Abstract
Epidemiologic studies have demonstrated an association of elevated plasma homocysteine levels with greater bone resorption and fracture risk. Vitamins B12, B6 and folic acid are cofactors in homocysteine metabolism, and supplementation with B vitamins is effective in lowering homocysteine levels in humans. However, randomized trials of supplemental B vitamins for reduction of fracture risk have been limited. Therefore, we performed an ancillary study to the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), a large randomized trial of women with preexisting cardiovascular disease or 3 or more coronary risk factors, to test whether a daily B vitamin intervention including folic acid (2.5 mg/day), vitamin B6 (50 mg/day), and vitamin B12 (1 mg/day) reduces non-spine fracture risk over 7.3 years of treatment and follow-up. Among 4810 women, we confirmed 349 non-spine fracture cases by centralized review of medical records. In a substudy of 300 women (150 in treatment group and 150 controls) with paired plasma samples at randomization and follow-up (7.3 years later), we measured two bone turnover markers, including C-terminal cross-linking telopeptide of type I collagen (CTX) and intact type I procollagen N-propeptide (P1NP). In Cox proportional hazards models based on intention-to-treat, we found no significant effects of B vitamin supplementation on non-spine fracture risk (Relative Hazard = 1.08; 95% confidence interval 0.88 – 1.34). In a nested case-cohort analysis, there were no significant effects of B vitamins on fracture risk among women with elevated plasma homocysteine levels, or low levels of vitamins B12 or B6, or folate at baseline. Furthermore, treatment with B vitamins had no effect on change in markers of bone turnover. We found no evidence that daily supplementation with B vitamins reduces fracture risk or rates of bone metabolism in middle aged and older women at high risk of CV disease.
Keywords: osteoporosis, fracture prevention, B vitamins, biochemical markers of bone turnover, therapeutics, nutrition
Introduction
Osteoporotic fractures are a leading cause of disability in older adults, and a major contributor to medical care costs around the world(1). Therefore, the identification of modifiable risk factors for osteoporosis is of high importance to improve public health. It is especially important to identify inexpensive and effective treatments for osteoporosis that are available even to those with limited resources.
Dietary supplementation with the nutrients folic acid, vitamin B12, and vitamin B6 has been proposed as one such candidate treatment. These three vitamins are cofactors in homocysteine metabolism, and studies have shown that as many as two thirds of cases of elevated homocysteine are associated with low plasma folate or vitamin B12 (2). Randomized trials in adults have demonstrated that treatment with folic acid and B vitamins can significantly lower plasma homocysteine levels(3, 4), although the extent of homocysteine-lowering may depend on the prevalence of poor vitamin intake.(5)
Results from several epidemiologic studies have demonstrated an association between elevated plasma homocysteine levels and increased risk of osteoporotic fractures(6–8), including hip fractures(9). Furthermore, high homocysteine levels have been associated with increased bone resorption(10–12). Additionally, a recent study based on data and samples from the NHANES (National Health and Nutrition Examination Survey) study demonstrated that elevated serum homocysteine and lower folate levels (but not B12 levels) correlated cross-sectionally with lower lumbar and total body bone mineral density (BMD)(13).
Despite relatively strong epidemiologic evidence, results of prior clinical trials to determine if administration of B vitamins can reduce fracture risk have been inconclusive(14–17). For example, in the B-PROOF (B-Vitamins for the PRevention Of Osteoporotic Fractures) study, over 2900 older adults with elevated homocysteine levels were randomized to receive daily B-12 and folic acid supplementation or placebo for a period of 2 years. No significant differences in fracture incidence were observed overall, though there was a suggestion of protective effect among compliant individuals over 80 years old(14). Two other studies, conducted among individuals at high risk of cardiovascular disease(15) or recent stroke(17), found no significant association of homocysteine-lowering therapy and fracture risk. Only one study among older Japanese persons with ischemic stroke reported a significant reduction of fracture risk in those taking daily folic acid and vitamin B12 compared to placebo(16). However, this study by Sato et al, was recently retracted, invalidating those positive findings(18).
