ABSTRACT
The polyphyletic nature of many formae speciales of Fusarium oxysporum prevents molecular identification of newly encountered strains based on conserved, vertically inherited genes. Alternative molecular detection methods that could replace labor- and time-intensive disease assays are therefore highly desired. Effectors are functional elements in the pathogen-host interaction and have been found to show very limited sequence diversity between strains of the same forma specialis, which makes them potential markers for host-specific pathogenicity. We therefore compared candidate effector genes extracted from 60 existing and 22 newly generated genome assemblies, specifically targeting strains affecting cucurbit plant species. Based on these candidate effector genes, a total of 18 PCR primer pairs were designed to discriminate between each of the seven Cucurbitaceae-affecting formae speciales. When tested on a collection of strains encompassing different clonal lineages of these formae speciales, nonpathogenic strains, and strains of other formae speciales, they allowed clear recognition of the host range of each evaluated strain. Within Fusarium oxysporum f. sp. melonis more genetic variability exists than anticipated, resulting in three F. oxysporum f. sp. melonis marker patterns that partially overlapped with the cucurbit-infecting Fusarium oxysporum f. sp. cucumerinum, Fusarium oxysporum f. sp. niveum, Fusarium oxysporum f. sp. momordicae, and/or Fusarium oxysporum f. sp. lagenariae. For F. oxysporum f. sp. niveum, a multiplex TaqMan assay was evaluated and was shown to allow quantitative and specific detection of template DNA quantities as low as 2.5 pg. These results provide ready-to-use marker sequences for the mentioned F. oxysporum pathogens. Additionally, the method can be applied to find markers distinguishing other host-specific forms of F. oxysporum.
IMPORTANCE Pathogenic strains of Fusarium oxysporum are differentiated into formae speciales based on their host range, which is normally restricted to only one or a few plant species. However, horizontal gene transfer between strains in the species complex has resulted in a polyphyletic origin of host specificity in many of these formae speciales. This hinders accurate and rapid pathogen detection through molecular methods. In our research, we compared the genomes of 88 strains of F. oxysporum with each other, specifically targeting virulence-related genes that are typically highly similar within each forma specialis. Using this approach, we identified marker sequences that allow the discrimination of F. oxysporum strains affecting various cucurbit plant species through different PCR-based methods.
KEYWORDS: cucurbits, genome analysis, host range, molecular markers, pathogen detection, pathogenic fungi
INTRODUCTION
Accurate and rapid pathogen detection is necessary to take appropriate action against plant diseases. Fusarium oxysporum is a soilborne fungus that includes both nonpathogenic and plant-pathogenic strains. Pathogenic strains of F. oxysporum cause vascular wilt and cortical rot disease in a wide variety of agricultural crop species. They are classified into host-specific forms (formae speciales) and are often further subdivided into races based on their capacity to infect different cultivars of a plant species (1–3).
Fusarium wilt and root rot in cucurbits are among the most prominent and destructive diseases affecting this plant family (4–6). In total, seven cucurbit-infecting formae speciales have been described: Fusarium oxysporum f. sp. cucumerinum, Fusarium oxysporum f. sp. melonis, Fusarium oxysporum f. sp. niveum, Fusarium oxysporum f. sp. radicis-cucumerinum, Fusarium oxysporum f. sp. lagenariae, Fusarium oxysporum f. sp. momordicae, and Fusarium oxysporum f. sp. luffae (Table 1). The last three are mostly restricted to Southeast Asia (7), while the formae speciales affecting cucumber, melon, and watermelon are globally distributed and more important from an economic standpoint (4, 7, 8).
TABLE 1.
Formae speciales of F. oxysporum affecting members of the Cucurbitaceae family
| Forma specialis | Abbreviation in strain designations | Host | Reference(s) |
|---|---|---|---|
| F. oxysporum f. sp. cucumerinum | Focuc | Cucumber (Cucumis sativus) | 66 |
| F. oxysporum f. sp. melonis | Fomln | Muskmelon (Cucumis melo) | 67 |
| F. oxysporum f. sp. niveum | Foniv | Watermelon (Citrullus lanatus) | 68 |
| F. oxysporum f. sp. radicis-cucumerinum | Forcu | Multiple cucurbits (including cucumber, melon, watermelon, and gourd) | 69 |
| F. oxysporum f. sp. momordicae | Fomom | Bitter gourd (Momordica charantia) | 70 |
| F. oxysporum f. sp. lagenariae | Folag | Calabash gourd (Lagenaria spp.) | 32, 71 |
| F. oxysporum f. sp. luffae | Foluf | Sponge gourd (Luffa cylindrica) | 7 |
Currently, there are no effective curative treatments for Fusarium disease (9). Use of resistant varieties or rootstocks is the only practical measure for controlling the disease in the field (10–12). In greenhouses, soil sterilization by fumigation with methyl bromide can be performed (10, 13). Most efforts are directed toward prevention of the disease. Routine methods that provide reliable subspecific identification, sensitive detection, and accurate quantification of F. oxysporum are of high importance (14) and could prevent unnecessary efforts to suppress harmless fungal populations (15). Development of these types of markers has thus far been complicated by the polyphyletic nature of most formae speciales of F. oxysporum (14).
As many F. oxysporum strains have been found to be nonpathogenic, endophytic, or even applicable as biocontrol strains (16–18), discrimination between pathogenic and abundantly present nonvirulent strains is very important (19). Discrimination of F. oxysporum formae speciales and races is routinely done through labor- and time-intensive disease assays (20–22). Molecular detection methods are therefore highly desired.
Formae speciales are often of polyphyletic origin (23), and pathogenic strains may share a higher level of sequence similarity of conserved genes with strains that are nonpathogenic or pathogenic toward another host (24, 25). Diagnostics based on genes like that encoding translation elongation factor 1-alpha (EF1α) or the ribosomal intergenic spacer (IGS) are therefore only useful to discriminate between fungal species (26, 27). In several cases they have been suggested for subspecies discrimination, but these often prove to be unreliable for this purpose (8, 27, 28).
Several molecular markers for the cucurbit-infecting F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. radicis-cucumerinum, F. oxysporum f. sp. niveum, and F. oxysporum f. sp. luffae have been developed. These are all based on random amplified polymorphic DNA (RAPD) fragments, resulting in sequence-characterized amplified region (SCAR) markers. SCAR markers are suboptimal for forma specialis discrimination because they are based on genomic regions that are not necessarily required for virulence. Furthermore, as they can be localized anywhere on the genome, there are often little to no sequence data available in public databases for comparison with other sequences. The robustness of the markers can be verified only by screening against a large collection of strains (14).
Interestingly, closer inspection of previously developed forma specialis-distinguishing SCAR markers showed that the selected sequences were often (part of) a transposable element, such as Fot1 (Fusarium oxysporum f. sp. albedinis, Fusarium oxysporum f. sp. chrysanthemi, and Fusarium oxysporum f. sp. dianthi) and Folyt1 (F. oxysporum f. sp. radicis-cucumerinum) and Impala (Fusarium oxysporum f. sp. ciceris and F. oxysporum f. sp. dianthi) (29), or pathogenicity-associated genes like FTF1 (Fusarium oxysporum f. sp. phaseoli) (30). A race 1-specific Fusarium oxysporum f. sp. lactucae marker was developed by amplifying and cloning regions between long terminal repeats of retrotransposons in the genome (31). For F. oxysporum f. sp. lagenariae, F. oxysporum f. sp. momordicae, and F. oxysporum f. sp. melonis, only DNA fingerprinting results have been described thus far (32).
It was recently shown that host specificity is associated with the suite of effector genes present in the genomes of F. oxysporum strains (33). Both presence-absence polymorphisms and the sequence type of individual effector genes turned out to be predictive for a strain's host range. These genes therefore form the most solid base for discrimination of formae speciales within the F. oxysporum species complex (FOSC) (14, 20, 25). Indeed, use of virulence genes to identify fungal plant pathogens has proven successful in the past for other Fusarium species (34, 35). Within the FOSC, this approach has been applied to distinguish Fusarium oxysporum f. sp. cubense tropical race 4 by targeting a candidate effector gene (36). Additionally, Fusarium oxysporum f. sp. lycoperici and F. oxysporum f. sp. cubense can be discriminated from other formae speciales through the use of PCR primers designed to detect specific secreted in xylem (SIX) effector gene sequences (15, 25, 37). At the time of these studies, however, no (or limited) comparative genomics analyses could be performed due to the lack of available genome sequences. All SIX genes have homologs in other host-pathogenic forms of F. oxysporum (e.g., SIX1, SIX5, and SIX6 [33, 38–40]). For these, marker specificity could not be evaluated beforehand and cross-reaction with nontarget formae speciales was found (25).
Since it is not yet viable to sequence every individual strain encountered, we decided to design effector candidate-based markers. In this way, we aimed to be able to distinguish cucurbit-affecting formae speciales from (i) each other, (ii) other formae speciales, and (iii) nonpathogenic strains. Therefore, we used whole-genome sequences of a number of representative cucurbit-infecting F. oxysporum strains as a starting point and identified putative effector genes suitable as markers. An advantage of using molecular markers over whole-genome sequencing is that they can also be applied to infected soil or plant tissue samples; the fungus does not need to be isolated and cultured (28). Techniques such as TaqMan real-time PCR even allow for a quantitative evaluation of pathogen abundance, e.g., on DNA isolated from soil (41).
The genetic bases for host specificity of FOSC strains toward plants belonging to the Cucurbitaceae family are similar (33), making these formae speciales relatively difficult to separate. This means that this is a good test case for host specificity discrimination, and the results presented here can be exemplary for application to other plant species where disease caused by F. oxysporum is a pressing problem.
RESULTS
Several cucurbit-infecting formae speciales have a polyphyletic origin.
In order to be able to select forma specialis-wide marker sequences, it is necessary to collect the genetic variety for that forma specialis as completely as possible. We made use of 66 previously published genome sequences and added de novo genome assemblies generated from Illumina paired-end read data of 22 new strains (see Data S2 in the supplemental material).
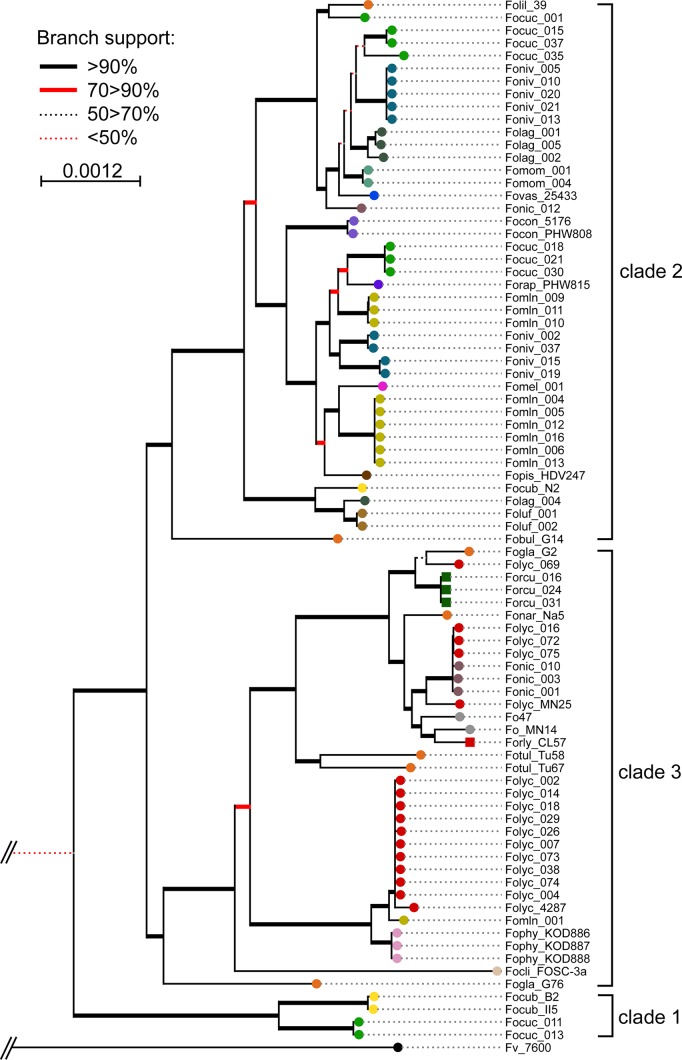
In order to assess the genetic diversity of the formae speciales under investigation, we generated a phylogenetic tree based on over 400 core genomic gene sequences from each of their genomes (Fig. 1). This showed that F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. melonis, F. oxysporum f. sp. niveum, and F. oxysporum f. sp. lagenariae occupied multiple clades in the tree (5, 3, 3, and 3, respectively), indicating that they belong to different clonal lines. In our set of strains, we have 6 of 7 described F. oxysporum f. sp. cucumerinum vegetative compatibility groups (VCGs) (6, 42), 3 of 8 F. oxysporum f. sp. melonis VCGs (43), all 3 F. oxysporum f. sp. niveum VCGs (43), and both F. oxysporum f. sp. radicis-cucumerinum VCGs (43). For F. oxysporum f. sp. lagenariae (3 VCGs described [44]), F. oxysporum f. sp. momordicae (4 VCGs described [44]), and F. oxysporum f. sp. luffae (unknown number of VCGs), no VCG information was available for our strains, although they group into three, one, and one clade(s), respectively (Fig. 1).
