Abstract
Background
The European Randomized Study of Screening for Prostate Cancer (ERSPC) found screening reduced prostate cancer (PC) mortality, but the Prostate, Lung, Colorectal, and Ovarian trial (PLCO) found no reduction.
Objective
To evaluate whether effects of screening on PC mortality relative to no screening differed between the ERSPC and PLCO.
Design
Cox regression of PC death in each trial arm adjusted for age and trial, and extended analyses that accounted for increased incidence due to screening and diagnostic workup on each arm via mean lead times (MLTs). MLTs were estimated empirically and using analytic or microsimulation models.
Setting
Randomized controlled trials in Europe and the US.
Participants
Men aged 55–69 (ERSPC) or 55–74 (PLCO) at randomization.
Intervention
Prostate cancer screening.
Measurements
PC incidence and survival from randomization; PC incidence in the US before screening began.
Results
Estimated MLTs were similar in the ERSPC and PLCO intervention arms but were longer in the PLCO control arm than the ERSPC control arm. Extended analyses found no evidence that effects of screening differed between trials (P=0.37–0.47, range across MLT estimation approaches) but strong evidence that benefit increased with MLT (P=0.0027–0.0032). Screening was estimated to confer a 7–9% reduction in PC death per year of MLT. This translated into an estimated 25–31% and 27–32% lower risk of PC death under screening as performed in the ERSPC and PLCO intervention arms, respectively, relative to no screening.
Limitations
MLT is a simple metric of screening and diagnostic workup.
Conclusion
After accounting for differences in implementation and settings, the ERSPC and PLCO provide compatible evidence that screening reduces PC mortality.
Keywords: efficacy, screening, prostate-specific antigen, prostatic neoplasms, randomized controlled trials
INTRODUCTION
More than two decades after prostate-specific antigen (PSA) screening for prostate cancer entered clinical practice, in 2012 the US Preventive Services Task Force (USPSTF) determined there was “very low probability of preventing a death from prostate cancer in the long term” and recommended against routine use of the test (1). Since then, PSA screening rates and prostate cancer incidence rates in the United States have declined significantly (2, 3).
The USPSTF recommendation relied heavily on results from the European Randomized Study of Screening for Prostate Cancer (ERSPC; ISRCTN49127736) and the Prostate, Lung, Colorectal, and Ovarian cancer screening trial (PLCO; NCT00002540). However, the trials produced apparently conflicting results, with the ERSPC reporting a 21% reduction in prostate cancer mortality (4–6) and the PLCO finding no mortality difference between the trial arms (7–9). Interpreting results of these trials is complicated by differences in their implementations, including design and adherence, and practice settings. The PLCO used shorter screening intervals (annual screening versus every 2–4 years in the ERSPC), had a higher PSA threshold for biopsy referral (4.0 μg/L versus 3.0 μg/L in most ERSPC centers and rounds), and stopped regular screening after 6 rounds. Prostate cancer incidence in the US was higher than in Europe before the trials started, reflecting different populations and clinical diagnosis patterns. The US practice setting also contributed to a higher frequency of screening in the control arm and a lower frequency of biopsy compared with the ERSPC. Consequently, the PLCO compared effects of an organized screening program relative to opportunistic screening rather than effects of screening versus no screening (8–10). Nonetheless, the PLCO results have been viewed as more relevant to the US setting (11).
The objectives of this study are to (1) formally test whether the effects of screening on prostate cancer mortality differed between the ERSPC and PLCO after accounting for differences in implementation and practice settings and (2) to estimate the effects of screening in both trials relative to no screening.
METHODS
Overview
Our study used individual records from both trials in a collaboration between trial investigators and the Cancer Intervention and Surveillance Modeling Network (CISNET) prostate cancer working group. In the intervention arms, these records included age and year of randomization, enrollment center, dates and results of PSA tests and rectal exams, whether biopsy was performed, date of cancer diagnosis, and date and cause of death. In the control arms, the records included age and year of randomization, enrollment center, date of cancer diagnosis, and date and cause of death. For consistency with prior publications, ERSPC data included men aged 55–69 years at randomization (12), while PLCO data included men aged 55–74 years at randomization (13).
