 43 |
12 wk | Predialysis strength training Intradialytic Exercise counseling |
A 5- to 10-min warm-up and cool-down; a 20-min exercise program including calisthenics, steps, flexibility, and low weight resistance training Cycling 20–30 min 2–3 times per wk The techniques based on the transtheoretical model, motivational interviewing, and health counseling |
Borg scale 12–16 (< 60% maximal capacity) |
Exercise tolerance (VO2 peak) Lower extremity muscle strength (STS) QoL |
| Wilund et al. (2010)58 | USA | Ex: 60.8 (3.2) Con: 59.0 (4.9) |
Ex: 5.3 (8.7) Con: 3.7 (1.0) |
Ex: 7 Con: 8 |
4 months | Intradialytic | Endurance exercise training for 45 min using cycle ergometer 3 times per wk | Borg scale 12–14 | Walking ability (shuttle walk test) |
| Wu et al. (2014)49 | China | Ex: 45 Con: 44 |
Ex: 4.6 (3.1) Con: 3.3 (2.5) |
Ex: 32 Con: 33 |
12 weeks | Intradialytic | 15–20 min of recumbent cycling (including 5-min warm-up) | Energy consumption of 70–100 calories, Borg scale 12–16 and an increase in heart rate of 20 beats/min (optimum individualized exercise load) | Grip strength Lower extremity muscle strength (STS) Walking ability (6MWT) QoL |
ADL, activities of daily living; Con, control; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; Ex, exercise; HD, hemodialysis; HRmax, maximum heart rate; MRI, magnetic resonance imaging; PImax, maximum inspiratory pressure; RM, repetition maximum; QoL, quality of life; SPPB, short physical performance battery; STS, sit-to-stand; TUG, timed up & go test; VO2max, maximum oxygen consumption; VT, ventilatory threshold; 6MWT, 6-minute walk test.
Thera-Band tubing is manufactured by Performance Health (Akron, OH).
Treatment Outcomes
Exercise Tolerance
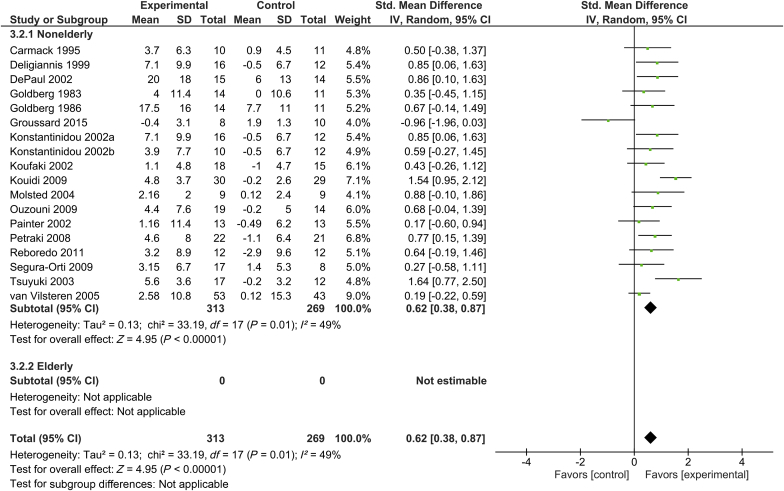
Eighteen trials included measurement of peak/maximum VO2, with a total of 313 subjects in the intervention group and 269 control subjects.17, 23, 26, 27, 30, 31, 35, 36, 37, 38, 39, 40, 43, 44, 45, 47, 48 Supervised exercise training was shown to significantly increase exercise tolerance in the total patient population. The standardized mean difference (SMD) of peak/maximum VO2 was 0.62 (95% confidence interval [CI], 0.38–0.87; P < 0.001) in the total patient population. There was a moderate degree of heterogeneity in exercise tolerance across studies (I2 = 49%). However, because there was no study involving elderly participants, we were not able to analyze the efficacy of exercise training on exercise tolerance among elderly patients undergoing hemodialysis (Figure 2).
Figure 2.
Forest plot showing the effects of supervised exercise training compared with usual care on changes in exercise tolerance (peak/maximum oxygen consumption). CI, confidence interval; IV, inverse variance; Std., standard.