FIG 1.
F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. melonis, F. oxysporum f. sp. niveum, and F. oxysporum f. sp. lagenariae are of polyphyletic origin. A total of 441 conserved core genes from all genomes were extracted, aligned, and concatenated into a multiple-sequence alignment. Phylogeny was inferred with 100 bootstrap iterations. All strains fall within the three main clades of the FOSC. Focuc, F. oxysporum f. sp. cucumerinum; Fomln, F. oxysporum f. sp. melonis; Foniv, F. oxysporum f. sp. niveum; Forcu, F. oxysporum f. sp. radicis-cucumerinum; Folag, F. oxysporum f. sp. lagenariae; Foluf, F. oxysporum f. sp. luffae; Fomom, F. oxysporum f. sp. momordicae. For abbreviations of other formae speciales, see Data S1.
Candidate effector gene phylogenies display clear grouping of host specificity.
Unlike conserved core genes, virulence-related genes tend to be identical across members belonging to the same polyphyletic forma specialis of F. oxysporum (15, 33). For this reason, they have predictive value for a strain's host range. Forma specialis markers are essentially the smallest possible set of effector genes that is shared by all strains of a forma specialis and absent or different in sequence (at least as a set) in all other strains (33).
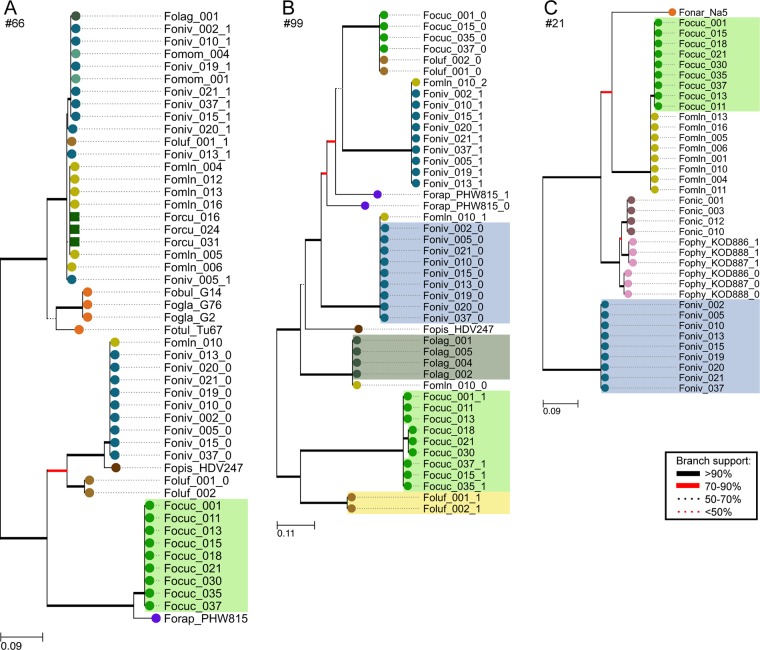
We extracted the sequences of the candidate effector genes from the work of van Dam et al. (33) from all assemblies and generated a multiple-sequence alignment (MSA; see Data S1 in the supplemental material) and phylogenetic tree for each of them (three examples in Fig. 2 and continued in Data S2). A custom python script identified those genes in which all members of a forma specialis grouped together in a separate clade. From the genes displaying such grouping, the genes that facilitated the best discrimination were selected based on manual inspection of the MSA to come to a final selection of marker sequences per forma specialis (Table 2).
FIG 2.
Phylogenetic trees of three genes selected as markers for F. oxysporum f. sp. cucumerinum: 66 (A), 99 (B), and 21 (C). Separation of a clade that includes all strains belonging to a forma specialis indicates sequence similarity within and sequence dissimilarity between formae speciales. Colored areas in the tree reflect the target forma specialis of the marker. Hypothetical protein-encoding genes 99 and 21 are used as markers for multiple formae speciales.
TABLE 2.
Selected marker genes and their respective target formae speciales
| Gene IDa | Target gene | Target forma specialisb | Gene tree illustration |
|---|---|---|---|
| Positive control | FEM1 | Positive control | |
| 94 | HPEGc | All cucurbit-infecting formae speciales | Data S2A |
| 13 | SIX13 | radicis-cucumerinum | Data S2B |
| 70 | HPEG | radicis-cucumerinum | Data S2C |
| 66 | HPEG | cucumerinum | Fig. 2A |
| 99 | HPEG | cucumerinum | Fig. 2B |
| 21 | Fom effector 7 | cucumerinum | Fig. 2C |
| 1 | SIX1 | melonis | Data S2D |
| 20 | Fom effector 6 | melonis | Data S2E |
| 18 | Fom effector 3 | melonis + niveum | Data S2G |
| 99 | HPEG | niveum | Fig. 2B |
| 100 | HPEG | niveum | Data S2H |
| 21 | Fom effector 7 (pseudogenized) | niveum | Fig. 2C |
| 98 | HPEG | momordicae | Data S2I |
| 130 | HPEG | momordicae | Data S2J |
| 1 | SIX1 | lagenariae + momordicae | Data S2D |
| 71 | HPEG | lagenariae | Data S2F |
| 99 | HPEG | lagenariae | Fig. 2B |
| 99 | HPEG | luffae | Fig. 2B |
ID, identifier.
All formae speciales listed are F. oxysporum formae speciales; to save space, a shortened form of the organism name is used.
HPEG, hypothetical protein-encoding gene.
Some of the selected genes show multiple forma specialis-specific clades; therefore, multiple markers targeting different forma specialis could be designed on these genes. An example is candidate effector 99, a hypothetical protein-encoding gene that is used as a marker for F. oxysporum f. sp. niveum, F. oxysporum f. sp. lagenariae, F. oxysporum f. sp. cucumerinum, and F. oxysporum f. sp. luffae (Fig. 2B). F. oxysporum f. sp. melonis strain Fomln010 possesses a copy identical to both candidate effector 99 homologs present in the F. oxysporum f. sp. niveum strains as well as a copy identical to the F. oxysporum f. sp. lagenariae gene sequence. To still be able to distinguish these formae speciales from one another, it is therefore of importance to use multiple markers for each forma specialis.
Discrimination of cucurbit-infecting formae speciales by PCR.
PCR primers were designed specifically on polymorphic regions of the selected DNA sequences (Table 2; see also Data S1), aiming to generate a PCR product sized above 120 and below 700 nucleotides (nt) for quick and reliable application. The Fusarium extracellular matrix 1 gene (FEM1) (45) was taken along as a positive control. To verify the applicability of the markers, PCRs were executed for each of the primer pairs on a subset of the strains that were used for marker design, i.e., of which the host range has been confirmed and the genome had been sequenced. This included strains belonging to the cucurbit-infecting formae speciales, several other formae speciales (Fusarium oxysporum f. sp. vasinfectum, Arabidopsis infecting, F. oxysporum f. sp. lycopersici, Fusarium oxysporum f. sp. radicis-lycopersici, Fusarium oxysporum f. sp. nicotianae, Fusarium oxysporum f. sp. melongenae, Physalis infecting, F. oxysporum f. sp. cubense, Fusarium oxysporum f. sp. pisi, Fusarium oxysporum f. sp. tulipae, and Fusarium oxysporum f. sp. gladioli) and two nonpathogenic F. oxysporum strains: Fo47 (16) and MN14 (33). The strains were selected based on their differential phylogenetic distribution in Fig. 1 as well as the presence and absence of selected marker sequences in their genome assembly.
All except one of the forma specialis-specific PCR markers behaved like expected (Table 3), showing PCR products only in the expected combinations of genomic DNA and marker primers. One false-positive PCR product was found, in the combination of F. oxysporum f. sp. pisi HDV247 and marker 130 (F. oxysporum f. sp. momordicae). In the genome assembly of HDV247, this gene was found to be present with 97% sequence similarity, although the downstream region of this gene provided sufficient sequence diversity for primer design (Data S1).
TABLE 3.
PCR markers allowing discrimination of cucurbit-affecting formae speciales of F. oxysporuma
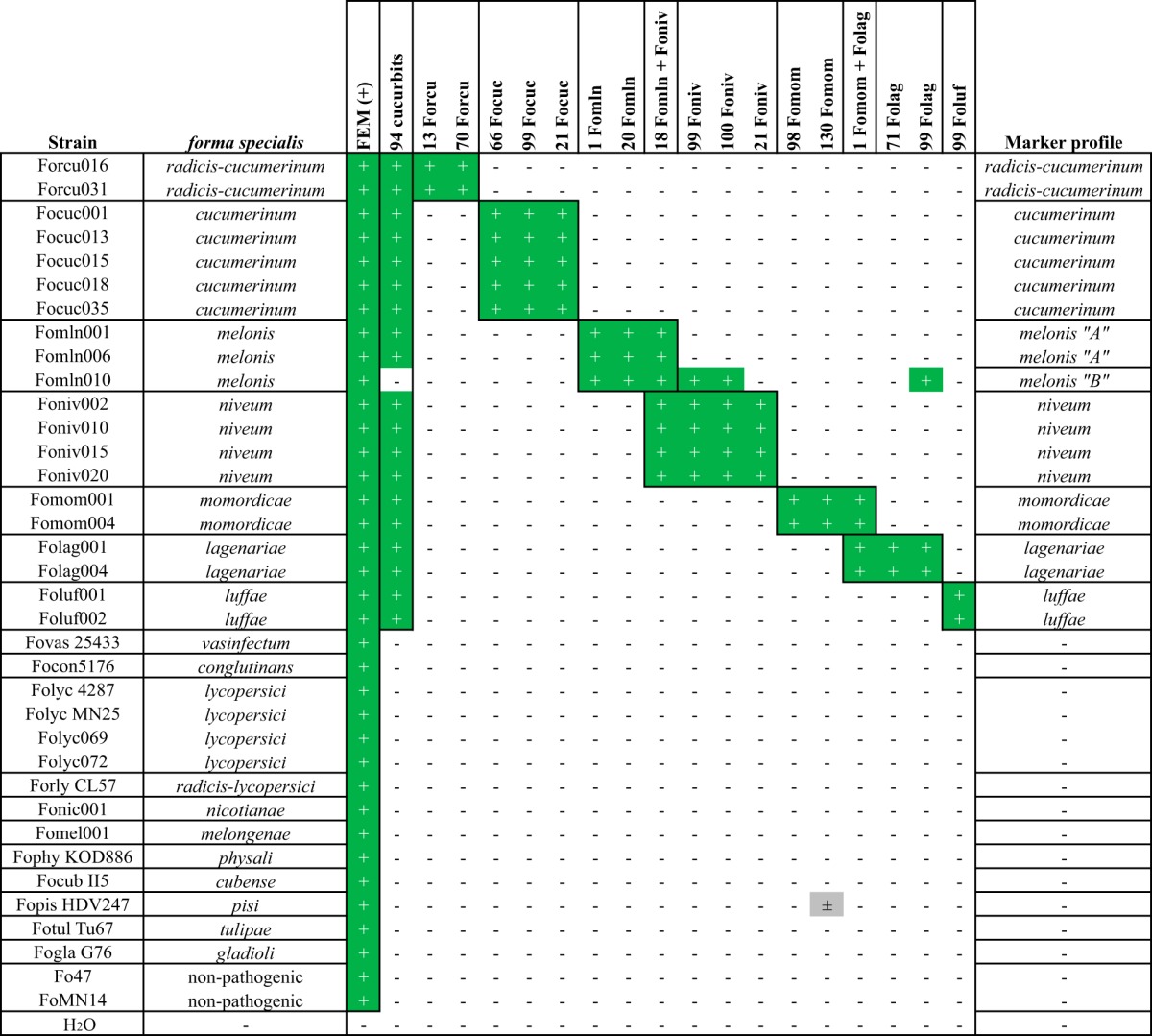
Symbols: +, positive test result; −, negative test result; ±, weak positive test result (very faint PCR product of the expected size present).
