Abstract
Developers of gene therapy products (GTPs) must adhere to additional regulation beyond that of traditional small-molecule therapeutics, due to the unique mechanism-of-action of GTPs and the subsequent novel risks arisen. We have provided herein a summary of the regulatory structure under which GTPs fall in the United States, the European Union, and Japan, and a comprehensive overview of the regulatory guidance applicable to the developer of GTP. Understanding the regulatory requirements for seeking GTP market approval in these major jurisdictions is crucial for an effective and expedient path to market. The novel challenges facing GTP developers is highlighted by a case study of alipogene tiparvovec (Glybera).
Keywords: advanced therapeutic medicinal product, ATMP, Japan, MHLW, FDA, EMA, gene therapy, regulation
Introduction
In the last decade, the cost of genome sequencing has dropped from $100,000,000 to $1,000 per genome [1]. This five-log decrease over ten years, initiated in part by a scientific arms race in the late 1990s to first sequence the human genome, has yielded unprecedented access to the genetic code of our cells [2]. The unwinding of the human genome has created myriad new therapeutic opportunities for previously untreated diseases. Genome sequencing is complemented and its potential enhanced by the development of molecular techniques for artificial genomic delivery and modification, including viral-based transfection mechanisms and the CRISPR/Cas9 system [3].
The specific terminology for a gene therapy differs slightly in our jurisdictions of interest (Table 1); for the purpose of this review gene therapies will be generally referred to as a “gene therapy product” (GTP). GTPs face significant additional regulatory challenges when pursuing market approval due to the risks and concerns unique to gene therapies which must be accounted for during the regulatory process. These challenges are amplified by the novelty of gene therapies; the first gene therapy in either the European Union (EU) or United States of America (US) alipogene tiparvovec (Glybera, uniQure; Amsterdam NL) was approved in 2012, and at time of publication only two more have received market approval since. In comparison, the first recombinant protein drug Humulin was approved 35 years ago (FDA), the first monoclonal antibody Orthoclone OKT3 31 years ago (FDA), and the first synthetic drug chloral hydrate was discovered 148 years ago (Germany) [4-6]. As the first rudimentary pre-market approval process for drugs in the US was established in 1938, small molecule drugs pre-date regulatory structures [7].
Table 1. Definitions of gene therapy products in EU, US, and Japan.
| Jurisdiction | Name | Gene Therapy Definition |
| EMA (EU) | Gene therapy medicinal product (GTMP) | “a biological medicinal product (excluding vaccines) that: |
| (a) Contains an active substance which contains or consists of a recombinant nucleic acid used in or administered to human beings with a view to regulating, repairing, replacing, adding or deleting a genetic sequence and; | ||
| (b) Its therapeutic, prophylactic or diagnostic effect relates directly to the recombinant nucleic acid sequence it contains, or to the product of genetic expression of this sequence. | ||
| Gene therapy medicinal products shall not include vaccines against infectious diseases.” | ||
| Directive 2001/83/EC, Annex I, Part IV, as amended in Directive 107 2009/120/EC) | ||
| FDA (USA) | Gene therapy product | “Gene therapy is a medical intervention based on modification of the genetic material of living cells. Cells may be modified ex vivo for subsequent administration to humans, or may be altered in vivo by gene therapy given directly to the subject. When the genetic manipulation is performed ex vivo on cells which are then administered to the patient, this is also a form of somatic cell therapy. The genetic manipulation may be intended to have a therapeutic or prophylactic effect, or may provide a way of marking cells for later identification. Recombinant DNA materials used to transfer genetic material for such therapy are considered components of gene therapy and as such are subject to regulatory oversight.” |
| FDA. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. March 1998 | ||
| MHLW (Japan) | Gene therapy product | “The term “Regenerative Medicinal Products” (as “SAISEI-IRYOUTOU-SEIHIN” in Japanese) used in this act refers to the articles (excluding quasi-drugs and cosmetics) specified in the following items which are specified by the cabinet order. |
| […] | ||
| (2) The articles which are intended to be used in the treatment of disease in humans or animals, and are transgened to express in human or animal cells.” | ||
| Act on Pharmaceutical and Medical Devices, Chapter 1 Article 2-9* | ||
| *translation from [24] |
Understanding the regulatory framework that a gene therapy must navigate to achieve market approval is critical for both developers and scientists. The former need to plan their path to market accordingly and assess candidates’ translational viability, but scientists also need to understand the regulatory framework in order to inform their work in improving and optimizing gene therapies in the laboratory and ensure that they are translatable.
This review will cover the relevant regulatory considerations for the US, EU, and Japan. The US and EU were chosen due to their inherent relevance to Western drug developers. Japan is a significant jurisdiction and an interesting case study for the topic of this review due to their unique regulatory pathways available only to regenerative medicines, including gene therapies. Additionally, the US, EU, and Japan are the founding members of the International Conference of Harmonization, an international organization which develops scientific and regulatory documents on drug development for widespread international adoption. Therefore, these jurisdictions’ regulatory outlook is especially influential on worldwide therapeutics development. The formal definition of a gene therapy in the jurisdictions of interest is given in Table 1. Within the general terminology, gene therapies are divided into two broad classes: in vivo and ex vivo. In vivo gene therapy is the direct modification or insertion of genetic information to cells within a tissue body; ex vivo gene therapy is the genetic manipulation of cells in vitro which are then delivered into the patient. As ex vivo gene therapy necessitates the introduction of significantly manipulated cellular products into the patient, these products must generally comply with both cell-based medicinal product and gene therapy product guidelines and regulation. Only regulation directly relevant to gene therapy will be covered in this review, however, ex vivo GTPs are also subject to cell therapy considerations and regulations. The regulation of cell-based medicinal products has been recently reviewed [8-10].
