Abstract
Cognitive decline is one of the major causes of disability among the aging population. The aim of this study was to explore the relationship between oral health parameters (number of teeth, chewing ability, and presence of a denture) and cognitive function in the elderly across the UAE. Fifty persons (age ≥ 60; 71.26 ± 10.23) were enrolled in the study. Cognitive status was assessed using the standardized mini-mental state examination (SMMSE) and accordingly, cognitively normal subjects scoring ≥24 were considered as the control group and cognitively impaired individuals scoring ≤23 were considered as the low scoring group. Chewing ability was examined, number of teeth was noted, and demographical data was collected. The results of this pilot study showed that individuals with low SMMSE scores were significantly less educated (P < 0.01) and had fewer number of remaining teeth (P < 0.05) and impaired chewing ability (P < 0.05). These results demonstrate a significant link between the number of teeth, chewing ability, and cognitive function. However, this pilot study had its limitations and was the first of its kind in the UAE and Gulf region; therefore, future research addressing the limitations is needed to further explore this association.
1. Introduction
On a global scale, according to the United Nations, by mid-century, the number of people over 60 years will represent 32% of the world population [1]. Considering the UAE, the proportion of elderly persons aged 60 years and above in the year 2000 was 5.1% and is expected to increase to 23.6% by 2025 [2]. The UAE provides a unique population in which the UAE nationals make up 11.6% of the population while the other 88.4% are expatriates [3], many of whom have been born in the UAE or have lived there for generations. Thus, it provides a controlled environment in which we can study both UAE nationals and expatriates. This, in addition to the rise in life expectancy from 74 years to 78 years [2], means there is an increased need to address age-related chronic diseases within the UAE.
Dementia, a neurocognitive disorder, is a broad term used to describe a range of symptoms associated with a decline in cognitive function and is one of the most common age-associated diseases. It is characterized by memory loss, neurological symptoms, disorientation, impaired judgement, personality changes, and loss of motor function [4]. Diagnosing dementia remains a challenge for physicians due to the symptom overlap with many other conditions such as depression, vitamin deficiencies, and thyroid dysfunction [5]. There is no cure for dementia and treatment remains to address the patients' symptoms and attempts to improve their quality of life and that of their families. Therefore, an understanding of early decline in cognitive function and the associated predisposing risk factors is becoming more important, shifting the focus towards prevention and delayed onset of disease.
Among the literature, links have been established between certain systemic factors such as diabetes [6, 7] and cardiovascular diseases [8] and their bidirectional association with poor dental and periodontal health. The links are increasingly being investigated as a possible means to address risk factors. Accordingly, the potential relationship between oral health and cognitive function has become a topic of interest. A wide range of studies have been conducted to assess the link between oral health conditions such as tooth loss [5, 9–19], impaired chewing ability [6, 20–26], and the absence of a denture [27, 28] in relation to cognitive impairment. The rationale is based upon the sensory and motor cortical remapping hypothesis, relating tooth loss and impaired masticatory ability to neuroanatomical and chemical changes that occur in the brain due to the reduction in sensory input and cortical blood flow [25, 26]. Additionally, studies have linked dental status and the use of dentures to improved nutritional intake [27, 29–38] and have established a relationship between low dietary intake of omega-3 fatty acids [38, 40], antioxidants [41, 42], and vitamin B12 [40–42] and an increased risk for cognitive decline [43–45]. Systemic factors such as hypertension [46–48], obesity [49, 50], and hypercholesterolemia [39] were also found to be risk factors. Periodontal disease and periodontal inflammatory blood markers have also been investigated in relation to cognitive decline [51].
To the best of our knowledge, no similar studies have been conducted within the Middle East or Gulf region. Therefore, the objectives of this pilot study are to (1) examine the relationship between the number of remaining teeth and cognitive ability, (2) examine the relationship between chewing status and cognitive ability, (3) examine the relationship between the presence of a denture and cognitive ability, and (4) test whether other demographic characteristics may be related to cognitive function.