Marker 94, targeting all cucurbit-affecting formae speciales, gave a band of the correct size for all cucurbit-affecting isolates tested, except Fomln010. Furthermore, strain Fomln010 displayed an atypical F. oxysporum f. sp. melonis marker pattern, as it yielded PCR products that were not seen in the other F. oxysporum f. sp. melonis isolates for F. oxysporum f. sp. niveum markers 99 and 100 and F. oxysporum f. sp. lagenariae marker 99. This pattern, designated pattern B in Table 3, was not unexpected, since presence of identical sequences for these three markers as well as absence of the gene encoding hypothetical protein 94 had been observed in the genome assembly of Fomln010 (sequence similarity of marker 99 shown in Fig. 2B). The presence of identical effector candidate sequences across formae speciales affecting similar plant species was not surprising since they share part of their genetic toolset allowing for pathogenic colonization of these plants (33). It does, however, make marker selection more challenging. While screening for specific differentiation of, for instance, F. oxysporum f. sp. melonis and F. oxysporum f. sp. niveum, it is therefore important to check multiple markers.
Evaluating forma specialis classification using markers.
After testing of the markers on sequenced strains to verify that they worked as anticipated, an extended set of strains originating from around the world (strain information in Data S1) was screened. Most strains were isolated from Fusarium-affected cucurbit plants and were described as one of the pathogenic forms listed in Table 1. The aim was to either confirm or reject their reported host specificity with our markers. A number of strains isolated from noncultivated soil samples was also taken along. The expectation was that these nonspecialized strains do not possess many effector genes and therefore would test negative for all of the 18 markers.
As can be seen from Table 4, marker analysis confirmed the reported forma specialis of most strains that were tested. However, some strains behaved differently than expected. For example, PCR products were identified for Fomln017, Fomln021, Fomln024, and Fomln026 for cucumerinum markers 66 and 21, as well as F. oxysporum f. sp. momordicae/F. oxysporum f. sp. lagenariae marker 1. Intriguingly, none of the F. oxysporum f. sp. melonis markers tested positive in these strains (marker pattern C in Table 4). Additionally, a third F. oxysporum f. sp. melonis pattern was observed with strain Fomln023 that was nearly identical to the pattern of Fomln010 (pattern B in Table 3). Finally, F. oxysporum f. sp. melonis marker 1 cross-reacted with Foniv041 genomic DNA, showing that this marker is not 100% specific for F. oxysporum f. sp. melonis.
TABLE 4.
PCR testing of the markers on a set of 48 worldwide isolates for verification of their reported formae speciales
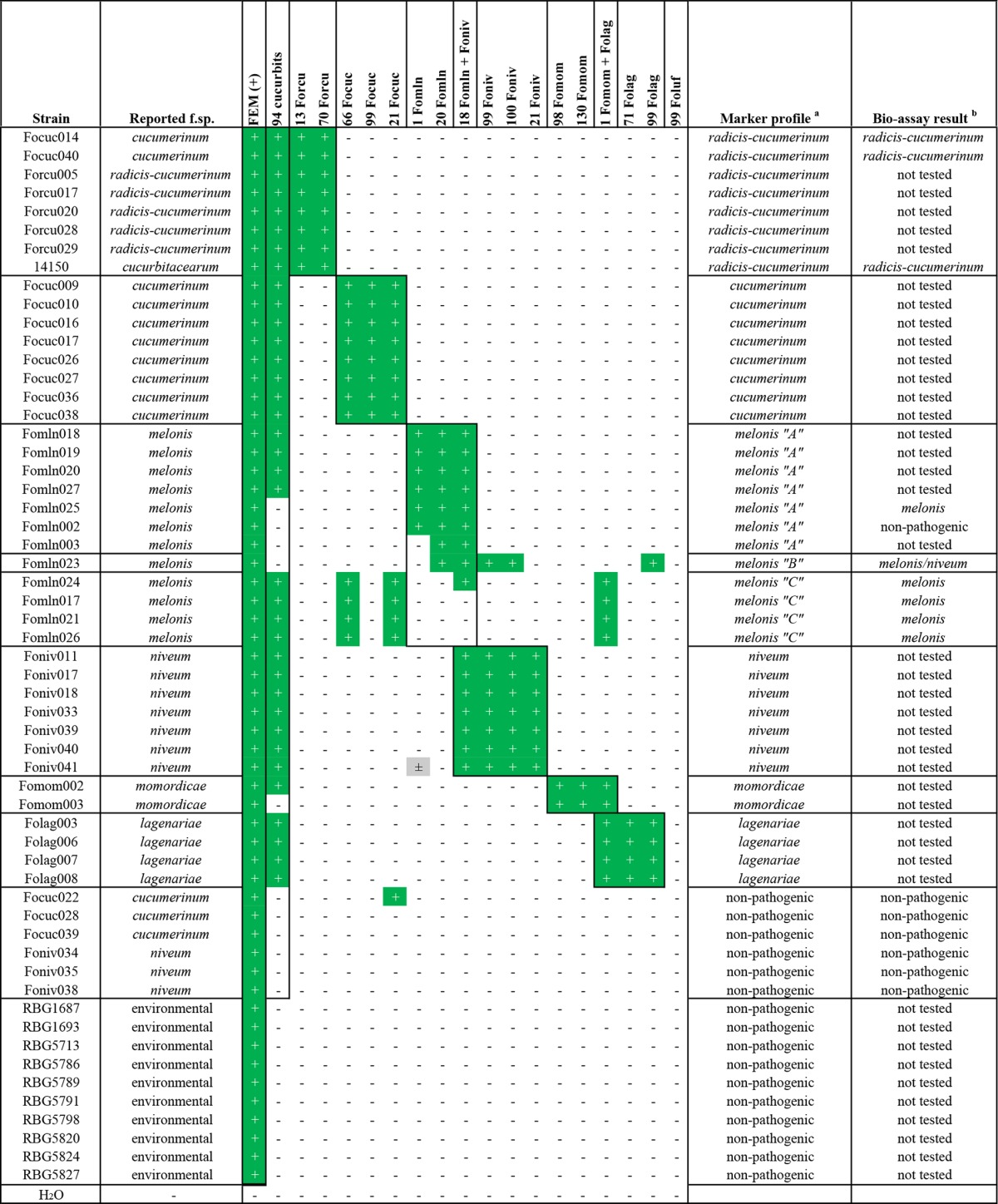
“Non-pathogenic” means not pathogenic toward any of the seven hosts listed in Table 1.
“Non-pathogenic” means no symptom development in susceptible plant hosts of the originally reported forma specialis.
Cucurbit marker 94 did not test positive for four individual F. oxysporum f. sp. melonis strains and one F. oxysporum f. sp. momordicae strain. However, while it does not detect all cucurbit-infecting strains, it did not result in false positives (Tables 3 and 4), meaning that it can still be used in addition to the other forma specialis-specific markers.
Several strains showed a marker pattern typically observed for another forma specialis, indicating that their reported host specificities might not be accurate. Strains Focuc014 and Focuc040, reported as F. oxysporum f. sp. cucumerinum, clearly showed a positive result for both F. oxysporum f. sp. radicis-cucumerinum markers and an absence of all three F. oxysporum f. sp. cucumerinum markers, suggesting that they are in fact F. oxysporum f. sp. radicis-cucumerinum strains. Another interesting candidate was strain 14150, reportedly an isolate belonging to “F. oxysporum f. sp. cucurbitacearum,” a forma specialis proposed to encompass all formae speciales affecting cucurbits (46). This strain also showed the marker pattern typically observed for F. oxysporum f. sp. radicis-cucumerinum. Four strains (one reported as F. oxysporum f. sp. cucumerinum and three as F. oxysporum f. sp. niveum) displayed an absence of all 18 markers tested, while another strain (reported as F. oxysporum f. sp. cucumerinum) tested positive only for F. oxysporum f. sp. cucumerinum marker 21, suggesting that they are not capable of infecting any of the cucurbit plants. As expected, each of the environmental strains tested negative for all of the markers.
Disease assays confirm marker predictions.
The strains of which the reported forma specialis did not match the marker pattern were tested in a bioassay on susceptible cucurbit varieties to evaluate their actual host range (Table 4, rightmost column; Data S3). Strains Focuc014, Focuc040, and 14150 caused severe crown rot symptoms in both cucumber and melon, meaning that they are in fact F. oxysporum f. sp. radicis-cucumerinum strains, as predicted by our PCR analysis. The strains that were predicted to be nonpathogenic based on their marker patterns indeed did not cause symptom development when tested on susceptible cucumber (Focuc028 and Focuc039) or watermelon (Foniv034, Foniv035, and Foniv038) plants. Strains Fomln017, Fomln021, Fomln024, and Fomln026 (profile C) as well as Fomln023 (profile B) were all able to cause disease in susceptible musk melon plants, even though their marker pattern was different from the most common profile in our set of isolates (profile A [Tables 3 and 4]). Fomln023, which tested positive for two of the three F. oxysporum f. sp. niveum markers, was also tested on susceptible watermelon plants. This strain was found to also be capable of causing disease in these plants, whereas Fomln010, with an almost identical marker pattern, was not (33). Fomln002 did not cause symptoms in susceptible melon plants, showing that possessing effector gene sequences alone is not always sufficient for pathogenicity and false positives may show up.
The fact that the bioassay data confirmed the suspected forma specialis predicted by the reported markers indicates that they provide a robust tool for identifying whether an isolate indeed belongs to the suspected forma specialis or not. PCR cross-reaction between F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. melonis, and F. oxysporum f. sp. niveum markers and the cross-pathogenicity of strain Fomln023 suggest a shared evolutionary origin of the formae speciales affecting cucumber, melon, and watermelon.
Specific detection of F. oxysporum f. sp. niveum using a TaqMan assay.
TaqMan real-time PCR has added benefits over traditional PCR: samples can easily be multiplexed, the fluorescent probe provides additional sequence specificity, and the technique allows for quantification of a target DNA sequence, for example, on DNA isolated from soil or diseased plant tissue. A TaqMan experiment was conducted using two of the marker genes in this study, F. oxysporum f. sp. niveum markers 21 and 100. These markers showed good specificity and displayed no cross-reaction with nontarget strains in the PCRs (Tables 3 and 4). TaqMan-specific primers and probes were designed in such a way that 116- and 138-bp F. oxysporum f. sp. niveum-specific amplicons were formed, respectively. As a fluorescent dye, hexachlorofluorescein (HEX; λemission = 556 nm), was used for marker 21 and 6-carboxyfluorescein (FAM; λemission = 518 nm) was used for marker 100. As an internal control for sample/DNA quality that would allow for normalization of the tested markers during multiplexing experiments, a set of primers and a probe with a different fluorescent dye (6-carboxytetramethylrhodamine [TAMRA]; λemission = 580 nm) was designed on a region of EF1α conserved in all F. oxysporum strains. To test the efficiency of the primers and probe sets, a dilution series of F. oxysporum genomic DNA was made and used as the template in a TaqMan assay.
A linear relationship was found between Foniv002 genomic DNA concentration and real-time quantification cycles (Data S4; R2marker21 = 0.999; R2marker100 = 0.998; R2EF1α = 0.999). The pathogen could be detected at template concentrations as low as ∼2.5 pg (Data S4).