Similarly, results of most previous trials testing the effects of B vitamins on markers of bone turnover(19–22) have also failed to demonstrate significant effects. However, these studies have also suffered from small sample size and most were relatively short-term trials of 3–6 months(20–22).
To test the hypothesis that treatment with a regimen of folic acid, vitamin B6, and vitamin B12 would reduce the risk of incident fractures and influence levels of bone turnover markers pre- and post treatment in women, we performed an ancillary study to the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), a large, double-blind, randomized placebo-controlled trial originally designed to test the effects of antioxidants and B vitamins on cardiovascular disease outcomes in women at high CVD risk. We further hypothesized that women with elevated homocysteine levels, or low serum levels of folic acid and vitamins B6 or B12 would experience the greatest benefits of treatment.
Study Population
The Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS) was a randomized, double-blind, placebo-controlled trial, originally designed to test the effects of antioxidant vitamins (vitamin C, vitamin E, and ß-carotene) for the prevention of cardiovascular events among female health professionals aged ≥ 40 years at enrollment (mean age 60.6 years, range 43 to 90 years), who had either pre-existing cardiovascular disease (CVD) or ≥ 3 coronary risk factors(3, 23, 24). The sample was approximately 94% Caucasian(23).
Initially, the WAFACS cohort included 8,171 participants who were randomly assigned in a 2×2×2 factorial design to vitamin C, vitamin E, ß-carotene, or placebo in 1995(23, 24). Additional funding was received to add the folic acid (2.5 mg/d), vitamin B6, (50 mg/d), and vitamin B12 (1 mg/d) intervention to the study and 5,442 willing participants were further randomized to either treatment with combination B vitamins or placebo in April 1998(3). The design of the study is illustrated in Figure 1. The B vitamin intervention was designed with the objective of lowering homocysteine levels, thereby reducing the risk for cardiovascular disease in this population of high risk women. A blood substudy of a random sample of 300 women (150 in each treatment group) confirmed that those receiving the B vitamin supplementation had higher folate levels, and lower homocysteine levels relative to baseline levels, and significantly more so than the placebo group(3). To participate in the B vitamin intervention, women had to forego the use of B vitamin supplements at levels greater than the recommended dietary allowance (RDA) for the duration of the intervention(3).
Figure 1.
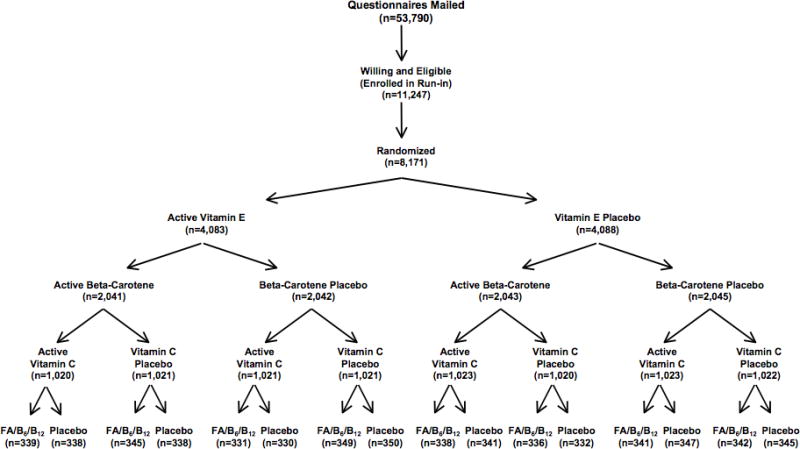
Randomization scheme for the women’s Antioxidant and Folic Acid Cardiovascular Study
Treatment and follow-up of the B vitamin intervention were concluded in July 2005, with mean follow-up at study closeout of approximately 7.3 years. As previously published, all CVD risk factors, medication use, multivitamin use, and median intake of the relevant nutrients (folate, B6, and B12) were very similar between randomization arms, with no significant differences. (3)
Fracture Assessment
Throughout the treatment and follow-up period, annual follow-up questionnaires were mailed to participants to collect self-reported information on study outcomes including fractures that occurred during the preceding 12-month period. Women who reported a fracture were contacted by WAFACS staff who requested detailed information on each fracture reported during the B vitamin intervention/follow-up period. Clinical documentation to confirm fractures,, including medical and radiographic reports, were requested.