2. Materials and Method
2.1. Participants
The Medical Ethics Committee of the University of Sharjah, UAE, approved data collection for this study. The sample consisted of 50 participants, 25 males and 25 females, all 60 years and above within the UAE. Written informed consent was obtained from each of the participants prior to their involvement in the study. The exclusion criteria included any disorders interfering with psychometric assessment such as severe blindness or terminal illness and/or conditions such as depression or history of cerebrovascular accident.
2.2. Assessment of Cognitive Mental Status
The Standardized Mini-Mental State Examination test (SMMSE) [52] was translated into Arabic using a forward-backward approach and was used to measure participants' global cognitive status. The SMMSE (score range 0–30) is the most common instrument used as a screening tool for cognitive function. It tests orientation, registration, short-term memory, language use, comprehension, and basic motor skills. According to the examination guidelines, participants were considered part of the low scoring group at a score of 23 or below, while those scoring 24 and above represented normal cognitive function (SMMSE ≤ 23 or SMMSE ≥ 24, resp.).
2.3. Assessment of Oral Health Parameters
Three parameters were explored and data was collected for each participant, number of teeth present, presence/absence of denture, and chewing ability using the Index of Chewing Ability (ICA) [53] which was translated into Arabic using a forward-backward approach. The ICA consists of five yes/no questions (score range 0–5) based on the ability to chew certain foods. Accordingly, those scoring less than 5 were considered to have impaired chewing ability while a score of 5 indicated competent chewing ability (ICA ≤ 4 or ICA = 5, resp.).
2.4. Other Recorded Variables
Questionnaires were administered through interviews to collect the data. Demographic variables (age, gender, and education level), lifestyle variables (smoking status), and the presence of chronic medical diseases such as cancer, cerebrovascular disease, myocardial infarction, diabetes mellitus, and hypertension were noted.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS® Ver. 24.0 for Mac OS X. Clinical variables were analysed using independent t-tests and the chi-square test. The Pearson correlation was conducted to analyse correlations between cognitive impairment, dental health status, chewing ability, and denture presence. Two-tailed P values were calculated in all the analyses. Differences were considered statistically significant at P < 0.05.
3. Results
The subjects were divided into two groups according to their SMMSE scores (low scoring, SMMSE ≤ 23, n = 31 or SMMSE ≥ 24, n = 19; control). Table 1 shows the demographic characteristics of the subjects included in the study and the summary statistics of oral health variables and SMMSE scores are listed in Table 2. The subjects with low SMMSE scores were found to be significantly less educated, had fewer remaining teeth, and, according to the ICA, had impaired chewing ability. It was also noted among nationals. No other differences were observed in all the other characteristics.
Table 1.
Demographic characteristics of the subjects.
|
|
Control | Low SMMSE score | Pearson's r | P value |
|---|---|---|---|---|
| n = 19 | n = 31 | |||
| Age | 67.7 ± 5.4 | 73.5 ± 11.8 | −0.277 | 0.052∗ |
| Gender | ||||
| Male | 11 (57.9) | 14 (45.2) | 0.124 | 0.382† |
| Female | 8 (42.1) | 17 (54.8) | ||
| Nationality | ||||
| UAE | 4 (16.0) | 21 (84.0) | −0.453 | 0.001† |
| Expatriate | 15 (60.0) | 10 (40.0) | ||
| Education | ||||
| Uneducated | 3 (10.3) | 24 (16.7) | 0.600 | 0.001† |
| Educated | 16 (8.7) | 1 7 (14.3) | ||
| Health | ||||
| Hypertension | 7 (30.4) | 16 (69.6) | −0.144 | 0.387† |
| Diabetes | 7 (29.2) | 17 (70.8) | −0.175 | 0.255† |
| Smoking | ||||
| Nonsmoker | 4 (21.1) | 15 (16.0) | 0.108 | 0.459† |
| Smoker | 4 (5.0) | 27 (26.0) |
SMMSE: Standardized mini-mental state examination; †Chi Square, as no., %; ∗Unpaired T-Test, as mean, standard deviation.
