Abstract
Background
Human cytomegalovirus (HCMV) infection has been associated with inflammatory bowel disease (IBD). Numerous studies have been conducted to analyze the association between HCMV infection and risk of IBD and steroid-resistant IBD, but no clear consensus had been reached.
Objectives
The aim of this study was to confirm this relationship precisely by doing a systematic review and meta-analysis.
Study design
We identified relevant studies through a search of PubMed and Embase. Studies were eligible for inclusion if they 1) evaluated the association between HCMV infection and IBD disease; 2) evaluated the association between HCMV infection and steroid-resistant IBD disease; 3) were case–control studies or nested case–control studies; 4) provided the numbers (or percentage) of positivity for HCMV infection in cases and controls, respectively. Data were extracted and analyzed independently by two investigators.
Results and conclusion
A total of 18 studies including 1,168 patients and 951 health groups was identified, and HCMV infection was distinctly confirmed as a risk factor for the occurrence and development of IBD. When involving 17 studies including 1,306 IBD patients, a total of 52.9% of patients in the cytomegalovirus (CMV)-positive groups were observed to have steroid resistance, compared with 30.2% of patients in the CMV-negative groups. There was a significant difference in the risk of steroid resistance between people exposed to HCMV infection and those not exposed HCMV infection in IBD patients. This meta-analysis suggested that HCMV infection is associated with an increased risk for IBD and steroid-resistant IBD.
Keywords: cytomegalovirus, infection, inflammatory bowel disease, Crohn’s disease, ulcerative colitis, meta-analysis
Background
Inflammatory bowel disease (IBD) is a group of chronic inflammatory diseases of the gastrointestinal tract with no specific etiology, and it includes ulcerative colitis and Crohn’s disease, and other chronic, nonspecial inflammatory diseases of the gut with unknown etiology. The diagnosis is based on clinical, radiological, endoscopic, and histopathological studies. Several attempts have been made to uncover a possible infectious agent as a cause of IBD. But to date, such efforts have failed to prove any suspected pathogen.
Even though the etiology of IBD is unknown, recent studies have shown that the pathogenesis of IBD is related to susceptible genes, immune dysregulation, and microbiota of the gut.1 IBD is characterized by alternating phases of exacerbation and remission, and cytomegalovirus (CMV) infection is one prominent cause of exacerbation. In addition, CMV infection is an emerging and controversial complication in patients with IBD.2 There are many case reports which suggest that IBD patients are particularly vulnerable to CMV infection.
CMV is a β herpes virus with double-stranded DNA. Worldwide, the current infective rate ranges between 40% and 100%. CMV infection is a type of opportunistic infection, as the virus is reactivated and results in active CMV infection with characteristics of viremia and visceral injury (for example pneumonia or colitis) in immunocompromised patients. During the past few decades, abundant observational research has produced data on CMV prevalence in patients with IBD.3 Accordingly, previous studies recommended that CMV infection be suspected in IBD patients with resistance to steroid treatment.4 However, it is still uncertain whether CMV infection has an active role in IBD or whether it exists coincidentally.
The prevalence of CMV can be assessed in several ways. Serological CMV IgG testing provides data about latent infection, while IgM testing provides information on recent infection or, as is more likely in adults, reactivation. Other techniques used to demonstrate the virus include immunohistochemistry with antibodies to CMV early antigen or pp65, in situ hybridization to detect CMV mRNA, and polymerase chain reaction (PCR) for CMV DNA,5,6 and each has been used to attempt to quantify the infection.
The aims of this study were to clarify the association between CMV infection and IBD and to identify the relationship between CMV infection and steroid-resistant IBD.
Study design
Search strategy
We searched PubMed and Embase and for eligible studies to include in the present meta-analysis, using the terms “human cytomegalovirus” and “inflammation bowel disease” or “Crohn’s disease” or “ulcerative colitis.” An upper date limit of June 10, 2017 was applied; we used no lower date limit. Papers published in English were included. We reviewed citations in the retrieved papers to search for additional relevant studies. The retrieved studies were then read in their entirety to assess their appropriateness for the inclusion in this meta-analysis.
Inclusion/exclusion criteria
Studies were eligible for inclusion if they 1) evaluated the association between human cytomegalovirus (HCMV) infection and IBD; 2) evaluated the association between HCMV infection and steroid-resistant IBD; 3) were case–control studies or nested case–control studies; 4) and provided the numbers (or percentage) of positivity for HCMV infection in cases and controls, respectively. Conference abstracts, case reports, editorials, review articles, and letters were excluded.
Search selection and data extraction
Two authors (Ya-li Lv and Fei-fei Han) extracted data and reached a consensus on all of the eligibility items according to the aforementioned inclusion criteria. The following data were collected from each study: first author’s surname, the year of publication, source of cases, HCMV detection method, total numbers of cases and controls, and the positivity for HCMV infection in cases and controls or the incidence of steroid resistance in HCMV positive and negative patients, respectively.