Walking Ability
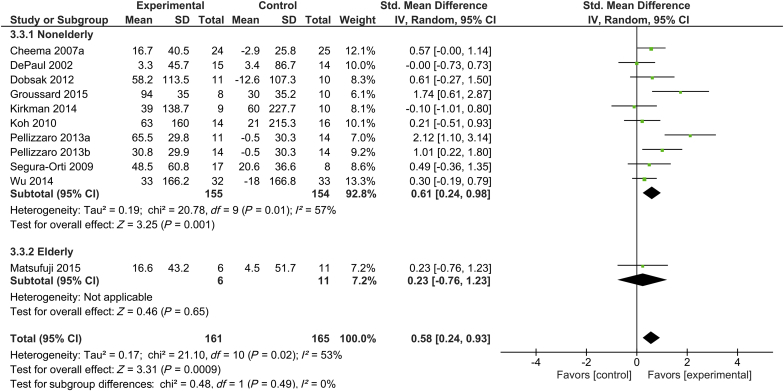
Ten trials assessed 6-minute walk distance with a total of 161 subjects in the intervention group and 165 subjects in the control group.16, 17, 24, 27, 33, 34, 42, 45, 49, 53 Only 1 of these 11 trials included elderly participants.16 Supervised exercise training was shown to significantly increase walking ability, as determined by 6-minute walking test, in subjects undergoing hemodialysis, with SMD of 0.58 (95% CI, 0.24–0.93; P < 0.001) in the total patient population. There was a moderate degree of heterogeneity across studies in walking ability (I2 = 53%). In elderly subjects undergoing hemodialysis, exercise training did not significantly increase 6-minute walking distance (SMD, 0.23; 95% CI, -0.76 to 1.23; P = 0.65) (Figure 3).
Figure 3.
Forest plot showing the effects of supervised exercise training compared with usual care on changes in walking ability (6-minute walking distance). CI, confidence interval; IV, inverse variance; Std., standard.
Muscle Strength
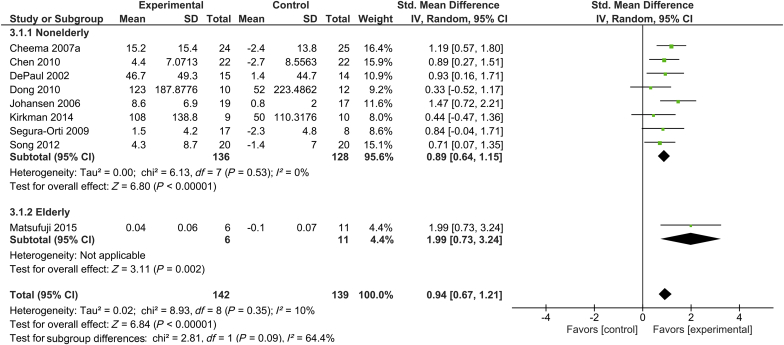
In 9 trials with 142 subjects in the intervention group and 139 control subjects, lower extremity muscle strength was measured by using a dynamometer.16, 24, 25, 27, 28, 32, 33, 45, 46 Only 1 of these 9 trials included elderly participants.16 Supervised exercise training was shown to significantly increase lower extremity muscle strength in patients undergoing hemodialysis, with SMD of 0.94 (95% CI, 0.67–1.21; P < 0.001) in the total patient population. There was a low degree of heterogeneity across studies for muscle strength (I2 = 10%). In elderly subjects undergoing hemodialysis, exercise training was shown to significantly increase muscle strength (SMD, 1.99; 95% CI, 0.73–3.24; P = 0.002) (Figure 4).
Figure 4.
Forest plot showing the effects of supervised exercise training compared with usual care on changes in muscle strength (lower-extremity muscle strength). CI, confidence interval; IV, inverse variance; Std., standard.
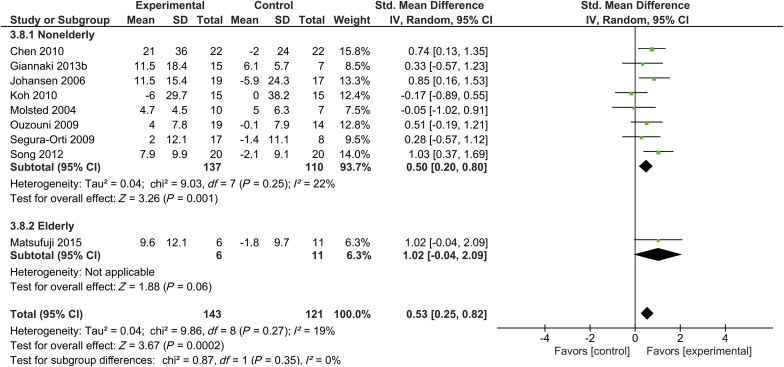
Quality of Life
Nine trials with 143 subjects in the intervention group and 121 subjects in the control group assessed the physical component summary of the short-form health survey.16, 25, 29, 32, 34, 38, 39, 45, 46 Only 1 of these 9 trials included elderly participants.16 Supervised exercise training was shown to significantly increase physical component summary score in patients undergoing hemodialysis, with SMD of 0.53 (95% CI, 0.52–0.82; P < 0.001) in the total patient population. There was only a low level of heterogeneity across studies for the physical component summary (I2 = 19%). Exercise training was not shown to significantly increase the physical component summary score in elderly subjects undergoing hemodialysis (SMD, 1.02; 95% CI, -0.04 to 2.09; P = 0.06) (Figure 5).
Figure 5.
Forest plot showing the effects of supervised exercise training compared with usual care on changes in quality of life (short-form health survey: physical component summary). CI, confidence interval; IV, inverse variance; Std., standard.