The primary endpoint was confirmed incident non-spine fracture during the B vitamin intervention trial. Spine fractures and pathological fractures were excluded. Radiographic reports or other clinical documentation for each reported fracture were reviewed and adjudicated centrally at the San Francisco Coordinating Center, without knowledge of treatment assignment. Women with inadequate fracture documentation were excluded from the main analysis (n=632).
Case-Cohort Fracture Study by Baseline Plasma Levels of Homocysteine and B Vitamins
A case-cohort study design was used to test whether the effects of treatment on fracture risk differed among those with elevated baseline homocysteine levels, or low levels of Vitamin B6, Vitamin B12 or folate.(25) Approximately 75% of participants in the B vitamin intervention had provided EDTA plasma samples at baseline. Following completion of the fracture adjudication, among those with baseline plasma samples available, we selected a case-cohort sample of 833 participants independent of active vs. placebo treatment status, which included all 274 participants with confirmed incident non-spine fractures, and a random cohort sample of 675 participants selected regardless of fracture status. Among the 675 women in the random cohort sample, 116 were fracture cases, and 559 did not have incident adjudicated non-spine fracture and served as the ‘control’ group for analyses, resulting in an approximate control to case ratio of 2:1.
Archived blood specimens were assayed for biochemical parameters by the Nutrition Evaluation Laboratory at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University. Plasma total homocysteine (tHcy) was measured by high-performance liquid chromatography using fluorescence detection (Empower HPLC System, Waters Corporation, Milford, MA 01757)(26). The detection limit of the tHcy assay is 1 nM/mL and the intra- and inter-assay CVs are 4% and 7% respectively. The reference range is 4.6–11.0 nM/mL. Folate and vitamin B12 were measured by a competitive binding protein radioimmunoassay (Quantaphase II RIA kit, Bio-Rad Laboratories, Hercules, CA). The detection limit for the folate assay is <0.1 ng/mL and the intra- and inter-assay CVs are 3.8–5.2% and 4.1-.8.7% respectively. The reference range is 1.5–20.6 ng/mL. The detection limit of the vitamin B12 assay is 20 pg/mL and the intra- and inter-assay CVs are 4.0–5.9% and 4.5–6.8% respectively. The reference range is 200–1200 pg/mL. Pyridoxal 5′-phosphate (PLP: vitamin B6) was determined by enzymatic radioimmunoassay using tyrosine decarboxylase apoenzyme(27). The detection limit of the vitamin B6 assay is 10 nmol/L and the intra- and inter-assay CVs are 5% and 9% respectively. The reference range is 20–100 nmol/L.
Markers of Bone Turnover
In a separate previous substudy(3), a total of 300 women (150 in the active treatment and 150 in the placebo group) provided a fasting plasma sample at randomization for the B vitamin intervention, and at the end of randomized treatment and follow-up 7.3 years later. C-terminal cross-linking telopeptide of type I collagen (CTX; Roche Diagnostics), and marker of bone resorption, and intact type I procollagen N-propeptide (P1NP; Roche Diagnostics, Mannheim, Germany), a marker of bone formation, were measured using automated platforms at a specialized laboratory (CCBR-Synarc, Lyon, France). Intra- and interassay CVs are <4.4% for PINP and <5.7% for CTX.