All articles reporting data for HCMV prevalence based on enzyme-linked immunosorbent, PCR detection methods, and immunohistochemistry with antibodies to CMV early antigen or pp65 since April 12, 1975, were selected.
Statistical analysis
All statistical analyses were performed using Review Manager (Review Manager 5.0 software; Cochrane, London, England, UK) and Stata/MP 11.0. Cochran’s w2 test and the inconsistency index (I2) were used to evaluate heterogeneity across the included studies. Random-effects model was applied in all the analysis. Odds ratio (OR) and corresponding 95% confidence intervals (CIs) were estimated. Z-test was performed to determine the statistical significance of pooled OR, and was considered significant when P<0.05. Statistical heterogeneity test was performed by using the χ2 and I2 statistics, and I2 value of more than 50% was considered as substantial heterogeneity.7
Results
Search results
Our search identified 705 potentially relevant studies, 30 of which met the predefined study criteria and were included in our meta-analysis. Figure 1 summarizes the process of study identification, inclusion, and exclusion. There were 245 abstracts from the PubMed search and 460 articles from Embase search. After duplicates were removed, 667 records were assessed for eligibility, and 30 records were finally included in the analysis.1,3,8–35
Figure 1.
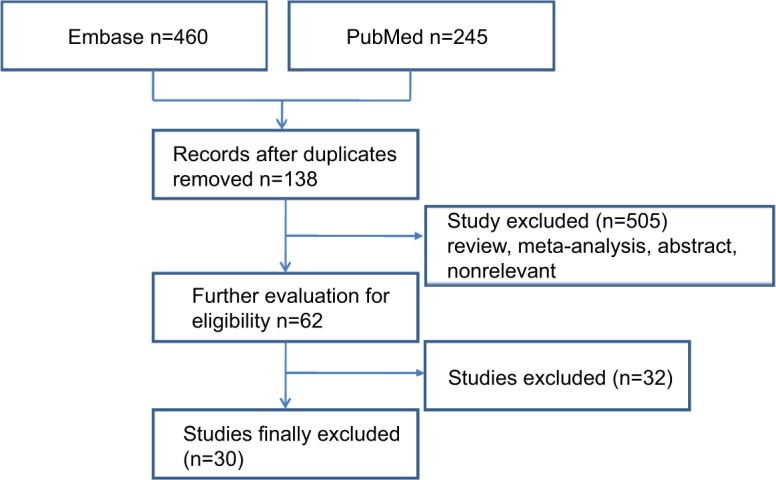
Flow diagram of selected studies.
Characteristics of included studies
The characteristics of included studies and patients are presented in Table 1, including 18 studies, and Table 2 including 17 researches; there were 5 duplicates studies among these, so 30 records were included in the final analysis. Table 1 shows the characteristics and variables collected for the prevalence of CMV infection in IBD disease. And Table 2 displays the characteristics of the relationship between CMV infection and steroid-resistant IBD.
Table 1.
Main characteristics of the studies included in the meta-analysis
| Study | Source of cases | Study design type | Disease type | Definition of disease | Detection of HCMV | Case
|
Control
|
||
|---|---|---|---|---|---|---|---|---|---|
| HCMV+ | Total | HCMV+ | Total | ||||||
| Rahbar et al,8 2003 | Sweden | Case–control | UC and CD (IBD) | WHO | DNA | 22 | 23 | 0 | 10 |
| Wakefiels et al,10 1992 | United Kingdom | Case–control | IBD | WHO | DNA | 36 | 50 | 6 | 21 |
| Verdonk et al,9 2006 | The Netherlands | Case–control | IBD | WHO | pp65 | 12 | 31 | 17 | 53 |
| Marszatek et al,11 2011 | Poland | Case–control | IBD | WHO | DNA IgG IgM |
13 9 0 |
32 | 8 5 0 |
15 |
| Nahar et al,12 2015 | Japan | Case–control | UC | WHO | DNA | 26 | 71 | 6 | 188 |
| Domenech et al,13 2008 | Spain | Case–control | UC | WHO | IgG | 66 | 94 | 19 | 25 |
| Sipponen et al,14 2011 | Finland | Case–control | IBD | WHO | pp65 | 64 | 79 | 6 | 15 |
| Knosel et al,15 2009 | Germany | Case–control | CD | WHO | DNA | 2 | 56 | 0 | 10 |
| Lavagna et al,16 2006 | Italy | Case–control | UC | WHO | IE DNA |
1 3 |
24 | 0 | 20 |
| Dimitroulia et al,17 2006 | Greece | Case–control | IBD | WHO | pp65 DNA |
10 23 |
85 | 0 5 |
42 |
| Van Kruiningen et al,18 2007 | USA | Case–control | CD | WHO | DNA | 1 | 70 | 1 | 41 |
| Kuwabara et al,19 2007 | Japan | Case–control | IBD | WHO | IE | 18 | 34 | 3 | 31 |
| Yi et al,1 2013 | People’s Republic of China | Case–control | IBD | WHO | DNA IgG IgM |
190 172 4 |
226 | 173 147 1 |
290 |
| Ciccocioppo et al,20 2015 | Italy | Case–control | IBD | WHO | IE | 9 | 40 | 2 | 40 |
| Ciccocioppo et al,34 2016 | Italy | Case–control | IBD | WHO | IE | 51 | 64 | 7 | 25 |
| Criscuoli et al,21 2015 | Italy | Case–control | UC | WHO | DNA Pp65 |
11 8 |
24 | 2 2 |
24 |
| Taherkhani et al,22 2015 | Iran | Case–control | UC | WHO | DNA | 12 | 98 | 0 | 67 |
| Thörn et al,23 2016 | Sweden | Case–control | IBD | WHO | IgM DNA |
14 12 |
67 | 1 0 |
34 |
Abbreviations: CD, Crohn’s disease; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; IE, immediate-early; UC, ulcerative colitis; WHO, World Health Organization.