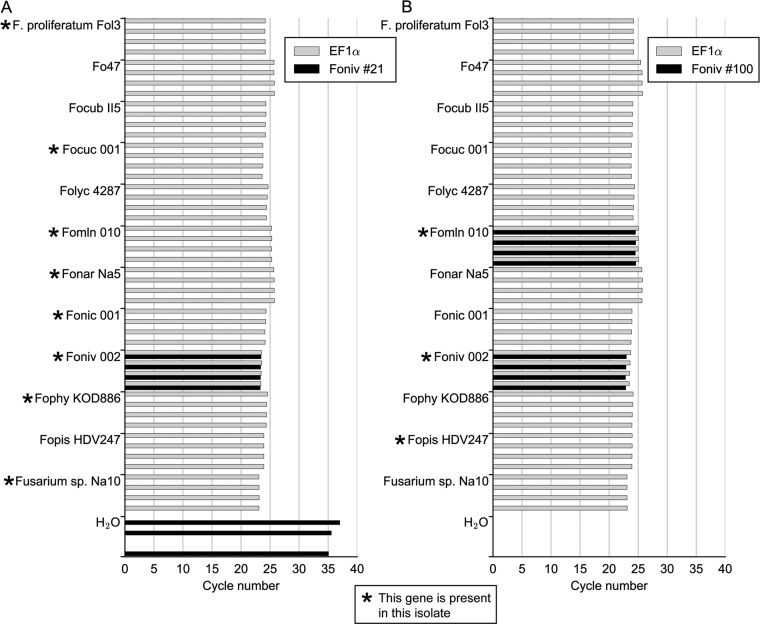
The TaqMan assay was performed on isolates for which marker genes 21 and 100 were identified in the genome assembly (Fig. 2C; see also Data S2H). Each sequence type was included, with the addition of strains of Fusarium proliferatum and a Fusarium sp. that were identified to have candidate effector 100 in a recent study (47). F. oxysporum f. sp. lycopersici 4287, F. oxysporum f. sp. cubense II5, and biocontrol strain Fo47 were included as negative controls, since these do not have either of the marker genes. No cross-reactions were found, except in the case of Fomln010, which possesses a gene sequence identical to marker 100 in F. oxysporum f. sp. niveum isolates (Fig. 3). These results show the applicability of the TaqMan assay for specific detection of F. oxysporum f. sp. niveum DNA in very small quantities.
FIG 3.
TaqMan primer-probe combinations show amplification of F. oxysporum f. sp. niveum DNA (Foniv002) when markers 21 (A) and 100 (B) are tested in duplex with EF1α. No amplification of these markers was detected in any of the non-F. oxysporum f. sp. niveum strains, with the exception of Fomln010, which has an identical gene sequence for hypothetical protein-encoding gene 100. High threshold cycle (CT) values (≥35 cycles) under the detection threshold in the water control of marker 21 are probably caused by primer-dimer formation. Four technical replicates were used per sample, each represented by a bar.
DISCUSSION
In the current study, we tried to make use of comparative genomics to design robust markers based on candidate effector genes. Effectors are functional elements in the pathogen-host interaction and have been found to show very limited sequence diversity between members of the same forma specialis (48, 49). This means that they form ideal targets for marker design (20). Effector gene sequences are often different between formae speciales, although several cases of identical gene sequences have been found in a previous study in our lab (33). For example, the SIX6 and SIX11 homologs present in some isolates belonging to F. oxysporum f. sp. niveum, F. oxysporum f. sp. melonis, and F. oxysporum f. sp. radicis-cucumerinum have 100% nucleotide identity. These sequences can therefore not be used for differentiation of these formae speciales. They do, however, give insight into the evolutionary history of pathogenicity of F. oxysporum toward cucurbits; the presence of sequences that are completely identical between relatively distantly related strains implies recent horizontal transfer of genetic material.
The benefit of using comparative genomics for marker design is that the specificity of the designed markers can directly be evaluated in other genome assemblies (as opposed to RAPD-derived marker sequences). Within the FOSC, one study has reported the use of comparative genomics for forma specialis marker development. This resulted in markers based on unique (random) sequences distinguishing F. oxysporum f. sp. conglutinans from 19 other formae speciales of F. oxysporum (50).
Our goal was to differentiate between formae speciales affecting the Cucurbitaceae family. The respective hosts are highly similar to each other, and incidental cross-pathogenicity between these formae speciales has been described (51–53). We designed a set of 18 primer pairs aiming to discriminate seven cucurbit-infecting formae speciales from each other as well as from other host-specific forms and nonpathogenic strains of F. oxysporum. We found that for F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. radicis-cucumerinum, F. oxysporum f. sp. niveum, F. oxysporum f. sp. lagenariae, F. oxysporum f. sp. momordicae, and F. oxysporum f. sp. luffae, the marker sets allowed clear recognition of the host range of each evaluated strain. Marker 94, designed on a gene encoding a hypothetical protein present in all cucurbit-infecting formae speciales, was positive for all target strains, with the exception of several F. oxysporum f. sp. melonis strains and one F. oxysporum f. sp. momordicae strain. This gene was not identified in the genome sequence of Fomln010.
Within F. oxysporum f. sp. melonis strains, more genetic variability exists than what had been taken into account as a starting point used for marker design (the 10 F. oxysporum f. sp. melonis strains with a sequenced genome). Indeed, F. oxysporum f. sp. melonis has been described as a highly heterogeneous forma specialis, encompassing at least eight VCGs (33, 43, 54). Several F. oxysporum f. sp. melonis strains showed overlap in their effector gene contents with cucurbit-infecting F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. niveum, F. oxysporum f. sp. momordicae, and/or F. oxysporum f. sp. lagenariae (Table 4). So far, no SCAR or other marker sequences have been reported for F. oxysporum f. sp. melonis, possibly due to its heterogeneous nature. Two marker patterns were observed that were different from the marker patterns found in the majority of our F. oxysporum f. sp. melonis strains. Two strains (Fomln010 and Fomln023) tested positive for F. oxysporum f. sp. melonis as well as F. oxysporum f. sp. niveum markers (pattern B). Interestingly, Fomln023 was capable of causing severe wilting symptoms both in melon and in watermelon, while Fomln010 was not (33). This raises the question of whether the separation of these formae speciales is justified, similar to the question of whether strains pathogenic toward both cucumber and melon should be regarded as F. oxysporum f. sp. cucumerinum or F. oxysporum f. sp. melonis. Cafri et al. (53) decided in their study that since the F. oxysporum f. sp. cucumerinum strains they tested were more aggressive toward cucumber than melon and no cross-pathogenicity was found the other way around, these formae speciales should indeed remain distinct. In the case of strain Fomln023 in the current study, disease severities were comparable between watermelon and melon plants, indicating that this strain is a “bridging” forma specialis, and its marker gene pattern reflects this. Isolates bridging multiple host species are not commonly described in the literature, although most isolates admittedly are not tested against a large variety of plant species to confirm host specificity. It would be interesting to compare the genomes of strains with a wider host range with those that are highly specific to one plant species, which may have implications for the current nomenclature system within the FOSC.
We recently demonstrated that clustering isolates based on patterns of presence or absence of candidate effector genes divided F. oxysporum f. sp. cucumerinum into two groups, separated from each other by F. oxysporum f. sp. melonis and F. oxysporum f. sp. niveum strains (33). The cucurbit-infecting isolates formed a supercluster from other formae speciales, indicating that they share a significant number of effector genes between them. Not much is known regarding the evolution of host specificity of F. oxysporum toward cucurbits, but Fomln023 might contain accessory genetic material originating from both an F. oxysporum f. sp. niveum and an F. oxysporum f. sp. melonis strain. Likewise, strains Fomln017, Fomln021, Fomln024, and Fomln026 tested positive for two F. oxysporum f. sp. cucumerinum markers and the F. oxysporum f. sp. melonis markers used all tested negative (pattern C). This indicates that these F. oxysporum f. sp. melonis and F. oxysporum f. sp. cucumerinum strains also share accessory genetic material. However, Fomln017 is, like most F. oxysporum f. sp. melonis isolates, highly specific to melon plants. It would be interesting to further investigate these strains, for example, through long-read sequencing of their genomes and analysis of their pathogenicity chromosome(s) compared to those of other F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. melonis, and F. oxysporum f. sp. niveum strains. This could shed light on how pathogenicity toward cucurbits has evolved in the FOSC.
Horizontal gene and chromosome transfer has been described as an important contributor to genetic diversity and the generation of new (pathogenic) clonal lines in fungi (55, 56). The different effector sequences and presence/absence patterns between and even within some cucurbit-infecting formae speciales suggest that it is possible that multiple horizontal transfers of accessory genome material have taken place in the evolutionary trajectory, resulting in pathogenicity of F. oxysporum toward cucurbits. This is in contrast to the case with F. oxysporum f. sp. lycopersici: the four clonal lines that were tested in the work of van Dam et al. all have nearly identical sets of effectors and effector gene sequences (25, 33).
Minor cross-reaction (a much lighter band) was found with one of the markers (F. oxysporum f. sp. momordicae marker 130) with an unrelated forma specialis. F. oxysporum f. sp. pisi HDV247 (as well as F. oxysporum f. sp. raphani PHW815) indeed possesses this gene, although its downstream flank on which the reverse primer (FP7336) was designed was deemed to be sufficiently different from the copy in F. oxysporum f. sp. momordicae; only the four 5′ nucleotides matched between these two sequences. The forward primer contained only a single nucleotide polymorphism (SNP), meaning that it probably binds in the nontarget sequence of F. oxysporum f. sp. pisi, too. A similar observation was made for F. oxysporum f. sp. melonis marker 1 and Foniv041 genomic DNA. For this isolate, however, no genome sequence is available. Through quantitative PCR techniques such as TaqMan, (cross-)reactions with a significantly smaller amount of product can probably be distinguished from genuine positives.
As a proof of concept, a TaqMan test was developed for two of the markers. The TaqMan real-time PCR technique has several advantages over traditional PCR. Since it makes use of a sequence-specific fluorescently tagged probe in addition to the primer sequences, marker specificity is potentially higher. Additionally, the technique allows for quantification of the targeted DNA sequence (and thus of the pathogen in soil or infected plant tissue). Quantification of pathogenic F. oxysporum propagules in soil, seeds, or plant tissues may aid in deciding if and when to take action. Also, it is possible to test multiple markers by multiplexing, using several different fluorescent dyes at once (57–59). The markers that were tested in duplex for F. oxysporum f. sp. niveum behaved like expected: no amplification was identified in other strains (except Fomln010 with marker 100), even those that do possess the target gene. The technique allows for identification of sequences slightly different from the target sequence; the cycle number of a single copy marker with SNPs would be distinguishably higher than that of a positive-control single-copy gene like EF1α.
These findings illustrate the hurdles that can be experienced in the process of designing forma specialis-specific markers based on candidate effector genes, specifically if the formae speciales infect members of the same plant family and possibly arose through a shared and recent evolutionary history. Nonetheless, the combination of marker sequences described here can be used with relatively high fidelity to discriminate the seven cucurbit-affecting formae speciales, particularly when multiple markers are tested simultaneously in the analysis. It is possible—perhaps even likely—that more diversity exists among the seven formae speciales targeted in this study, since for several of the formae speciales not all VCGs were sampled for genome sequencing due to unavailability of these strains. This means that the markers might require revision in the future. The availability of more whole-genome sequences like the ones generated in this study will allow easier marker design and comparison in the future.
MATERIALS AND METHODS
Fungal strains and accession numbers.
An overview of the strains that were used in this study and their respective genome assembly or nucleotide sequence accession numbers can be found in Table 5.
TABLE 5.