We first examined a traditional statistical analysis that combined data from both trials and compared hazards of prostate cancer death in the intervention versus control arms adjusting for participant age and trial setting. However, this analysis is questionable due to remaining differences in implementation between trials. To overcome this limitation, we also examined extended analyses that accounted for variable screening and diagnostic workup (hereafter “screening intensity”) in each trial arm, which we operationalized using mean lead times (MLTs). The MLTs reflect the magnitude of increased prostate cancer incidence relative to a baseline level expected in the absence of screening, thus capturing differences in both design and adherence (see below). We estimated the MLTs both empirically and using analytic or microsimulation models; using multiple approaches allowed us to assess robustness of results to this uncertain quantity.
Estimating mean lead times
The MLT is usually defined as the average time by which diagnosis is advanced by screening relative to the date of diagnosis without screening. Under complete follow-up (i.e., where all pre-clinical cases are eventually diagnosed in the no-screening setting), the MLT corresponds to the difference in areas under two “survival curves” for time from randomization to diagnosis: one in the absence of screening minus one in the presence of screening. Under limited follow-up, we can define a restricted version of the MLT as an analogous difference in areas under survival curves up to a specified time point (14). Restricting to the duration of the trial recognizes that events after the trial period cannot affect the mortality during the trial. To make estimates between trials comparable, follow-up was restricted to 11 years.
Note that our estimates of the MLTs are different from other estimates in the literature that can be interpreted as the average time by which screening advances diagnosis among cases that would have been clinically diagnosed (15). Our MLTs are designed to represent proxies for the intensity of screening and diagnosis, with higher values reflecting higher attendance rates at screening exams, more frequent screening exams, less conservative criteria for biopsy referral, and/or higher frequencies of biopsy. Thus, accounting for variable MLTs across trial arms captures in a single measure important differences in the trial screening protocols, participant adherence to those protocols in the intervention arms, and control arm screening.
We estimated the MLTs empirically, without any model assumptions about cancer progression and diagnosis, and also using three models of cancer natural history and diagnosis. The empirical approach estimated the MLTs by calculating the difference between “survival curves” for observed time from randomization to diagnosis in each trial arm relative to an assumed baseline level. The assumed baseline probability of diagnosis in the absence of screening was derived using incidence rates from the Surveillance, Epidemiology, and End Results (SEER) program in 1986, just before PSA screening began in the US, adjusted to reflect distributions of age at randomization in each trial. Additionally, one (University of Michigan [UMICH]) analytic model and two (Fred Hutchinson Cancer Research Center [FHCRC] and Erasmus University Medical Center MIcrosimulation SCreening ANalysis [MISCAN]) simulation models estimated times from randomization to diagnosis in the absence and presence of screening based on cancer progression and diagnosis rates, which were estimated using individual patient attendance, screening, and incidence data. The fitted models then estimated MLTs as in the empirical approach but using projected instead of observed incidence rates. Each MLT was then scaled by the corresponding fraction of individuals diagnosed within the 11-year follow-up period projected so that it could be interpreted as an average interval among cancers detected on the relevant trial arm. Further details are described in the Supplementary Materials.
Statistical analysis
We used Cox regression to model survival from randomization to prostate cancer death, censoring individuals who died of other causes or were alive at last follow-up. We examined both a traditional statistical analysis and extended analyses that incorporated the measure of screening intensity captured by the estimated MLTs. Both types of analysis included participant age at randomization and a trial setting indicator (PLCO versus ERSPC), which allowed for a different baseline risk of prostate cancer death in the absence of screening between the two trial settings.
Traditional statistical analysis
We first conducted a traditional analysis to test whether the effect of screening differed between trials. Specifically, we tested the effect of being randomized to the intervention arm (relative to the control arm) on the risk of prostate cancer death. The exponential of the coefficient for the trial arm indicator is the hazard ratio for prostate cancer death in the intervention arm relative to the control arm; in other words, it reflects the effect of screening on prostate cancer mortality in an intent-to-screen analysis. We fitted this model with and without allowing separate effects of screening in each trial (i.e., with and without interaction between the trial arm and trial indicator), then used a likelihood ratio test to evaluate evidence of differential effects of screening between trials.