Table 2.
Main characteristics of the relationship between CMV infection and steroid-resistant IBD
| Study | Source of cases | Study design type | Disease type | Definition of disease | Detection of HCMV | HCMV+
|
HCMV−
|
||
|---|---|---|---|---|---|---|---|---|---|
| Steroid resistant/refractory | Total | Steroid resistant/refractory | Total | ||||||
| Lévêque et al,24 2010 | France | Case | IBD | WHO | DNA | 0 | 5 | 8 | 29 |
| Domènech et al,13 2008 | Spain | Case | UC | WHO | IgG | 13 | 29 | 6 | 14 |
| Iida et al,3 2013 | Japan | Case | UC | WHO | IgG IgM |
62 | 141 | 20 | 46 |
| Wada et al,25 2003 | Japan | Case | UC | WHO | pp65 | 13 | 16 | 9 | 31 |
| Kambham et al,26 2004 | USA | Case | UC | WHO | CMV inclusions IE |
12 | 13 | 28 | 67 |
| Maconi et al,27 2005 | Italy | Case | UC | WHO | IE | 15 | 17 | 40 | 60 |
| Kuwabara et al,19 2007 | Japan | Case | IBD | WHO | IE | 13 | 14 | 2 | 8 |
| Maher et al,28 2009 | Egypt | Case | IBD | WHO | IgM IE |
8 | 9 | 15 | 63 |
| Criscuoli et al,29 2011 | Italy | Case | UC | WHO | CMV inclusions IE DNA Pp65 |
6 | 28 | 11 | 57 |
| Mccurdy et al,30 2015 | USA | Case | IBD | WHO | CMV inclusions IE |
40 | 68 | 61 | 202 |
| Ciccocioppo et al,20 2015 | Italy | Case | IBD | WHO | IE | 4 | 9 | 13 | 31 |
| Ciccocioppo et al,34 2016 | Italy | Case | IBD | WHO | IE | 25 | 51 | 0 | 13 |
| Cottone et al,31 2001 | Italy | Case | IBD | WHO | DNA | 7 | 7 | 12 | 55 |
| Criscuoli et al,31 2004 | Italy | Case | IBD | WHO | pp65 DNA |
4 | 9 | 8 | 33 |
| Criscuoli et al,21 2015 | Italy | Case | UC | WHO | CMV inclusions IE DNA pp65 |
5 | 11 | 7 | 13 |
| Kim et al,35 2012 | Korea | Case | UC | WHO | DNA | 14 | 31 | 7 | 41 |
| Ormeci et al,33 2016 | Turkey | Case | IBD | WHO | DNA | 8 | 13 | 5 | 72 |
Abbreviations: HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; IE, immediate-early; CMV, cytomegalovirus; UC, ulcerative colitis; WHO, World Health Organization.
As shown in Table 1, data were available for a total of 1,168 patients and 951 healthy people; containing 14 studies from a Caucasian subgroup, 2 from an Asian subgroup, and 1 from a subgroup of other ethnicities. As can be seen in Table 2, data were available for a total of 1,306 patients, containing 11 studies from a Caucasian subgroup, 4 from an Asian subgroup, and 2 from a subgroup of other ethnicities.
Risk of CMV infection in patients with IBD
Meta-analysis results
A meta-analysis of 18 studies is reported in Table 1 and Figures 2–6, dealing with the association between HCMV infection and IBD disease. When involving 18 studies including 1,168 patients and 951 health groups, a total HCMV positive ratio of 69.6% in IBD patients (247/352) and 51.82% in control groups (171/330) based HCMV IgG tests, 5.5% in IBD patients (18/325) and 0.59% in control groups (28/895) based HCMV IgM tests, 42.5% in IBD patients (351/826) and 26.4% in control groups (201/762) based HCMV DNA tests, 48.8% in IBD patients (79/162) and 10.3% in control groups (12/116) based on HCMV immediate-early (IE) tests, and 40.4% in IBD patients (92/228) and 6.6% in control groups (8/121) based on HCMV pp65 tests was observed.
Figure 2.
