ABSTRACT
Vegetable seeds contaminated with bacterial pathogens have been linked to fresh-produce-associated outbreaks of gastrointestinal infections. This study was undertaken to observe the physiological behavior of Salmonella enterica and enterohemorrhagic Escherichia coli (EHEC) cells artificially internalized into vegetable seeds during the germination process. Surface-decontaminated seeds of alfalfa, fenugreek, lettuce, and tomato were vacuum-infiltrated with four individual strains of Salmonella or EHEC. Contaminated seeds were germinated at 25°C for 9 days, and different sprout/seedling tissues were microbiologically analyzed every other day. The internalization of Salmonella and EHEC cells into vegetable seeds was confirmed by the absence of pathogens in seed-rinsing water and the presence of pathogens in seed homogenates after postinternalization seed surface decontamination. Results show that 317 (62%) and 343 (67%) of the 512 collected sprout/seedling tissue samples were positive for Salmonella and EHEC, respectively. The average Salmonella populations were significantly larger (P < 0.05) than the EHEC populations. Significantly larger Salmonella populations were recovered from the cotyledon and seed coat tissues, followed by the root tissues, but the mean EHEC populations from all sampled tissue sections were statistically similar, except in pregerminated seeds. Three Salmonella and two EHEC strains had significantly larger cell populations on sprout/seedling tissues than other strains used in the study. Salmonella and EHEC populations from fenugreek and alfalfa tissues were significantly larger than those from tomato and lettuce tissues. The study showed the fate of internalized human pathogens on germinating vegetable seeds and sprout/seedling tissues and emphasized the importance of using pathogen-free seeds for sprout production.
IMPORTANCE The internalization of microorganisms into vegetable seeds could occur naturally and represents a possible pathway of vegetable seed contamination by human pathogens. The present study investigated the ability of two important bacterial pathogens, Salmonella and enterohemorrhagic Escherichia coli (EHEC), when artificially internalized into vegetable seeds, to grow and disseminate along vegetable sprouts/seedlings during germination. The data from the study revealed that the pathogen cells artificially internalized into vegetable seeds caused the contamination of different tissues of sprouts/seedlings and that pathogen growth on germinating seeds is bacterial species and vegetable seed-type dependent. These results further stress the necessity of using pathogen-free vegetable seeds for edible sprout production.
KEYWORDS: alfalfa, EHEC, fenugreek, lettuce, Salmonella, seedlings, sprouts, tomato, vegetable seeds
INTRODUCTION
The consumption of vegetable seed sprouts has become popular worldwide in recent decades due to the shift in consumers' preferences to more healthy and nutritious foods. However, the seed germination process has made sprouts highly susceptible to microbial contamination; thus, concerns have been raised about the safety of seeds used for sprout production (1). The contamination of sprout seeds by Salmonella enterica and enterohemorrhagic Escherichia coli (EHEC) has been confirmed as the cause of several sprout-associated outbreaks of infections (2). In 1995, an international outbreak in Finland and in 17 states in the United States was traced to alfalfa seeds contaminated with S. enterica subsp. enterica serovar Stanley (3). In 1997, simultaneous outbreaks of E. coli O157:H7 infections in Michigan and Virginia were linked to the consumption of alfalfa sprouts grown from the same lot of seeds (4). In 2003, two E. coli O157 outbreaks associated with alfalfa sprouts in Colorado and Minnesota were linked to a common seed source (5). In a 2009 sprouts-related multistate outbreak of S. enterica subsp. enterica serovar Saintpaul infections, seeds from a single grower were identified as the source of contamination (6).
The treatment of sprout seeds with 20,000 ppm calcium hypochlorite before germination is recommended by the U.S. Food and Drug Administration (FDA) to minimize the risk of microbial contamination (7). However, the efficacy of this sanitation protocol is highly variable due to the inability of chlorine to make contact with pathogen cells that are located in the internal spaces of vegetable seeds (8). Pathogenic bacteria can naturally infiltrate cracks, crevices, and intercellular spaces of seeds when a negative pressure is created across the seed coat by changes in environmental temperature and the hydraulic status of seed surfaces (9). The precise incidence of pathogen infiltration into vegetable seeds in the natural environment is unknown, but it is assumed to be extremely low. After reaching internal seed tissues, the fate of the pathogens during sprout and seedling production is largely unknown. Several studies have described the growth of S. enterica and E. coli during seed germination when the pathogen cells were inoculated onto the surfaces of seeds and seedlings (10–12). However, it is not yet clear if human pathogens internalized into vegetable seeds would behave in a similar fashion during germination, as the chemical and biological environments may differ within and outside the germinating seeds (13). This study was undertaken to observe the physiological behavior of selected bacterial pathogens artificially internalized into vegetable seeds during the germination process and to understand whether pathogen behaviors on various tissues of sprouting vegetable seeds is bacterial species, pathogen strain/serotype, and seed-type dependent.
RESULTS
Among the 512 sprout/seedling samples analyzed in the Salmonella or EHEC experiment, 317 (62%) tested positive for Salmonella and 343 (67%) tested positive for EHEC (detailed data not shown). Table 1 shows the mean populations of all four Salmonella or EHEC strains recovered from different sampling points, tissue sections, or vegetable seed types. The mean population of each S. enterica or EHEC strain is the mean value recovered from all four types of vegetable sprout/seedling tissues at all sampling points. It is evident that the mean Salmonella population recovered from all sprout/seedling tissue sections increased from the initial 1.45 log CFU/g to 3.20 log CFU/g at the end of a 9-day germination process, and the EHEC population increased from 0.88 log CFU/g to 2.30 log CFU/g. The average Salmonella population increased significantly from day 3 to day 5, and there was no significant change in the population before and after these two sample points. In contrast to that of Salmonella, a significant increase in the EHEC population was not observed until 7 to 9 days into the germination process. Cotyledon and seed coat tissues had the largest populations of Salmonella cells. On average, more Salmonella cells were recovered from the root tissues than from the stem tissues and pregerminated seeds. The Salmonella population from seed coat/cotyledon tissues was larger than that from pregerminated seeds but was not significantly different from the cell populations from the stem and root tissues. On average, the largest EHEC population was found on the cotyledon tissues, but this population was not significantly different from that on other sprout/seedling tissues except on pregerminated seeds (P < 0.05). Among the four Salmonella strains included in the study, S. enterica subsp. enterica serovar Montevideo had the smallest cell population, and the populations of the other three Salmonella strains were not significantly different. The average population of E. coli F4546 was significantly smaller than the populations of E. coli 4492 and E. coli 1730. The mean populations of both Salmonella and EHEC recovered from fenugreek and alfalfa sprout tissues were significantly larger (P < 0.05) than those from tomato and lettuce seedling tissues.