General Regulatory Environment
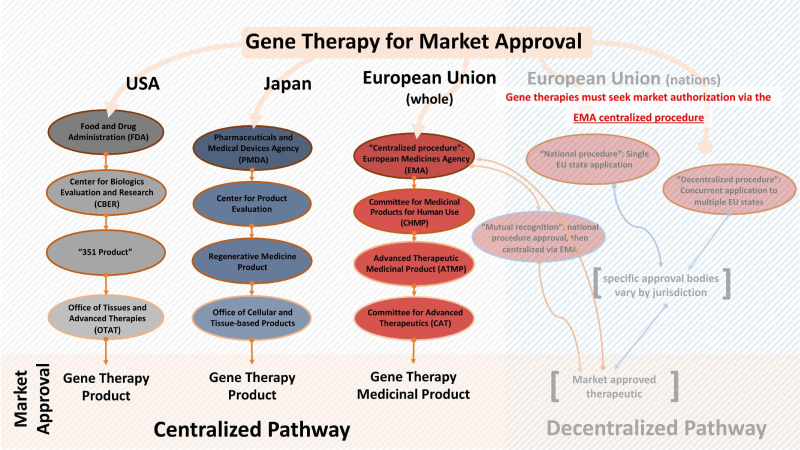
Within the EU and US, GTPs are regulated within the same general regulatory framework as other types of therapeutics [11]. Japan’s regulatory agency, the Ministry of Health, Labour, and Welfare (MHLW), has a special regulatory framework for “regenerative medicinal products” under which gene therapy products are categorized and regulated (not to be confused with “regenerative medicine,” a separate category defined in the Japanese regulation solely for academic or clinical research products where market approval will not be sought). As the Japanese regulation of GTPs differs significantly from the US and EU, further specifics of this jurisdiction’s practices will be discussed separately in this review under Japanese Regenerative Medicinal Products. An overview of the regulatory hierarchy for GTPs in the jurisdictions of interest may be found in Figure 1.
Figure 1.
The regulatory structure of the US, EU, and Japanese regulatory bodies as relevant to gene therapies. Unlike the FDA and MHLW, the EMA is not a completely centralized approval body – developers may pursue market approval also via the “national procedure” (single EU state application), “decentralized procedure” (concurrent application to multiple EU states) or “mutual recognition” (first via a single EU state, then centralized via the EMA). However, ATMPs including GTs are mandated to seek approval under the centralized EMA pathway, along with specific therapeutics of certain modalities and indications.
The United States of America
The U.S. Food and Drug Administration (FDA) has jurisdiction over a variety of products including food, tobacco, vaccines, and therapeutics in the US. Within the FDA, therapeutics are regulated under either the Center for Drug Evaluation and Research (CDER) or the Center for Biologics Evaluation and Research (CBER). CBER is responsible for the regulation of human gene therapy products [12]. Within CBER, oversight of gene therapy products falls to the Office of Tissues and Advanced Therapies (OTAT), which before restructuring in October 2016 was known as The Office of Cellular, Tissue, and Gene Therapies (OCTGT) [13]. In addition to regulatory review, OTAT also releases regulatory policy and guidance documents, many of which will be referenced in this publication [14].
Biologics, and therefore GTPs, are regulated under Section 351 of the Public Health Services Act and are therefore commonly referred to as “351 Products.” A 351 Product is defined by exclusion; it is a biologic which does not meet the criteria of 361 Products as defined in the same legislation. 361 products cover products including tissue and bone transplants and are referred to as “human cells, tissues, and cellular and tissue-based products” (HCT/Ps) [15]. They must meet the following requirements: minimal manipulation; homologous use; not combined with another article; either no systemic effect and do not rely on metabolic effect of living cells; or have a system effect and rely on metabolic effect of living cells but are for autologous, first or second-degree blood relative or reproductive use [16]. The mechanism of genetically modifying a cell precludes 361 Product classification, as genetic modification has a systemic effect and depends on the metabolic activity of living cells. Therefore, GTPs are regulated as 351 Products. As a 351 Product, GTPs must receive pre-market approval and meet Good Manufacturing Practice (GMP) requirements, among others.