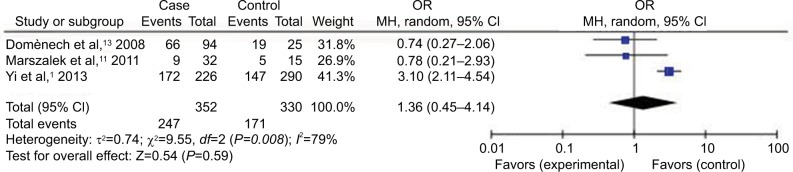
Forest plot (random-effect model) of the association between HCMV infection and IBD stratified by the study design based on HCMV IgG tests.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; MH, Mantel–Haenszel; OR, odds ratio.
Figure 3.
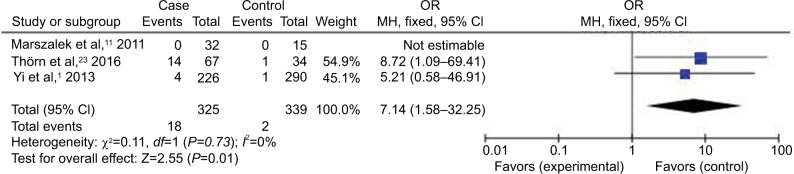
Forest plot (random-effect model) of the association between HCMV infection and IBD stratified by the study design based on HCMV IgM tests.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; MH, Mantel–Haenszel; OR, odds ratio.
Figure 4.
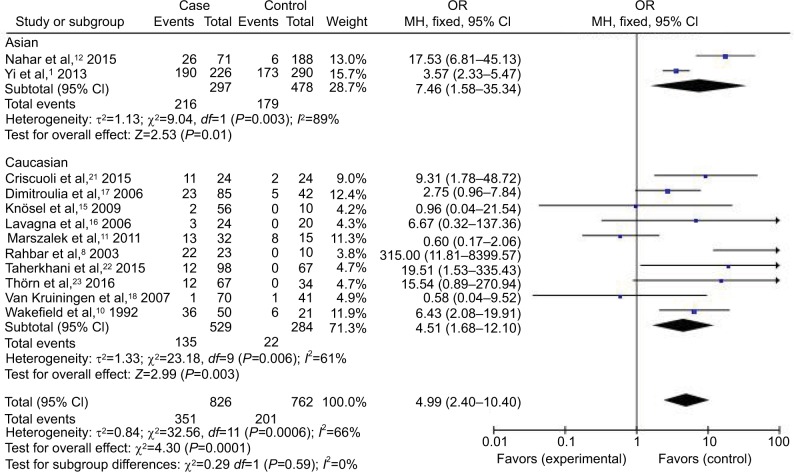
Forest plot (random-effect model) of the association between HCMV infection and IBD stratified by the study design based on HCMV DNA tests.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; MH, Mantel–Haenszel; OR, odds ratio.
Figure 5.
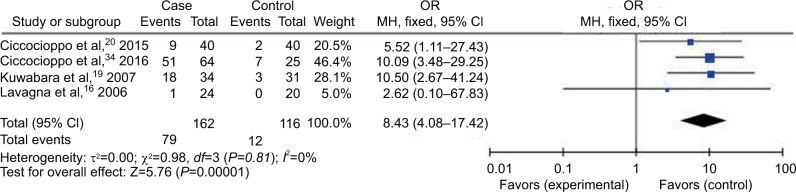
Forest plot (random-effect model) of the association between HCMV infection and IBD stratified by the study design based on HCMV IE tests.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; IE, immediate-early; MH, Mantel–Haenszel; OR, odds ratio.
Figure 6.
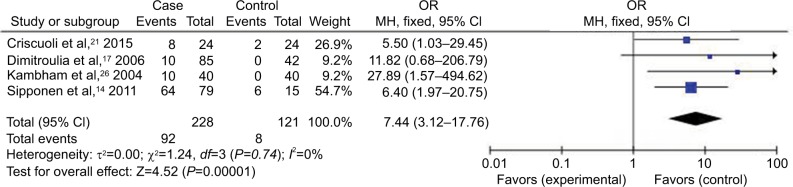
Forest plot (random-effect model) of the association between HCMV infection and IBD stratified by the study design based on HCMV pp65 tests.
Abbreviations: CI, confidence interval; HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; MH, Mantel–Haenszel; OR, odds ratio.
Compared with the control groups, people exposed to HCMV infection had higher risk than those not exposed to CMV. The ORs of HCMV infection rate in IBD patients compared to the control groups were, respectively, 7.14 [95% CI =1.58–32.25, p=0.01] based on HCMV IgM tests, 4.99 [95% CI =2.40–10.40, p<0.0001] based on HCMV DNA tests, 8.43 [95% CI =4.08–17.42, p<0.00001] based on HCMV IE tests, and 7.44 [95% CI =3.12–17.76, p<0.00001] based on HCMV pp65 tests. However, there was no difference in the risk of HCMV infection rate in IBD patients compared to the control groups based on HCMV IgG tests [OR =1.36, 95% CI =0.45–4.14, p=0.59].