TABLE 1.
Overall mean populations of Salmonella enterica and EHEC recovered at different sampling points during germination and from different types and tissue sections of sprouts/seedlings
| Sampling | Mean population (log CFU/g)a |
|
|---|---|---|
| BSA or NASMACb | NATSAc | |
| Salmonella | ||
| Trial (n = 256 each) | ||
| 1 | 2.79 A | 3.00 A |
| 2 | 2.78 A | 2.95 A |
| Germination day | ||
| 9 (n = 128) | 3.20 A | 3.57 A |
| 7 (n = 128) | 3.19 A | 3.40 A |
| 5 (n = 128) | 3.13 A | 3.30 A |
| 3 (n = 96) | 1.63 B | 1.67 B |
| 1 (n = 32) | 1.45 B | 1.48 B |
| Strain (n = 128 each) | ||
| S. Stanley | 3.09 A | 3.52 A |
| S. Baildon | 3.30 A | 3.37 A |
| S. Cubana | 3.16 A | 3.24 A |
| S. Montevideo | 1.55 B | 1.75 B |
| Tissue sections of sprouts/seedlings | ||
| Cotyledon (n = 96) | 3.63 A | 3.82 A |
| Seed coat (n = 96) | 3.52 A | 3.80 A |
| Root (n = 128) | 2.69 B | 2.96 B |
| Seed coat/cotyledon (n = 32) | 2.11 B,C | 2.13 C |
| Stem (n = 128) | 2.17 C | 2.30 C |
| Pregerminated seeds (n = 32) | 1.45 D | 1.48 D |
| Vegetable seed type (n = 128 each) | ||
| Fenugreek | 4.08 A | 4.36 A |
| Alfalfa | 4.21 A | 4.30 A |
| Tomato | 1.47 B | 1.68 B |
| Lettuce | 1.35 B | 1.55 B |
| EHEC | ||
| Trial (n = 256 each) | ||
| 1 | 1.60 A | 1.93 A |
| 2 | 1.54 A | 1.89 A |
| Germination day | ||
| 9 (n = 128) | 2.30 A | 2.91 A |
| 7 (n = 128) | 1.76 B | 2.27 B |
| 5 (n = 128) | 1.43 B | 1.54 C |
| 3 (n = 96) | 1.11 B,C | 1.15 C,D |
| 1 (n = 32) | 0.88 C | 1.03 D |
| Strain (n = 128 each) | ||
| E. coli K4492 | 1.70 A | 1.91 A |
| E. coli H1730 | 1.72 A | 1.93 A |
| E. coli BAA-2326 | 1.49 A,B | 1.90 A |
| E. coli F4546 | 1.37 B | 1.91 A |
| Tissue sections of sprouts/seedlings | ||
| Cotyledon (n = 96) | 1.83 A | 2.19 A |
| Seed coat/cotyledon (n = 32) | 1.66 A | 2.18 A,B |
| Seed coat (n = 96) | 1.67 A | 1.95 A,B |
| Root (n = 128) | 1.53 AB | 1.85 A,B |
| Stem (n = 128) | 1.44 AB | 1.68 B |
| Pregerminated seeds (n = 32) | 1.11 B | 1.15 C |
| Vegetable seed type (n = 128 each) | ||
| Alfalfa | 3.31 A | 3.70 A |
| Fenugreek | 1.93 B | 2.27 B |
| Lettuce | 0.67 C | 1.54 C |
| Tomato | 0.38 C | 1.15 D |
Mean populations of the same bacterial pathogen within a column and the same comparative category that are not followed by the same letter are significantly different (P < 0.05).
BSA, bismuth sulfite agar; NASMAC, sorbitol MacConkey agar supplemented with nalidixic acid.
NATSA, tryptic soy agar supplemented with nalidixic acid.
The average cell populations of four individual Salmonella or EHEC strains from all tissue sections of each type of vegetable seeds over the 9-day germination period are summarized in Tables 2 and 3, respectively. Higher numbers of Salmonella and EHEC cells were recovered from fenugreek and alfalfa than from tomato and lettuce tissues, except E. coli F4546 (Tables 2 and 3).
TABLE 2.
Mean populations of individual Salmonella strains recovered from different types of sprouts/seedlings over the 9-day germination period
| Seed type | Mean population (log CFU/g)a |
|||
|---|---|---|---|---|
| S. Stanley | S. Baildon | S. Cubana | S. Montevideo | |
| Fenugreek | 4.44 A* | 4.02 A* | 4.12 A* | 1.59 B* |
| Alfalfa | 3.88 A* | 4.14 A* | 4.10 A* | 2.01 B* |
| Tomato | 1.49 A† | 1.32 A† | 1.18 A,B† | 0.88 B† |
| Lettuce | 0.90 B,C† | 1.99 A† | 1.27 B† | 0.67 C† |
Mean values within a column that are not followed by the same symbol (* or †) are significantly different (P < 0.05). Mean values within a row that are not followed by the same uppercase letter are significantly different (P < 0.05).
TABLE 3.