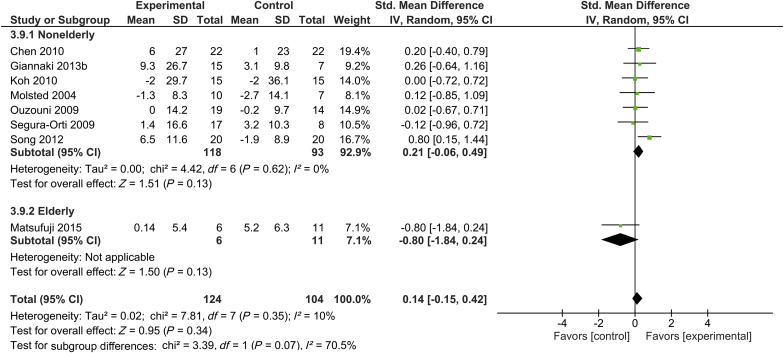
The mental component summary score of short-form health survey was measured in 8 trials, which included 124 subjects in the intervention group and 104 subjects in the control group.16, 25, 29, 34, 38, 39, 45, 46 Only 1 of these 8 studies included elderly participants.16 There were no increases in the mental component summary score associated with supervised exercise training in elderly, nonelderly, or all subjects undergoing hemodialysis (P = 0.13, P = 0.13, and P = 0.34, respectively). There was a low degree of heterogeneity with regard to the mental component summary score across studies (I2 = 10%) (Figure 6).
Figure 6.
Forest plot showing the effects of supervised exercise training compared with usual care on changes in quality of life (short-form health survey: mental component summary). CI, confidence interval; IV, inverse variance; Std., standard.
Assessment of Risks of Bias and Publication Bias
The risks of bias were frequently high or unclear in the studies (Table 2).16, 17, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 In 10 studies (33.3%) low-risk methods for random sequence generation were reported, and allocation was adequately concealed in 8 studies (26.7%). The assessor was blinded to patient allocation in 5 studies (16.7%), and both participants and investigators were masked and blinded in only 1 study (3.3%). Outcome data were incomplete or were reported only selectively in 4 (13.3%) and 7 (23.3%) studies, respectively. In 3 studies (10.0%) the analyses were reported as intention-to-treat. The total Tool for the assEssment of Study qualiTy and reporting in EXercise score, study quality score, and study reporting score of the studies were 7.9 ± 2.3, 2.5 ± 1.1, and 5.4 ± 1.5, respectively.
Table 2.
Summary of risk of bias assessment
| Studies | The Cochrane Collaboration Tool |
TESTEX |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Total score (/15) | Study quality score (/5) | Study reporting score (/10) | |
| Akiba et al. (1995)50 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 4 | 1 | 3 |
| Carmack et al. (1995)23 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 6 | 3 | 4 |
| Carney et al. (1987)51 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 7 | 3 | 5 |
| Cheema et al. (2007)24 | Low bias | Low bias | High bias | High bias | Low bias | Low bias | Unclear | 10 | 3 | 7 |
| Chen et al. (2010)25 | Unclear | Low bias | High bias | Low bias | High bias | High bias | Low bias | 13 | 4 | 8 |
| de Lima et al. (2013)52 | Low bias | Low bias | Unclear | Unclear | High bias | Unclear | Low bias | 9 | 4 | 5 |
| Deligiannis et al. (1999)26 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 6 | 3 | 4 |
| DePaul et al. (2002)27 | Low bias | Low bias | High bias | Low bias | Low bias | Unclear | Unclear | 10 | 4 | 6 |
| Dobsak et al. (2012)53 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 6 | 1 | 5 |
| Dong et al. (2011)28 | Low bias | Low bias | Unclear | Unclear | Low bias | Low bias | Low bias | 9 | 4 | 5 |
| Giannaki et al. (2013)29 | Unclear | Low bias | High bias | Unclear | Unclear | Low bias | Unclear | 10 | 3 | 7 |
| Giannaki et al. (2013)29 | Unclear | Unclear | High bias | Unclear | Low bias | Low bias | Unclear | 7 | 3 | 5 |