Extended statistical analysis
Next we replaced the trial arm indicator with the corresponding MLT estimated empirically or using a model-based approach. The exponential of the coefficient for the MLT represents the hazard ratio for prostate cancer death per additional year of MLT; in other words, it reflects screening efficacy standardized by screening intensity. As in the traditional analysis, we fitted this model with and without allowing separate effects of screening on prostate cancer mortality in each trial (i.e., with and without interaction between the MLT and trial indicator), then used a likelihood ratio test to evaluate evidence of differential effects of screening between trials.
Our extended analyses are consistent with the analyses in the trial publications (4, 7) with two important differences. First, rather than relying on an intent-to-treat effect of screening determined by the assigned arm in a single trial, we explicitly included a covariate (MLT) to capture the intensity of screening in each arm. This represents a transition from thinking about screening as all or nothing, corresponding to an intervention and control arm, to a continuous metric of screening intensity, with resulting coefficient estimates interpreted relative to a no-screening setting (i.e., a setting where MLT=0). Second, we used combined data from both trials in a single analysis, adding an indicator for trial to capture differences between trials in baseline cancer-specific survival without screening and an interaction term to test whether screening efficacy (per year of MLT) differed between trials.
Role of the funding source
This study was supported was supported by the National Cancer Institute, which had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
RESULTS
Table 1 summarizes participants, follow-up, and prostate cancer cases and deaths in the two trials using all available follow-up and restricted to 11 years of follow-up. The data under all available follow-up differ modestly from published results (5, 8) due to additional cleaning and updating. Nonetheless, the cleaned and updated data restricted to 11 years of follow-up yielded values similar to published prostate cancer incidence rate ratios (PLCO: 1.12 vs 1.12; ERSPC: 1.68 vs 1.63) and mortality rate ratios (PLCO: 1.02 vs 1.09; ERSPC: 0.79 vs 0.79) and preserved the greater effects of screening on prostate cancer incidence and mortality rates in the ERSPC relative to the PLCO.
Table 1.
Summary of participants, follow-up, and prostate cancer cases and deaths in the ERSPC and PLCO under all available follow-up or restricted to 11 years of follow-up
| ERSPC | PLCO | |||
|---|---|---|---|---|
|
| ||||
| Control | Screening | Control | Screening | |
|
| ||||
| No. of participants | 88,921 | 72,473 | 38,343 | 38,340 |
| Age at randomization (years) | ||||
| median | 59 | 60 | 62 | 62 |
| range | 55–69 | 55–69 | 55–74 | 55–74 |
| All available follow-up | ||||
| Follow-up from randomization (years) | ||||