Mean populations of individual EHEC strains recovered from different types of sprouts/seedlings over the 9-day germination period
| Seed type | Mean population (log CFU/g)a |
|||
|---|---|---|---|---|
| E. coli K4492 | E. coli H1730 | E. coli BAA-2326 | E. coli F4546 | |
| Alfalfa | 2.52 B* | 2.83 B* | 1.59 C† | 3.72 A* |
| Fenugreek | 1.64 B† | 1.74 A,B† | 2.46 A* | 0.21 C† |
| Lettuce | 0.59 A,B‡ | 0.82 A‡ | 0.07 B‡ | 0.51 A,B† |
| Tomato | 0.42 A‡ | 0.00 B‡ | 0.58 A‡ | 0.06 B† |
Mean values within a column that are not followed by the same symbol (*, †, or ‡) are significantly different (P < 0.05). Mean values within a row that are not followed by the same uppercase letter are significantly different (P < 0.05).
The mean populations of S. Montevideo on fenugreek and alfalfa tissues were significantly smaller (P < 0.05) than the cell populations of the other three Salmonella strains (Table 2). On tomato seedling tissues, the average populations of S. enterica subsp. enterica serovars Stanley and Baildon were significantly larger than the population of S. Montevideo, and these three populations were not significantly different from the mean population of S. enterica subsp. enterica serovar Cubana. The average S. Baildon population on lettuce tissues was significantly larger than the populations of the other 3 Salmonella strains, and the mean population of S. Stanley was not significantly different from the average populations of S. Cubana and S. Montevideo.
E. coli BAA-2326 and F4546 had the smallest and largest cell populations, respectively, on alfalfa samples (Table 3), and the cell populations of the other two E. coli strains were not statistically different. In contrast to that from alfalfa samples, the average population of E. coli F4546 on fenugreek sprouts was significantly smaller (P < 0.05) than the other three E. coli strains. The E. coli BAA-2326 population on lettuce seedlings was significantly smaller than that of E. coli H1730, which was undetectable from tissues of tomato seedlings.
The average cell populations of all 4 Salmonella and 4 EHEC strains from different tissue sections of each type of sprout/seedling over the 9-day germination period are shown in Tables 4 and 5, respectively. Similar to the result shown in Tables 2 and 3, the average Salmonella and EHEC populations on individual tissue sections of alfalfa and fenugreek sprouts were significantly larger (P < 0.05) than those from tomato and lettuce seedlings except for the population from seed coats/cotyledons of fenugreek sprouts and tomato seedlings (Tables 4 and 5).
TABLE 4.
Mean populations of all 4 S. enterica strains recovered from different tissue sections of each type of sprouts/seedlings over the 9-day germination period
| Seed type | Mean population (log CFU/g)a |
|||||
|---|---|---|---|---|---|---|
| Cotyledon | Seed coat | Root | Seed coat/cotyledon | Stem | Pregerminated seeds | |
| Fenugreek | 4.38 A* | 4.24 A* | 3.23 B* | 2.52 B,C† | 2.61 B* | 1.59 C† |
| Alfalfa | 4.21 A* | 4.18 A* | 2.99 B* | 4.73 A* | 2.68 B* | 3.14 B* |
| Tomato | 1.59 A† | 1.21 A† | 1.56 A† | 1.14 A,B†‡ | 0.86 B† | 0.00 B‡ |
| Lettuce | 1.75 A*† | 1.44 A† | 1.45 A† | 0.00 B‡ | 0.85 B† | 0.00 B‡ |
Mean values within a column that are not followed by the same symbol (*, †, or ‡) are significantly different (P < 0.05). Mean values within a row that are not followed by the same uppercase letters are significantly different (P < 0.05).
TABLE 5.
Mean populations of all 4 EHEC strains recovered from different types of sprouts/seedlings over the 9-day germination period
| Seed type | Mean population (log CFU/g)a |
|||||
|---|---|---|---|---|---|---|
| Cotyledon | Seed coat/cotyledon | Seed coat | Root | Stem | Pregerminated seed | |
| Alfalfa | 3.18 A,B* | 3.14 A,B* | 3.03 A* | 2.56 A,B* | 2.01 B* | 3.36 A* |
| Fenugreek | 1.87 A† | 2.29 A* | 1.58 A† | 1.55 A† | 1.38 A,B† | 0.30 B† |
| Lettuce | 0.51 A,B‡ | 0.00 B† | 0.50 A,B‡ | 0.68 A‡ | 0.49 A,B‡ | 0.00 B‡ |
| Tomato | 0.34 A,B‡ | 0.15 A,B† | 0.06 A,B‡ | 0.46 A‡ | 0.27 A,B‡ | 0.00 B‡ |
Mean value within a column that are not followed by the same symbol (*, †, or ‡) are significantly different (P < 0.05). Mean values within a row that are not followed by the same uppercase letter are significantly different (P < 0.05).
The cotyledons and seed coats of all 4 types of sprouts/seedlings had higher Salmonella counts (Table 4). The root tissues of tomato and lettuce seedlings and seed coat/cotyledon tissues of alfalfa sprouts also had higher Salmonella counts than other tissues of corresponding sprouts/seedlings (Table 4). Lower Salmonella counts were associated with stem tissues and pregerminated seeds. However, the growth trend of EHEC on tissues developed from each type of seeds was not as clear as that of Salmonella (Table 5).
Daily changes in Salmonella and EHEC populations on alfalfa, fenugreek, tomato, and lettuce sprout/seedling samples during the germination process are shown in Fig. 1 and 2, respectively. In general, cell populations of Salmonella and EHEC on all samples increased as the germination time increased.
FIG 1.
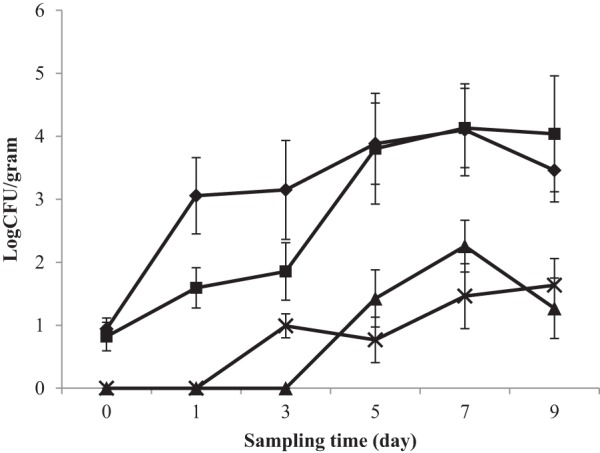
Growth of S. enterica on sprouting seeds of alfalfa (◆), fenugreek (■), tomato (×) and lettuce (▲). Values are averages from S. Montevideo, S. Stanley, S. Cubana, and S. Baildon populations recovered from various tissues of different types of germinating seeds.