Subgroup analysis
In the stratified analysis by ethnicity, through the experimental results (Figure 4), HCMV infection was distinctly confirmed as a risk factor for the occurrence and development of IBD in Asian and Caucasian groups based on HCMV DNA tests, with ORs of 7.46 [95% CI =1.58–35.34, p=0.01] and 4.51 [95% CI =1.68–12.10, p=0.003], respectively.
Risk of CMV infection in patients with steroid-resistant IBD
Meta-analysis results
When involving 17 studies including 1,306 IBD patients, a total of 52.9% of patients in the CMV-positive groups were observed to have steroid resistance, compared with 30.2% of patients in the CMV-negative groups. There was a significant difference in the risk of steroid resistance between people exposed to HCMV infection and those not exposed HCMV infection for IBD patients [OR =3.63, 95% CI =1.99–6.62, p<0.0001].
Subgroup analysis
In the stratified analysis by ethnicity and CMV detection method, HCMV infection was distinctly confirmed as a risk factor for the occurrence of steroid-resistant IBD through the experimental results (Table 3).
Table 3.
Main characteristics of the studies included in the meta-analysis
| Characteristics | Subgroup | N (Study) | N (Subject) | OR | 95% CI | I2 (%) |
|---|---|---|---|---|---|---|
| All ethnicity | 17 | 1,306 | 3.84 | 2.12–6.97 | 67 | |
| Caucasian | 11 | 220 | 3.47 | 1.43–8.41 | 65 | |
| Asian | 4 | 140 | 4.75 | 1.16–19.52 | 80 | |
| Other | 2 | 141 | 5.43 | 1.22–24.17 | 55 | |
| CMV detection | IgG | 1 | 43 | 1.08 | 0.30–3.92 | Not applicable |
| DNA | 4 | 253 | 6.40 | 1.13–36.33 | 71 | |
| IE | 4 | 203 | 5.86 | 1.17–29.37 | 61 | |
| pp65 | 1 | 47 | 10.59 | 2.42–46.33 | Not applicable | |
| CMV inclusion + IE | 2 | 350 | 5.43 | 1.22–24.17 | 55 | |
| IgG + IgM | 1 | 187 | 1.02 | 0.52–2.00 | Not applicable | |
| IgM + IE | 1 | 72 | 25.60 | 2.96–221.58 | Not applicable | |
| pp65 + DNA | 1 | 42 | 2.50 | 0.54–11.63 | Not applicable | |
| CMV inclusion + IE + DNA + pp65 | 2 | 109 | 0.98 | 0.39–2.45 | 0 |
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; IE, immediate-early; OR, odds ratio.
Sensitivity analysis
The purpose of sensitivity analysis is to estimate the stability of the results. When any single study was omitted, the pooled results showed a lack of change. This means that in the overall meta-analysis, no single study substantially affected the stability of the results. Therefore, the results of this meta-analysis were stable and reliable.
Publication bias
The publication bias of the included studies was investigated with the Begg’s funnel plot and Egger’s test. The results are presented in Figures 7 and 8. The shapes of the funnel plots showed some dissymmetry. Egger’s test was used to provide statistical evidence of funnel plot symmetry, and there was no evidence of publication bias.
Figure 7.
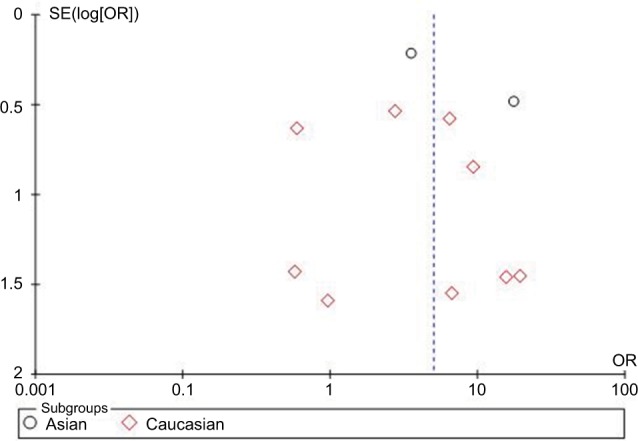
Begg’s funnel plot of the association between HCMV infection and IBD based on HCMV DNA tests.
Abbreviations: HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; OR, odds ratio; SE, standard error of the mean.
Figure 8.
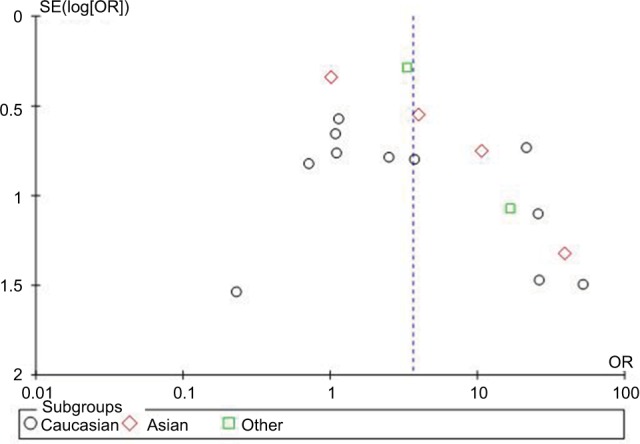
Begg’s funnel plot of the association between HCMV infection and steroid-resistant IBD.