Table 2.
Summary statistics of oral health variables and SMMSE scores.
| Control | Low SMMSE score | Pearson's r | P value | |
|---|---|---|---|---|
| n = 19 | n = 31 | |||
| Teeth remaining | ||||
| (0–10) | 3 (13.6) | 19 (86.4) | 0.465 | 0.04† |
| (11–21) | 5 (45.5) | 6 (54.5) | ||
| (22–32) | 11 (64.7) | 6 (35.3) | ||
| Chewing ability | ||||
| Competent | 13 (52.4) | 11 (45.8) | 0.320 | 0.04† |
| Impaired | 6 (23.1) | 20 (76.9) | ||
| Denture | ||||
| Present | 2 (28.6) | 5 (71.4) | 0.078 | 0.695† |
| Absent | 17 (39.5) | 26 (60.5) |
SMMSE: Standardized mini-mental state examination; †Chi Square, as no., %.
Figure 1 shows the prevalence of a normal or low SMMSE score and the number of teeth remaining. The number of remaining teeth (range: 0–32) was categorized into 3 (22–32, 11–21, and 0–10). The number of remaining teeth was significantly associated with low SMMSE scores (r = +0.465, P < 0.05). The prevalence of low SMMSE score was 86.4% in subjects with 0–10 remaining teeth, 54.5% in those with 11–21 teeth remaining, and 35.3% in those with 22–32 remaining teeth.
Figure 1.
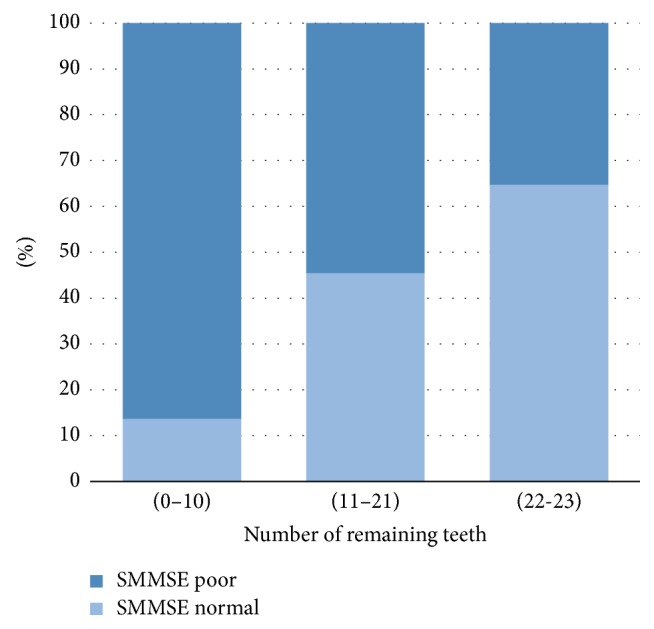
Prevalence of normal SMMSE or low SMMSE score according to the number of remaining teeth.
Figure 2 shows the prevalence of a normal or low SMMSE score and chewing ability. Individuals' chewing ability was classified as either competent or impaired. A significant association was observed between chewing ability and the prevalence of low SMMSE scores (r = 0.320, P < 0.05). The prevalence of low SMMSE score was 45.8% in persons with competent chewing ability and 76.9% in those with impaired chewing ability.
Figure 2.