FIG 2.
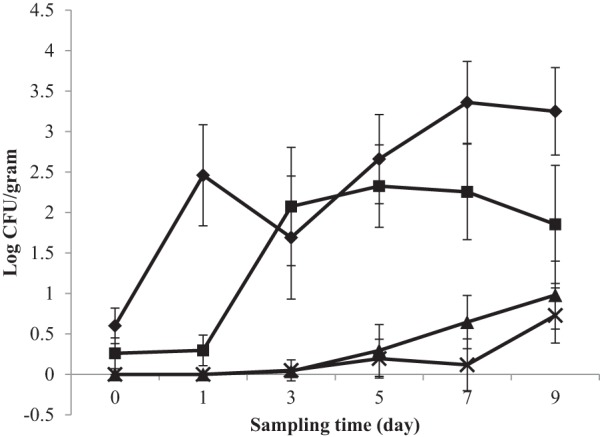
Growth of EHEC on sprouting seeds of alfalfa (◆), fenugreek (■), tomato (×), and lettuce (▲). Values are averages from E. coli F4546, E. coli K4492, E. coli H1730, and E. coli ATCC BAA-2326 populations recovered from various tissues of different types of germinating seeds.
DISCUSSION
The infiltration of human and plant pathogens into vegetable seeds can occur naturally at various stages during seed production (9). When the water pressure overcomes the internal gas pressure of seeds and the hydrophobic nature of seed surfaces, bacterial cells can infiltrate through cracks, crevices, and intercellular spaces of the vegetable seeds (14). Vacuum infiltration, first described by Boosalis (15), has been used to mimic this natural process and inoculate microorganisms into plant and seed tissues. Under vacuum, the air trapped in the intercellular spaces of seed tissue is removed to yield a lower internal gas pressure. A subsequent sudden release of the vacuum creates a positive pressure difference toward the internal seed tissues, which allows bacterial cells in a suspension to be drawn into the seeds (16). The efficacy of seed inoculation by vacuum infiltration was previously discussed by Prathuangwong and coworkers (17) who found that a 20-min vacuum at 20 lb/in.2 was as effective as direct micropyle injections in inoculating Xanthomonas campestris pv. glycines into soybean seeds. Thus, the technique has been used routinely to study seed-microorganism interactions (11, 16, 18–20). In the present study, vacuum infiltration was used to introduce Salmonella and EHEC cells into alfalfa, fenugreek, lettuce, and tomato seeds.
During vacuum infiltration, the surfaces of vegetable seeds might be simultaneously contaminated. As a control measure, seed surface decontamination was performed after the vacuum infiltration process in the present study. The treatment of vacuum-infiltrated vegetable seeds with sodium hypochlorite successfully inactivated Salmonella or EHEC cells on seed surfaces, as no pathogen cells were detected from the seed-rinsing water. However, a low number (0.26 to 0.95 log CFU) of pathogen cells was recovered from the homogenates of alfalfa and fenugreek seeds. These results indicate that vacuum infiltration successfully introduced S. enterica and EHEC cells into the vegetable seeds used in the study.
The growth and dissemination of internalized S. enterica and EHEC cells to different tissue sections of sprouts/seedlings were demonstrated in the present study (Table 1). The precise mechanism for the observed phenomenon is not known. However, Cooley et al. (21) reported that Salmonella and E. coli could migrate along plant surfaces by either diffusion or active movement. Kroupitski et al. (22) found that cells of some Salmonella strains sensed and moved toward specific chemoattractants, such as sucrose, on plant surfaces. Since cotyledon and root tissues and seed coats are closely related to seed/seedling exudation, abundant nutrients and water exuded from these tissues can support the growth of microorganisms (23). Klerks et al. (24) observed the active movement of S. enterica subsp. enterica serovar Dublin toward root exudates of lettuce seedlings in soil and glass capillary tubes. Effective chemoattractants of E. coli, such as d-glucose, fructose, and maltose (25), are frequently present in vegetable seed and root exudates (26). As Salmonella populations in seed coats and roots and Salmonella and E. coli populations in cotyledon were larger than those in other tissue sections according to the results of the present study (Table 1), it is likely that chemotaxis is one of the forces that drove Salmonella and E. coli cells into these locations.
It was observed that pathogen growth was less significant on lettuce and tomato seedling tissues compared to those on alfalfa and fenugreek sprouts (Table 1). The reason for this difference is currently unknown. However, differences in the initial bacterial population (data not shown) on various types of infiltrated vegetable seeds might be partially responsible for the observed phenomenon. Vegetable seeds used in the study varied in mass and size, as well as in their chemical and physical surface properties, which may have influenced the efficacy of vacuum infiltration.
The chemical compositions of vegetable seeds and seed exudates might also affect the growth of bacterial cells on sprouts/seedlings during germination (27–29). Generally, seeds and seed exudates that contain more essential nutrients and fewer growth inhibitors better support the growth of microorganisms (30). Previous studies have shown that alfalfa and fenugreek seeds contain arabinose (31, 32), which is absent in tomato (33) and lettuce seeds (34). Arabinose may serve as a carbon source to support the growth of Salmonella and EHEC, as it can be utilized by the cells of most S. enterica and EHEC strains (35, 36). Furthermore, l-arabinose is an active regulator of Salmonella pathogenicity island 1, which has been suggested to play a role in the interactions between Salmonella and plant hosts (37). In addition to arabinose, the abundance of threonine differs in the four types of vegetable seeds used in the study (38). The amounts of threonine per unit weight of alfalfa and fenugreek seeds are higher than those in tomato and lettuce seeds (31–34). Although arabinose could be a good carbon source and threonine is an essential amino acid for bacterial growth, it is not known whether they have contributed to the differential growth of Salmonella and EHEC on tissues of alfalfa and fenugreek sprouts versus on lettuce and tomato seedlings in the present study.
