Introduction
Osmotic demyelination is the major complication to prevent when treating a patient with chronic hyponatremia.1 It is particularly critical for pediatric patients because they are more vulnerable to brain damage than adults.2 If the patient is symptomatic (e.g., seizures), plasma sodium concentration must be rapidly increased by 4 to 6 mmol/l by infusing hypertonic saline.3 Once the patient is stable (or in asymptomatic patients), the aim of therapy is to slowly raise the plasma sodium concentration by 6 to 8 mmol/l daily.3 The history and clinical characteristic of the patient must be carefully reviewed to establish the most likely etiology4, 5 and to assess the level of renal function. Renal replacement therapy may be required for patients with severe hyponatremia and concomitant renal failure. This poses difficulties due to the fixed sodium concentrations in peritoneal dialysis fluid or continuous renal replacement therapy (CRRT) replacement solutions. Furthermore, the range of possible sodium concentrations is narrow with conventional hemodialysis machines.6
We report on an 8-month-old female infant who presented with severe hyponatremia and previously undiagnosed chronic kidney disease. The primary diagnosis was suspected and later confirmed to be primary hyperoxaluria type 1 (PH1). A CRRT protocol previously applied to hyponatremic adult patients with renal failure was used to successfully normalize her serum sodium and manage her chronic kidney disease.7 To our knowledge, this is the first pediatric patient treated with dialysis/replacement fluid bags customized to the daily target plasma sodium concentration.
Case Presentation
Case
An 8-month-old girl presented to the emergency department with a 9-day history of vomiting and progressive oliguria. She was born at term following an uneventful pregnancy to healthy non-consanguineous parents. Antenatal ultrasounds in the second and third trimester were normal. The history was remarkable for a 5-month period of failure to thrive managed expectantly, without investigations. Physical examination revealed an afebrile, nondysmorphic, and nonedematous child who was mildly irritable, but easily consolable. Her vital signs showed a pulse of 156 bpm and a blood pressure of 83/43 mm Hg. Her weight was 6.6 kg (sixth percentile) and length 67 cm (23th percentile), with a body surface area of 0.34 m2. Cardiac, pulmonary, and abdominal examinations were unremarkable. No crystals were seen on formal ophthalmological examination.
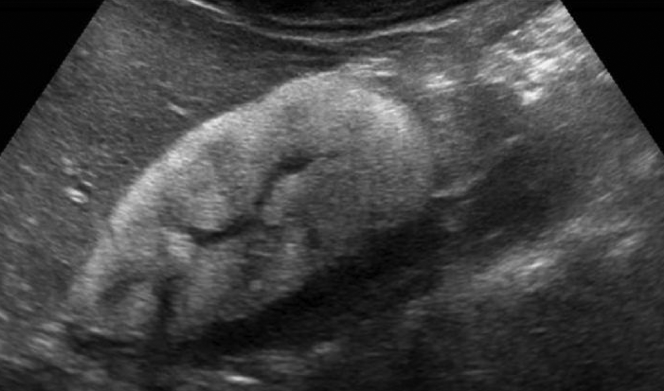
Her initial laboratory results showed severe hyponatremia, anemia, and renal failure (Table 1). Investigations performed on a few drops of urine revealed an elevated urine protein to creatinine ratio and undetectable urine sodium (Table 1). Urine microscopy showed 20 to 50 erythrocytes and 1 to 2 leukocytes per high power field. No casts or crystals were seen. Abdominal ultrasound showed hyperechogenic kidneys with size within normal limits for age (Figure 1). Chest and abdominal radiographic studies were unremarkable.
Table 1.