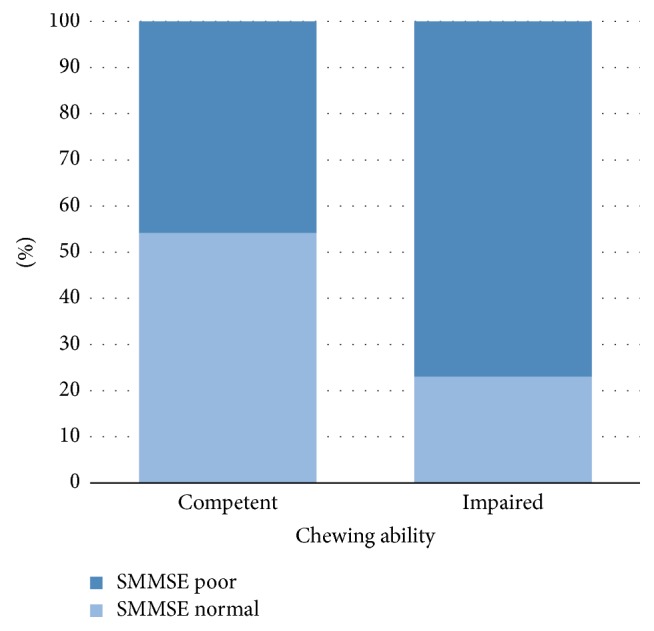
Prevalence of normal SMMSE or low SMMSE score according to chewing ability.
4. Discussion
This cross-sectional pilot study was designed to explore the relationship between number of remaining teeth, chewing ability, denture presence, and cognitive function in an elderly UAE population.
The study revealed that the prevalence of a low SMMSE score or poor cognition was significantly greater in association with fewer teeth remaining (P < 0.05) (Figure 1). These results were consistent with the literature supporting the association between tooth loss and decreased cognitive function [5, 9–19]. According to Stewart and Hirani (2007) the localized inflammatory reaction associated with periodontal disease could lead to a state of chronic low-grade systemic infection and an increase in the cytokines reaching the brain [16]. Moreover, it has been noted that individuals with fewer teeth are at a greater risk of developing nutritional deficiencies, especially B vitamins, which play a major role in the pathogenesis of dementia and cognitive decline [5, 40–42]. According to animal models studies, loss of teeth was associated with neuroanatomical and chemical changes that may eventually have a negative effect on learning and memory [18, 19]. It is important to consider the likelihood of a reversed casualty, whereby individuals with poor cognition may have a lower ability to exact oral hygiene measures, such as tooth brushing and denture care, which ultimately leads to poor oral health [54, 55].
Additionally, a greater prevalence of low SMMSE scores was observed in persons with impaired chewing ability (P < 0.05) (Figure 2). With the use of functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), multiple studies have observed an increase in cortical blood flow [20–26] and a rise in oxygen level in the prefrontal cortex and hippocampus [6, 9] during mastication. Chewing has also been noted to stimulate increased cardiac activity, suggesting greater sympathetic stimulation, which increases blood glucose levels and arousal when undertaking a cognitive task [25, 26]. Furthermore, according to Teixeira et al. there was an increased performance in relation to memory retrieval when elderly people aged 60–70 years used chewing gum [26]. In conjunction with the former, animal model studies revealed a causal relationship; occlusal hypofunction caused degenerative changes in periodontal mechanoreceptors, which in effect lead to the suppression of sensory stimulation from the periodontal ligaments during mastication and poor performance in memory and learning tests [56–59].
Although a lower number of remaining teeth and decreased chewing ability were significantly correlated with lower SMMSE scores, it must be noted that individuals from the elderly population usually present with other risk factors which may have affected their cognitive function. Hence, the decreased number of remaining teeth and impaired chewing ability may not alone lead to cognitive decline but may be markers of comorbidities [60, 61].
In this pilot study, no significant relationship was found between denture presence and cognitive function (P > 0.05), which may be justified by the insignificant number of denture wearing individuals within the study sample.
A significant difference in SMMSE scores was noted between nationals and expatriates (P > 0.05), which may be related to the nationals' difficulty in accessing schools until the late 1950's [62].
Education was significantly correlated with higher SMMSE scores (P < 0.05). These results are in accordance with the cognitive reserve hypothesis, which assumes some aspects of life experiences such as education and knowledge act as markers of cognitive reserve and protect against age-associated cognitive decline in later life [6].