In this blood subgroup, the concentration of baseline total homocysteine was previously determined by enzymatic assay using the Hitachi 917 analyzer, Roche Diagnostics, Basel, Switzerland. Baseline and follow-up samples were assayed in blinded fashion in the same analytical run(3).
Other Variables
Information regarding body mass index (BMI), menopausal status, menopausal hormone therapy (HT) use, multivitamin use, personal use of folic acid and other B vitamin supplements, smoking status (current, past and never), physical activity (kcal/day) and history of prior fracture were collected by self-reported questionnaire at the time of the randomization in the B vitamin intervention(3, 23).
Statistical Analysis
Characteristics of the B vitamin treatment and placebo groups were compared using t-tests for continuous variables and chi-square statistics for categorical variables.
Following intention-to-treat analysis, the effect of B vitamin therapy vs. placebo on fracture risk was assessed using Cox proportional hazards models. The primary outcome was adjudicated non-spine fracture. However, given that a relatively large number (n=632) had inadequate fracture documentation, self-reported non-spine fracture was analyzed as a sensitivity analysis. Pre-specified subgroup analyses were performed stratifying on adherence (defined as those who took at least two thirds of their assigned pills at all follow-up time points) (Y/N), age (≤65, >65 years), baseline use of B vitamin supplements (Y/N), current smoking (Y/N), and current HT use (Y/N). Because multivitamins often contain up to 100% of the recommended dietary allowance for some B vitamins, we performed a sensitivity analysis excluding those reporting use of B vitamins or multivitamins at baseline.
The case-cohort adaptation to the Cox proportional hazards model was used for analysis of the effects of treatment on risk of non-spine fracture among those with elevated baseline levels of homocysteine (defined as highest quartile), or low B6, B12 or folate levels (defined as lowest quartile). Because power for this analysis was limited, in sensitivity analyses we also explored the median as the cutpoint for these analyses.
For change in bone marker analysis, linear regression models were used to assess effects of the treatment of interest on percent change in untransformed P1NP and CTX which was calculated by 100* [(follow-up – baseline)/baseline]. Subgroup analyses were done by HT use status, menopause status, and baseline homocysteine level.
All analyses were conducted using SAS (Version 9.4).
Results
Among 5442 participants who were enrolled in the vitamin B intervention component of WAFACS, 1229 reported incident non-spine fractures on one or more annual questionnaires. Of these, 632 had inadequate fracture documentation (319 in the B vitamin group and 313 in the placebo group) and were excluded from analyses. An additional 248 reported fractures were adjudicated as non-fractures. The analytic cohort was therefore comprised of a total of 4810 women with 349 adjudicated non-spine fractures (including 22 hip fractures, and 67 wrist fractures). The vitamin and placebo groups were well matched at baseline with regards to age, postmenopausal status, previous fracture and HT use (Table 1). Approximately two thirds of women reported >66% compliance with their assigned intervention at all follow-up timepoints during the trial.
Table 1.