The European Union
The European Medicines Agency (EMA) is the centralized regulatory body of the EU. Within the EMA, The Committee for Advanced Therapies (CAT) provides an expert opinion on the drug’s application dossier and gives a recommendation for approval or rejection. This decision is reviewed and ratified by the Committee for Medicinal Products for Human Use (CHMP). GTs are classified as Advanced Therapeutic Medicinal Products (ATMP) which are governed under the ATMP Regulation (Directive 2001/83/EC, as amended by Regulation (EC) No. 1394/2007). ATMP Regulation covers gene therapy medicinal products (GTMP), Somatic Cell Therapy Medicinal Products (CTMP), Tissue Engineered Products (TEP), and Combined ATMPs, which are a combination of a medical device with one or more of the previous categories. ATMPs must comply with EU pharmaceutical regulations and therefore receive pre-market approval. A non-pharmaceutical product meets the following criteria: “It is not substantially manipulated; cells are used for the same essential function in the donor and recipient (sometimes called ‘homologous use’); it is not combined with a medical device or an active implantable medical device.” If these criteria are not met, the product is regulated as an ATMP [17]. The classification is tasked to CAT, which provides a non-binding recommendation as to whether a specific product should be considered an ATMP. As with 351/361 Product designation, any GTP would be considered a gene therapy medicinal product (GTMP), and therefore an ATMP, due to the mechanism and cellular effect of gene therapeutics. The regulation of a Combined ATMP is determined by its components, following a categorical hierarchy defined by the EMA: GTMP over TEP over CTMP. Therefore, an ex vivo gene therapy would be classified as a GTMP, even though the final therapeutic product would be cell-based like CTMPs [17].
Unlike the FDA and MHLW, the EMA is not a completely centralized approval body. Developers may pursue market authorization in individual EU member states; GTPs are mandated to seek approval under the centralized EMA pathway, as are all other ATMPs are along with other therapeutics of certain modalities and indications. (Figure 1) [18]. However, within the ATMP Regulation there is a special provision entitled hospital exemption, which allows small scale custom ATMP treatments for rare, specialized diseases to be used in patients without market authorization. The product must be prepared on a non-routine basis, and be used within the same hospital in which it is produced under the exclusive supervision of a medical practitioner. A controversial example is the work of Dr. Paolo Macchiarini. At Karolinska Instituet in Stockholm, Sweden, Dr. Macchiarini implanted tissue-engineered tracheas combined with cellular products into patients with cancer and other forms of esophageal damage under the hospital exemption clause. Numerous issues with the experimental treatment were later revealed, including that animal studies were not robust nor sufficiently supportive of human trials, inadequate patient consent procedures, and inconsistent and perhaps not experimentally justified material use for the synthetic tracheas. The failure of the grafts is implicated in the death of multiple patients, including those who were not in critical condition and therefore likely would have had fewer complications should they not have received the transplant at all [19].
Guidances On Pre-Clinical and Clinical Development of GTPs
GTPs present challenges and risks in their development and use in patients. The manufacturing process must be defined and validated to ensure product standardization, batch comparability and sterility. As many GTPs are dependent on viral delivery mechanisms, meeting these requirements may be more complicated than with small molecule therapeutics and other biologics. Toxicity and immune response of the component parts of the GTP, even if the part does not confer the therapeutic effect, should be addressed. Immune responses are a significant concern with some types of GTPs, tragically highlighted by the death of Jesse Gelsinger. In 1999, Mr. Gelsinger received an experimental GTP consisting of the corrective gene for ornithine transcarbamoylase deficiency delivered via recombinant adenoviral vector, the attenuated cold virus. He later died of a massive, systemic immune response to the vector [20]. The safety of the delivery mechanism and the gene therapy itself must be thoroughly evaluated during the development of a new GTP. This includes rigorous product definition and standardization, toxicity, and immune response studies to all component parts of the GTP, shedding studies, and genomic integration sites. Even after market approval, long-term structured monitoring of some types of GTPs is necessary due to the unique long-term risks posed by GTPs, especially those which have the propensity to integrate in the genome and therefore may inadvertently induce deleterious mutations.
To address GTP-specific concerns and ensure product safety, homogeneity, and compliance, the EMA/CAT and FDA/OTAT have made available multiple resources for developers (Table 2, Table 3). Relevant FDA and EMA documents are referenced for each section. For the purposes of this review the FDA guidance documents have been labeled FDA-1 to 9 for further reference to Table 2.
Table 2. FDA Guidance Available for Gene Therapy Products. Only includes regulation and guidance specific to gene therapy products. Reference abbreviation provided is not official nomenclature and is only for the purposes of this review.
| Title | Reference (non-official) | Year |
| Recommendations for Microbial Vectors used for Gene Therapy | FDA-1 | 2016 |
| Determining the Need for and Content of Environmental Assessments for Gene Therapies, Vectored Vaccines, and Related Recombinant Viral or Microbial Products | FDA-2 | 2015 |
| Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products | FDA-3 | 2015 |
| Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products | FDA-4 | 2015 |
| Preclinical Assessment of Investigational Cellular and Gene Therapy Products | FDA-5 | 2013 |
| Potency Tests for Cellular and Gene Therapy Products | FDA-6 | 2011 |
| Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs) | FDA-6 | 2008 |
| Supplemental Guidance on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors | FDA-7 | 2006 |
| Gene Therapy Clinical Trials – Observing Subjects for Delayed Adverse Events | FDA-8 | 2006 |
| Guidance for Human Somatic Cell Therapy and Gene Therapy (superseded by 2013 guidance) | FDA-9 | 1998 |
Table 3. EMA Guidance Available for Gene Therapy Products. Only includes regulation and guidance specific to gene therapy products.