Limitations of the present study merit consideration. The primary limitation arises due to the nature of cross-sectional study; hence a casual inference is difficult to make regarding the relationship between teeth remaining, chewing ability, denture presence, and poor cognitive function. Future studies with longitudinal designs would help investigate these associations. Additionally, cognitive function was assessed using only the SMMSE and although it is the most widely used means for examining cognitive function, the education level of an individual may influence it. Finally, due to the small sample size and since most of the participants were volunteers residing in elderly homes, the results of this pilot study may not represent the general population.
5. Conclusion
In conclusion, this pilot study revealed a significant finding whereby persons with fewer number of remaining teeth and impaired chewing ability demonstrated poor cognitive ability. The results draw attention to the possible role a person's oral health may play on their cognitive function. However, the interpretation of the results was hindered by the lack of longitudinal observation and the use of a single examination for cognitive function. Therefore, future research investigating the associations is required and the limitations need to be addressed.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.United Nations. World population ageing 1950–2050. New York: United Nations, 2007.
- 2.Alshaali A., Al Jaziri A. Health Profile of Elderly Patients Registered in the Elderly Home Based Primary Care, Dubai, United Arab Emirates. Middle East Journal of Age and Ageing. 2015;12(1):13–19. doi: 10.5742/MEAA.2015.92609. [DOI] [Google Scholar]
- 3.The World Factbook (n.d.) https://www.cia.gov/library/publications/resources/the-world-factbook/geos/print_ae.html.
- 4.Chertkow H., Feldman H. H., Jacova C., Massoud F. Definitions of dementia and predementia states in Alzheimer's disease and vascular cognitive impairment: Consensus from the Canadian conference on diagnosis of dementia. Alzheimer’s Research & Therapy. 2013;5(1, article no. S2) doi: 10.1186/alz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito Y., Sugawara N., Yasui-Furukori N., Takahashi I., Nakaji S., Kimura H. Cognitive function and number of teeth in a community-dwelling population in Japan. Annals of General Psychiatry. 2013;12(1, article no. 20) doi: 10.1186/1744-859X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mummolo S., Ortu E., Necozione S., Monaco A., Marzo G. Relationship between mastication and cognitive function in elderly in L'Aquila. International Journal of Clinical and Experimental Medicine. 2014;7(4):1040–1046. [PMC free article] [PubMed] [Google Scholar]
- 7.Lamster I. B., Lalla E., Borgnakke W. S., Taylor G. W. The relationship between oral health and diabetes mellitus. The Journal of the American Dental Association. 2008;139(10):19–24. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 8.Grudyanov A. I., Tkacheva O. N., Avraamova T. V. Correlation of chronic periodontal disease and cardiovascular disease. Stomatologiya. 2017;96(1):p. 4. doi: 10.17116/stomat20179614-7. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto N., Morikawa M., Okamoto K., et al. Relationship of tooth loss to mild memory impairment and cognitive impairment: findings from the fujiwara-kyo study. Behavioral and Brain Functions. 2010;6, article 77 doi: 10.1186/1744-9081-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes-Ortiz C. A., Luque J. S., Eriksson C. K., Soto L. Self-reported tooth loss and cognitive function: Data from the Hispanic established populations for epidemiologic studies of the elderly (Hispanic EPESE) Colombia Médica. 2013;44(3):139–145. [PMC free article] [PubMed] [Google Scholar]
- 11.Stein P. S., Desrosiers M., Donegan S. J., Yepes J. F., Kryscio R. J. Tooth loss, dementia and neuropathology in the Nun Study. The Journal of the American Dental Association. 2007;138(10):1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 12.Sabbah W., Sheiham A. The relationships between cognitive ability and dental status in a national sample of USA adults. Intelligence. 2010;38(6):605–610. doi: 10.1016/j.intell.2010.08.003. [DOI] [Google Scholar]