| Goldberg et al. (1983)31 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 4 | 1 | 3 |
| Goldberg et al. (1986)30 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 5 | 1 | 4 |
| Groussard et al. (2015)17 | Unclear | Unclear | High bias | Unclear | Unclear | Unclear | Low bias | 6 | 3 | 4 |
| Guadalupe et al. (2016)54 | Low bias | Low bias | Low bias | Low bias | Unclear | Unclear | Low bias | 10 | 5 | 5 |
| Johansen et al. (2006)32 | Unclear | Low bias | Unclear | Unclear | Unclear | Unclear | Unclear | 9 | 3 | 7 |
| Kirkman et al. (2014)33 | Unclear | Unclear | High bias | Unclear | Unclear | Low bias | Low bias | 7 | 3 | 5 |
| Koh et al. (2010)34 | Low bias | Low bias | High bias | High bias | High bias | High bias | Low bias | 10 | 4 | 6 |
| Konstantinidou et al. (2002)35 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 8 | 3 | 6 |
| Koufaki et al. (2002)36 | Low bias | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 9 | 3 | 6 |
| Kouidi et al. (2009)37 | Low bias | Unclear | Unclear | Low bias | Unclear | Low bias | Low bias | 13 | 4 | 8 |
| Kouidi et al. (1997)55 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 8 | 3 | 6 |
| Matsufuji et al. (2015)16 | Low bias | Low bias | Unclear | Unclear | High bias | Low bias | Unclear | 8 | 4 | 4 |
| Matsumoto et al. (2007)56 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 7 | 3 | 5 |
| Molsted et al. (2004)38 | Low bias | Unclear | Unclear | Low bias | Unclear | Unclear | Unclear | 10 | 4 | 6 |
| Ouzouni et al. (2009)39 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 8 | 3 | 6 |
| Painter et al. (2002)40 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 7 | 3 | 5 |
| Parsons et al. (2004)41 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 6 | 3 | 4 |
| Pellizzaro et al. (2013)42 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Low bias | 8 | 3 | 6 |
| Petraki et al. (2008)43 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 7 | 3 | 5 |
| Reboredo et al. (2011)44 | Unclear | Unclear | Unclear | Unclear | Unclear | Low bias | Unclear | 8 | 3 | 6 |
| Roboredo et al. (2015)57 | Unclear | Unclear | Unclear | Unclear | Unclear | Low bias | Low bias | 7 | 3 | 5 |
| Segura-Orti et al. (2009)45 | Low bias | Unclear | Low bias | Low bias | Unclear | Unclear | Unclear | 11 | 3 | 8 |
| Song and Sohng (2012)46 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 7 | 3 | 5 |
| Tsuyuki et al. (2003)47 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | 3 | 1 | 1 |
| van Vilsteren et al. (2005)48 | Unclear | Unclear | Unclear | Unclear | High bias | Unclear | Low bias | 8 | 1 | 7 |
| Wilund et al. (2010)58 | Unclear | Unclear | Unclear | Low bias | High bias | Unclear | Low bias | 9 | 3 | 6 |
| Wu et al. (2014)49 | Low bias | Low bias | Unclear | Unclear | Unclear | Unclear | Low bias | 10 | 4 | 6 |
TESTEX, Tool for the assEssment of Study qualiTy and reporting in Exercise.
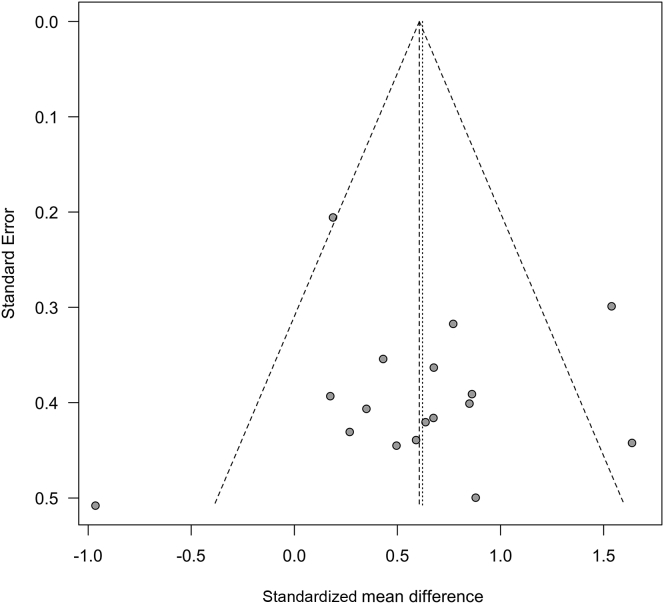
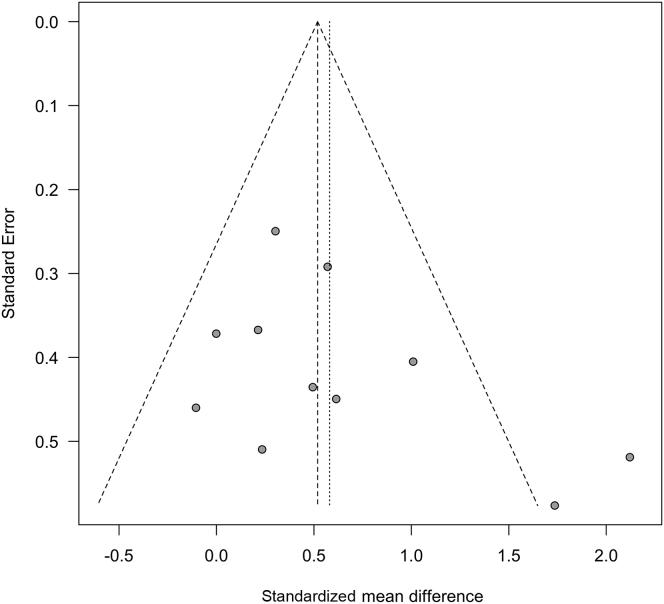
Egger’s regression test for publication bias was not significant for exercise tolerance (P = 0.27) or walking ability (P = 0.93). Funnel plots were symmetrical for each outcome (Figures 7 and 8), and we did not detect evidence of publication bias for other outcomes because fewer than 10 studies dealt with muscle strength and QoL.