Baseline Characteristics by Treatment Group Among WAFACS Women with Valid Fracture Info (N=4810)
| Variable | Folate/Vitamin B Group (N=2402) |
Placebo Group (N=2408) |
P value |
|---|---|---|---|
| Age | 62.6 ± 8.7 | 62.5 ± 8.7 | 0.65 |
| BMI | 30.5 ± 6.6 | 30.6 ± 6.7 | 0.55 |
| Post-menopause status Yes No Unsure |
2193 (91.3%) 149 (6.2%) 60 (2.5%) |
2204 (91.5%) 156 (6.5%) 48 (2.0%) |
0.47 |
| Smoking status Never Past Current |
1094 (45.6%) 1046 (43.6%) 262 (10.9%) |
1035 (43.0%) 1071 (44.5%) 302 (12.5%) |
0.03 |
| Current HT use | 1245 (51.8%) | 1248 (51.8%) | 0.99 |
| Taking multivitamin | 555 (23.1%) | 546 (22.7%) | 0.73 |
| Taking folate/B6/B12 supplement | 184 (7.7%) | 166 (6.9%) | 0.31 |
| Taking folate supplement | 64 (2.7%) | 60 (2.5%) | 0.71 |
| Taking B6 supplement | 81 (3.4%) | 80 (3.3%) | 0.92 |
| Taking B12 supplement | 90 (3.8%) | 75 (3.1%) | 0.23 |
| Taking other supplement with high folate/B6/B12 | 51 (2.1%) | 57 (2.4%) | 0.57 |
| Prior fracture | 891 (37.1%) | 903 (37.5%) | 0.77 |
| Physical activity – kcal per week from exercise | 1259 ± 1779 | 1168 ± 1670 | 0.07 |
| Adherent to treatment* | 1600 (67.2%) | 1636 (68.6%) | 0.30 |
Adherence is defined as having 100% of annual follow-up questionnaires in which participant reports 67% or more study medications were taken
Fracture outcome
The risk of any non-spine fracture was similar among those randomized to vitamin or placebo (hazard ratio (HR)=1.08, 95% confidence interval (CI): 0.88, 1.34), as were risks of hip (HR=0.99, 95% CI: 0.43, 2.29), wrist (HR=1.30, 95% CI: 0.80, 2.11), and other fracture (HR=1.03, 95% CI: 0.82, 1.30) (Table 2). In a sensitivity analysis, results were also similar for the outcome of self-reported (unadjudicated) non-spine fracture (HR=1.06, 95% CI: 0.93, 1.20)(Table 2). Results were also unchanged by further adjustment for treatment assignment to Vitamin C, E, or beta-carotene (results not shown).
Table 2.
Incident Fractures by Treatment Group during 7.3 Years of Treatment/Follow-up
| Variable | Folate/Vitamin B Group (N=2402) |
Placebo Group (N=2408) |
Relative Hazard (95% CI) | P value* |
|---|---|---|---|---|
| Non-spine Fracture | 182 (7.6%) | 167 (6.9%) | 1.08 (0.88, 1.34) | 0.39 |
| Hip Fracture | 11 (0.5%) | 11 (0.5%) | 0.99 (0.43, 2.29) | 0.99 |
| Wrist Fracture | 38 (1.6%) | 29 (1.2%) | 1.30 (0.80, 2.11) | 0.26 |
| Other Non-spine fracture | 146 (6.1%) | 142 (5.9%) | 1.03 (0.82, 1.30) | 0.79 |
| Self-reported (unadjudicated) non-spine fracture | 628 (23.1%) | 601 (22.1%) | 1.06 (0.93, 1.20) | 0.38 |
p-value from chi-square test
Non-spine fracture results were similar among those who were considered adherent to study medications during the follow-up period, and among baseline subgroups defined by age, use of B vitamin supplements, HT use, and smoking status (all interaction p values>0.05) (Table 3). There were similarly no significant effects of treatment observed after excluding those who reported baseline use of either B vitamin supplements or multivitamins (HR=1.07, 95% CI: 0.83, 1.36).
Table 3.