Overview of fungal strains used in this study and their NCBI genome accession numbers
| Strain | Original designation | Forma specialis or description | VCG | Race | Origin of strain | Source or referencea | GenBank assembly accession no. |
|---|---|---|---|---|---|---|---|
| Folyc 4287 | NRRL34936 | lycopersici | 0030 | 2 | Spain | Broad Institute | GCA_000149955.2 |
| Focon 5176 | Fo5176 | On Brassica | Australia | Broad Institute | GCA_000222805.1 | ||
| Folyc MN25 | NRRL54003 | lycopersici | 0033 | 3 | USA | Broad Institute | GCA_000259975.2 |
| Fopis HDV247 | NRRL37622 | pisi | Broad Institute | GCA_000260075.2 | |||
| Forly CL57 | NRRL26381 | radicis-lycopersici | 0094 | USA (Florida) | Broad Institute | GCA_000260155.3 | |
| Fovas 25433 | NRRL25433 | vasinfectum | China | Broad Institute | GCA_000260175.2 | ||
| Focub II-5 | NRRL54006 | cubense | 01213 | TR4 | Indonesia | Broad Institute | GCA_000260195.2 |
| Focon PHW808 | NRRL54008 | conglutinans | 0101 | 2 | Broad Institute | GCA_000260215.2 | |
| Forap PHW815 | NRRL54005 | raphani | 0102 | Broad Institute | GCA_000260235.2 | ||
| Fomln001 | NRRL26406 | melonis | 0136 | 1 | Mexico | Broad Institute | GCA_000260495.2 |
| Fo47 | NRRL54002 | Nonpathogen, biocontrol | France | Broad Institute | GCA_000271705.2 | ||
| Focli FOSC 3-a | NRRL32931 | Clinical isolate, from human blood | USA (Massachusetts) | Broad Institute | GCA_000271745.2 | ||
| Focub N2 | N2 | cubense | 1 | China | 72 | GCA_000350345.1 | |
| Focub B2 | B2 | cubense | 4 | China | 72 | GCA_000350365.1 | |
| Focuc013 | 9904-1 | cucumerinum | 0186 | China | 22 | MABJ01000000 | |
| Focuc015 | 9906-3 | cucumerinum | 0184 | China | 22 | MABK01000000 | |
| Focuc021 | ATCC 16416 | cucumerinum | 0180 | USA (Florida) | 22 | MABL01000000 | |
| Focuc018 | Afu-50(B) | cucumerinum | 0180 | Crete, Greece | 22 | MABM01000000 | |
| Focuc030 | FOCU-22P | cucumerinum | 0180 | Israel | 22 | MABN01000000 | |
| Focuc035 | NETH 11179 | cucumerinum | 0181 | Netherlands | 22 | MABO01000000 | |
| Focuc037 | Tf-213 | cucumerinum | 0185 | Japan | 22 | MABP01000000 | |
| Forcu016 | 33 | radicis-cucumerinum | 0260 | Canada | 22 | MABQ02000000 | |
| Forcu024 | Afu-11(A) | radicis-cucumerinum | 0260 | Crete, Greece | 22 | MABR01000000 | |
| Forcu031 | AK-2 | radicis-cucumerinum | 0261 | Crete, Greece | 22 | MABS01000000 | |
| Focuc011 | 9903-1 | cucumerinum | 0186 | China | 22 | MABT01000000 | |
| Fomln005 | Fom 0123 | melonis | 0134 | 1 | Spain | 73 | MAKY01000000 |
| Focuc001 | Foc-1 | cucumerinum | 0183 | Japan | B.L. | MAKZ01000000 | |
| Fomln004 | Fom 0122 | melonis | 0134 | 0 | Spain | 73 | MALA01000000 |
| Fomln006 | Fom 0124 | melonis | 0134 | 2 | Spain | 73 | MALB01000000 |
| Fomln009 | melonis | 0135 | 2 | Israel | 73 | MALC01000000 | |
| Fomln010 | melonis | 1 | Israel | 73 | MALD01000000 | ||
| Fomln012 | ML2 | melonis | 0134 | 0 | 73 | MALE01000000 | |
| Fomln016 | Fom26 | melonis | 0134 | 1 | 73 | MALF01000000 | |
| Fomln013 | melonis | 0134 | 2 | Spain | 73 | MALG01000000 | |
| Folyc004 | IPO1530/B1 | lycopersici | 0030 | 1 | Netherlands | 74 | MALH01000000 |
| Folyc007 | D2 | lycopersici | 0030 | 2 | France | 74 | MALI01000000 |
| Folyc014 | LSU-3 | lycopersici | 0030 | 1 | USA (Louisiana) | 74 | MALJ01000000 |
| Folyc026 | BRIP 14844 (M1943) | lycopersici | 0030 | 3 | Australia | 74 | MALK01000000 |
| Folyc018 | LSU-7 | lycopersici | 0030 | 2 | USA (Louisiana) | 74 | MALL01000000 |
| Folyc016 | BFOL-51 | lycopersici | 0031 | 1 | USA (Louisiana) | 74 | MALM01000000 |
| Folyc029 | 5397 | lycopersici | 0030 | 3 | USA (Florida) | 74 | MALN01000000 |
| Folyc038 | CA92/95 | lycopersici | 0030 | 3 | USA (California) | 25 | MALO01000000 |
| Folyc069 | DF0-23 | lycopersici | 0035 | 2 | USA (California) | 75 | MALP01000000 |
| Folyc072 | DF0-38 | lycopersici | 0031 | 2 | USA (California) | 75 | MALQ01000000 |
| Folyc073 | DF0-40 | lycopersici | 0030 | 2 | USA (California) | 75 | MALR01000000 |
| Folyc074 | DF0-41 | lycopersici | 0030 | 3 | USA (California) | 75 | MALS01000000 |
| Folyc075 | DF0-62 | lycopersici | 0031 | 2 | USA (California) | 75 | MALT01000000 |
| FoMN14 | MN-14 | Nonpathogen, from tomato plant | USA (California) | 76 | MALU01000000 | ||
| Foniv002 | CBS 418.90 | niveum | Israel | 22 | MALX01000000 | ||
| Foniv005 | TX-471-1 | niveum | 0080 | 0 | USA (Texas) | 51 | MALY01000000 |
| Foniv010 | F-016-1 | niveum | 0082 | 1 | USA (Maryland) | 51 | MALZ01000000 |
| Foniv013 | F-014-2 | niveum | 0082 | 2 | USA (Maryland) | 51 | MAMA01000000 |
| Foniv015 | F-063-1 | niveum | 0082 | 2 | USA (Maryland) | 51 | MAMB01000000 |
| Foniv019 | TX-X1D | niveum | 0082 | 2 | USA (Texas) | 51 | MAMC01000000 |
| Foniv020 | F-099-1 | niveum | 0083 | 2 | USA (Delaware) | 51 | MAMD01000000 |
| Foniv021 | MD-ZE622 | niveum | 3 | USA (Maryland) | 51 | MAME01000000 | |
| Foniv037 | NRRL38539 | niveum | Israel | 77 | MAMF01000000 | ||
| Folyc002 | WCS862/E241 | lycopersici | 0030 | 2 | Netherlands | 74 | MAMG01000000 |
| Fomln011 | melonis | 0 | Israel | 73 | MAMH01000000 | ||
| Fogla G14 | G14 | gladioli | 0341 | Netherlands | 78 | NJCM01000000 | |
| Fogla G2 | G2 | gladioli | 0340 | France | 78 | NJCL01000000 | |
| Fogla G76 | G76 | gladioli | 0343 | Italy | 78 | NJCK01000000 | |
| Folag001 | 01-03008 | lagenariae | Japan | 32 | NJCJ01000000 | ||
| Folag002 | 03-05118 | lagenariae | Japan | 32 | NJCI01000000 | ||
| Folag004 | Lag:3-1 (JCM9293) | lagenariae | Japan | 32 | NJCH01000000 | ||
| Folag005 | Lag:1-1 | lagenariae | Japan | 32 | NJCG01000000 | ||
| Folil Fol39 | Fol39 | lilii | Netherlands | 79; J.V.D. | NJCF01000000 | ||
| Foluf001 | Fol-114 | luffae | Taiwan | 25 | NJCE01000000 | ||
| Foluf002 | Fol-167 | luffae | Taiwan | 25 | NJCD01000000 | ||
| Fomel001 | J-71 | melongenae | IPO | NJCC01000000 | |||
| Fomom001 | NRRL26413 | momordicae | Taiwan | ARS | NJCB01000000 | ||
| Fomom004 | 90NF2-1 (JCM9292) | momordicae | Japan | 32 | NJCA01000000 | ||
| Fonar Na5 | Na5 | narcissi | 2 | Netherlands | 79; J.V.D. | NJCV01000000 | |
| Fonic001 | FON-1 | nicotianae | USA (Connecticut) | 80 | NJBZ01000000 | ||
| Fonic003 | 10913 | nicotianae | 0373 | USA (Maryland) | 80 | NJBY01000000 | |
| Fonic010 | Ft-Rob | nicotianae | 0378 | USA (North Carolina) | 80 | NJBX01000000 | |
| Fonic012 | Ft-1512 | nicotianae | USA (North Carolina) | 80 | NJCU01000000 | ||
| Fophy KOD886 | KOD886 | physali | USA (California) | K.O. | NJBW01000000 | ||
| Fophy KOD887 | KOD887 | physali | USA (California) | K.O. | NJBV01000000 | ||
| Fophy KOD888 | KOD888 | physali | USA (California) | K.O. | NJBU01000000 | ||
| Fo Tu58 | Tu58 | Nonpathogenic, from symptomatic tulip) | Netherlands | 79; J.V.D. | NJBT01000000 | ||
| Fotul Tu67 | Tu67 | tulipae | Netherlands | 79; J.V.D. | NJBS01000000 | ||
| 14150 | 14150 | cucurbitacearum (redesignated radicis-cucumerinum) | Netherlands | NAKT | Not available | ||
| Focuc009 | 0020 | cucumerinum | 0187 | China | 22 | Not available | |
| Focuc010 | 9901-2 | cucumerinum | 0186 | China | 22 | Not available | |
| Focuc014 | 9906-2 | cucumerinum (redesignated radicis-cucumerinum) | 0184 | China | 22 | Not available | |
| Focuc016 | 9909-2 | cucumerinum | 0185 | China | 22 | Not available | |
| Focuc017 | 9909-3 | cucumerinum | 0186 | China | 22 | Not available | |
| Focuc022 | ATCC 36330 | cucumerinum (redesignated a nonpathogen) | 0180 | Israel | 22 | Not available | |
| Focuc026 | ATCC 201950 | cucumerinum | 0180 | USA (Florida) | 22 | Not available | |
| Focuc027 | Cu:4-1 Koma 4 | cucumerinum | 0181 | Japan | 22 | Not available | |
| Focuc028 | Cu:5-0 Koma 5 | cucumerinum (redesignated a nonpathogen) | 0183 | Japan | 22 | Not available | |
| Focuc036 | NRRL26437 | cucumerinum | USA (South Carolina) | ARS | Not available | ||
| Focuc038 | NRRL26744 | cucumerinum (redesignated a nonpathogen) | Japan | ARS | Not available | ||
| Focuc039 | NRRL38591 | cucumerinum | New Zealand | ARS | Not available | ||
| Focuc040 | 07-08969 | cucumerinum (redesignated radicis-cucumerinum) | Netherlands | NAKT | Not available | ||
| Folag003 | 07-27503 | lagenariae | Japan | 32 | Not available | ||
| Folag006 | Lag:7-1 | lagenariae | Kumamoto, Japan | 32 | Not available | ||
| Folag007 | No. 87 | lagenariae | Tochigi, Japan | 32 | Not available | ||
| Folag008 | No. 134 | lagenariae | Tochigi, Japan | 32 | Not available | ||
| Fomln002 | CBS 420.90 | melonis | Israel | 22 | Not available | ||
| Fomln003 | CBS 423.90 | melonis | Israel | 22 | Not available | ||
| Fomln017 | NRRL22518 | melonis | USA | 81 | Not available | ||
| Fomln018 | NRRL22519 | melonis | France | 81 | Not available | ||
| Fomln019 | NRRL22520 | melonis | USA | 81 | Not available | ||
| Fomln020 | NRRL22521 | melonis | Belgium | 81 | Not available | ||
| Fomln021 | NRRL26172 | melonis | China | ARS | Not available | ||
| Fomln023 | NRRL26174 | melonis | China | ARS | Not available | ||
| Fomln024 | NRRL26745 | melonis | Japan | ARS | Not available | ||
| Fomln025 | NRRL26746 | melonis | Japan | ARS | Not available | ||
| Fomln026 | NRRL38516 | melonis | New Zealand | ARS | Not available | ||
| Fomln027 | NRRL38524 | melonis | New Zealand | ARS | Not available | ||
| Fomom002 | NRRL26748 | momordicae | Japan | 82 | Not available | ||
| Fomom003 | 90NF1-2 | momordicae | Kagoshima, Japan | 32 | Not available | ||
| Foniv011 | F-086-1 | niveum | 0082 | 1 | USA (Maryland) | 83 | Not available |
| Foniv017 | F-097-2 | niveum | 0082 | USA (Delaware) | 51 | Not available | |
| Foniv018 | F-100-2 | niveum | 0082 | 2 | USA (Delaware) | 51 | Not available |
| Foniv033 | NRRL26747 | niveum | Japan | ARS | Not available | ||
| Foniv034 | NRRL36275 | niveum (redesignated a nonpathogen) | ARS | Not available | |||
| Foniv035 | NRRL38278 | niveum (redesignated a nonpathogen) | USA | ARS | Not available | ||
| Foniv036 | NRRL38503 | niveum | New Zealand | ARS | Not available | ||
| Foniv038 | NRRL38552 | niveum (redesignated a nonpathogen) | Israel | ARS | Not available | ||
| Foniv039 | LB | niveum | 0 | NAKT | Not available | ||
| Foniv040 | IPO 30288 | niveum | 1 | IPO | Not available | ||
| Foniv041 | CBS 419.90 | niveum | Israel | 22 | Not available | ||
| Forcu005 | 14 | radicis-cucumerinum | Canada | 22 | Not available | ||
| Forcu017 | 34 | radicis-cucumerinum | Canada | 22 | Not available | ||
| Forcu020 | 38 | radicis-cucumerinum | France | 22 | Not available | ||
| Forcu028 | Afu-58 | radicis-cucumerinum | 0260 | Crete, Greece | 22 | Not available | |
| Forcu029 | Afu-68(A) | radicis-cucumerinum | 0260 | Crete, Greece | 22 | Not available | |
| RBG1687 | RBG1687 | Nonpathogen, from Wollemia nobilis seedling leaves | Australia | M.L. | Not available | ||
| RBG1693 | RBG1693 | From flannel flower roots | Australia | M.L. | Not available | ||
| RBG5713 | RBG5713 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5786 | RBG5786 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5789 | RBG5789 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5791 | RBG5791 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5798 | RBG5798 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5820 | RBG5820 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5824 | RBG5824 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| RBG5827 | RBG5827 | Nonpathogen, from soil | Australia | 49 | Not available | ||
| F. proliferatum Fol3 | Fol3 | From a Lilium sp. | Netherlands | 47 | NJCT01000000 | ||
| Fusarium sp. strain Na10 | Na10 | From a Narcissus sp. | Netherlands | 47 | NJCS01000000 |
Abbreviations and initials: NAKT, NAKtuinbouw, Netherlands Inspection Service for Horticulture, Roelofarendsveen, Netherlands; B.L., Bart Lievens, Scientia Terrae Research Institute, Belgium; J.V.D., Joop van Doorn, PPO Research Centre, Lisse, Netherlands; M.L., Matthew Laurence, Plant Disease Diagnostic Unit of the Royal Botanic Gardens and Domain Trust, Sydney, Australia; ARS, Agricultural Research Service, USDA, USA; IPO, Plant Research International (formerly Instituut voor Planteziektenkundig Onderzoek), Wageningen, Netherlands; K.O., Kerry O'Donnell, USDA ARS, Peoria, IL.