Tu (39) compared the seed and early root exudates of 19 crop species and reported significantly varied abundances in amino acids, amines, amides, and reducing sugars. The researchers recovered only trace amounts of reducing sugars and amino acids that could be utilized by microorganisms from tomato seedlings after 5 days of germination. In addition, the exudates of tomato seeds and seedlings contained one order of magnitude more organic acids than sugars, and had a weakly acidic pH of 5.5 (40, 41). Neumann et al. (42) reported the presence of benzoic and lauric acids, which were natural antimicrobial agents, in the root exudates of lettuce seedlings.
Although in lower numbers, Salmonella and EHEC cells disseminated from contaminated seeds to lettuce and tomato seedlings. However, whether the presence of the pathogen on the seedlings would impose any health risks to fresh produce safety largely depends on the fate of the pathogens at later stages of plant development. Deering et al. (43) recovered E. coli O157:H7 cells in tomato fruits grown from seeds artificially inoculated with the pathogen. Gu et al. (44) examined tomato fruits grown from seeds extracted from tomato fruits infested with Salmonella but did not recover the pathogen from the second-generation fruits. Cooley et al. (21) observed a temporal reduction in the contamination frequency of plants grown from contaminated Arabidopsis thaliana seeds during 30 days of cultivation.
Howard and Hutcheson (18) previously reported that the growth of S. enterica on germinating alfalfa seeds is serotype independent. The researchers inoculated alfalfa seeds with nine different serotypes of S. enterica and observed no difference in growth among the tested strains after 24 and 48 h of germination. However, the present study reveals that the average population of S. Montevideo recovered from different sprout/seedling tissues was significantly smaller than the other Salmonella strains used in the study (Table 1 and 2). This indicates that the S. Montevideo strain might be poorly adapted for growth in germinating seeds. Interestingly, S. Montevideo was reported in early studies as better adapted to growth on tomato plants than nine other S. enterica serotypes, such as S. enterica subsp. enterica serovars Newport, Dublin, and Typhimurium (45, 46). In contrast to previous studies that used mature tomato plants (47), germinating tomato seeds were used in the present study. Furthermore, only four S. enterica strains were tested in the current study, whereas approximately 100 documented pathogenic Salmonella serotypes are frequently associated with human infections (48). More strains/serotypes should be tested before a conclusion can be drawn with respect to the serovar dependency of Salmonella growth on germinating vegetable seeds.
We observed, in the present study, that the population of EHEC recovered from sprouts/seedling tissues was significantly smaller than that of Salmonella. Similar results were reported by Charkowski et al. (11), who compared the growth of five S. enterica strains and six E. coli O157:H7 strains on germinating alfalfa seeds for 2 days. The population of S. enterica was 0.5 to 2.0 log CFU/sprout higher than that of E. coli O157:H7. The authors ascribed the lower growth rate of E. coli on alfalfa sprouts to poor adaptation to alfalfa seed exudates and/or the inability of the pathogen to firmly attach to sprout surfaces. Roy et al. (49) reported that relative to S. enterica, E. coli O157:H7 triggered a stronger stomatal immunity and elevated the expression of plant defense-related marker genes, such as PR1 in A. thaliana and lettuce plants.
In conclusion, this study found that Salmonella and E. coli artificially internalized into vegetable seeds caused the contamination of different tissues of sprouts/seedlings and that pathogen growth on germinating seeds is bacterial species and vegetable seed-type dependent. The average Salmonella populations recovered from different tissue sections of sprouts/seedlings were larger than the E. coli populations. Alfalfa and fenugreek sprout tissues had, on average, larger pathogen populations than lettuce and tomato seedling tissues. Whether pathogen cells on vegetable seedlings, such as those of lettuce and tomato, will impose any risk to fresh produce safety largely depends on the fate of the pathogens at later stages of plant development. However, sprouts of alfalfa and fenugreek seeds are often consumed as raw or minimally processed products and have the potential to impose health risks to the consumers once they are contaminated with bacterial pathogens.
MATERIALS AND METHODS
Bacterial strains.
Four Salmonella strains (S. Baildon, S. Cubana, S. Montevideo, and S. Stanley), three E. coli O157:H7 strains (F4546, H1730, and K4492) and one E. coli O104:H4 strain (ATCC BAA-2326) that were isolated from fresh produce-associated outbreaks of human gastrointestinal infections were used in this study (50). Nalidixic acid (NA)-resistant mutants of each bacterial strain were selected on tryptic soy agar (Becton, Dickinson and Company, Sparks, MD) supplemented with 50 μg/ml of NA (NATSA). Bacterial inocula were prepared by transferring a loop of each overnight culture to Luria-Bertani no-salt broth supplemented with 50 μg/ml of NA and incubated for 16 to 18 h at 37°C. The resulting bacterial cultures were diluted in sterile water to approximate cell concentrations of 104 CFU/ml. The exact cell populations in the inocula were determined using a standard plate count assay on NATSA.
Inoculation of vegetable seeds.