Figure 7.
Funnel plot test exploring publication bias (exercise tolerance: peak/maximum oxygen consumption).
Figure 8.
Funnel plot test exploring publication bias (walking ability: 6-minute walk distance).
Discussion
We conducted a systematic review of the literature to evaluate the effects of supervised exercise training on exercise tolerance, walking ability, muscle strength, and QoL in elderly people undergoing hemodialysis and to provide an update of recent studies regarding the effects of exercise training on functional status. Only 1 study targeted people aged 60 years and older undergoing hemodialysis, and thus we could not perform a meta-analysis to confirm the effects of exercise training in elderly people undergoing hemodialysis. There is still insufficient evidence regarding the effectiveness of exercise training for elderly people undergoing hemodialysis. Further RCTs will be needed to clarify the effectiveness of exercise training on exercise tolerance, walking ability, muscle strength, and QoL in elderly people undergoing hemodialysis. On the other hand, our findings suggest that supervised exercise training has significant beneficial effects on exercise tolerance, walking ability, muscle strength, and QoL (physical component summary score) in the general hemodialysis population.
In 2016, the European Renal Best Practice Guideline Development Group published new clinical practice guidelines for elderly patients with chronic kidney disease,59 recommending the use of physical functional assessment tools and interventions aimed at increasing functional status in older patients with renal failure. Given the importance of these recommendations in clinical settings, the present study assessed the impact of supervised exercise on functional status in elderly patients undergoing hemodialysis.
Although the findings of the present study were generally in agreement with those of previous meta-analyses,13, 14, 60 our analysis of the effectiveness of exercise training in elderly people as compared with nonelderly people was somewhat inconclusive. In particular, we found no studies in which the association between exercise training and exercise tolerance was evaluated in elderly people undergoing hemodialysis. Groussard et al.17 reported that an intradialytic aerobic exercise training program significantly improved 6-minute walking distance in middle-aged and elderly people, although no changes were observed in peak VO2. They postulated that this discrepancy was due to the short duration of the intervention program and the use of aerobic training alone, rather than a combination of aerobic and strength training. Moreover, people undergoing hemodialysis might not achieve maximum VO2 because of functional limitations caused by bone, joint, and/or muscle pain and muscle fatigue. Because elderly patients are likely to experience difficulty participating in combined, prolonged exercise training, peak or maximum VO2 evaluated by cardiopulmonary exercise testing might not provide appropriate outcome measurements in most elderly patients. On the other hand, 6-minute walking distance—which has proven relative and absolute reliability in elderly people undergoing hemodialysis,61 is used in clinical settings as an index of exercise tolerance, and provides prognostic information comparable to that of peak VO2 in elderly patients with heart failure62—is an appropriate outcome measure for exercise training in elderly people undergoing hemodialysis.
The prevalence of frailty is higher among elderly people with end-stage renal disease compared with community-dwelling elderly people. In a previous study, 85.9% of elderly people undergoing hemodialysis were found to be frail or intermediately frail.63 Given that muscle weakness is an important component of frailty, our review of the effects of exercise training on physical function in these populations could be of clinical significance.
Matsufuji et al. evaluated the effects of chair stand exercise on physical performance among elderly people (≥60 years old) undergoing hemodialysis and reported improvements in their activities of daily living by strengthening the quadriceps.16 Chair stand exercise is suitable for elderly patients, because it does not require any special equipment or place. Low-intensity strength training with ankle weights was also shown to improve physical performance in elderly patients.25 Because reduced physical performance is a strong predictor of poor prognosis in people undergoing hemodialysis,7, 8 participation in chair stand exercise or low-intensity weight training may not only increase QoL but also improve prognosis in elderly people undergoing hemodialysis. In a recent multicenter RCT, Zoccali et al.64 revealed that a low-intensity, home-based walking program improved functional status compared with usual care in patients with end-stage renal disease. These interventions are inexpensive, safe, and feasible for elderly people undergoing hemodialysis.