Whole-genome sequencing and de novo assembly.
F. oxysporum genomic DNA was isolated through phenol-chloroform extraction from freeze-dried mycelium that was harvested from 5-day-old NO3 medium (0.17% yeast nitrogen base, 3% sucrose, 100 mM KNO3) cultures as described in detail in reference 33. Library preparation of insert size 550 bp and Illumina HiSeq 2500 paired-end sequencing were performed at Keygene N.V. (Wageningen, Netherlands).
Sequencing reads were trimmed for quality and to remove adapter sequences with FastqMcf v1.04.676 (https://github.com/ExpressionAnalysis/ea-utils/blob/wiki/FastqMcf.md; quality threshold = 20). De novo assemblies were generated using CLC-workbench 8.0. Default settings were used, except “minimum contig length = 500.”
For generating a core phylogeny, homologs of 15,956 Fol4287 core genes (including introns) were searched in all genomes using BLASTN with default parameters. We selected all sequences that overlapped >70% with the query sequence and with more than 80% identity to the query. We then selected query genes for which we found only a single hit in each genome, leaving us with 440 genes. We used ClustalO (60) to construct a multiple-sequence alignment for each gene and a custom python script to concatenate these alignments. This alignment was subsequently trimmed using trimAl-strictplus. We used PhyML v20120412 (61) with 100 bootstraps to infer phylogeny and ETE v3.0.0b35 (62) to visualize the tree.
Marker discovery and primer design.
A custom python script was written to extract the sequence (plus 150 bp up- and downstream) of candidate effector genes from each of the genome assemblies using BLASTN with default parameters. MUSCLE v3.8.31 (63) was used to generate alignments of each gene, and phylogeny was inferred using PhyML v20120412 with 100 bootstraps. Another python script was used to traverse the tree in ETE v3 to identify instances where all isolates belonging to a forma specialis were clustering together in a separate clade, indicating sequence similarity that could potentially be used for primer design. Highlighting, drawing, and rendering of the gene trees were done using ETE v3. Visual inspection of each of the gene trees allowed for the selection of a final set of marker genes per forma specialis. Scripts are available upon request.
Primers were designed manually based on the sequence alignment per gene (see Data S1 in the supplemental material). In cases where only a few SNPs were identified to separate host specificity of isolates, we aimed to target the mismatching nucleotides toward the 3′ end of the primer, as described in reference 64.
DNA isolation.
Genomic DNA isolation for testing of markers was performed using 10- to 20-day-old mycelium scraped off a peptone-dextrose agar (PDA) plate as starting material. The tissue was disrupted by shaking it in a TissueLyser (Qiagen) for 2 min at 30 Hz in the presence of 400 μl of Tris-EDTA (TE), 300 μl of phenol-chloroform (1:1), and glass beads. The aqueous phase was transferred to a fresh tube, and an equal volume of chloroform was added. The DNA in the aqueous phase was transferred to a fresh tube and diluted 10× with sterile Milli-Q water prior to use in PCR. DNA quantity was estimated for the TaqMan standard curve using a Qubit fluorometer (Thermo Fisher Scientific).
PCR.
PCR was executed using Sphaero-Q Super Taq (Gorinchem, Netherlands) in 20-μl reaction volumes which included the following components (final concentration): 1× Sphaero-Q Super Taq buffer, 0.25 U of Sphaero-Q Super Taq, 5 pmol of each primer, deoxynucleoside triphosphates (dNTPs; 0.2 mM each), and 1 μl of template DNA. The following PCR program was used: 2 min at 94°C; 35 cycles of 30 s at 94°C, 30 s at annealing temperature (Tann), and 40 s at 72°C; 5 min at 72°C; and a pause at 16°C. The PCR primer sequences and corresponding annealing temperatures are listed in Table 6. Fusarium FEM1 primers were used as a positive control and sterile Milli-Q was used as a negative control for each of the primer combinations instead of template DNA.
TABLE 6.
Primers and annealing temperatures used in this study
| Gene ID | Target gene | Target forma specialis | Primer name | Primer sequence (5′–3′) | Tann (°C) | Product size (nt) |
|---|---|---|---|---|---|---|
| + | FEM1 | Positive control | fp157 | ATGAAGTACACTCTCGCTACC | 54 | 274 |
| fp158 | GGTGAAAGTGAAAGAGTCACC | |||||
| 94 | HPEG | All cucurbit infecting | fp7304 | GCCTCATTGAAGTTTCAACA | 54 | 346 |
| fp7321 | TGGTAAAGGACACGACCATT | |||||
| 13 | SIX13 | radicis-cucumerinum | fp7305 | TTGCCCAAAATGGCATGTTT | 56 | 328 |
| fp7322 | CATTGACACTGTAAGTGGG | |||||
| 70 | HPEG | radicis-cucumerinum | fp7306 | TACAACCTCTCTCTTTCCTT | 54 | 454 |
| fp7323 | GCTGAATTCTAGCAGAGAAT | |||||
| 66 | HPEG | cucumerinum | fp7307 | CCGTTATGGCCAGAGATC | 54 | 425 |
| fp7324 | CCAACAAACAGAGCAAAACTAA | |||||
| 99 | HPEG | cucumerinum | fp7308 | CTACCAATCTCTCCTGAGTG | 54 | 445 |
| fp7325 | GTCGATTGCAGTGCTAGTCT | |||||
| 21 | Fom effector 7 | cucumerinum | fp7309 | CAGTCTAACCCTGTCTCATT | 54 | 381 |
| fp7326 | CGCCAATAGATAGTGATGGA | |||||
| 1 | SIX1 | melonis | fp7310 | CCTCTCAGTCCTTGGGTCT | 54 | 397 |
| fp7327 | ACTCGCTTCAGCTTACCGA | |||||
| 20 | Fom effector 6 | melonis | fp7406 | TGAAAGTCTTGGCGGGTGT | 56 | 305 |
| fp7328 | TCCTCTCCATCCTCATCAGT | |||||
| 18 | Fom effector 3 | melonis + niveum | fp7312 | TTAGTGCAGCTTTTCTCCTC | 54 | 299 |
| fp7329 | AGTGGTTAGTCAAGTGGTAA | |||||
| 99 | HPEG | niveum | fp7313 | TGCCGGGCTAGTTAATATAGT | 54 | 406 |
| fp7330 | ACCATTTTTCTGTTGGGGTTG | |||||
| 100 | HPEG | niveum | fp7314 | ATTTTGCTAGCTTCAGCAGTT | 54 | 482 |
| fp7331 | ATCCTGAACGGTGACTAGAG | |||||
| 21 | Fom effector 7 | niveum | fp7315 | CGCTCGCTATAATTCAAACG | 54 | 139 |
| fp7332 | GGAGGAGCACTACAACTAAT | |||||
| 71 | HPEG | lagenariae | fp7407 | TAGTCCAATCTGCCTCAGCAA | 54 | 270 |
| fp7410 | GGAAGTGAGCATTCTTCCGTA | |||||
| 99 | HPEG | lagenariae | fp7408 | TCGTATCTCTCAGTAGTATGG | 54 | 367 |
| fp7411 | AATGGATACCTTATAAGGGCT | |||||
| 1 | SIX1 | lagenariae + momordicae | fp7409 | TTGGGATTGCGGCTTATGCT | 56 | 463 |
| fp7412 | AAAGTGGTACACTCCGTGC | |||||
| 98 | HPEG | momordicae | fp7318 | AGGTGCAGCGTTTTTAGGT | 60 | 469 |
| fp7335 | GAGGGCTGGTTGAGAACTA | |||||
| 130 | HPEG | momordicae | fp7319 | TCTACGCTTCGAGGATGGTA | 56 | 368 |
| fp7336 | TCGTTTAGACGACTACAACC | |||||
| 99 | HPEG | luffae | fp7320 | TACTCTCCTAGAGTCAGTCT | 54 | 606 |
| fp7337 | CACGCCATCATCCTTTATTC |
TaqMan real-time PCR.
TaqMan reverse transcription-PCRs (RT-PCRs) were performed on a QuantStudio 3 system (Thermo Fisher Scientific). Primers and probes were designed using Primer3web v4.0.0 (http://primer3.ut.ee/), and their sequences can be found in Table 7. A total volume of 10 μl of the reaction mixture included the following components (final concentrations): 1× Sphaero-Q Super Taq buffer, 0.25 U of Sphaero-Q Super Taq (Gorinchem, Netherlands), 3 pmol of each primer, 1 pmol of each probe, dNTPs (0.2 mM each), 0.1× ROX reference dye (Thermo Fisher Scientific), and 1 μl of template DNA. Four simultaneous amplifications were performed for each sample to confirm the reproducibility of the results. A negative-control sample consisted of sterile Milli-Q substituted for the DNA template. The PCR program was set as follows: 2 min at 94°C and 40 cycles of 30 s at 94°C, 48 s at 60°C, and 12 s at 60°C (data collection).
TABLE 7.
Primers and probes used for the TaqMan experiments
| Gene ID | Target gene | Target forma specialis | Primer name | Sequence (5′–3′) | Product size (nt) |
|---|---|---|---|---|---|
| 21 | Fom effector 7 | niveum | fp7589 | CCGGTACCCCAGCTTTATGT | 116 |
| fp7590 | CAGCAACGTTCTGAAAGCGT | ||||
| probe_3 | HEX-TGCAGGTTGGCAGGCCCCTG-BHQ1 | ||||
| 100 | HPEG | niveum | fp7591 | CACCAACAACTATGCGGCAC | 138 |
| fp7592 | GCAATTGACCCAGCTGCAAT | ||||
| probe_4 | FAM-AGTCGCCGGCCACCACATTGA-BHQ1 | ||||
| EF1α | Elongation factor 1 α | All strains | fp7710 | CGCTGAGCTCGGTAAGGG | 97 |
| fp7711 | CCAGAGAGCAATATCGATGGTGA | ||||
| probe_7 | TAMRA-ACGCCTGGGTTCTTGACAAGCTCA-BHQ2 |
Disease assays.