| Title | EMA Reference | Year |
| Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products | EMA/CAT/80183/2014 | 2015 |
| Management of clinical risks deriving from insertional mutagenesis | CAT/190186/2012 | 2013 |
| Risk-based approach according to Annex I, part IV of Directive 2001/83/EC applied to Advanced Therapy Medicinal Products | CAT/CPWP/686637/2011 | 2013 |
| Design modifications of gene therapy medicinal products during development | CAT/GTWP/44236/2009 | 2012 |
| Quality, nonclinical and clinical aspects of medicinal products containing genetically modified cells | CHMP/GTWP/671639/2008 | 2012 |
| CHMP/CAT position statement on Creutzfeldt-Jakob disease and advanced therapy medicinal products | CHMP/CAT/BWP/353632/2010 | 2011 |
| Quality, non-clinical and clinical issues relating specifically to recombinant adeno-associated viral vectors | CHMP/GTWP/587488/07 | 2010 |
| Questions and answers on gene therapy | CHMP/GTWP/212377/08 | 2009 |
| ICH Considerations General Principles to Address Virus and Vector Shedding | CHMP/ICH/449035/09 | 2009 |
| ICH Considerations - Oncolytic Viruses | CHMP/GTWP/607698/08 | 2009 |
| Follow-up of patients administered with gene therapy medicinal products | CHMP/GTWP/60436/07 | 2009 |
| Non-clinical studies required before first clinical use of gene therapy medicinal products | CHMP/GTWP/125459/06 | 2008 |
| Scientific Requirements for the Environmental Risk Assessment of Gene Therapy Medicinal Products | CHMP/GTWP/125491/06 | 2008 |
| Guideline On Safety and Efficacy Follow-Up - Risk Management of Advanced Therapy Medicinal Products | EMEA/149995/2008 | 2008 |
| Non-Clinical testing for Inadvertent Germline transmission of Gene Transfer Vectors | EMEA/273974/05 | 2006 |
| Development and Manufacture of Lentiviral Vectors | CHMP/BWP/2458/03 | 2005 |
| Quality, Preclinical and Clinical Aspects of Gene Transfer Medicinal Products (superseded by EMA/CAT/80183/2014) | CPMP/BWP/3088/99 | 2001 |
Product Quality
(FDA-6; EMA/CAT/80183/2014, EMA/CHMP/BWP/187338/2014)
Standard Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) requirements apply to GTPs, with some additional considerations tailored to GTPs. Starting materials should be controlled, including using viral seeds if appropriate. Potency can be challenging to determine for GTPs. Potency is defined by the FDA as “the specific ability or capacity of the product...to effect a given result.” Additional challenges may be due to factors such as the inherent variability of the starting materials, lack of reference standards, multiple active agents, and the possibility for these to interfere or synergize in an unpredictable or undesirable manner, complex mechanism(s) of action, and the in vivo fate of the product, which can be challenging to determine and variable. Special care should be taken to detect and avoid contamination from adventitious agents, for example extraneous viral contamination.
Pre-Clinical Assessment
(FDA-5, FDA-9; EMEA/CHMP/GTWP /125459/2006).
The FDA OTAT evaluates the following GTP products: non-viral vectors, replication-deficient viral vectors, replication-competent oncolytic vectors, microbial vectors, and ex vivo genetically modified cells. The following should be detailed and justified in a pre-clinical program design: animal species selection and the model of disease or injury; proof-of-concept studies, toxicology, product delivery considerations, GLP, adherence to reduction, refinement, and replacement of animals, and development of the product for later phase trials such as further safety and changes in formulation of the product. Animal selection should take into consideration the permissiveness/susceptibility of the candidate species to infection and replication of the viral vector and the response of the specific species to the expressed transgene. The document also provides a list of safety considerations specific to GTPs:
“a. Toxicities due to the components of the final formulation (e.g., liposomes and various excipients/contaminants).
b. Toxicities due to the ROA [route of administration] used.
c. Aberrant localization to non-target cells/tissues.
d. Level and persistence of vector and expressed transgene.
e. Level of viral replication in non-target cells/tissues.
f. Immune activation or suppression.
g. Immune response directed against the vector.
h. Phenotype/activation state of target cell(s).
i. Potential for insertional mutagenesis or oncogenicity.
j. Potential for germline transmission.
k. Potential horizontal transmission of replication competent vectors from the patient to family members and health care providers (i.e., shedding).”
The EMA requires at minimum the following additional pre-clinical studies for GTMPs: integration (if applicable), germline transmission (see: EMEA/273974/2005), target tissue selectivity, immunogenicity and immunotoxicity, delivery devices, and excipients contribution, reproductive toxicology, oncogenicity/tumorigenicity, and environmental risk/shedding. These requirements can vary depending on the type of GTP product and the variant risks inherent to them.
Clinical Development
(FDA-3; CHMP/GTWP/212377/08, EMEA/CHMP/GTWP/587488/2007, EMA/CAT/GTWP/44236/2009, CHMP/EWP/83561/2005, EMA/CAT/80183/2014)
In general, GTPs follow the same standard principles of any therapeutic entering clinical trials in the EU or US. Dependent on their mechanism of action, GTPs may require additional risk consideration when undertaking human studies due to their unique mechanism of action. Specific risks include uncontrolled delivery of the gene, interference of normal function of cells and tissue, and the long-term integration of the gene into the genome. In GTPs utilizing an integrating vector, these adverse events may lead to the subsequent potential inactivation or activation of neighboring genes; of particular concern is the activation of proto-oncogenes (see: Delayed Adverse Effects). Developers should heed special monitoring considerations including: immunogenicity, duration of the product in the body, shedding, clonal outgrowths if applicable, and the effect on children linear development and maturation. Inherited genetic disorders are often already expressed in children, for example, Duchenne’s muscular dystrophy. Therefore, it is critical to understand the potential effect on pediatric patients. Differential vulnerabilities to the vector may exist between special patient populations, e.g. pregnant women, children, and the elderly.