Alfalfa (Medicago sativa), fenugreek (Trigonella foenum-graecum), tomato (Solanum lycopersicum [Roma]), and lettuce (Lactuca sativa [iceberg]) seeds were purchased from Twilley seed company (Hodges, SC) and stored at 10°C as instructed by the distributer. These seed species were selected based on the fact that alfalfa and fenugreek sprouts and tomato (fruit) and lettuce (leafy green) are commonly consumed fresh produce, which have all been previously linked to outbreaks of human gastrointestinal infections. Before pathogen inoculation, each vegetable seed type (2 g) was surface disinfected in 20 ml of 20,000 ppm sodium hypochlorite at room temperature for 10 min with agitation at 120 rpm on a platform shaker (Orbit shake; Lab-Line Instruments, Inc.). Residual chlorine on vegetable seeds was neutralized with 20 ml Dey-Engley neutralization broth (BD) for 10 min. Disinfected seeds were rinsed twice, each with 20 ml sterilized distilled water, and were vigorously vortexed for 20 s. Vacuum infiltration was performed according to a procedure previously described by Darrasse et al. (51) with modifications. Specifically, the seeds were placed in 20 ml of Salmonella or E. coli inoculum at room temperature for 30 min before being exposed to a vacuum of 25 in. Hg for 10 min. The vacuum was then broken to create a negative pressure. The draw and release of vacuum were repeated three times (52), and inoculated vegetable seeds were collected and dried overnight in a biological safety cabinet (class II type A/B 3; Nuaire, Plymouth, MN). Salmonella or E. coli cells on seed surfaces were inactivated using another sodium hypochlorite treatment as described above. Successful inactivation of Salmonella or E. coli cells on seed surfaces was confirmed by plating the final seed rinse water on bismuth sulfate agar (BSA; BD) or sorbitol MacConkey agar (SMAC; BD) supplemented with 50 μg/ml NA (NASMAC), respectively. Both types of samples were also plated on NATSA plates. The detection limit of the plating assay was 10 CFU/ml. Twenty seeds of each inoculated and disinfected seed type were individually homogenized in 0.5 ml of phosphate-buffered saline (PBS) and plated on NATSA and BSA or NASMAC in duplicates, as described above, to determine the initial bacterial loads.
Germination of vegetable seeds.
For seed germination, 1.0% (wt/vol) water agar was prepared in sterile 100 mm × 100 mm square plates (Fisher Scientific, CA). Twenty seeds of each type inoculated with an individual bacterial strain were placed on a water agar plate with moderate spacing. The plates were then placed in germination boxes at 25°C in the dark, and samples were taken every other day for microbiological analysis. The experiment was conducted twice.
Sampling and microbiological analyses.
On each sampling day, 10 contaminated seeds or sprouts/seedlings were aseptically removed from water agar plates using sterilized forceps. Intact vegetable seeds were collected on day 1, and on the rest of the sampling days, sprouts/seedlings were dissected into multiple tissue sections, including seed coat, cotyledon, stem, and root, and each tissue section was sampled individually for Salmonella and EHEC populations. At the 3rd day of germination, seed coats and cotyledons were sampled as “seed coat/cotyledon” due to the difficulty of separating the two tissue sections. Samples were homogenized in 2.0 ml PBS (pH 7.4), and the resulting homogenates were serially diluted as necessary. Homogenates obtained from Salmonella-contaminated samples were plated on BSA and NATSA and those from E. coli-contaminated samples were plated on NASMAC and NATSA. All plates were spread plated in duplicates and incubated at 37°C for 24 h before colonies were enumerated. The enrichment of samples with negative plating results was performed according to the FDA's bacteriological analytical manual (53, 54).
Statistical analysis.
Data were analyzed by analysis of variance (ANOVA) and Fisher's least significant difference (LSD) test using the R 3.2.2 software. Cell populations of Salmonella or EHEC strains recovered at different sampling points and from different seed types and sprout/seedling tissue sections were compared. For all comparisons, P values of less than 0.05 were considered significant.
ACKNOWLEDGMENTS
This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2014-67017-21705.
REFERENCES
- 1.National Advisory Committee on Microbiological Criteria for Foods. 1999. Microbiological safety evaluations and recommendations on sprouted seeds. Int J Food Microbiol 52:123–153. doi: 10.1016/S0168-1605(99)00135-X. [DOI] [PubMed] [Google Scholar]
- 2.De Roever C. 1998. Microbiological safety evaluations and recommendations on fresh produce. Food Control 9:321–347. doi: 10.1016/S0956-7135(98)00022-X. [DOI] [Google Scholar]
- 3.Mahon BE, Pönkä A, Hall WN, Komatsu K, Dietrich SE, Siitonen A, Cage G, Hayes PS, Lambert-Fair MA, Bean NH. 1997. An international outbreak of Salmonella infections caused by alfalfa sprouts grown from contaminated seeds. J Infect Dis 175:876–882. doi: 10.1086/513985. [DOI] [PubMed] [Google Scholar]
- 4.Como-Sabetti K, Reagan S, Allaire S, Parrott K, Simonds CM, Hrabowy S, Ritter B, Hall W, Altamirano J, Martin R, Downes F, Jennings G, Barrie R, Dorman MF, Keon N, Kucab M, Al Shab A, Robinson-Dunn B, Dietrich S, Moshur L, Reese L, Smith J, Wilcox K, Tilden J, Wojtala G, Park JD, Winnett M, Petrilack L, Vasquez L, Jenkins S, Barrett E, Linn M, Woolard D, Hackler R, Martin H, McWilliams D, Rouse B, Willis S, Rullan J, Miller G Jr, Henderson S, Pearson J, Beers J, Davis R, Saunders D. 1997. Outbreaks of Escherichia coli O157:H7 infection associated with eating alfalfa sprouts–Michigan and Virginia, June–July 1997. MMWR Morb Mortal Wkly Rep 46:741–744. [Google Scholar]