This study has several limitations. First, because our literature searches were restricted to studies published in English, some articles might have been overlooked. Second, the number of studies that included elderly people (≥60 years old) undergoing hemodialysis was too small for performance of meta-analysis. Barriers to exercise (e.g. self-efficacy, discomfort, disability, fear of injury, habits, environmental factors, cognitive decline, and fatigue)15 could explain why elderly patients were not often recruited for exercise trials. Studies targeting elderly patients can be helpful in designing exercise programs and exercise goals that take into consideration patient barriers. Although the optimal program has yet to be identified, it might be more effective to implement programs such as chair stand exercise16 and electromyostimulation53 that are affordable and more feasible for older patients. A recent non-RCT showed that low-intensity physical exercise improved muscle strength, functional capacity, and QoL in subjects aged 80 years and older.65 Further RCTs in elderly people undergoing hemodialysis will be necessary to confirm these findings. Third, the present review focused on the effects of supervised exercise training, without taking into consideration the effects of home-based exercise training. However, Konstantinidou et al. compared the effects of home-based exercise and supervised exercise training in people undergoing hemodialysis and reported that the former did not show a greater improvement in exercise tolerance compared with the latter.35 On the other hand, another study suggested greater benefits of independent home-based exercise compared with intradialytic exercise in people undergoing hemodialysis. Therefore further studies will be needed to compare the effectiveness of home-based exercise and supervised exercise training in this patient population.
In conclusion, our meta-analysis confirmed the positive effects of supervised exercise training on exercise tolerance, walking ability, muscle strength, and QoL in the general hemodialysis population. However, there still is insufficient evidence regarding the effectiveness of exercise training for elderly people undergoing hemodialysis, despite a strong rationale for the use of exercise in the population. Future studies should investigate whether supervised exercise training leads to similar improved outcomes in elderly people undergoing hemodialysis and identify the most favorable exercise program for this patient population.
Disclosure
All the authors declared no competing interests. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Acknowledgments
This study was supported by a JSPS KAKENHI (Grant Number 16K16466). We thank all of the investigators and contributors to our study. Author contributions are as follows: conception or design; RM, YK; analysis and interpretation of data, or both: MH, TW; drafting the article or revising it: RM, KH, TS; providing intellectual content of critical importance to the work described: KH, YS; final approval of the version to be published: AM.
Footnotes
Appendix S1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist.
Appendix S2. Search strategy.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist.
Search strategy.
References
- 1.Hamer R.A., El Nahas A.M. The burden of chronic kidney disease. BMJ. 2006;332:563–564. doi: 10.1136/bmj.332.7541.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canaud B., Tong L., Tentori F. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2011;6:1651–1662. doi: 10.2215/CJN.03530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager K.J., van Dijk P.C., Dekker F.W. The epidemic of aging in renal replacement therapy: an update on elderly patients and their outcomes. Clin Nephrol. 2003;60:352–360. doi: 10.5414/cnp60352. [DOI] [PubMed] [Google Scholar]
- 4.Kurella M., Covinsky K.E., Collins A.J. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146:177–183. doi: 10.7326/0003-4819-146-3-200702060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Nakai S., Hanafusa N., Masakane I. An overview of regular dialysis treatment in Japan (as of 31 December 2012) Ther Apher Dial. 2014;18:535–602. doi: 10.1111/1744-9987.12281. [DOI] [PubMed] [Google Scholar]
- 6.Johansen K.L., Chertow G.M., Jin C. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa R., Matsunaga A., Wang G. Habitual physical activity measured by accelerometer and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:2010–2016. doi: 10.2215/CJN.03660412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuzawa R., Matsunaga A., Wang G. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Phys Ther. 2014;94:947–956. doi: 10.2522/ptj.20130270. [DOI] [PubMed] [Google Scholar]
- 9.Johansen K.L., Dalrymple L.S., Glidden D. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11:626–632. doi: 10.2215/CJN.03710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallenberg M.H., Kleinveld H.A., Dekker F.W. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD-a systematic review. Clin J Am Soc Nephrol. 2016;11:1624–1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Labra C., Guimaraes-Pinheiro C., Maseda A. Effects of physical exercise interventions in frail older adults: a systematic review of randomized controlled trials. BMC Geriatr. 2015;15:154. doi: 10.1186/s12877-015-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 13.Smart N., Steele M. Exercise training in haemodialysis patients: a systematic review and meta-analysis. Nephrology (Carlton) 2011;16:626–632. doi: 10.1111/j.1440-1797.2011.01471.x. [DOI] [PubMed] [Google Scholar]