| median | 11.0 | 11.1 | 12.5 | 12.5 |
| range | 0.4–17.5 | 0.4–17.3 | 0.0–13.0 | 0.0–13.0 |
| No. of prostate cancer cases | 5,398 | 6,967 | 4,040 | 4,430 |
| Person-years of follow-up for incidence | 933,854 | 740,775 | 403,955 | 400,008 |
| No. of deaths | 17,019 | 13,652 | 7,149 | 6,940 |
| other causes | 16,557 | 13,353 | 7,003 | 6,788 |
| prostate cancer | 462 | 299 | 146 | 152 |
| Person-years of follow-up for mortality | 990,678 | 827,148 | 426,720 | 427,824 |
| Restricted to 11 years of follow-up | ||||
| Follow-up from randomization (years) | ||||
| median | 11.0 | 11.0 | 11.0 | 11.0 |
| range | 0.4–11.0 | 0.4–11.0 | 0.0–11.0 | 0.0–11.0 |
| No. of prostate cancer cases | 4,961 | 6,586 | 3,641 | 4,038 |
| Person-years of follow-up for incidence | 868,834 | 686,766 | 368,844 | 365,129 |
| No. of deaths | 13,207 | 10,397 | 5,880 | 5,798 |
| other causes | 12,822 | 10,150 | 5,771 | 5,687 |
| prostate cancer | 385 | 247 | 109 | 111 |
| Person-years of follow-up for mortality | 890,581 | 725,997 | 387,027 | 387,861 |
ERSPC=European Randomized Study of Screening for Prostate Cancer; PLCO=Prostate, Lung, Colorectal, and Ovarian cancer screening trial
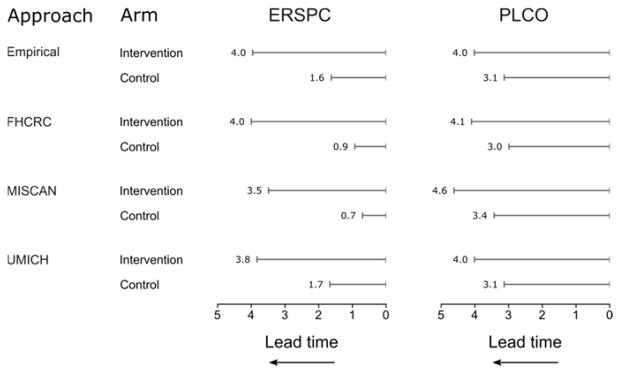
To compare the screening intensity in the intervention and control arms of the two trials, Figure 1 illustrates MLTs estimated empirically or using a model-based approach. All estimation approaches found similar ordering and relative magnitudes of MLTs across trial arms. The ERSPC and PLCO intervention arms had similar MLTs, but the PLCO control arm had substantially longer MLTs than the ERSPC control arm, consistent with more intensive screening (i.e., greater “contamination”) in the PLCO control arm.
Figure 1. Estimated mean lead times (years) in the intervention and control arms of the ERSPC and PLCO relative to a hypothetical no-screening setting (where lead time is always zero).
Estimated MLTs are visualized as increasing to the left to suggest the extent to which prostate cancer diagnosis is advanced by more intensive screening and diagnostic workup.
ERSPC=European Randomized Study of Screening for Prostate Cancer; PLCO=Prostate, Lung, Colorectal, and Ovarian cancer screening trial; FHCRC=Fred Hutchinson Cancer Research Center; MISCAN=Erasmus University Medical Center MIcrosimulation SCreening ANalysis; UMICH=University of Michigan
Table 2 reports results of the traditional analysis. A likelihood ratio test associated with this analysis modestly suggested different effects of screening on mortality between trials (P=0.09). Under a common effect of screening, screening was estimated to reduce the risk of prostate cancer death by 16% (95% CI 4–27%; P=0.01) after accounting for different baseline risks of prostate cancer death in the PLCO setting relative to the ERSPC setting and participant age at randomization. This result essentially corresponds to a weighted average of the effect in each trial with the relative sizes of the trials as weights.
Table 2.