Abbreviations: HCMV, human cytomegalovirus; IBD, inflammatory bowel disease; OR, odds ratio; SE, standard error of the mean.
Discussion
CMV is an opportunistic pathogenic microorganism that was discovered in immunocompetent individuals in the 1960s. As a β-herpesvirus, it is an important pathogen in many patients and widely exists in the majority of the population. After primary infection, the virus persists in the body and is located mainly in white blood cells and endothelial cells. CMV also has wide tissue tropism and is able to infect multiple organs, including the intestine.36 CMV was first found to be related with IBD in 1961.37 Since then, research on the effect of CMV infection on IBD activation has attracted broad attention and interest among clinicians. In IBD patients, immunosuppressive therapy, impaired absorption of nutrients, and dysfunction of the immune system render them susceptible to CMV infection.38 However, the role of CMV infection in patients with IBD is not confirmed yet. This meta-analysis sought to address unresolved debate regarding the relationship between CMV infection and IBD, especially CMV infection and steroid-resistant IBD.
When analyzing the relationship between CMV infection and IBD, our meta-analysis involved 19 studies including 1,218 patients and 972 healthy groups. Compared with the control groups, people exposed to HCMV infection had higher risk than those not exposed to CMV. Among 19 included studies, anti-CMV IgG, IgM, DNA, IE, and pp65 were used to detect CMV infection in IBD patients and healthy controls. Compared to the control groups, anti-CMV IE and pp65 were significantly higher in IBD patients, and the ORs of HCMV infection rate in IBD patients were, respectively, 8.43 [95% CI =4.08–17.42, p<0.00001] and 7.44 [95% CI =3.12–17.76, p<0.00001]. This suggested that CMV infection might be nearly 7~8 times higher in IBD patients than in control groups, thereby indicating the association between IBD and CMV. Our results are in agreement with the previous reports39,40 suggesting that immunosuppressive medications might be an important risk factor for CMV infection. Significant heterogeneity of HCMV prevalence was noted when analyzed by ethnic subgroup. Our findings indicated that HCMV infection in Asia was the highest. Positive rates in Asian studies were 72.7% in the IBD population and 37.4% in the control population based on HCMV DNA tests, which might be the cause of the increased risk of IBD in the Asian population. However, there are limited included studies and insufficient participants in our research, so it needs further study in order to validate the relationship between ethnic difference and HCMV infection in IBD risk.
When analyzing the relationship between CMV infection and steroid resistance in IBD patients, our meta-analysis involved 17 studies including 1,306 IBD patients. Compared to CMV-negative groups, the OR in CMV-positive groups was 3.63 [95% CI =1.99–6.62, p<0.0001], which suggested that CMV infection might cause an approximately fourfold risk of steroid resistance in IBD patients. When analyzed by ethnic subgrouping, the percentage of steroid-resistant patients in the CMV-positive groups was higher than in the CMV-negative groups regardless of the ethnicity. And when analyzed by CMV detection method, the percentage of steroid resistance in CMV-positive groups was also higher than that in CMV-negative groups for the majority of the CMV detection methods. These results thus showed a positive correlation between CMV infection and steroid resistance in IBD patients. Previous studies have also reported that local reactivation of CMV is closely related with severe and steroid-refractory IBD in patients.3,41,42
Previous studies had linked CMV infection with IBD and its resistance to steroids; however, those researches failed to provide a strong and systematic relationship between CMV infection and IBD.1,34,42 In this research, we carried out a systematic meta-analysis assessing the effect of CMV infection on IBD and its resistance to steroids. Our results showed that there exists a significant association between CMV infection with IBD and its resistance to steroids. However, our study has several limitations. First, the number of articles included in this research is relatively small, ie, there were only 33 studies. Second, there have been some differences in the method of CMV detection and protocol, this may be because we only included published studies and did not include unpublished ones. An additional limitation in this research may be that there is a potential publication bias. In order to provide a more precise estimation on the basis of adjustment for confounders, more well-designed studies should be performed. Further prospective controlled trials will help to confirm these findings and identify subgroups of patients who might suffer from CMV infection.
In summary, our meta-analysis strongly suggests that CMV infection might be a probable cause of IBD and its resistance to steroids.