- 13.Park H., Suk S.-H., Cheong J.-S., et al. Tooth loss may predict poor cognitive function in community-dwelling adults without dementia or stroke: The PRESENT project. Journal of Korean Medical Science. 2013;28(10):1518–1521. doi: 10.3346/jkms.2013.28.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsakos G., Watt R. G., Rouxel P. L., De Oliveira C., Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. Journal of the American Geriatrics Society. 2015;63(1):91–99. doi: 10.1111/jgs.13190. [DOI] [PubMed] [Google Scholar]
- 15.Naorungroj S., Schoenbach V. J., Wruck L., et al. Tooth loss, periodontal disease, and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) study. Community Dentistry and Oral Epidemiology. 2015;43(1):47–57. doi: 10.1111/cdoe.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart R., Hirani V. Dental health and cognitive impairment in an english national survey population. Journal of the American Geriatrics Society. 2007;55(9):1410–1414. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 17.Bergdahl M., Habib R., Bergdahl J., Nyberg L., Nilsson L.-G. Natural teeth and cognitive function in humans. Scandinavian Journal of Psychology. 2007;48(6):557–565. doi: 10.1111/j.1467-9450.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 18.Onozuka M., Watanabe K., Fujita M., Tomida M., Ozono S. Changes in the septohippocampal cholinergic system following removal of molar teeth in the aged SAMP8 mouse. Behavioural Brain Research. 2002;133(2):197–204. doi: 10.1016/S0166-4328(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 19.Kato T., Usami T., Noda Y., Hasegawa M., Ueda M., Nabeshima T. The effect of the loss of molar teeth on spatial memory and acetylcholine release from the parietal cortex in aged rats. Behavioural Brain Research. 1997;83(1-2):239–242. doi: 10.1016/S0166-4328(97)86078-0. [DOI] [PubMed] [Google Scholar]
- 20.Momose T., Nishikawa J., Watanabe T., et al. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Archives of Oral Biolog. 1997;42(1):57–61. doi: 10.1016/S0003-9969(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 21.Ono Y., Yamamoto T., Kubo K., Onozuka M. Occlusion and brain function: mastication as a prevention of cognitive dysfunction. Journal of Oral Rehabilitation. 2010;37(8):624–640. doi: 10.1111/j.1365-2842.2010.02079.x. [DOI] [PubMed] [Google Scholar]
- 22.Onozuka M., Fujita M., Watanabe K., et al. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. Journal of Dental Research. 2002;81(11):743–746. doi: 10.1177/154405910208101104. [DOI] [PubMed] [Google Scholar]
- 23.Onozuka M., Hirano Y., Tachnibana A., Kim W., Ono Y., Sasaguri K., et al. Interactions between chewing and brain activities in humans. In: Onozuka M., Yen C. T., editors. Novel trends in brain science. Tokyo: Springer; 2007. pp. 99–113. [Google Scholar]
- 24.Hirano Y., Obata T., Kashikura K., et al. Effects of chewing in working memory processing. Neuroscience Letters. 2008;436(2):189–192. doi: 10.1016/j.neulet.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya K., Narita N., Iwaki S., Bencharit S. Improved Prefrontal Activity and Chewing Performance as Function of Wearing Denture in Partially Edentulous Elderly Individuals: Functional Near-Infrared Spectroscopy Study. PLoS ONE. 2016;11(6):p. e0158070. doi: 10.1371/journal.pone.0158070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira F., Melo Pereira Fernandes de., Noronha L. P., et al. Masticatory Deficiency as a Risk Factor for Cognitive Dysfunction. Proceedings of the International Journal Of Medical Sciences; 2014; pp. 209–214. http://dx.doi.org/10.7150/ijms.6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall T. A., Warren J. J., Hand J. S., Xie X.-J., Stumbo P. J. Oral health, nutrient intake and dietary quality in the very old. The Journal of the American Dental Association. 2002;133(10):1369–1379. doi: 10.14219/jada.archive.2002.0052. [DOI] [PubMed] [Google Scholar]