Additionally, it should be noted that determining safe and optimal dosing can be more challenging in GTPs. Dose scaling from animal models to humans may not be as predictable as they are with small-molecule therapeutics, therefore extra care should be taken to minimize risk of dosage errors in humans.
Some GTPs utilize viral-based delivery technologies, for example adeno-associated virus. Briefly, AAV is a low immune-reactive with a predictive integration pattern in chromosome 19. Numerous serotypes of AAV exist, which vary in their inherent tissue tropism and immunogenicity. The further described GTP Glybera utilized AAV1 to deliver the gene product to the patient [21]. These may necessitate or benefit from the concomitant use of immunosuppressive agents, whose choice and efficacy should also be validated clinically. There is some allowance for modifying a GTP during an ongoing clinical trial without invalidating previously-acquired data. This is possible if the new GTP shares critical properties with the previous version, such as the gene product and tissue tropism.
Often GTPs are indicated for orphan diseases, in which case developers should follow guidance available for designing clinical trials for small populations (CHMP/EWP/83561/2005: Guideline on Clinical Trials in Small Populations).
Shedding
(FDA-2, FDA-4; EMA/CAT/80183/2014, EMEA/CHMP/GTWP/125491/2006)
Shedding studies evaluate how a viral or bacterial based GTP is excreted or released from the body, with concern towards contamination of others and the environment due to this shedding. These studies should be conducted to determine the risk of a GTP being transferred from the patient to an untreated person in close contact, such as a health care professional. Developers should prepare a shedding report for delivery to the FDA, as outlined in the guidance document. Additionally, some types of GTPs may require the submission of an Environmental Assessment to determine the risk of the GTP on the environment.
Delayed Adverse Effects
(FDA-8; EMA/CAT/190186/2012, EMEA/ 149995/2008, EMEA/CHMP/GTWP/60436/2007)
GTPs are prone to delayed adverse effects due to the persistent biology activity of the genetic material. Additionally, insertional mutagenesis may cause the development of a malignancy years after treatment. Such serious adverse events have been previously reported in GTP clinical trials. Five patients treated with genetically-modified autologous HSC transformed with a gamma retroviral vector for the treatment of X-linked severe combined immunodeficiency developed T-cell lymphoid leukemia 2 to 5.7 years after treatment [22-23].
A series of guidance questions can be used to determine whether a specific GTP has a high risk of delayed adverse events:
Question 1: “Is your gene therapy product only used for ex vivo modification of cells?”
Question 2: “Do pre-clinical study results show persistence of vector sequences?”
Question 3: “Are vector sequences integrated?”
Question 4: “Does the vector have potential for latency and reactivation?”
If the answer to Questions 2 or 4 is “no,” the risk of gene transfer-related adverse events is low and there may not be necessity for the developer to provide a structure for long-term monitoring of patients. If the answer to Questions 3 or 4 is “yes,” the developer should incorporate long-term monitoring into their design study.
For example, AAVs do not have the propensity to integrate and therefore GTPs dependent on this technology do not require long-term monitoring. Additionally, integration studies should be considered if the vector system has the capacity to permanently integrate into the cellular genome and has a long in vivo duration. Integration studies inform the developer and regulatory agency of the risk of insertional mutagenesis caused by for example the inadvertent activation of a proto-oncogene.
Regulatory Incentives and Support in the EU and US
The FDA and EMA offer programs aimed to expedite the development of critical medicines. The indications of interest to the developers of GTPs are often life threatening or debilitating and currently without an effective treatment, and are therefore often able to qualify for regulatory support mechanisms.
Japan, the US, and the EU offer orphan designation, and there is a common EMA/FDA application for developers pursuing orphan designation for their therapeutic candidate. Orphan designation is available for any drug which is indicated for a rare disease. A “rare disease or condition” is defined by the FDA as either affecting less than 200,000 people in the United States, or affecting a higher number but is without a reasonable expectation that a therapeutic for the indication will bring significant financial returns to justify its development [24]. Developers will receive benefits including tax credits equivalent to 50 percent of the clinical trial costs, market application fee waiver, and seven-year market exclusivity [25]. In the EU, a therapeutic candidate may be considered an “orphan medicinal product” if it is indicated for a life threatening or debilitating condition which is not more prevalent than 5 out of 10,000 people, or is more prevalent but unlikely to generate significant returns on the investment. The therapeutic candidate must offer a significant benefit over current treatment modalities. Benefits of orphan drug designation by the EMA include scientific advice, ten years of market exclusivity, and fee reductions [26]. Glybera, discussed further in this review, received orphan designation. In addition to standard orphan designation, Japan also offers a regenerative-medicine-specific orphan designation if the regenerative medicine product affects a patient population of less than 50,000 and offers significant potential medical benefit. These therapeutics receive financial and advisory benefits and priority review by the MHLW [27].