Acknowledgments
This study was supported by the Beijing Natural Science Foundation (grant nos. 7151004 and 7153165) and Natural Science Foundation of China (grant nos. 81500336). Thanks are due to Zhuoling An, Song Yang, Lingling Xuan, and Wen Zhang for drafting the article and revising it critically for important intellectual content.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yi F, Zhao J, Luckheeram RV, et al. The prevalence and risk factors of cytomegalovirus infection in inflammatory bowel disease in Wuhan, Central China. Virol J. 2013;10:43. doi: 10.1186/1743-422X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16(9):1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 3.Iida T, Ikeya K, Watanabe F, et al. Looking for endoscopic features of cytomegalovirus colitis: a study of 187 patients with active ulcerative colitis, positive and negative for cytomegalovirus. Inflamm Bowel Dis. 2013;19(6):1156–1163. doi: 10.1097/MIB.0b013e31828075ce. [DOI] [PubMed] [Google Scholar]
- 4.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443–468. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Sager K, Alam S, Bond A, Chinnappan L, Probert CS. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(8):725–733. doi: 10.1111/apt.13124. [DOI] [PubMed] [Google Scholar]
- 6.Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011;16(11):2001–2008. doi: 10.1038/ajg.2011.202. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahbar A, Boström L, Lagerstedt U, Magnusson I, Södergerg-Naucler C, Sundqvist VA. Evidence of active cytomegalovirus infection and increased production of IL-6 in tisseu specimens obtained from patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2003;9(3):154–161. doi: 10.1097/00054725-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Verdonk RC, Haagsma EB, Van Den Berg AP, et al. Inflammatory bowel disease after liver transplantation: a role for cytomegalovirus infection. Scand J Gastroenterol. 2006;41(2):205–211. doi: 10.1080/00365520500206293. [DOI] [PubMed] [Google Scholar]
- 10.Wakefield AJ, Fox JD, Sawyerr AM, et al. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn’s disease using the nested polymerase chain reaction. J Med Virol. 1992;38(3):183–190. doi: 10.1002/jmv.1890380306. [DOI] [PubMed] [Google Scholar]
- 11.Marszalek A, Marciniak R, Szkaradkiewicz A, et al. Inflammatory bowel disease - is there something new in the immunological background? Folia Histochem Cytobiol. 2011;49(2):357–362. doi: 10.5603/fhc.2011.0049. [DOI] [PubMed] [Google Scholar]
- 12.Nahar S, Iraha A, Hokama A, et al. Evaluation of a multiplex PCR assay for detection of cytomegalovirus in stool samples from patients with ulcerative colitis. World J Gastroenterol. 2015;21(44):12667–12675. doi: 10.3748/wjg.v21.i44.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domènech E, Vega R, Ojanguren I, et al. Azathioprine without oral ciclosporin in the long-term maintenance of remission induced by intravenous ciclosporin in severe, steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2008;14(10):1373–1379. [Google Scholar]
- 14.Sipponen T, Turunen U, Lautenschlager I, Nieminen U, Arola J, Halme L. Human herpesvirus 6 and cytomegalovirus in ileocolonic mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2011;46(11):1324–1333. doi: 10.3109/00365521.2011.605466. [DOI] [PubMed] [Google Scholar]
- 15.Knösel T, Schewe C, Petersen N, Dietel M, Petersen I. Prevalence of infectious pathogens in Crohn’s disease. Pathol Res Pract. 2009;205(4):223–230. doi: 10.1016/j.prp.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Lavagna A, Bergallo M, Daperno M, et al. The hazardous burden of Herpesviridae in inflammatory bowel disease: The case of refractory severe ulcerative colitis. Dig Liver Dis. 2006;38(12):887–893. doi: 10.1016/j.dld.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Dimitroulia E, Spanakis N, Konstantinidou AE, Legakis NJ, Tsakris A. Frequent detection of cytomegalovirus in the intestine of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(9):879–884. doi: 10.1097/01.mib.0000231576.11678.57. [DOI] [PubMed] [Google Scholar]
- 18.Van Kruiningen HJ, Poulin M, Garmendia AE, et al. Search for evidence of recurring or persistent viruses in Crohn’s disease. APMIS. 2007;115(8):962–968. doi: 10.1111/j.1600-0463.2007.apm_564.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuwabara A, Okamoto H, Suda T, Ajioka Y, Hatakeyama K. Clinicopathologic characteristics of clinically relevant cytomegalovirus infection in inflammatory bowel disease. J Gastroenterol. 2007;42(10):823–829. doi: 10.1007/s00535-007-2103-3. [DOI] [PubMed] [Google Scholar]