- 28.Listl S. Oral health conditions and cognitive functioning in middle and later adulthood. BMC Oral Health. 2014;14(1, article no. 70) doi: 10.1186/1472-6831-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papas A. S., Palmer C. A., Rounds M. C., Russell R. M. The effects of denture status on nutrition. Special Care in Dentistry. 1998;18(1):17–25. doi: 10.1111/j.1754-4505.1998.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 30.Fontijn-Tekamp F. A., vant Hof M. A., Slater A. P., van Waas M. A. The state of dentition in relation to nutrition in elderly Europeans in the SENECA study of 1993. European Journal of Clinical Nutrition. 1993:S117–22. [PubMed] [Google Scholar]
- 31.Hutton B., Feine J., Morais J. Is there an association between edentulism and nutritional state? Journal of the Canadian Dental Association. 2002;68(3):182–187. [PubMed] [Google Scholar]
- 32.Cousson P. Y., Bessadet M., Nicolas E., Veyrune J.-L., Lesourd B., Lassauzay C. Nutritional status, dietary intake and oral quality of life in elderly complete denture wearers. Gerodontology. 2012;29(2):e685–e692. doi: 10.1111/j.1741-2358.2011.00545.x. [DOI] [PubMed] [Google Scholar]
- 33.McKenna G., Allen P. F., Flynn A., et al. Impact of tooth replacement strategies on the nutritional status of partially-dentate elders. Gerodontology. 2012;29(2):e883–e890. doi: 10.1111/j.1741-2358.2011.00579.x. [DOI] [PubMed] [Google Scholar]
- 34.Prakash N., Kalavathy N., Sridevi J., Premnath K. Nutritional status assessment in complete denture wearers. Gerodontology. 2012;29(3):224–230. doi: 10.1111/j.1741-2358.2011.00620.x. [DOI] [PubMed] [Google Scholar]
- 35.Sheiham A., Steele J. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutrition. 2001;4(3):797–803. doi: 10.1079/PHN2000116. [DOI] [PubMed] [Google Scholar]
- 36.Nurk E., Drevon C. A., Refsum H., et al. Cognitive performance among the elderly and dietary fish intake: The Hordaland Health Study. American Journal of Clinical Nutrition. 2007;86(5):1470–1478. doi: 10.1093/ajcn/86.5.1470. [DOI] [PubMed] [Google Scholar]
- 37.van Gelder B. M., Tijhuis M., Kalmijn S., Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen elderly study. American Journal of Clinical Nutrition. 2007;85(4):1142–1147. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 38.Kim J.-M., Stewart R., Prince M., et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. International Journal of Geriatric Psychiatry. 2007;22(9):850–855. doi: 10.1002/gps.1750. [DOI] [PubMed] [Google Scholar]
- 39.Zambón D., Quintana M., Mata P., Alonso R., Benavent J., Cruz-Sánchez F., et al. Higher incidence of mild cognitive impairment in familial hypercholesterolemia. The American Journal of Medicine. 2010;123(3):267–274. doi: 10.1016/j.amjmed.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker K. L., Qiao N., Scott T., Rosenberg I., Spiro A., III High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. American Journal of Clinical Nutrition. 2005;82(3):627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 41.Kim H., Kim G., Jang W., Kim S. Y., Chang N. Association between intake of B vitamins and cognitive function in elderly Koreans with cognitive impairment. Nutrition Journal. 2014;13(1, article 118):1–11. doi: 10.1186/1475-2891-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeill G., Jia X., Whalley L. J., et al. Antioxidant and B vitamin intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. European Journal of Clinical Nutrition. 2011;65(5):619–626. doi: 10.1038/ejcn.2011.2. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez L. J., Barbagallo M. The relevance of nutrition for the concept of cognitive frailty. Current Opinion in Clinical Nutrition & Metabolic Care. 2017;20(1):61–68. doi: 10.1097/MCO.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 44.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nature Reviews Neuroscience. 