- 5.Ferguson D, Scheftel J, Cronquist A, Smith K, Woo-Ming A, Anderson E, Knutsen J, De A, Gershman K. 2005. Temporally distinct Escherichia coli O157 outbreaks associated with alfalfa sprouts linked to a common seed source–Colorado and Minnesota, 2003. Epidemiol Infect 133:439–447. doi: 10.1017/S0950268804003589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanning IB, Nutt J, Ricke SC. 2009. Salmonellosis outbreaks in the United States due to fresh produce: sources and potential intervention measures. Foodborne Pathog Dis 6:635–648. doi: 10.1089/fpd.2008.0232. [DOI] [PubMed] [Google Scholar]
- 7.Winthrop K, Palumbo M, Farrar J, Mohle-Boetani J, Abbott S, Beatty M, Inami G, Werner S. 2003. Alfalfa sprouts and Salmonella Kottbus infection: a multistate outbreak following inadequate seed disinfection with heat and chlorine. J Food Prot 66:13–17. doi: 10.4315/0362-028X-66.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Ding H, Fu TJ, Smith MA. 2013. Microbial contamination in sprouts: how effective is seed disinfection treatment? J Food Sci 78:R495–R501. doi: 10.1111/1750-3841.12064. [DOI] [PubMed] [Google Scholar]
- 9.Buck J, Walcott R, Beuchat L. 2003. Recent trends in microbiological safety of fruits and vegetables. Plant Health Prog doi: 10.1094/PHP-2003-0121-01-RV. [DOI] [Google Scholar]
- 10.Liao CH. 2008. Growth of Salmonella on sprouting alfalfa seeds as affected by the inoculum size, native microbial load and Pseudomonas fluorescens 2-79. Lett Appl Microbiol 46:232–236. doi: 10.1111/j.1472-765X.2007.02302.x. [DOI] [PubMed] [Google Scholar]
- 11.Charkowski A, Barak J, Sarreal C, Mandrell R. 2002. Differences in growth of Salmonella enterica and Escherichia coli O157:H7 on alfalfa sprouts. Appl Environ Microbiol 68:3114–3120. doi: 10.1128/AEM.68.6.3114-3120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warriner K, Spaniolas S, Dickinson M, Wright C, Waites W. 2003. Internalization of bioluminescent Escherichia coli and Salmonella Montevideo in growing bean sprouts. J Appl Microbiol 95:719–727. doi: 10.1046/j.1365-2672.2003.02037.x. [DOI] [PubMed] [Google Scholar]
- 13.Kigel J. 1995. Seed development and germination, p 277–298. CRC Press, Boca Raton, FL. [Google Scholar]
- 14.Beuchat LR. 2002. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect 4:413–423. doi: 10.1016/S1286-4579(02)01555-1. [DOI] [PubMed] [Google Scholar]
- 15.Boosalis M. 1950. A partial-vacuum technique for inoculating seedlings with bacteria and fungi. Phytopathology 40:2–3. [Google Scholar]
- 16.Dutta B, Gitaitis R, Smith S, Langston D Jr. 2014. Interactions of seedborne bacterial pathogens with host and non-host plants in relation to seed infestation and seedling transmission. PLoS One 9:e99215. doi: 10.1371/journal.pone.0099215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prathuangwong S, Khandej K. 1998. Development of the new methods for ecological study of soybean bacterial pustule: an artificial inoculation method of soybean seed with Xanthomonas campestris pv. glycines for inducing disease. Nat Sci 32:84–89. [Google Scholar]
- 18.Howard MB, Hutcheson SW. 2003. Growth dynamics of Salmonella enterica strains on alfalfa sprouts and in waste seed irrigation water. Appl Environ Microbiol 69:548–553. doi: 10.1128/AEM.69.1.548-553.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bashan Y, Okon Y, Henis Y. 1982. Long-term survival of Pseudomonas syringae pv. tomato and Xanthomonas campestris pv. vesicatoria in tomato and pepper seeds. Phytopathology 72:1143–1144. doi: 10.1094/Phyto-72-1143. [DOI] [Google Scholar]
- 20.Dutta B, Avci U, Hahn M, Walcott R. 2012. Location of Acidovorax citrulli in infested watermelon seeds is influenced by the pathway of bacterial invasion. Phytopathology 102:461–468. doi: 10.1094/PHYTO-10-11-0286-R. [DOI] [PubMed] [Google Scholar]
- 21.Cooley MB, Miller WG, Mandrell RE. 2003. Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacter asburiae. Appl Environ Microbiol 69:4915–4926. doi: 10.1128/AEM.69.8.4915-4926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroupitski Y, Golberg D, Belausov E, Pinto R, Swartzberg D, Granot D, Sela S. 2009. Internalization of Salmonella enterica in leaves is induced by light and involves chemotaxis and penetration through open stomata. Appl Environ Microbiol 75:6076–6086. doi: 10.1128/AEM.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolswinkel P, Ammerlaan A. 1985. Characteristics of sugar, amino acid and phosphate release from the seed coat of developing seeds of Vicia faba and Pisum sativum. J Exp Bot 36:359–368. doi: 10.1093/jxb/36.3.359. [DOI] [Google Scholar]
- 24.Klerks MM, Franz E, van Gent-Pelzer M, Zijlstra C, Van Bruggen AH. 2007. Differential interaction of Salmonella enterica serovars with lettuce cultivars and plant-microbe factors influencing the colonization efficiency. ISME J 1:620–631. doi: 10.1038/ismej.2007.82. [DOI] [PubMed] [Google Scholar]
- 25.Adler J. 1973. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol 74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 26.Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg B. 2005. Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7:1809–1817. doi: 10.1111/j.1462-2920.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- 27.Green SJ, Michel FC, Hadar Y, Minz D. 2007. Contrasting patterns of seed and root colonization by bacteria from the genus Chryseobacterium and from the family Oxalobacteraceae. ISME J 1:291–299. doi: 10.1038/ismej.2007.33. [DOI] [PubMed] [Google Scholar]
- 28.El-Gali ZI. 2015. Influence of seeds and roots extracts and exudates of bean plant on growth of some pathogenic fungi. OALib 2:1. doi: 10.4236/oalib.1101666. [DOI] [Google Scholar]