Results of traditional and extended Cox regression analyses of prostate cancer death and estimated mortality reductions in the settings of the ERSPC and PLCO intervention arms relative to no screening
| Estimated mortality reduction relative to no screening | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cox regression analysis | Setting of ERSPC intervention arm | Setting of PLCO intervention arm | ||||||||
|
| ||||||||||
| Covariate | HR | 95% CI | P | MLT | Reduction | 95% CI | MLT | Reduction | 95% CI | |
| Traditional analysis | ||||||||||
|
| ||||||||||
| PLCO setting | 0.53 | (0.45–0.62) | <0.0001 | |||||||
| Age | 1.13 | (1.11–1.14) | <0.0001 | |||||||
| Intervention arm | 0.84 | (0.73–0.96) | 0.0099 | n/a | 16% | (4–27%) | n/a | 16% | (4–27%) | |
|
| ||||||||||
| Extended analyses | ||||||||||
|
| ||||||||||
| Empirical | PLCO setting | 0.57 | (0.48–0.67) | <0.0001 | ||||||
| Age | 1.13 | (1.11–1.14) | <0.0001 | |||||||
| MLT | 0.92 | (0.87–0.97) | 0.0027 | 3.96 | 29% | (11–43%) | 4.02 | 29% | (11–44%) | |
| FHCRC | PLCO setting | 0.58 | (0.49–0.69) | <0.0001 | ||||||
| Age | 1.13 | (1.11–1.14) | <0.0001 | |||||||
| MLT | 0.93 | (0.88–0.97) | 0.0029 | 4.00 | 27% | (10–40%) | 4.10 | 27% | (10–41%) | |
| MISCAN | PLCO setting | 0.63 | (0.51–0.77) | <0.0001 | ||||||
| Age | 1.13 | (1.11–1.14) | <0.0001 | |||||||
| MLT | 0.92 | (0.87–0.97) | 0.0032 | 3.49 | 25% | (9–38%) | 4.62 | 32% | (12–47%) | |
| UMICH | PLCO setting | 0.57 | (0.48–0.68) | <0.0001 | ||||||
| Age | 1.13 | (1.11–1.14) | <0.0001 | |||||||
| MLT | 0.91 | (0.85–0.97) | 0.0029 | 3.83 | 31% | (12–45%) | 4.01 | 32% | (12–47%) | |
ERSPC=European Randomized Study of Screening for Prostate Cancer; PLCO=Prostate, Lung, Colorectal, and Ovarian cancer screening trial; HR=hazard ratio; CI=confidence interval; PLCO setting=indicator of PLCO setting relative to the ERSPC setting to account for differential baseline risk of prostate cancer death; Age=participant age at randomization (continuous); Intervention arm=indicator of randomization to intervention arm; MLT=mean lead time (continuous) estimated in each trial arm by the specified estimation approach; FHCRC=Fred Hutchinson Cancer Research Center; MISCAN=Erasmus University Medical Center MIcrosimulation SCreening ANalysis; UMICH=University of Michigan
Table 2 also presents our extended analyses, which account for the MLT in each trial arm estimated empirically or using a model-based approach. The analyses are highly consistent and indicate no evidence of different effects of screening on mortality between trials (P=0.37–0.47 for interaction, range across estimation approaches). Under a common effect of screening, all approaches indicated strong evidence that a longer MLT was associated with a lower risk of prostate cancer death after accounting for differential baseline risks of prostate cancer death between trial settings and participant age at randomization (P=0.0027–0.0032). These analyses showed that screening was estimated to confer a 7–9% lower risk of prostate cancer death per year of MLT. Using the formula 1–HRMLT, this would translate into an estimated 25–31% and 27–32% reduction in the expected risk of prostate cancer death in the setting of screening as performed in the ERSPC and PLCO intervention arms, respectively, over 11 years of follow-up relative to no screening.
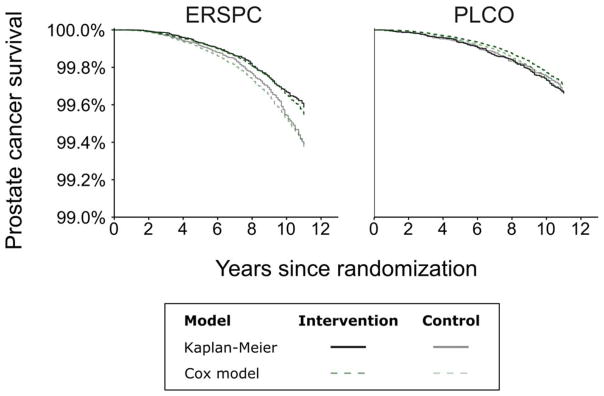
Figure 2 illustrates prostate cancer survival from randomization in each trial arm obtained by Kaplan-Meier estimation and predicted under a common effect of screening given MLTs estimated by the empirical approach. Predictions obtained using MLTs estimated by the model-based approaches (not shown) are similar. The predicted curves closely reproduce observed differences in prostate cancer survival between the intervention and control arms in both trials, showing that screening intensity as captured by the MLT is highly informative about between-arm differences in risks of prostate cancer death in both trials.