2008;9(7):568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Pinilla F., Tyagi E. Diet and cognition: Interplay between cell metabolism and neuronal plasticity. Current Opinion in Clinical Nutrition & Metabolic Care. 2013;16(6):726–733. doi: 10.1097/MCO.0b013e328365aae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faraco G., Iadecola C. Hypertension: A harbinger of stroke and dementia. Hypertension. 2013;62(5):810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ninomiya T., Ohara T., Hirakawa Y., et al. Midlife and late-life blood pressure and dementia in japanese elderly: the hisayama study. Hypertension. 2011;58(1):22–28. doi: 10.1161/HYPERTENSIONAHA.110.163055. [DOI] [PubMed] [Google Scholar]
- 48.Köhler S., Baars M. A. E., Spauwen P., Schievink S., Verhey F. R. J., Van Boxtel M. J. P. Temporal evolution of cognitive changes in incident hypertension: Prospective cohort study across the adult age span. Hypertension. 2014;63(2):245–251. doi: 10.1161/HYPERTENSIONAHA.113.02096. [DOI] [PubMed] [Google Scholar]
- 49.Atti A. R., Palmer K., Volpato S., Winblad B., De Ronchi D., Fratiglioni L. Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. Journal of the American Geriatrics Society. 2008;56(1):111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 50.Kivipelto M., Ngandu T., Fratiglioni L., et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. JAMA Neurology. 2005;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 51.Noble J. M., Borrell L. N., Papapanou P. N., Elkind M. S. V., Scarmeas N., Wright C. B. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(11):1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molloy D. W., Standish T. I. M. A guide to the standardized Mini-Mental State Examination. International Psychogeriatrics. 1997;9(1):87–94. doi: 10.1017/S1041610297004754. [DOI] [PubMed] [Google Scholar]
- 53.Leake J. L. An Index of Chewing Ability. Journal of Public Health Dentistry. 1990;50(4):262–267. doi: 10.1111/j.1752-7325.1990.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 54.Philip P., Rogers C., Kruger E., Tennant M. Oral hygiene care status of elderly with dementia and in residential aged care facilities. Gerodontology. 2012;29(2):e306–e311. doi: 10.1111/j.1741-2358.2011.00472.x. [DOI] [PubMed] [Google Scholar]
- 55.Chen X., Clark J. J., Chen H., Naorungroj S. Cognitive impairment, oral self-care function and dental caries severity in community-dwelling older adults. Gerodontology. 2015;32(1):53–61. doi: 10.1111/ger.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakata M. Masticatory function and its effects on general health. International Dental Journal. 1998;48(6):540–548. doi: 10.1111/j.1875-595X.1998.tb00489.x. [DOI] [PubMed] [Google Scholar]
- 57.Ekuni D., Tomofuji T., Irie K., et al. Occlusal disharmony increases amyloid-β in the rat hippocampus. NeuroMolecular Medicine. 2011;13(3):197–203. doi: 10.1007/s12017-011-8151-0. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T., Hirayama A. Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Research. 2001;902(2):255–263. doi: 10.1016/S0006-8993(01)02410-6. [DOI] [PubMed] [Google Scholar]
- 59.Muramoto T., Takano Y., Soma K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Archives of Histology and Cytology. 2000;63(4):369–380. doi: 10.1679/aohc.63.369. [DOI] [PubMed] [Google Scholar]
- 60.Bunn F., Burn A.-M., Goodman C., et al. Comorbidity and dementia: A scoping review of the literature. BMC Medicine. 2014;12(1, article no. 192) doi: 10.1186/s12916-014-0192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Formiga F., Fort I., Robles M. J., et al. Comorbidity and clinical features in elderly patients with dementia: differences according to dementia severity. The Journal of Nutrition, Health & Aging. 2009;13(5):423–427. doi: 10.1007/s12603-009-0078-x. [DOI] [PubMed] [Google Scholar]
- 62.Alhebsi A., Pettaway L. D., Waller L. A history of education in the United Arab Emirates and trucial sheikdoms. The Global eLearning Journal. 2015;4(1) [Google Scholar]