- 20.Ciccocioppo R, Racca F, Paolucci S, et al. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: Need for mucosal viral load measurement. World J Gastroenterol. 2015;21(6):1915–1926. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criscuoli V, Rizzuto MR, Gallo E, Orlando A, Cottone M. Toxic megacolon and human cytomegalovirus in a series of severe ulcerative colitis patients. J Clin Virol. 2015;66:103–106. doi: 10.1016/j.jcv.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Taherkhani R, Farshadpour F, Makvandi M, et al. Determination of cytomegalovirus prevalence and glycoprotein B genotypes among ulcerative colitis patients in Ahvaz, Iran. Jundishapur J Microbiol. 2015;8(2):e17458. doi: 10.5812/jjm.17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thörn M, Rorsman F, Rönnblom A, et al. Active cytomegalovirus infection diagnosed by real-time PCR in patients with inflammatory bowel disease: a prospective, controlled observational study*. Scand J Gastroenterol. 2016;51(9):1075–1080. doi: 10.3109/00365521.2016.1156154. [DOI] [PubMed] [Google Scholar]
- 24.Lévêque N, Brixi-Benmansour H, Reig T, et al. Low frequency of cytomegalovirus infection during exacerbations of inflammatory bowel diseases. J Med Virol. 2010;82(10):1694–1700. doi: 10.1002/jmv.21877. [DOI] [PubMed] [Google Scholar]
- 25.Wada Y, Matsui T, Matake H, et al. Intractable ulcerative colitis caused by cytomegalovirus infection: A prospective study on prevalence, diagnosis, and treatment. Dis Colon Rectum. 2003;46(Suppl 10):S59–S65. doi: 10.1097/01.DCR.0000087486.21981.C6. [DOI] [PubMed] [Google Scholar]
- 26.Kambham N, Vij R, Cartwright CA, Longacre T. Cytomegalovirus infection in steroid-refractory ulcerative colitis: A Case-Control Study. Am J Surg Pathol. 2004;28(3):365–373. doi: 10.1097/00000478-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Maconi G, Colombo E, Zerbi P, et al. Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig Liver Dis. 2005;37(6):418–423. doi: 10.1016/j.dld.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Maher MM, Nassar MI. Acute cytomegalovirus infection is a risk factor in refractory and complicated inflammatory bowel disease. Dig Dis Sci. 2009;54(11):2456–2462. doi: 10.1007/s10620-008-0639-6. [DOI] [PubMed] [Google Scholar]
- 29.Criscuoli V, Rizzuto MR, Montalbano L, Gallo E, Cottone M. Natural history of cytomegalovirus infection in a series of patients diagnosed with moderate-severe ulcerative colitis. World J Gastroenterol. 2011;17(5):633–638. doi: 10.3748/wjg.v17.i5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurdy JD, Enders FT, Jones A, et al. Detection of cytomegalovirus in patients with inflammatory bowel disease: where to biopsy and how many biopsies? Inflamm Bowel Dis. 2015;21(12):2833–2838. doi: 10.1097/MIB.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 31.Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol. 2001;96(3):773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 32.Criscuoli V, Casà A, Orlando A, et al. Severe acute colitis associated with CMV: A prevalence study. Digest Liver Dis. 2004;36(12):818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Ormeci AC, Akyuz F, Baran B, et al. Steroid-refractory inflammatory bowel disease is a risk factor for CMV infection. Eur Rev Med Pharm Sci. 2016;20(5):858–865. [PubMed] [Google Scholar]
- 34.Ciccocioppo R, Racca F, Scudeller L, et al. Differential cellular localization of Epstein-Barr virus and human cytomegalovirus in the colonic mucosa of patients with active or quiescent inflammatory bowel disease. Immunol Res. 2016;64(1):191–203. doi: 10.1007/s12026-015-8737-y. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Kim YH, Kim JS, et al. The prevalence and efficacy of ganciclovir on steroid-refractory ulcerative colitis with cytomegalovirus infection: a prospective multicenter study. J Clin Gastroenterol. 2012;46(1):51–56. doi: 10.1097/MCG.0b013e3182160c9c. [DOI] [PubMed] [Google Scholar]
- 36.Cho BS, Yahng SA, Kim JH. Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy. Ann Hematol. 2013;92(4):497–504. doi: 10.1007/s00277-012-1632-x. [DOI] [PubMed] [Google Scholar]
- 37.Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis; report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- 38.Papadakis KA, Tung JK, Binder SW, et al. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001;96(7):2137–2142. doi: 10.1111/j.1572-0241.2001.03949.x. [DOI] [PubMed] [Google Scholar]
- 39.Kim YS, Kim YH, Kim JS, et al. IBD Study Group of the Korean Association for the Study of Intestinal Diseases(KASID), Korea Cytomegalovirus infection in patients with new onset ulcerative colitis: a prospective study. Hepatogastroenterology. 2012;59(116):1098–1101. doi: 10.5754/hge10217. [DOI] [PubMed] [Google Scholar]
- 40.Kishore J, Ghoshal UC, Krishnani N, Kumar S, Singh M, Ayyagari A. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical signifi cance and outcome. J Med Microbiol. 2004;53(Pt 11):1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- 41.Ayre K, Warren BF, Jeffery K, Travis SP. The role of CMV in steroid-resistant ulcerative colitis: A systematic review. J Crohns Colitis. 2009;3(3):141–148. doi: 10.1016/j.crohns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Wu XW, Wu L, Ji HZ, Wang FY. Relationship between cytomegalovirus infection and steroid resistance in inflammatory bowel disease: a meta-analysis. Dig Dis Sci. 2015;60(11):3203–3208. doi: 10.1007/s10620-015-3733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]