- 29.Emmert EA, Milner JL, Lee JC, Pulvermacher KL, Olivares HA, Clardy J, Handelsman J. 1998. Effect of canavanine from alfalfa seeds on the population biology of Bacillus cereus. Appl Environ Microbiol 64:4683–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barak JD, Schroeder BK. 2012. Interrelationships of food safety and plant pathology: the life cycle of human pathogens on plants. Annu Rev Phytopathol 50:241–266. doi: 10.1146/annurev-phyto-081211-172936. [DOI] [PubMed] [Google Scholar]
- 31.Kylen AM, McCready RM. 1975. Nutrients in seeds and sprouts of alfalfa, lentils, mung beans and soybeans. J Food Sci 40:1008–1009. doi: 10.1111/j.1365-2621.1975.tb02254.x. [DOI] [Google Scholar]
- 32.Naidu MM, Shyamala B, Naik JP, Sulochanamma G, Srinivas P. 2011. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. Lebenson Wiss Technol 44:451–456. doi: 10.1016/j.lwt.2010.08.013. [DOI] [Google Scholar]
- 33.Abdel-Rahman A-HY. 1982. The chemical constituents of tomato seeds. Food Chem 9:315–318. doi: 10.1016/0308-8146(82)90084-X. [DOI] [Google Scholar]
- 34.Xu F, Zou G, Liu Y, Aisa H. 2012. Chemical constituents from seeds of Lactuca sativa. Chem Nat Compd 48:574–576. doi: 10.1007/s10600-012-0314-1. [DOI] [Google Scholar]
- 35.Abbott SL, Hanson DF, Felland TD, Connell S, Shum AH, Janda JM. 1994. Escherichia coli O157:H7 generates a unique biochemical profile on MicroScan conventional gram-negative identification panels. J Clin Microbiol 32:823–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendriksen R. April 2003. A global Salmonella surveillance and laboratory support project of the World Health Organization: laboratory protocols level 1 training course identification of Salmonella, 4th ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 37.López-Garrido J, Puerta-Fernández E, Cota I, Casadesús J. 2015. Virulence gene regulation by l-arabinose in Salmonella enterica. Genetics 200:807–819. doi: 10.1534/genetics.115.178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hume M, Nisbet D, DeLoach J. 1997. In vitro 14C-amino acid fermentation by CF3, a characterized continuous-flow competitive exclusion culture of caecal bacteria. J Appl Microbiol 83:236–242. doi: 10.1046/j.1365-2672.1997.00224.x. [DOI] [PubMed] [Google Scholar]
- 39.Tu C-C. 1972. Seed and root exudations in relation to rhizoctonia damping-off of various crops. Taiwan Agr Res J 1972:128–138. [Google Scholar]
- 40.Lugtenberg BJ, Kravchenko LV, Simons M. 1999. Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ Microbiol 1:439–446. doi: 10.1046/j.1462-2920.1999.00054.x. [DOI] [PubMed] [Google Scholar]
- 41.Kamilova F, Kravchenko LV, Shaposhnikov AI, Makarova N, Lugtenberg B. 2006. Effects of the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol Plant Microbe Interact 19:1121–1126. doi: 10.1094/MPMI-19-1121. [DOI] [PubMed] [Google Scholar]
- 42.Neumann G, Bott S, Ohler M, Mock H-P, Lippmann R, Grosch R, Smalla K. 2014. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front Microbiol 5:2. doi: 10.3389/fmicb.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deering AJ, Jack DR, Pruitt RE, Mauer LJ. 2015. Movement of Salmonella serovar Typhimurium and E. coli O157:H7 to ripe tomato fruit following various routes of contamination. Microorganisms 3:809–825. doi: 10.3390/microorganisms3040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu G, Cevallos-Cevallos JM, Vallad GE, van Bruggen AH. 2013. Organically managed soils reduce internal colonization of tomato plants by Salmonella enterica serovar Typhimurium. Phytopathology 103:381–388. doi: 10.1094/PHYTO-04-12-0072-FI. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J, Allard S, Reynolds S, Millner P, Arce G, Blodgett RJ, Brown EW. 2013. Colonization and internalization of Salmonella enterica in tomato plants. Appl Environ Microbiol 79:2494–2502. doi: 10.1128/AEM.03704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi X, Wu Z, Namvar A, Kostrzynska M, Dunfield K, Warriner K. 2009. Microbial population profiles of the microflora associated with pre- and postharvest tomatoes contaminated with Salmonella Typhimurium or Salmonella Montevideo. J Appl Microbiol 107:329–338. doi: 10.1111/j.1365-2672.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 47.Kus JV, Zaton K, Sarkar R, Cameron RK. 2002. Age-related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae. Plant Cell 14:479–490. doi: 10.1105/tpc.010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen SJ, Bishop R, Brenner FW, Roels TH, Bean N, Tauxe RV, Slutsker L. 2001. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987–1997. J Infect Dis 183:753–761. doi: 10.1086/318832. [DOI] [PubMed] [Google Scholar]
- 49.Roy D, Panchal S, Rosa BA, Melotto M. 2013. Escherichia coli O157:H7 induces stronger plant immunity than Salmonella enterica Typhimurium SL1344. Phytopathology 103:326–332. doi: 10.1094/PHYTO-09-12-0230-FI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taormina PJ, Beuchat LR, Slutsker L. 1999. Infections associated with eating seed sprouts: an international concern. Emerging Infect Dis 5:626–634. doi: 10.3201/eid0505.990503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darrasse A, Darsonval A, Boureau T, Brisset M-N, Durand K, Jacques M-A. 2010. Transmission of plant-pathogenic bacteria by nonhost seeds without induction of an associated defense reaction at emergence. Appl Environ Microbiol 76:6787–6796. doi: 10.1128/AEM.01098-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtis IS. 2004. Transgenic crops of the world: essential protocols, p 445. Springer Science & Business Media, New York, NY. [Google Scholar]
- 53.Andrews W, Hammack T, Amaguana R. 2007. Bacteriological analytical manual. Methods for specific pathogens: chapter 5, Salmonella. U.S. Food and Drug Administration, Silver Spring, MD: http://www.cfsan.fda.gov/ebam/bam-5.htm Accessed 27 July 2017. [Google Scholar]
- 54.Feng P, Weagant S, Jinneman K. 2011. Bacteriological analytical manual. Diarrheagenic Escherichia coli, chapter 4A. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070080.htm Accessed 11 December 2011. [Google Scholar]