Results of the laboratory investigations
| Blood | Results |
Normal ranges | |
|---|---|---|---|
| Day 0 | Day 1 | ||
| pH: venous | 7.32 | 7.27 | 7.32–7.42 |
| Bicarbonate | 9 | 11 | 22–30 mmol/l |
| Sodium | 106 | 113 | 135–143 mmol/l |
| Potassium | 5.2 | 4.6 | 3.7–5.0 mmol/l |
| Chloride | 74 | 86 | 99–111 mmol/l |
| Calcium | 2.13 | 1.92 | 2.22–2.54 mmol/l |
| Phosphorus | 3.39 | 2.72 | 1.41–2.17 mmol/l |
| Oxalate | 179.8 | 161.4 | <1.8 μmol/l |
| Creatinine | 634 | 562 | 13–33 μmol/l |
| Urea | 47 | 44.5 | 3.4–8.1 mmol/l |
| Albumin | 44 | 33 | 35–47 g/l |
| WBC | 12.8 | 7.7 | 5.0–12.0 ×109/l |
| Hemoglobin | 72 | 83 | 110–140 g/l |
| Platelets | 425 | 220 | 150–400 × 109/l |
| Urine | |||
| Creatinine | 1.405 | 1.938 | mmol/l |
| Oxalate | Done (NSQ) | 0.311 | mmol/l |
| Oxalate/creatinine | 0.16 | <0.26 mmol/mmol | |
| Protein/creatinine | 1629 | < 50 mg/mmol | |
| Sodium | < 20 | mmol/l | |
| FENa | 0.01 | % | |
| Urine hyperoxaluria profilea | Oxalate 668 Glycolate 348 Glycerate 23.2 Glyocylate 2.6 |
<352 mmol/mmol creatinine <89 mmol/mmol creatinine <60 mmol/mmol creatinine <8 mmol/mmol creatinine |
|
FENa, fractional excretion of sodium; NSQ, not sufficient quantity; WBC, white blood count.
Done on day 7 when we had enough urine volume to do the whole hyperoxaluria profile.
Figure 1.
Sonographic appearance of the right kidney. A longitudinal view demonstrates a diffuse increase of echogenicity with loss of corticomedullary differentiation. There was no evidence of hydronephrosis. The right kidney measured 5.87 cm, and the left kidney measured 5.79 cm. The left kidney had a similar sonographic appearance.
The clinical, biochemical, and ultrasound findings were in keeping with end-stage renal disease with a presumptive diagnosis of PH1, which was subsequently confirmed on the basis of a markedly elevated plasma oxalate (Table 1), and elevated urine glycolic acid and oxalic acid levels. Genetic testing revealed compound heterozygous mutations in the gene encoding for the enzyme alanine-glyoxylate aminotransferase (AGXT), which was predicted to be pathogenic. The first mutation was an apparently novel missense mutation (c.560C>A; p.S187Y) absent from the Human Gene Mutation Database (HGMD) and Exome Aggregation Consortium (ExAC) database. The second was a deletion of exon 9, which is also likely to be novel because the only other deletion of exon 9 reported has been in a Hispanic patient.8 Parental carrier testing has not yet been done.
Because of the history and absence of serious neurological symptoms, the hyponatremia was presumed to be chronic rather than acute. The goal was slow gradual correction of the serum sodium to avoid cerebral damage caused by osmotic demyelination. Furthermore, the severity of her renal failure and her degree of oligoanuria strongly influenced our choice of therapy to correct the sodium.
CRRT was chosen as the safest way to correct the metabolic abnormalities of the patient while also removing oxalate.9 Because of mild irritability, an i.v. infusion of 38 ml of 3% sodium chloride (19 mmol) was given to acutely raise the serum sodium from 106 to 110 mmol/l over the first 5 hours. Her fluid infusion was then changed to D5W 0.2% sodium chloride at 400 ml/m2 per day to replace her estimated daily insensible water losses. She received 2 doses of sodium bicarbonate (1 mmol/kg) to treat ongoing metabolic acidosis.
Using sonographic and fluoroscopic guidance, and with the patient under general anesthesia, an 8-Fr × 125-mm double lumen GamCath (GAMBRO, Hechingen, Germany) nontunneled central venous line was inserted in the left internal jugular vein 6 hours after deciding to start CRRT. The decision to place a temporary line was based on the clinical condition of the patient. The left side was used to preserve the contralateral side in case a long-term hemodialysis catheter was needed later. The tip of the line was positioned at the upper portion of the right atrium/superior vena cava junction, obtaining good flows from both lumens on the procedure table. She received 1 dose of i.v. desmopressin (0.3 μg/kg) to help prevent bleeding due to uremia.10
Continuous venovenous hemofiltration (CVVH) began using the Prismaflex machine loaded with a HF20 polyarylethersulfone filter (Gambro Lundia AB, Lund, Sweden). A blood prime was necessary because this device includes a total extracorporeal circuit of 90 ml (65-ml circuit and 25-ml HotLine water bath blood warmer; Smith Medical ASD, Inc. Rockland, Massachusetts, USA). A bolus of 10 U/kg of unfractionated heparin was used to initiate anticoagulation, followed by an infusion of 10 U/kg per hour. The monitoring was with activated clotting time, with a target between 140 and170 seconds.