Effect of Randomized Treatment Assignment on the Primary Outcome in Pre-specified Subgroups
| No. of Patients | No. of Events | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Active | Placebo | Active | Placebo | RR (95% CI) | P value*** |
|
| ||||||
| Overall | 2402 | 2408 | 182 | 167 | 1.08 (0.88, 1.34) | |
|
| ||||||
| Age, y | ||||||
| ≤65 | 1417 | 1415 | 104 | 107 | 0.95 (0.73, 1.25) | 0.14 |
| >65 | 985 | 993 | 78 | 60 | 1.32 (0.94, 1.85) | |
|
| ||||||
| Taking Supplements* | ||||||
| Yes | 184 | 166 | 12 | 16 | 0.64 (0.30, 1.35) | 0.15 |
| No | 2218 | 2242 | 170 | 151 | 1.13 (0.91, 1.41) | |
|
| ||||||
| Current HRT use | ||||||
| Yes | 1245 | 1248 | 78 | 88 | 0.87 (0.64, 1.18) | 0.052 |
| No | 1157 | 1160 | 104 | 79 | 1.33 (0.99, 1.78) | |
|
| ||||||
| Current Smoker | ||||||
| Yes | 262 | 302 | 13 | 14 | 1.06 (0.50, 2.25) | 0.94 |
| No | 2140 | 2106 | 169 | 153 | 1.08 (0.87, 1.35) | |
|
| ||||||
| Adherent** | ||||||
| Yes | 1600 | 1636 | 129 | 124 | 1.05 (0.82, 1.35) | 0.62 |
| No | 782 | 750 | 53 | 43 | 1.19 (0.79, 1.77) | |
Taking folic acid, B6, B12 and any other supplements which were above the US recommended daily allowance for folic acid, B6, and B12 at baseline (400 μg folic acid, 2 mg B6, and 6 ug B12)
Adherence is defined as having 100% of annual follow-up questionnaires in which participant reports 67% or more study medications were taken.
p-value for interaction
In the case-cohort fracture substudy, there was no evidence of an interaction of treatment assignment with baseline plasma levels of homocysteine, B12 or folate (Table 4). However, there was a significant interaction observed between baseline B6 levels and treatment (interaction p-value = 0.04). Among those in the lowest quartile of plasma B6 levels, there was a non-significant protective effect of treatment (relative hazard (RH)=0.69; 95% CI 0.39, 1.22), whereas there was a significant increased risk observed among those with normal B6 levels (RH=1.39; 95% CI 1.01, 1.91). In separate sensitivity analyses, we found no significant interactions of treatment assignment and baseline plasma levels of homocysteine, B12, folate, or B6 (nor did we observe any specific effects of treatment within strata) when we stratified based on median concentrations instead of high-risk quartile versus others.
Table 4.
Case-Cohort Analysis: Effect of Randomized Treatment Assignment on the Non-Spine Fracture Risk by Baseline Plasma Levels of Homocysteine, B6 and Folate*
| No. of Patients | No. of Events | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Active | Placebo | Active | Placebo | RR (95% CI) | P value** |
|
| ||||||
| Homocysteine | ||||||
| High (Q4) | 94 | 109 | 34 | 36 | 1.11 (0.64, 1.94) | 0.82 |
| Normal (Q1–Q3) | 309 | 321 | 107 | 97 | 1.20 (0.87, 1.65) | |
|
| ||||||
| Vitamin B12 | ||||||
| Low (Q1) | 109 | 101 | 37 | 37 | 0.91 (0.53, 1.56) | 0.31 |
| Normal (Q2–Q4) | 294 | 329 | 104 | 96 | 1.27 (0.92, 1.76) | |
|
| ||||||
| Folate | ||||||
| Low (Q1) | 87 | 101 | 33 | 37 | 1.28 (0.74, 2.22) | 0.73 |
| Normal (Q2–Q4) | 316 | 312 | 108 | 96 | 1.15 (0.83, 1.58) | |
|
| ||||||
| Vitamin B6 | ||||||
| Low (Q1) | 92 | 110 | 25 | 38 | 0.69 (0.39, 1.22) | 0.04 |
| Normal (Q2–Q4) | 311 | 321 | 116 | 95 | 1.39 (1.01, 1.91) | |
High homocysteine is > 15.1 nM/mL; Low B12 is ≤ 334 pg/mL; Low folate is ≤ 6.4 ng/mL; Low B6 is ≤ 34.1 nmol/L
p-value for interaction
Bone turnover markers outcome
There were no differences between treatment and placebo groups regarding baseline and follow-up bone turnover marker measurements and change in bone markers (Table 5). Change in bone markers were also similar with active and placebo treatment after stratifying for baseline HRT use status (all p>0.3). There were similarly no significant interactions between treatment and menopause status or baseline homocysteine levels.