- 14.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Nied R.J., Franklin B. Promoting and prescribing exercise for the elderly. Am Fam Physician. 2002;65:419–426. [PubMed] [Google Scholar]
- 16.Matsufuji S., Shoji T., Yano Y. Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr. 2015;25:17–24. doi: 10.1053/j.jrn.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Groussard C., Rouchon-Isnard M., Coutard C. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl Physiol Nutr Metab. 2015;40:550–556. doi: 10.1139/apnm-2014-0357. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuzawa R., Hoshi K., Yoneki K. Evaluating the effectiveness of exercise training on elderly patients who require haemodialysis: study protocol for a systematic review and meta-analysis. BMJ Open. 2016;6:e010990. doi: 10.1136/bmjopen-2015-010990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savovic J., Weeks L., Sterne J.A. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3:37. doi: 10.1186/2046-4053-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smart N.A., Waldron M., Ismail H. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P., Thompson S.G., Deeks J.J. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmack C.L., Amaral-Melendez M., Boudreaux E. Exercise as a component in the physical and psychological rehabilitation of hemodialysis patients. Int J Rehabil Health. 1995;1:13–23. [Google Scholar]
- 24.Cheema B., Abas H., Smith B. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 25.Chen J.L., Godfrey S., Ng T.T. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25:1936–1943. doi: 10.1093/ndt/gfp739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deligiannis A., Kouidi E., Tassoulas E. Cardiac effects of exercise rehabilitation in hemodialysis patients. Int J Cardiol. 1999;70:253–266. doi: 10.1016/s0167-5273(99)00090-x. [DOI] [PubMed] [Google Scholar]
- 27.DePaul V., Moreland J., Eager T. The effectiveness of aerobic and muscle strength training in patients receiving hemodialysis and EPO: a randomized controlled trial. Am J Kidney Dis. 2002;40:1219–1229. doi: 10.1053/ajkd.2002.36887. [DOI] [PubMed] [Google Scholar]
- 28.Dong J., Sundell M.B., Pupim L.B. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr. 2011;21:149–159. doi: 10.1053/j.jrn.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannaki C.D., Sakkas G.K., Karatzaferi C. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: a six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013;14:194. doi: 10.1186/1471-2369-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg A.P., Geltman E.M., Gavin J. Exercise training reduces coronary risk and effectively rehabilitates hemodialysis patients. Nephron. 1986;42:311–316. doi: 10.1159/000183694. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg A.P., Geltman E.M., Hagberg J.M. Therapeutic benefits of exercise training for hemodialysis patients. Kidney Int. 1983;24:S303–S309. [PubMed] [Google Scholar]
- 32.Johansen K.L., Painter P.L., Sakkas G.K. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 33.Kirkman D.L., Mullins P., Junglee N.A. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle. 2014;5:199–207. doi: 10.1007/s13539-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh K.P., Fassett R.G., Sharman J.E. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis. 2010;55:88–99. doi: 10.1053/j.ajkd.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 35.Konstantinidou E., Koukouvou G., Kouidi E. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med. 2002;34:40–45. doi: 10.1080/165019702317242695. [DOI] [PubMed] [Google Scholar]
- 36.Koufaki P., Mercer T.H., Naish P.F. Effects of exercise training on aerobic and functional capacity of end-stage renal disease patients. Clin Physiol Funct Imaging. 2002;22:115–124. doi: 10.1046/j.1365-2281.2002.00405.x. [DOI] [PubMed] [Google Scholar]
- 37.Kouidi E.J., Grekas D.M., Deligiannis A.P. Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: a randomized controlled trial. Am J Kidney Dis. 2009;54:511–521. doi: 10.1053/j.ajkd.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Molsted S., Eidemak I., Sorensen H.T. Five months of physical exercise in hemodialysis patients: effects on aerobic capacity, physical function and self-rated health. Nephron. 2004;96:c76–c81. doi: 10.1159/000076744. [DOI] [PubMed] [Google Scholar]
- 39.Ouzouni S., Kouidi E., Sioulis A. Effects of intradialytic exercise training on health-related quality of life indices in haemodialysis patients. Clin Rehabil. 2009;23:53–63. doi: 10.1177/0269215508096760. [DOI] [PubMed] [Google Scholar]
- 40.Painter P., Moore G., Carlson L. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis. 2002;39:257–265. doi: 10.1053/ajkd.2002.30544. [DOI] [PubMed] [Google Scholar]
- 41.Parsons T.L., Toffelmire E.B., King-VanVlack C.E. The effect of an exercise program during hemodialysis efficacy, blood pressure and quality of life in end-stage renal disease (ESRD) patients. Clin Nephrol. 2004;61:261–274. doi: 10.5414/cnp61261. [DOI] [PubMed] [Google Scholar]