Figure 2. Prostate cancer survival from randomization in the ERSPC and PLCO estimated by Kaplan-Meier or Cox regression model using mean lead time estimated using the empirical approach.
ERSPC=European Randomized Study of Screening for Prostate Cancer; PLCO=Prostate, Lung, Colorectal, and Ovarian cancer screening trial
DISCUSSION
The USPSTF is currently updating its recommendations about PSA screening, and it has looked at the ERSPC and PLCO as the main sources of evidence about screening benefit in the past. Primary publications from these high-quality randomized controlled trials are irreplaceable for evaluating causal effects of screening for prostate cancer. Yet analyses like the one in this article that attempt to overcome limitations of traditional statistical analyses critically complement the empirical trial findings, including informing about whether the evidence from the trials is compatible and about the expected reduction in prostate cancer mortality relative to no screening.
Rather than comparing the trial arms as if they represent screened and non-screened populations, this study estimated the intensity of screening in each arm relative to no screening. This framework allowed us to formally assess whether screening effects differed between the trials when accounting for differential screening intensity between arms in each trial. By decoupling screening intensity from trial arm labels and investigating how benefit depends on screening intensity, we concluded that differences between the ERSPC and PLCO results are largely attributable to differences in screening intensities between arms within each trial. Finding no evidence of different effects of screening on prostate cancer mortality between trials given the screening intensities, we estimated a common effect of screening on mortality using pooled data on 19,226 prostate cancer cases. The pooled estimate demonstrates a highly significant benefit of screening. This is the first time that data from both trials have been harnessed to estimate screening benefit.
It is possible that this analysis had insufficient power to detect a significant difference in screening efficacy between trials. Thus, while there is no evidence of different screening efficacies, we cannot unequivocally conclude they were identical. Nevertheless, our combined analysis of both trials permits the most powerful examination of this question to date.
Our analysis indicates that the baseline risk of prostate cancer death differed between trials. This could be due to different incidence, stage distributions, and treatment patterns in the trial populations in the absence of screening. A lower-than-expected mortality (relative to pre-PSA-era survival) was observed in the PLCO, possibly due to participants being healthier or reflecting an era with improved disease-specific survival (16). By quantifying screening efficacy as a function of screening intensity, we projected that screening lowered the expected risk of prostate cancer mortality in both PLCO arms.
We used multiple approaches to estimate screening intensity. The empirical approach reflects catch-all differences in the risk of prostate cancer diagnosis between arms and calculates the MLT most consistent with incidence in each arm relative to a common baseline level. In contrast, the model-based approaches explicitly account for trial protocols and practice setting details that are known or can be quantified, e.g., age distributions, enrollment and attendance patterns, and screening and biopsy frequencies within each ERSPC center. As expected, the estimates are shorter than in other studies (15) due to the different estimation approach and because we restricted to 11 years of follow-up. In general, results are highly consistent across estimation approaches and suggest robustness of our conclusions to these ways of estimating screening intensity. While quantifying the screening intensity through MLT is simple and natural for our problem, other more complicated measures are possible. These include various standardized measures derived from an excess hazard of cancer diagnosis in a screened population versus an unscreened population, using recent methods (17) and earlier literature on relative survival analysis.
The finding that effects of screening on mortality appear to be consistent between trials after accounting for differences in implementation and practice setting corroborate other analyses. For example, a prior investigation of the PLCO found that control arm screening substantially limited the power of that trial to detect a clinically important reduction in prostate cancer mortality (18). However, that study did not formally evaluate whether effects of screening on prostate cancer mortality differed between the ERSPC and PLCO when implementation and setting details are taken into account.
A limitation of this study is that we do not explicitly account for differences between trials in characteristics of cancer cases (e.g., clinical stage or Gleason score) or primary treatments. Any differences in these factors between trials will be accounted for in the trial-specific baseline risks of prostate cancer death. Also, the model-based approaches to estimate lead times assume that cancers are progressive, although they allow heterogeneity in progression risk across individuals. Ultimately, it is impossible to know whether some cancers could remain indolent indefinitely or regress spontaneously and permanently. However, all estimation approaches closely match incidence trends in each trial arm. We also assume that incidence in the absence of screening was constant across calendar years before and after the trials began. This too is a simplification. We consider only the mean lead time as a surrogate for screening intensity. It is possible that other metrics could have different associations with risk of prostate cancer death than we found. Finally, the estimated mortality reduction in each trial arm assumes that the risk of death from any cause is small during the follow-up period.