Pathogenicity tests were performed using the root dip method (65). In short, conidia were isolated from 5-day-old cultures in NO3 medium (0.17% yeast nitrogen base, 3% sucrose, 100 mM KNO3) by filtering through Miracloth (Merck; pore size of 22 to 25 μm). Spores were centrifuged, resuspended in sterile Milli-Q water, counted, and brought to a final concentration of 107/ml. When the first true leaves were emerging (after ~10 days), 5 to 8 seedlings per treatment were uprooted, inoculated, individually potted, and kept at 20°C (F. oxysporum f. sp. radicis-cucumerinum) or 25°C (all other formae speciales) in the greenhouse. The following plant cultivars were used: Cucumis sativus cv. Paraiso, Cucumis melo cv. Cha-T, and Citrullus lanatus cv. Black Diamond. Two weeks after inoculation, disease was scored using a disease index from 0 to 4 as described in detail by van Dam et al. (33).
Accession number(s).
Whole-genome shotgun projects for the newly sequenced strains of F. oxysporum have been deposited at GenBank under BioProject no. PRJNA389501. Raw sequence data have been deposited into the Sequence Read Archive under accession number SRP109253. All publicly available genome sequences that were used were obtained from GenBank. Their NCBI accession numbers can be found in Table 5.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the Horizon program (project 93512007) of the Netherlands Genomics Initiative (NGI) through a grant to M. Rep.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01868-17.
REFERENCES
- 1.Armstrong GM, Armstrong JK. 1978. Formae speciales and races of Fusarium oxysporum causing wilts of the Cucurbitaceae. Phytopathology 68:19. doi: 10.1094/Phyto-68-19. [DOI] [Google Scholar]
- 2.Del Mar Jiménez-Gasco M, Jiménez-Díaz RM. 2003. Development of a specific polymerase chain reaction-based assay for the identification of Fusarium oxysporum f. sp. ciceris and its pathogenic races 0, 1A, 5, and 6. Phytopathology 93:200–209. doi: 10.1094/PHYTO.2003.93.2.200. [DOI] [PubMed] [Google Scholar]
- 3.Inami K, Yoshioka-Akiyama C, Morita Y, Yamasaki M, Teraoka T, Arie T. 2012. A genetic mechanism for emergence of races in Fusarium oxysporum f. sp. lycopersici: inactivation of avirulence gene AVR1 by transposon insertion. PLoS One 7:e44101. doi: 10.1371/journal.pone.0044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martyn RD. 2014. Fusarium wilt of watermelon: 120 years of research. Hortic Rev 42:349–442. [Google Scholar]
- 5.Chen KS, Chang P-F, Liou TD, Huang JW. 2003. Identification of physiological races of Fusarium oxysporum f. sp. niveum and breeding for Fusarium wilt-resistant watermelon line. Plant Pathol Bull 12:247–254. [Google Scholar]
- 6.Vakalounakis DJ, Wang Z, Fragkiadakis GA, Skaracis GN, Li D-B. 2004. Isolates obtained from cucumber in China by pathogenicity, VCG, and RAPD. Plant Dis 88:645–649. doi: 10.1094/PDIS.2004.88.6.645. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Martyn RD, Magill CW. 1993. Mitochondrial DNA (mtDNA)-relatedness among formae speciales of Fusarium oxysporum in the Cucurbitaceae. Phytopathology 83:91–97. doi: 10.1094/Phyto-83-91. [DOI] [Google Scholar]
- 8.Zhang Z, Zhang J, Wang Y, Zheng X. 2005. Molecular detection of Fusarium oxysporum f. sp. niveum and Mycosphaerella melonis in infected plant tissues and soil. FEMS Microbiol Lett 249:39–47. doi: 10.1016/j.femsle.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Lievens B, Brouwer M, Vanachter ACRC, Cammue BPA, Thomma BPHJ. 2006. Real-time PCR for detection and quantification of fungal and oomycete tomato pathogens in plant and soil samples. Plant Sci 171:155–165. doi: 10.1016/j.plantsci.2006.03.009. [DOI] [Google Scholar]
- 10.Pavlou GC, Vakalounakis DJ, Ligoxigakis EK. 2002. Control of root and stem rot of cucumber, caused by Fusarium oxysporum f. sp. radicis-cucumerinum, by grafting onto resistant rootstocks. Plant Dis 86:379–382. doi: 10.1094/PDIS.2002.86.4.379. [DOI] [PubMed] [Google Scholar]
- 11.Cohen R, Tyutyunik J, Fallik E, Oka Y, Tadmor Y, Edelstein M. 2014. Phytopathological evaluation of exotic watermelon germplasm as a basis for rootstock breeding. Sci Hortic 165:203–210. doi: 10.1016/j.scienta.2013.11.007. [DOI] [Google Scholar]
- 12.Cohen R, Orgil G, Burger Y, Saar U, Elkabetz M, Tadmor Y, Edelstein M, Belausov E, Maymon M, Freeman S, Yarden O. 2015. Differences in the responses of melon accessions to fusarium root and stem rot and their colonization by Fusarium oxysporum f. sp. radicis-cucumerinum. Plant Pathol 64:655–663. doi: 10.1111/ppa.12286. [DOI] [Google Scholar]
- 13.Michielse CB, Rep M. 2009. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 10:311–324. doi: 10.1111/j.1364-3703.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lievens B, Thomma BPHJ. 2005. Recent developments in pathogen detection arrays: implications for fungal plant pathogens and use in practice. Phytopathology 95:1374–1380. doi: 10.1094/PHYTO-95-1374. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Does HC, Lievens B, Claes L, Houterman PM, Cornelissen BJC, Rep M. 2008. The presence of a virulence locus discriminates Fusarium oxysporum isolates causing tomato wilt from other isolates. Environ Microbiol 10:1475–1485. doi: 10.1111/j.1462-2920.2007.01561.x. [DOI] [PubMed] [Google Scholar]
- 16.Aimé S, Alabouvette C, Steinberg C, Olivain C. 2013. The endophytic strain Fusarium oxysporum Fo47: a good candidate for priming the defense responses in tomato roots. Mol Plant Microbe Interact 26:918–926. doi: 10.1094/MPMI-12-12-0290-R. [DOI] [PubMed] [Google Scholar]
- 17.Alabouvette C, Olivain C, Migheli Q, Steinberg C. 2009. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol 184:529–544. doi: 10.1111/j.1469-8137.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 18.Fravel D, Olivain C, Alabouvette C. 2003. Fusarium oxysporum and its biocontrol. New Phytol 157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Lin Y, Lin Y, Chung W. 2013. Modified primers for the identification of nonpathogenic Fusarium oxysporum isolates that have biological control potential against fusarium wilt of cucumber in Taiwan. PLoS One 8:e65093. doi: 10.1371/journal.pone.0065093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recorbet G, Steinberg C, Olivain C, Edel V, Trouvelot S, Dumas-Gaudot E, Gianinazzi S, Alabouvette C. 2003. Wanted: pathogenesis-related marker molecules for Fusarium oxysporum. New Phytol 159:73–92. doi: 10.1046/j.1469-8137.2003.00795.x. [DOI] [PubMed] [Google Scholar]
- 21.Covey PA, Kuwitzky B, Hanson M, Webb KM. 2014. Multilocus analysis using putative fungal effectors to describe a population of Fusarium oxysporum from sugar beet. Phytopathology 104:886–896. doi: 10.1094/PHYTO-09-13-0248-R. [DOI] [PubMed] [Google Scholar]
- 22.Lievens B, Claes L, Vakalounakis DJ, Vanachter ACRC, Thomma BPHJ. 2007. A robust identification and detection assay to discriminate the cucumber pathogens Fusarium oxysporum f. sp. cucumerinum and f. sp. radicis-cucumerinum. Environ Microbiol 9:2145–2161. doi: 10.1111/j.1462-2920.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 23.Baayen RP, O'Donnell K, Bonants PJ, Cigelnik E, Kroon LP, Roebroeck EJ, Waalwijk C. 2000. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90:891–900. doi: 10.1094/PHYTO.2000.90.8.891. [DOI] [PubMed] [Google Scholar]
- 24.Kistler HC. 1997. Genetic diversity in the plant-pathogenic fungus Fusarium oxysporum. Phytopathology 87:474–479. doi: 10.1094/PHYTO.1997.87.4.474. [DOI] [PubMed] [Google Scholar]
- 25.Lievens B, Houterman PM, Rep M. 2009. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol Lett 300:201–215. doi: 10.1111/j.1574-6968.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 26.Cenis JL, Tello J, Cifuentes D. 2003. Genetic relationships among seven specialized forms of Fusarium oxysporum determined by DNA sequencing of the ITS region and AFLPs. Spanish J Agric Res 1:55–63. doi: 10.5424/sjar/2003013-35. [DOI] [Google Scholar]
- 27.Haegi A, Catalano V, Luongo L, Vitale S, Scotton M, Ficcadenti N, Belisario A. 2013. A newly developed real-time PCR assay for detection and quantification of Fusarium oxysporum and its use in compatible and incompatible interactions with grafted melon genotypes. Phytopathology 103:802–810. doi: 10.1094/PHYTO-11-12-0293-R. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y-H, Chen K-S, Chang J-Y, Wan Y-L, Hsu C-C, Huang J-W, Chang P-FL. 2010. Development of the molecular methods for rapid detection and differentiation of Fusarium oxysporum and F. oxysporum f. sp. niveum in Taiwan. N Biotechnol 27:409–418. doi: 10.1016/j.nbt.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Lievens B, Rep M, Thomma BPHJ. 2008. Recent developments in the molecular discrimination of formae speciales of Fusarium oxysporum. Pest Manag Sci 64:781–788. doi: 10.1002/ps.1564. [DOI] [PubMed] [Google Scholar]
- 30.Alves-Santos FM, Ramos B, García-Sánchez MA, Eslava AP, Díaz-Mínguez JM. 2002. A DNA-based procedure for in planta detection of Fusarium oxysporum f. sp. phaseoli. Phytopathology 92:237–244. doi: 10.1094/PHYTO.2002.92.3.237. [DOI] [PubMed] [Google Scholar]
- 31.Pasquali M, Dematheis F, Gullino ML, Garibaldi A. 2007. Identification of race 1 of Fusarium oxysporum f. sp. lactucae on lettuce by inter-retrotransposon sequence-characterized amplified region technique. Phytopathology 97:987–996. doi: 10.1094/PHYTO-97-8-0987. [DOI] [PubMed] [Google Scholar]
- 32.Namiki F, Shiomi T, Kayamura T, Tsuge T. 1994. Characterization of the formae speciales of Fusarium oxysporum causing wilts of cucurbits by DNA fingerprinting with nuclear repetitive DNA sequences. Appl Environ Microbiol 60:2684–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dam P, Fokkens L, Schmidt SM, Linmans JHJ, Kistler HC, Ma L-J, Rep M. 2016. Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ Microbiol 18:4087–4102. doi: 10.1111/1462-2920.13445. [DOI] [PubMed] [Google Scholar]
- 34.Hogg AC, Johnston RH, Dyer AT. 2007. Applying real-time quantitative PCR to fusarium crown rot of wheat. Plant Dis 91:1021–1028. doi: 10.1094/PDIS-91-8-1021. [DOI] [PubMed] [Google Scholar]
- 35.Mbofung GCY, Fessehaie A, Bhattacharyya MK, Leandro LFS. 2011. A new TaqMan real-time polymerase chain reaction assay for quantification of Fusarium virguliforme in soil. Plant Dis 95:1420–1426. doi: 10.1094/PDIS-02-11-0120. [DOI] [PubMed] [Google Scholar]