The FDA offers four expedited programs for drugs which treat serious conditions where there is an unmet medical need: Fast Track designation, Breakthrough Therapy designation, Accelerated Approval, and Priority Review designation [28]. Fast Track provides the developer increased access to the FDA, rolling review of the Biologic License Application (BLA) or New Drug Application (NDA), and eligibility for Accelerated Approval and Priority Review. Breakthrough Therapies receive the Fast Track benefits in addition with intensive guidance during the drug development process from an early stage. Accelerated Approval grants market approval to drugs based on their effect on a surrogate endpoint, with the condition of meeting the requisite clinical endpoints in Phase 4 confirmatory trials. A surrogate endpoint is a biomarker or biomarker(s) which predict the meeting of a desired clinical endpoint in a clinical trial. Priority Review designation commits the FDA to reviewing the drug’s application within six months, in comparison to ten months under standard review. The details of these programs are provided in Guidance for Industry: Expedited Programs for Serious Conditions – Drugs and Biologics.
The EMA offers Priority Medicines (PRIME), Accelerated Assessment and Conditional Marketing Authorization. While the specifics of these programs vary, they are generally analogous to FDA Breakthrough Therapy designation, Priority Review, and Accelerated Approval, respectively.
Japanese Regenerative Medicinal Products
The Japanese government recently passed a set of legislations to promote the development of Japan as an international hub of medical research and therapeutics development, possibly due in part to the important role of Japanese scientists in the discovery of induced pluripotent stem cell (iPSC) [29]. Of specific relevance to regenerative medicines, and therefore GTPs, are the Regenerative Medicine Promotion Law (RMP, May 2013), the Act of Safety of Regenerative Medicine (RM Act, November 2013), and the Act on Pharmaceuticals and Medical Devices (PMD Act, November 2013). This legislation and statements from the MHLW have demonstrated their interest in promoting iPSC-based therapeutics development [30]. The RMP guarantees broad governmental protection and support of regenerative medicines at all stages and the RM Act gives more granular regulation on the clinical development of regenerative medicines, regulating logistical aspects to regenerative medicine development such as risk classification and processing facility requirements [11]. The PMD Act gives the strongest distinction of Japan’s regulatory environment from the US and EU. This act creates a therapeutic category “regenerative medicine products,” under which GTPs fall (Figure 1). Most interestingly, it creates separate drug approval pathway for these regenerative medicine products, effectively providing a shortcut through the clinical trials process [30]. Developers may seek conditional marketing approval after Phase II trials if their candidate has demonstrated safety and probable efficacy in a small sample size. Phase III data is collected while the drug is commercially available. The market approval is time-limited: within seven years from the initial approval, the drug will be re-analyzed and granted final market approval if the data is favorable.
Post-Market Challenges: Case Study Of Glybera
At time of publication, only four GTPs have received market approval in the either US or EU: Glybera (uniQure; Amsterdam, NL), Strimvelis (GlaxoSmithKline; Brentford, UK), Kymriah (Novartis; Basel, CH) and Yescarta (Kite Pharma; Los Angeles, CA, US). Additionally, Luxturna (Spark Therapeutics; Philadelphia, PA, US) is expected to receive market approval in early 2018. Glybera provides useful case studies for any developer of GTPs. Navigating the path to approval, in itself, does not guarantee a medicinal product’s success, and commercial, rather than regulatory, challenges have in some cases resulted in product failure. These too must be considered prior to and throughout development.
In 2012, Glybera (alipogene tiparvovec, see Figure 2) was recommended for approval, becoming the first GTP on market in either the EU or US [31]. Glybera is an in vivo AAV1 GTP indicated for lipoprotein lipase deficiency (LPLD). LPLD is an autosomal recessive disease caused by the loss-of-function of a critical protein. Patients with LPLD do not have a functional copy of the lipoprotein lipase enzyme and therefore cannot breakdown triglycerides, leading to a variety of health complications including possibly life-threatening pancreatitis [32]. The AAV1 delivers an intact DNA transcript of the deficient LPL protein to the patients’ cells. Both the CAT and the CHMP initially gave negative opinions on its market authorization candidacy before eventually approving the product [33]. As of late 2016, it had only been used commercially in one patient [34]. Glybera sponsor uniQure announced in early 2017 that the company would voluntarily withdraw the product from the EU market for logistical reasons [35]. Not only the first market approved GTP, Glybera was also the most expensive drug in the world, with an estimated cost of $1 million per treatment [36]. The large price tag in combination with a very small patient population, the curative action of the drug (therefore no repeat revenue from regular treatment), and inability to enter the US market due to FDA skepticism of the drug’s efficacy have proved Glybera financially unappetizing for uniQure [37].
Figure 2.
Case Study on Gene Therapy Product Glybera.