In conclusion, taken together, the data from the two screening trials do not provide evidence that screening efficacy (relative to no screening) differed between the ERSPC and PLCO after accounting for differences in implementation and setting. Our estimation results of the common effect of screening suggest that screening can significantly reduce the risk of prostate cancer death. However, as for all interventions, the benefit of screening must be weighed against its potential harms for informed clinical and shared decision making.
Supplementary Material
Acknowledgments
Funding Source: National Cancer Institute.
Grant Support: This work was supported by the National Cancer Institute Award Number U01CA157224.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Disclosures: Author forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-2586.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Source code or runs using the FHCRC model available from Mr. Gulati (rgulati@fredhutch.org), runs using the MISCAN model available from Dr. Heijnsdijk (e.heijnsdijk@erasmusmc.nl), and source code or runs using the UMICH model available from Dr. Tsodikov (tsodikov@umich.edu). Data set: PLCO data are available from the National Cancer Institute Cancer Data Access System (https://biometry.nci.nih.gov/cdas/). ERSPC data may be available from Dr. Moss (s.moss@qmul.ac.uk).
References
- 1.Moyer VA Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314(19):2054–61. doi: 10.1001/jama.2015.14905. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Ma J, Siegel R, Fedewa S, Brawley O, Ward EM. Prostate Cancer Incidence Rates 2 Years After the US Preventive Services Task Force Recommendations Against Screening. JAMA Oncol. 2016;2(12):1657–60. doi: 10.1001/jamaoncol.2016.2667. [DOI] [PubMed] [Google Scholar]
- 4.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine. 2012;366(11):981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andriole GL, Crawford ED, Grubb RLr, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriole GL. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinsky PF, Prorok PC, Yu K, Kramer BS, Black A, Gohagan J, et al. Extended mortality results for prostate cancer screening in the PLCO trial with median 15 years of follow-up. Cancer. 2017;123(4):592–9. doi: 10.1002/cncr.30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg CD. The Prostate, Lung, Colorectal and Ovarian cancer screening trial: The prostate cancer screening results in context. Acta Oncol. 2011;50(Suppl 1):12–7. doi: 10.3109/0284186X.2010.531283. [DOI] [PubMed] [Google Scholar]
- 11.Melnikow J, LeFevre ML, Wilt TJ, Moyer VA. Randomized Trials Provide the Strongest Evidence for Clinical Guidelines: The US Preventive Services Task Force and Prostate Cancer Screening. Medical Care. 2013;51(4):301–3. doi: 10.1097/MLR.0b013e31828a67d3. [DOI] [PubMed] [Google Scholar]
- 12.De Koning HJ, Hakulinen T, Moss SM, Adolfsson J, Smith PH, Alexander FE, et al. Monitoring the ERSPC trial. BJU International. 2003;92(Suppl 2):112–4. doi: 10.1111/j.1464-410x.2003.4410x.x. [DOI] [PubMed] [Google Scholar]
- 13.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 14.Uno H, Claggett B, Tian L, Inoue E, Gallo P, Miyata T, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–5. doi: 10.1200/JCO.2014.55.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. Journal of the National Cancer Institute. 2009;101(6):374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinsky PF, Miller A, Kramer BS, Church T, Reding D, Prorok P, et al. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165(8):874–81. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 17.Sasieni P, Brentnall AR. On standardized relative survival. Biometrics. 2016 doi: 10.1111/biom.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati R, Tsodikov A, Wever EM, Mariotto AB, Heijnsdijk EA, Katcher J, et al. The impact of PLCO control arm contamination on perceived PSA screening efficacy. Cancer Causes and Control. 2012;23(6):827–35. doi: 10.1007/s10552-012-9951-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.