Table 5.
Change in Bone Turnover Markers by Treatment Group Over 7.3 Years (pre- to post-treatment)*
| Variable | Active Group | Placebo Group | P value |
|---|---|---|---|
| Baseline CTX (ng/ml) | 0.21 ± 0.14 | 0.23 ± 0.15 | 0.17 |
| Baseline P1NP (ng/ml) | 32.3 ± 18.2 | 32.7 ± 16.3 | 0.85 |
| Follow-up CTX (ng/ml) | 0.28 ± 0.20 | 0.28 ± 0.19 | 0.93 |
| Follow-up P1NP (ng/ml) | 42.5 ± 31.7 | 39.9 ± 23.6 | 0.43 |
| % change in CTX | 113.2% ± 251.7% | 105.4% ± 230.9% | 0.78 |
| % change in P1NP | 67.7% ± 162.1% | 55.7% ± 114.7% | 0.46 |
Total N=300, including 150 in each treatment group.
Discussion
In this large randomized, placebo-controlled trial of women at high risk for CVD, we found no significant beneficial or harmful effects of daily B vitamin supplementation on non-spine fracture risk over 7.3.years of treatment and follow-up. In a case-cohort subsample, we similarly found no significant effects of B vitamins on non-spine fracture risk among those with elevated baseline plasma homocysteine levels. Results were similarly null in other pre-specified subgroup analyses based on adherence, age, HT use, baseline use of B vitamin supplements, and smoking status.
Overall, our results add to the growing body of evidence from randomized trials, demonstrating minimal effect of B vitamin supplementation on risk of fracture(14, 15, 17, 28). For example, our findings are consistent with the recently published negative results in the B-PROOF study, in which over 2900 older adults with elevated homocysteine levels were randomized to receive daily B-12 and folic acid supplementation or placebo for a period of 2 years(14). However, whereas the B-PROOF study reported a possible protective effect among compliant individuals older than 80, our pre-specified subgroup analyses revealed no significant interaction of treatment with age or compliance to assigned study pills. In contrast with the B-PROOF study, our intervention was longer (7.3 years versus 2 years in B-PROOF), and our study population comprised women at high risk of CVD, but were not otherwise selected for high baseline homocysteine levels. Nonetheless, our subgroup findings are consistent with those of B-PROOF, showing no significant effect of B vitamin supplementation in those with elevated baseline homocysteine levels(14). Of the four baseline plasma biomarkers (homocysteine, folic acid, B12, and B6), we observed a significant interaction with treatment for only one (B6), and therefore cannot rule out chance as an explanation for this finding.
Previous results from a Japanese study by Sato et al had suggested a significant protective benefit of B vitamin supplementation on fracture risk in patients with prior ischemic stroke(16). However, this study has since been retracted(18). To our knowledge, there are no randomized trials that have demonstrated significant effects of B vitamin supplementation for the prevention of fracture risk.
Finally, we found that a B vitamin intervention designed to lower homocysteine levels had no differential effects on changes in two contemporary markers of bone turnover over 7.3 years of treatment and follow-up. Results of previous trials, typically conducted over 1–2 year periods, have been mixed (19, 20, 22, 29–31), with most studies confirming our findings of no significant effects of B vitamin supplementation on changes in bone turnover markers, despite successful lowering of plasma homocysteine or B vitamin levels(19, 20, 30). To our knowledge, the only study to report significant changes in bone turnover markers was an intervention by Herrmann et al, in which B Vitamin supplementation was conducted in men and women aged > 54 for a period of 1 year(31). In this study, both treatment and placebo group received supplemental calcium and vitamin D. Those in the treatment group demonstrated significantly decreased levels of bone alkaline phosphatase, tartrate resistant acid phosphatase, and osteocalcin compared to those in the placebo group. We did not examine these bone biomarkers in our study, nor did we administer supplemental calcium or vitamin D.