Conclusion
Gene therapies offer the potential to improve prognosis and potentially cure certain diseases. However, the uncertainty and therefore risk surrounding this novel approach results in a regulatory landscape that in the view of some is overly stringent and complex. Ensuring that patient benefit is maximized requires both: i) that regulators remain flexible and reactive to the demands and challenges uniquely posed by GTPs, and ii) that those developing GTPs operate optimally within the constraints of the regulatory system. This article focusses on the latter, and hopes to serve as a guide for those interested in, or currently developing, gene therapies. An understanding of the regulations surrounding GTPs is essential to success in the area, and consideration of the core principles detailed in this article at the earliest possible stage of development will inevitably increase the likelihood of much needed gene therapies entering the clinic. Regulations are in place to ensure that the risk/benefit ratio of medicinal products is optimized, and this should also be the view of any entity developing GTP. GTP developers must act within the constraints imposed by regulatory bodies to improve the likelihood of their success; however, they should also remain aware of the shared goal, and work with regulators to define and improve the criteria for successful products. With a collaborative approach, regulations will evolve to best represent the needs of the stakeholders concerned – most importantly, the patients.
Acknowledgments
CLHH is funded by SRF-CASMI Alliance and thanks them for their continued support. JS is funded primarily by an MRC UK DPhil Studentship. JS also extends thanks to the SENS Research Foundation and CASMI for their intellectual and financial support. DB gratefully acknowledges personal funding from the Oxford Musculoskeletal National Institute for Health Research Biomedical Research Unit (NIHR BRU), the Saïd Foundation and the SENS Research Foundation.
Glossary
- GTP
Gene therapy product
- GMP
Good Manufacturing Practice
- GLP
Good Laboratory Practice
- AAV
adeno-associated virus
- iPSC
induced pluripotent stem cell
- LPLD
lipoprotein lipase deficiency
- US
United States of America
- FDA
The U.S. Food and Drug Administration
- CBER
Center for Biologics Evaluation and Research
- OTAT
Office of Tissues and Advanced Therapies (previously OCTGT)
- OCTGT
Office of Cellular, Tissue, and Gene Therapies
- CDER
Center for Drug Evaluation and Research
- HCT/Ps
Human cells, tissues, and cellular and tissue-based products
- BLA
Biologic License Application
- NDA
New Drug Application
- EU
European Union
- EMA
European Medicines Agency
- CAT
Committee for Advanced Therapeutics
- CMPH
Committee for Medicinal Products for Human Use
- ATMP
Advanced Therapeutic Medicinal Product
- GTMP
Gene Therapy Medicinal Product
- CTMP
Somatic Cell Therapy Medicinal Product
- TEP
Tissue Engineered Product
- MHLW
Ministry of Health, Labour, and Welfare
- RMP
Regenerative Medicine Promotion Law
- RM Act
the Act of Safety of Regenerative Medicine
- PMD
Act on Pharmaceuticals and Medical Devices
Author Contributions
CLHH: research and manuscript writing. JP: intellectual support, copy editing. JS: introduction and conclusion, copy editing. ZA: Glybera research and Figure 2. MS: gene therapy expert. DB: DPhil supervisor, editing RM: DPhil supervisor, editing.
Disclosures
CLHH is a consultant for Oisín Biotechnologies Inc. (San Francisco, CA), a biotechnology company developing senolytic and oncolytic therapeutics, and has consulted for Biolacuna Ltd (Charlbury UK). JGP is a shareholder and director of Apollo Ventures Holding, GmbH (Hamburg, Germany). MS is CEO of Immusoft Corp. (Seattle, WA), a biotechnology company developing gene therapeutics for a variety of indications, the President of Oisín Biotechnologies Inc., and Board Member at Sigma Genetics (Seattle, WA), a biotechnology company building cell transfection devices. JS has consulted with IP Asset Ventures Ltd. and Biolacuna Ltd. DB is a stockholder in Translation Ventures Ltd. (Charlbury, UK) and IP Asset Ventures Ltd. and Biolacuna Ltd., companies that among other services provide biomanufacturing, regulatory, and financial advice to pharmaceutical clients. DB also is subject to the CFA Institute’s codes, standards, and guidelines, so he must stress that this piece is provided for academic interest only and must not be construed in any way as an investment recommendation. Additionally, at time of publication, DB and the organizations with which he is affiliated may or may not have agreed to and/or pending funding commitments from the organizations named herein. REM is the academic founder and director of Nightstarx Ltd. (Oxford, UK), a biotechnology company specializing in gene therapy for retinal diseases.
References
- National Human Genome Research Institute [Internet]. The Cost of Sequencing the Human Genome. Available from: https://www.genome.gov/sequencingcosts/
- Abbott A. Human genome at ten: the human race. Nature. 2010;464:668–9. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science. 2013;339(6121):819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juod SW. Celebrating a Milestone: FDA’s Approval of First Genetically-Engineered Product. 2007. [Cited 3 July 2017] Available from: https://www.fda.gov/aboutfda/whatwedo/history/productregulation/selectionsfromfdliupdateseriesonfdahistory/ucm081964.htm
- Liu J. The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann Med Surg (Lond). 2014;3(4):113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW. Early drug discovery and the rise of pharmaceutical chemistry. Drug Test Anal. 2011;3(6):337–44. [DOI] [PubMed] [Google Scholar]
- Centennial Edition of FDA Consumer - Promoting Safe and Effective Drugs for 100 Years. U.S. Food and Drug Administration. [Cited 27 September 2017] Available from: https://www.fda.gov/AboutFDA/WhatWeDo/History/CentennialofFDA/CentennialEditionofFDAConsumer/ucm093787.htm
- Von Tigerstrom B. Revising the Regulation of Stem Cell-Based Therapies: Critical Assessment of Potential Models. Food Drug Law J. 2015;70(2):315–37. [PubMed] [Google Scholar]
- Yano K, Watanabe N, Tsuyuki K, et al. Regulatory approval for autologous human cells and tissue products in the United States, the European Union, and Japan. Regenerative Therapy. 2015;1:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez P, Clares B, Hmadcha A, Ruiz A, Soria B. Development of a cell-based medicinal product: regulatory structures in the European Union. Br Med Bull. 2013;105:85–105. [DOI] [PubMed] [Google Scholar]
- Nederland E. Study on the Regulation of Advanced Therapies in Selected Jurisdictions. 2016 Available from: https://ec.europa.eu/health//sites/health/files/human-use/docs/20147306_rfs_chafea_2014_health_24_060516.pdf .