Because the available CRRT solutions had a sodium concentration of 140 mmol/l, the final sodium concentration was adjusted to the daily serum sodium targets by adding specific volumes of sterile water to the bags. Our protocol closely follows the description published by Ostermann et al. who used this method to treat adult patients with chronic hyponatremia and renal insufficiency.7 Table 2 outlines the volume of sterile water required to achieve the target sodium and the effect on the concentration of other electrolytes in a 5-l bag of PrismaSOL 4 (Baxter Healthcare Corp., Deerfield, Illinois, USA).
Table 2.
Effect on electrolyte composition of adding different volumes of sterile water to a 5-l bag of PrismaSOL 4
| Volume of water added (ml) | Final volume of replacement fluid (l) | Sodium concentration (mmol/l) | Bicarbonate concentration (mmol/l)a | Potassium concentration (mmol/l) |
|---|---|---|---|---|
| Nil | 5 | 140 | 32 | 4.0 |
| 250 | 5.25 | 133 | 30 | 3.8 |
| 500 | 5.5 | 127 | 29 | 3.6 |
| 600 | 5.6 | 125 | 28.5 | 3.5 |
| 750 | 5.75 | 122 | 27.8 | 3.5 |
| 1000 | 6 | 117 | 26.6 | 3.3 |
Replacement fluid bag used was PrismaSOL 0 (without potassium) and PrismaSOL 4 (with potassium 4 mEq/l).
These bags contain 35 mmol/l of base: bicarbonate, 32 mmol/l, and lactate, 3 mmol/l (total of buffer 35 mmol/l).
Adapted from Ostermann M, Dickie H, Tovey L, et al. Management of sodium disorders during continuous haemofiltration. Crit Care. 2010;14:418.7
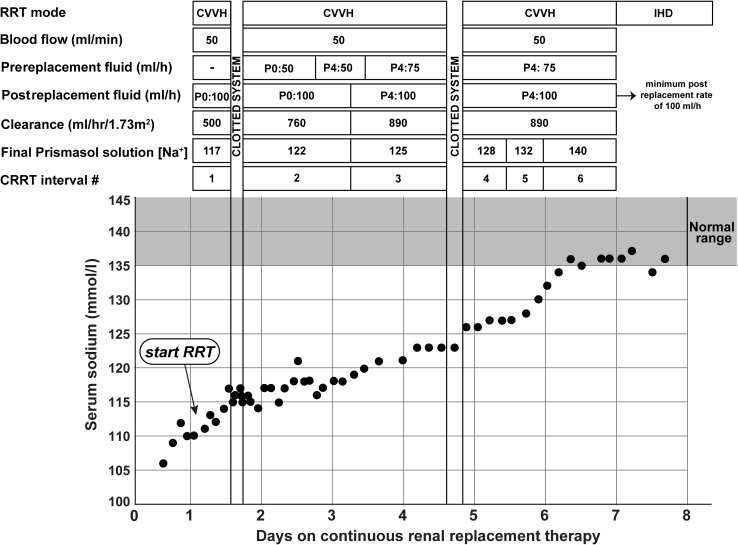
On day 1, 1 liter of sterile water was added to the CRRT solution bags (5-l PrismaSOL 0) to achieve a target sodium concentration of 117 mmol/l. The final sodium concentrations were confirmed by the biochemistry laboratory before use. On initiation of CVVH therapy, low clearances were used (500 ml/1.73 m2 per hour; urea reduction rate 33.7%) to reduce the likelihood of disequilibrium syndrome.11 Mannitol was not prescribed. The goal was to increase plasma sodium concentration by 7 mmol/l daily by systematically decreasing the volumes of free water added to the replacement fluid.