- 42.Pellizzaro C.O., Thome F.S., Veronese F.V. Effect of peripheral and respiratory muscle training on the functional capacity of hemodialysis patients. Ren Fail. 2013;35:189–197. doi: 10.3109/0886022X.2012.745727. [DOI] [PubMed] [Google Scholar]
- 43.Petraki M., Kouidi E., Grekas D. Effects of exercise training during hemodialysis on cardiac baroreflex sensitivity. Clin Nephrol. 2008;70:210–219. doi: 10.5414/cnp70210. [DOI] [PubMed] [Google Scholar]
- 44.Reboredo M.M., Neder J.A., Pinheiro B.V. Constant work-rate test to assess the effects of intradialytic aerobic training in mildly impaired patients with end-stage renal disease: a randomized controlled trial. Arch Phys Med Rehabil. 2011;92:2018–2024. doi: 10.1016/j.apmr.2011.07.190. [DOI] [PubMed] [Google Scholar]
- 45.Segura-Orti E., Kouidi E., Lisón J.F. Effect of resistance exercise during hemodialysis on physical function and quality of life: randomized controlled trial. Clin Nephrol. 2009;71:527–537. doi: 10.5414/cnp71527. [DOI] [PubMed] [Google Scholar]
- 46.Song W.J., Sohng K.Y. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J Korean Acad Nurs. 2012;42:947–956. doi: 10.4040/jkan.2012.42.7.947. [DOI] [PubMed] [Google Scholar]
- 47.Tsuyuki K., Kimura Y., Chiashi K. Oxygen uptake efficiency slope as monitoring tool for physical training in chronic hemodialysis patients. Ther Apher Dial. 2003;7:461–467. doi: 10.1046/j.1526-0968.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 48.van Vilsteren M., de Greef M.H.G., Huisman R.M. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in The Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant. 2005;20:141–146. doi: 10.1093/ndt/gfh560. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y., Yin X., He Q. Effect of individualized exercise during maintenance haemodialysis on exercise capacity and health-related quality of life in patients with uraemia. J Int Med Res. 2014;42:718–727. doi: 10.1177/0300060513509037. [DOI] [PubMed] [Google Scholar]
- 50.Akiba T., Matsui N., Shinohara S. Effects of recombinant human erythropoietin and exercise training on exercise capacity in hemodialysis patients. Artificial Organs. 1995;9:1262–1268. doi: 10.1111/j.1525-1594.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 51.Carney R.M., Templeton B., Hong B.A. Exercise training reduces depression and increases the performance of pleasant activities in hemodialysis patients. Nephron. 1987;47:194–198. doi: 10.1159/000184490. [DOI] [PubMed] [Google Scholar]
- 52.de Lima M.C., Cicotoste C.D., Cardoso K.D. Effect of exercise performed during hemodialysis: Strength versus aerobic. Renal Failure. 2013;35:697–704. doi: 10.3109/0886022X.2013.780977. [DOI] [PubMed] [Google Scholar]
- 53.Dobsak P., Homolka P., Svojanovsky J. Intra-dialytic electrostimulation of leg extensors may improve exercise tolerance and quality of life in hemodialyzed patients. Artif Organs. 2012;36:71–78. doi: 10.1111/j.1525-1594.2011.01302.x. [DOI] [PubMed] [Google Scholar]
- 54.Olvera-Soto M.G., Valdez-Ortiz R., Lopez Alvarenga J.C. Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on hemodialysis. J Renal Nutrition. 2016;26:53–60. doi: 10.1053/j.jrn.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Kouidi E., Iacovides A., Iordanidis P. Exercise renal rehabilitation program: psychosocial effects. Nephron. 1997;7:152–158. doi: 10.1159/000190266. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto Y., Furuta A., Furuta S. The impact of pre-dialytic endurance training on nutritional status and quality of life in stable hemodialysis patients (Sawada study) Renal Failure. 2007;29:587–593. doi: 10.1080/08860220701392157. [DOI] [PubMed] [Google Scholar]
- 57.Reboredo M.M., Neder J.A., Pinheiro B.V. Intra-dialytic training accelerates oxygen uptake kinetics in hemodialysis patients. European Journal of Preventive Cardiology. 2015;22:912–919. doi: 10.1177/2047487314543079. [DOI] [PubMed] [Google Scholar]
- 58.Wilund K.R., Tomayko E.J., Wu P.T. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant. 2010;25:2695–2701. doi: 10.1093/ndt/gfq106. [DOI] [PubMed] [Google Scholar]
- 59.Farrington K., Covic A., Aucella F. Clinical Practice Guideline on management of older patients with chronic kidney disease stage 3b or higher (eGFR <45 mL/min/1.73 m2) Nephrol Dial Transplant. 2016;31:ii1–ii66. doi: 10.1093/ndt/gfw356. [DOI] [PubMed] [Google Scholar]
- 60.Sheng K.X., Zhang P., Chen L.L. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol. 2014;40:478–490. doi: 10.1159/000368722. [DOI] [PubMed] [Google Scholar]
- 61.Overend T., Anderson C., Sawant A. Relative and absolute reliability of physical function measures in people with end-stage renal disease. Physiother Can. 2010;62:122–128. doi: 10.3138/physio.62.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forman D.E., Fleg J.L., Kitzman D.W. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McAdams-DeMarco M.A., Law A., Salter M.L. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manfredini F., Mallamaci F., D'Arrigo G. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28:1259–1268. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esteve Simo V., Junque Jimenez A., Moreno Guzman F. Benefits of a low intensity exercise programme during haemodialysis sessions in elderly patients. Nefrologia. 2015;35:385–394. doi: 10.1016/j.nefro.2015.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 checklist.
Search strategy.