- FDA. What Are “Biologics” Questions and Answers. 05 August 2015. [Cited 3 July 2017] Available from: https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cber/ucm133077.htm
- Information on CBER Restructuring U.S. Food and Drug Administration. 2016. [Cited 26 September 2017] Available from: https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm525907.htm
- Witten C. Office of Cellular, Tissue, and Gene Therapies Overview. PPMD Gene Therapy Forum. 22 February 2016. [Cited 3 July 2017] [slides] Available from: http://www.parentprojectmd.org/site/DocServer/PPMDGeneTherapyForum.pdf?docID=16785
- Public Health Service Act. Section 351. As Amended through P.L. 114-255. 13 December 2016.
- Coopman K. From production to patient: challenges and approaches for delivering cell therapies. StemBook [Internet]Cambridge (MA): Harvard Stem Cell Institute; 2014. [PubMed] [Google Scholar]
- Smith JA, Bravery CA, Hollander G, Brindley DA. Regenerative Medicine Regulations: Cell Therapy, Gene Therapy and Tissue Engineering. Rockville (MD): Regulatory Affairs Professional Society; 2015. [Google Scholar]
- EMA Authorisation of medicines. [Cited 3 July 2017] Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/about_us/general/general_content_000109.jsp
- Stockholms läs landsting. The Macchiarini Case: Investigation of the synthetic trachea transplantations at Karolinska University Hospital. Available from: http://www.sll.se/Global/Verksamhet/H%C3%A4lsa%20och%20v%C3%A5rd/Nyhet%20bilaga/The%20Macchiarini%20Case%20Summary%20(eng).pdf
- Sibbald B. Death but one unintended consequence of gene therapy trial. CMAJ. 2001;164(11):1612. [PMC free article] [PubMed] [Google Scholar]
- Samulski RJ, Muzyczka N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu Rev Virol. 2014;(1):427–51. [DOI] [PubMed] [Google Scholar]
- Hacein-Bay-Albina S, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of CID-X1. J Clin Invest. 2008;118(9):3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of CID-X1 patients. J Clin Invest. 2008;118(9):3143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Developing Products for Rare Diseases and Conditions. 29 June 2017. [Cited 3 July 2017] Available from: https://www.fda.gov/forindustry/developingproductsforrarediseasesconditions/ucm2005525.htm
- Reese JH. FDA Orphan Drug Designation 101. Worldwide Orphan Medicinal Designation Workshop. 10 March 2014. [Cited 3 July 2017] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2014/03/WC500164160.pdf
- Orphan Incentives EM. [[Cited 3 July 2017]]; Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000393.jsp&mid=WC0b01ac058061f017 .
- Kusakabe T. Regulatory perspectives of Japan. Biologicals. 2015;43(5):422–4. [DOI] [PubMed] [Google Scholar]
- Fast Track FD. Breakthrough Therapy, Accelerated Approval, Priority Review. 14 September 2015. [Cited 3 July 2017] Available from: https://www.fda.gov/forpatients/approvals/fast/ucm20041766.htm
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. [DOI] [PubMed] [Google Scholar]
- Azuma K. Regulatory Landscape of Regenerative Medicine in Japan. Current Stem Cell Rep. 2015;1(2):118–28. [Google Scholar]
- EMA European Medicines Agency recommends first gene therapy for approval. 20 July 2012. [Cited 3 July 2017] Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/07/news_detail_001574.jsp&mid=WC0b01ac058004d5c1
- Gaudet D, Méthot J, Déry S, et al. Efficacy and long-term safety of apilogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency Monthly report: Committee for Advanced Therapies October 2011 meeting. European Medicines Agency; 2011[Internet]. [Google Scholar]
- Touchot N, Flume M. Early Insights from Commercialization of Gene Therapies in Europe. Genes (Basel). 2017;8(78):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uniqure withdraws €1m Drug Glybera From Market. European Biotechnology. [Cited 21 April 2017] Available from: http://european-biotechnology.com/up-to-date/latest-news/news/uniqure-withdraws-eur1m-drug-glybera-from-market.html
- Morrison C. $1-million price tag set for Glybera gene therapy. Nat Biotechnol. 2015;33(3):217–8. [DOI] [PubMed] [Google Scholar]
- Taylor NP. UniQure abandons ambition to win FDA approval for €1.1M gene therapy. FierceBiotech. [Cited 1 December 2015] Available from: http://www.fiercebiotech.com/regulatory/uniqure-abandons-ambition-to-win-fda-approval-for-%E2%82%AC1-1m-gene-therapy