Laboratory investigations were done every 2 hours for the first 24 hours. Sampling intervals were gradually extended after we documented a slow and steady increase in plasma sodium. Solute clearance was increased daily, to a maximum of 890 ml/1.73 m2 per hour (Figure 2). Plasma phosphate and urea concentrations normalized after 2 and 5 days on CVVH, respectively. On day 3, the replacement fluid was changed to PrismaSOL 4 because of worsening hypokalemia. Figure 2 shows the dialysis prescriptions used and the concomitant rise in plasma sodium concentration achieved. The CVVH circuit clotted twice during the 7-day treatment period, which caused interruptions in therapy for 3.5 hours on day 1 and for 5 hours on day 4. Plasma sodium concentration normalized on CVVH without observable neurological sequelae. On day 7, the patient was switched to daily intermittent hemodialysis.
Figure 2.
Changes in serum sodium concentrations over time. CRRT, continuous renal replacement therapy; CVVH, continuous venovenous hemofiltration; IHD, intermittent hemodialysis; Na, sodium; P0 and P4, PrismaSOL solutions with 0 or 4 mmol/l of potassium; RRT, renal replacement therapy.
Plasma oxalate remained high while on hemodialysis almost daily (80–100 μmol/l; the last value at age 20 months was 84 μmol/l). Growth parameters progressively improved, but she has kept a moderate to severe failure to thrive due to her chronic renal disease. She never had any seizures after 20 months of follow-up. She has mild developmental delay, most likely related to her chronic disease, which was noted at her 18-month follow-up visit. The left nontunneled line was exchanged for a right-sided tunneled long-term hemodialysis catheter. She received a living donor liver transplantation from her mother at 16 months of age, with an uneventful post-transplantation course. Her plasma oxalic acid levels remained in the range of 74 to 109 μmol/l on 6 dialysis sessions per week. Renal transplantation is planned when she reaches an appropriate size for this procedure.
Discussion
We presented a case of an 8-month old girl with oliguric renal failure and severe hyponatremia. The literature is clear that rapid correction of serum sodium concentration is associated with osmotic demyelination, especially in the setting of severe chronic hyponatremia.1 As per guidelines, our plasma sodium correction target was 6 to 8 mmol/l per day.3
Hyponatremic patients with otherwise normal renal function are usually treated with i.v. salt solutions, controlled fluid intake, and frequent laboratory investigations.3, 4 This approach was inappropriate for our patient, who had oliguric/anuric chronic renal failure that require dialytic management. The use of customized dialysis fluid bags, first reported in adults by Ostermann et al.7 and replicated by others,6, 12 greatly facilitated our decision to proceed with CRRT. A similar approach was used for patients with chronic hypernatremia.13, 14 As a whole, these studies showed that custom CRRT therapy provides clinicians with great control over the direction, magnitude, and timing of serum sodium concentration correction. Intermittent hemodialysis was not considered a good alternative because it is not possible to change the dialysate sodium concentration to the range needed to safely correct severe dysnatremias.
We were confident that data from these adult studies would be applicable to our patient because CVVH therapy is scalable to the size of patients and thus age-independent. CRRT was an ideal therapy for our patient because it addressed the hyponatremia, severe metabolic disturbances of chronic renal failure, and the presumed high oxalate levels. Although this report focused on the management and outcome of a single pediatric patient, we believe the approach should be considered as the therapy of choice for children who present with a similar constellation of clinical findings, provided that there are no contraindications to CRRT.
Peritoneal dialysis (PD) is usually the preferred renal replacement therapy modality for infants because it contributes to preservation of residual renal function, does not require chronic i.v. access (preventing long-term central venous complications and/or sequelae), and is more compatible with family life.15 Similar to CRRT fluid bags, peritoneal dialysate could, in principle, be customized to any target sodium concentration. We elected not to proceed with PD because of the high probability that the underlying diagnosis was PH1, and PD is inferior to daily hemodialysis in the removal of plasma oxalate.9 PD should be considered as a reasonable choice for an infant with a similar clinical presentation who does not require aggressive solute clearance in the subacute phase.