- 36.Aguayo J, Mostert D, Fourrier-Jeandel C, Cerf-Wendling I, Hostachy B, Viljoen A, Ioos R. 2017. Development of a hydrolysis probe-based real-time assay for the detection of tropical strains of Fusarium oxysporum f. sp. cubense race 4. PLoS One 12:e0171767. doi: 10.1371/journal.pone.0171767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser-Smith S, Czislowski E, Meldrum RA, Zander M, O'Neill W, Balali GR, Aitker EAB. 2014. Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f. sp. cubense. Plant Pathol doi: 10.1111/ppa.12184. [DOI] [Google Scholar]
- 38.Thatcher LF, Gardiner DM, Kazan K, Manners JM. 2012. A highly conserved effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol Plant Microbe Interact 25:180–190. doi: 10.1094/MPMI-08-11-0212. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti A, Rep M, Wang B, Ashton A, Dodds P, Ellis J. 2011. Variation in potential effector genes distinguishing Australian and non-Australian isolates of the cotton wilt pathogen Fusarium oxysporum f. sp. vasinfectum. Plant Pathol 60:232–243. doi: 10.1111/j.1365-3059.2010.02363.x. [DOI] [Google Scholar]
- 40.Taylor A, Vagany V, Jackson AC, Harrison RJ, Rainoni A, Clarkson JP. 2016. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol Plant Pathol 17:1032–1047. doi: 10.1111/mpp.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C-H, Tsai R-T, Vallad GE. 2016. Development of a TaqMan real-time polymerase chain reaction assay for detection and quantification of Fusarium oxysporum f. sp. lycopersici in soil. J Phytopathol 164:455–463. doi: 10.1111/jph.12471. [DOI] [Google Scholar]
- 42.Vakalounakis DJ, Fragkiadakis GA. 1999. Genetic diversity of Fusarium oxysporum isolates from cucumber: differentiation by pathogenicity, vegetative compatibility, and RAPD fingerprinting. Phytopathology 89:161–168. doi: 10.1094/PHYTO.1999.89.2.161. [DOI] [PubMed] [Google Scholar]
- 43.Katan T. 1999. Current status of vegetative compatibility groups in Fusarium oxysporum. Phytoparasitica 27:51–64. doi: 10.1007/BF02980727. [DOI] [Google Scholar]
- 44.Cumagun J, Oribiana Z, Tolentino M, Relevante C, Balatero C. 2008. Vegetative compatibility among Fusarium oxysporum isolates from bitter gourd and bottle gourd in the Philippines. J Plant Prot Res 48:283–293. doi: 10.2478/v10045-008-0017-6. [DOI] [Google Scholar]
- 45.Schoffelmeer EA, Vossen JH, van Doorn AA, Cornelissen BJ, Haring MA. 2001. FEM1, a Fusarium oxysporum glycoprotein that is covalently linked to the cell wall matrix and is conserved in filamentous fungi. Mol Genet Genomics 265:143–152. doi: 10.1007/s004380000402. [DOI] [PubMed] [Google Scholar]
- 46.Gerlagh M, Blok WJ. 1988. Fusarium oxysporum f. sp. cucurbitacearum n.f. embracing all formae speciales of F. oxysporum attacking cucurbitaceous crops. Netherlands J Plant Pathol 94:17–31. [Google Scholar]
- 47.van Dam P, Rep M. 25 July 2017. The distribution of Miniature Impala elements and SIX genes in the Fusarium genus is suggestive of horizontal gene transfer. J Mol Evol doi: 10.1007/s00239-017-9801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon TR. 2017. Fusarium oxysporum and the Fusarium wilt syndrome. Annu Rev Phytopathol 55:23–29. doi: 10.1146/annurev-phyto-080615-095919. [DOI] [PubMed] [Google Scholar]
- 49.Rocha LO, Laurence MH, Ludowici VA, Puno VI, Lim CC, Tesoriero LA, Summerell BA, Liew ECY. 2016. Putative effector genes detected in Fusarium oxysporum from natural ecosystems of Australia. Plant Pathol 65:914–929. doi: 10.1111/ppa.12472. [DOI] [Google Scholar]
- 50.Ling J, Zhang J, Zeng F, Cao Y, Xie B, Yang Y. 2016. Comparative genomics provide a rapid detection of Fusarium oxysporum f. sp. conglutinans. J Integr Agric 15:822–831. doi: 10.1016/S2095-3119(15)61237-0. [DOI] [Google Scholar]
- 51.Zhou XG, Everts KL. 2007. Characterization of a regional population of Fusarium oxysporum f. sp. niveum by race, cross pathogenicity, and vegetative compatibility. Phytopathology 97:461–469. doi: 10.1094/PHYTO-97-4-0461. [DOI] [PubMed] [Google Scholar]
- 52.Mcmillan T. 1986. Cross pathogenicity studies with isolates of Fusarium oxysporum from either cucumber or watermelon pathogenic to both crop species. Ann Appl Biol 109:101–105. doi: 10.1111/j.1744-7348.1986.tb03188.x. [DOI] [Google Scholar]
- 53.Cafri D, Katan J, Katan T. 2005. Cross-pathogenicity between formae speciales of Fusarium oxysporum, the pathogens of cucumber and melon. J Phytopathol 153:615–622. doi: 10.1111/j.1439-0434.2005.01029.x. [DOI] [Google Scholar]
- 54.Mirtalebi M, Banihashemi Z. 2014. Genetic relationship among Fusarium oxysporum f. sp. melonis vegetative compatibility groups and their relatedness to other F. oxysporum formae speciales. J Agric Sci Technol 16:931–943. [Google Scholar]
- 55.Kang S, Demers J, del Mar Jimenez-Gasco M, Rep M. 2014. Fusarium oxysporum, p 99–119. In Dean RA, Lichens-Park A, Kole C (ed), Genomics of plant-associated fungi and oomycetes: dicot pathogens. Springer, Berlin, Germany. [Google Scholar]
- 56.Ma L-J, Geiser DM, Proctor RH, Rooney AP, O'Donnell K, Trail F, Gardiner DM, Manners JM, Kazan K. 2013. Fusarium pathogenomics. Annu Rev Microbiol 67:399–416. doi: 10.1146/annurev-micro-092412-155650. [DOI] [PubMed] [Google Scholar]
- 57.Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE. 2000. Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl Environ Microbiol 66:2853–2858. doi: 10.1128/AEM.66.7.2853-2858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agindotan BO, Shiel PJ, Berger PH. 2007. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan® real-time RT-PCR. J Virol Methods 142:1–9. doi: 10.1016/j.jviromet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Probert WS, Schrader KN, Khuong NY, Bystrom SL, Graves MH. 2004. Real-time multiplex PCR assay for detection of Brucella spp., B. abortus, and B. melitensis. J Clin Microbiol 42:1290–1293. doi: 10.1128/JCM.42.3.1290-1293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 62.Huerta-Cepas J, Serra F, Bork P. 2016. ETE 3: reconstruction, analysis, and visualization of phylogenomic data. Mol Biol Evol 33:1635–1638. doi: 10.1093/molbev/msw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu J, Huang S, Sun M, Liu S, Liu Y, Wang W, Zhang X, Wang H, Hua W. 2012. An improved allele-specific PCR primer design method for SNP marker analysis and its application. Plant Methods 8:34. doi: 10.1186/1746-4811-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wellman FL. 1939. A technique for studying host resistance and pathogenicity in tomato Fusarium wilt. Phytopathology 29:945–956. [Google Scholar]
- 66.Owen JH. 1956. Cucumber wilt, caused by Fusarium oxysporum f. cucumerinum. Phytopathology 46:153–157. [Google Scholar]
- 67.Leach JG, Currence TM. 1937. Fusarium wilt of muskmelons in Minnesota. University of Minnesota, Agricultural Experiment Station, St. Paul, MN. [Google Scholar]
- 68.Armstrong GM, Armstrong JK. 1981. Formae speciales and races of Fusarium oxysporum causing wilt diseases, p 391–399. In Nelson PE, Toussoun TA, Cook RJ (ed), Fusarium: diseases, biology, and taxonomy. Pennsylvania State University Press, University Park, PA. [Google Scholar]
- 69.Vakalounakis DJ. 1996. Root and stem rot of cucumber caused by Fusarium oxysporum f. sp. radicis-cucumerinum f. sp. nov. Plant Dis 80:313–316. [DOI] [PubMed] [Google Scholar]
- 70.Sun S, Huang J. 1982. A new Fusarium wilt of bitter gourd in Taiwan. Plant Dis 67:226–227. doi: 10.1094/PD-67-226. [DOI] [Google Scholar]
- 71.Matuo T, Yamamoto I. 1967. On Fusarium oxysporum f.sp. lagenariae n.f. causing wilt of Lagenaria vulgaris var. hispida. Trans Mycol Soc Japan 8:61–63. [Google Scholar]
- 72.Guo L, Han L, Yang L, Zeng H, Fan D, Zhu Y, Feng Y, Wang G, Peng C, Jiang X, Zhou D, Ni P, Liang C, Liu L, Wang J, Mao C, Fang X, Peng M, Huang J. 2014. Genome and transcriptome analysis of the fungal pathogen Fusarium oxysporum f. sp. cubense causing banana vascular wilt disease. PLoS One 9:e95543. doi: 10.1371/journal.pone.0095543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmidt SM, Lukasiewicz J, Farrer R, van Dam P, Bertoldo C, Rep M. 2016. Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon. New Phytol 209:307–318. doi: 10.1111/nph.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mes JJ, Weststeijn EA, Herlaar F, Lambalk JJ, Wijbrandi J, Haring MA, Cornelissen BJ. 1999. Biological and molecular characterization of Fusarium oxysporum f. sp. lycopersici divides race 1 isolates into separate virulence groups. Phytopathology 89:156–160. [DOI] [PubMed] [Google Scholar]
- 75.Cai G, Gale LR, Schneider RW, Kistler HC, Davis RM, Elias KS, Miyao EM. 2003. Origin of race 3 of Fusarium oxysporum f. sp. lycopersici at a single site in California. Phytopathology 93:1014–1022. doi: 10.1094/PHYTO.2003.93.8.1014. [DOI] [PubMed] [Google Scholar]
- 76.Gale LR, Katan T, Kistler HC. 2003. The probable center of origin of Fusarium oxysporum f. sp. lycopersici VCG 0033. Plant Dis 87:1433–1438. doi: 10.1094/PDIS.2003.87.12.1433. [DOI] [PubMed] [Google Scholar]
- 77.Hadar E, Katan J. 1989. The use of nitrate-nonutilizing mutants and a selective medium for studies of pathogenic strains of Fusarium oxysporum. Plant Dis 73:800. doi: 10.1094/PD-73-0800. [DOI] [Google Scholar]
- 78.Mes JJ, Doorn Van J, Roebroeck EJA, Van Egmond E, Van Aartrijk J, Boonekamp PM. 1994. Restriction fragment length polymorphisms, races and vegetative compatibility groups within a worldwide collection of Fusarium oxysporum f. sp. gladioli. Plant Pathol 43:362–370. doi: 10.1111/j.1365-3059.1994.tb02697.x. [DOI] [Google Scholar]
- 79.Breeuwsma SJ, De Boer M. 2004. Fusarium in bloembolgewassen: detectiemethoden en vruchtwisselingsproblematiek. PPO Bloembollen en Bomen. PPO project 320689. Praktijkonderzoek Plant & Omgeving, Lisse, Netherlands. [Google Scholar]
- 80.Clark CA, Hyun J, Hoy MW. 1998. Relationships among wilt-inducing isolates of Fusarium oxysporum from sweetpotato and tobacco. Plant Dis 82:530–536. doi: 10.1094/PDIS.1998.82.5.530. [DOI] [PubMed] [Google Scholar]
- 81.O'Donnell K, Cigelnik E, Nirenberg HI. 1998. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493. [Google Scholar]
- 82.Skovgaard K, Nirenberg HI, O'Donnell K, Rosendahl S. 2001. Evolution of Fusarium oxysporum f. sp. vasinfectum races inferred from multigene genealogies. Phytopathology 91:1231–1237. doi: 10.1094/PHYTO.2001.91.12.1231. [DOI] [PubMed] [Google Scholar]
- 83.Zhou XG, Everts KL. 2003. Races and inoculum density of Fusarium oxysporum f. sp. niveum in commercial watermelon fields in Maryland and Delaware. Plant Dis 87:692–698. doi: 10.1094/PDIS.2003.87.6.692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.