Conclusion
This is the case of an 8-month-old girl with severe chronic hyponatremia and renal failure that provides several learning points for students of pediatric nephrology (Table 3). The goal of the treatment was a slow gradual correction of the serum sodium to avoid osmotic demyelination. The patient was successfully managed without any complications. CRRT with customized dialysis and/or replacement fluid bags should be considered as a safe and efficacious treatment option for pediatric patients with severe hyponatremia in the context of renal failure and oligoanuria.
Table 3.
Learning points
|
|
|
|
Disclosure
All the authors declared no competing interests.
Acknowledgments
We wish to thank the patient and her family for agreeing to participate in this project. We also wish to thank the nursing staff that was responsible for the CRRT treatment for this patient.
References
- 1.Giuliani C., Peri A. Effects of Hyponatremia on the Brain. J Clin Med. 2014;3:1163–1177. doi: 10.3390/jcm3041163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moritz M.L., Ayus J.C. New aspects in the pathogenesis, prevention, and treatment of hyponatremic encephalopathy in children. Pediatr Nephrol. 2010;25:1225–1238. doi: 10.1007/s00467-009-1323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrogué H.J., Madias N.E. The challenge of hyponatremia. J Am Soc Nephrol. 2012;23:1140–1148. doi: 10.1681/ASN.2012020128. [DOI] [PubMed] [Google Scholar]
- 4.Adrogué H.J., Madias N.E. Hypernatremia. N Engl J Med. 2000;342:1493–1499. doi: 10.1056/NEJM200005183422006. [DOI] [PubMed] [Google Scholar]
- 5.Burton R., Post T.W. McGraw-Hill Education; New York: 2001. Clinical Physiology of Acid-Base and Electrolyte Disorders. [Google Scholar]
- 6.Vassallo D., Camilleri D., Moxham V., Ostermann M. Successful management of severe hyponatraemia during continuous renal replacement therapy. Clin Kidney J. 2012;5:155–157. doi: 10.1093/ckj/sfr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostermann M., Dickie H., Tovey L., Treacher D. Management of sodium disorders during continuous haemofiltration. Crit Care. 2010;14:418. doi: 10.1186/cc9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulter-Mackie M.B., Lian Q., Applegarth D., Toone J. The major allele of the alanine:glyoxylate aminotransferase gene: nine novel mutations and polymorphisms associated with primary hyperoxaluria type 1. Mol Genet Metab. 2005;86:172–178. doi: 10.1016/j.ymgme.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8:467–475. doi: 10.1038/nrneph.2012.113. [DOI] [PubMed] [Google Scholar]
- 10.Mannucci P.M., Remuzzi G., Pusineri F. Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308:8–12. doi: 10.1056/NEJM198301063080102. [DOI] [PubMed] [Google Scholar]
- 11.Tuchman S., Khademian Z.P., Mistry K. Dialysis disequilibrium syndrome occurring during continuous renal replacement therapy. Clin Kidney J. 2013;6:526–529. doi: 10.1093/ckj/sft087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yessayan L., Yee J., Frinak S., Szamosfalvi B. Treatment of severe hyponatremia in patients with kidney failure: role of continuous venovenous hemofiltration with low-sodium replacement fluid. Am J Kidney Dis. 2014;64:305–310. doi: 10.1053/j.ajkd.2014.01.451. [DOI] [PubMed] [Google Scholar]
- 13.Lin J.J., McKenney D.W., Price C., Morrison R.R., Novotny W.E. Continuous venovenous hemodiafiltration in hypernatremic hyperglycemic nonketotic coma. Pediatr Nephrol. 2002;17:969–973. doi: 10.1007/s00467-002-0947-6. [DOI] [PubMed] [Google Scholar]
- 14.Moss G.D., Primavesi R.J., McGraw M.E., Chambers T.L. Correction of hypernatraemia with continuous arteriovenous haemodiafiltration. Arch Dis Child. 1990;65:628–630. doi: 10.1136/adc.65.6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warady B.A., Neu A.M., Schaefer F. Optimal care of the infant, child, and adolescent on dialysis: 2014 update. Am J Kidney Dis. 2014;64:128–142. doi: 10.1053/j.ajkd.2014.01.430. [DOI] [PubMed] [Google Scholar]