Since we did not measure BMD in our study, we were unable to examine the effects of B vitamin supplementation on changes in BMD, although most previous randomized trials that have investigated this endpoint have been null(30, 32). Herrmann reported a significant improvement in lumbar spine BMD after a year of supplementation with a high-dose combination of folic acid, vitamins B6 and B12 among the small subset (n=8) with low total homocysteine concentrations at baseline(30).
There is rationale for an effect of supplemental B vitamins in fracture prevention and bone health. Prior studies demonstrated that elevated homocysteine may interfere with collagen cross-linking(33, 34). Collagen cross-links play an important role in determining the stability and strength of the collagen network so that if deficient, the bone matrix could be compromised, leading to an increased risk of fracture. Alternatively, homocysteine may promote oxidative damage leading to increased fractures. For example, when homocysteine undergoes oxidative metabolism to homocystine and homocysteine thiolactone, cytotoxic reactive oxygen species, including the superoxide anion radical and hydroxyl radical are produced, which in turn have been shown to initiate lipid peroxidation(35). Products of lipid and lipoprotein oxidation have been hypothesized to be risk factors for osteoporosis(36). Other plausible mechanisms may involve adverse effects of homocysteine on endothelial-derived nitric oxide (eNOS), which has been shown to negatively impact osteoblast function in mice.(37, 38). There may also be direct effects of B vitamin repletion on bone metabolism in vitamin-insufficient individuals, independently of homocysteine. With respect to the latter possibility, it is noteworthy that approximately 30% of participants were folate deficient at baseline (i.e. plasma folate < 7 ng/mL), but no deficiency was seen at the end of the trial in either the control or the placebo group (3). The improvement in the placebo group could have diminished our ability to detect a treatment effect. Nonetheless, results from randomized trials have largely conflicted with some animal studies as well as observational studies in humans showing an association between serum or plasma levels of homocysteine or B vitamins and bone health.
Our study has several strengths, given that it is a large, randomized placebo-controlled trial of B vitamin supplementation with centrally adjudicated non-spine fracture outcomes in middle-aged to older women. The availability of pre- and post-treatment markers of bone turnover is also a strength. Compared to prior studies, the treatment and follow-up period is relatively long at 7.3 years. Despite these major strengths, there are also some limitations. While power for overall fracture effects was adequate, we had less power to examine effects of treatment on fracture subtypes, or effects of treatment in certain subgroups such as older women, or those with high baseline homocysteine levels. Furthermore, we had limited power to examine the effects of B vitamin supplementation among women who may meet criteria for deficiency (for example, only 1.9% of women, 2.0% in the treatment group and 1.9% in the placebo group had Vitamin B12 levels < 200 pg/ml). Although our results are consistent with all other studies finding of no association of B vitamin intervention with fracture risk, we cannot dismiss the possibility that a different vitamin regimen or different dosages may yield different results. Some studies have suggested that relatively high doses of folic acid such as those used in our study may increase risk of cancer. However, previous analyses in WAFACS have found no significant effects of treatment assignment on risk of total invasive cancer or breast cancer. (39) Finally, our study was restricted to middle-aged to older women at high risk of CVD and may not be generalizable to younger women, men, or adults at high risk of fracture or usual risk for CVD. Since the primary outcome of the trial was CVD, we did not collect information on family history of fracture, nor is there information on sunlight exposure or calcium intake. Therefore we were unable to determine if the treatment groups were balanced with respect to these fracture risk factors, and it is not possible to rule out randomization bias with respect to such factors. Previous intervention studies to date have been very heterogeneous with respect to study duration, study population, fortification status and dosages.(40) The possibility of a benefit in terms of enhanced bone health for certain subgroups cannot be ruled out.
In a large randomized, placebo-controlled trial, we found no effects of long-term (7.3 year) B vitamin supplementation on risk of non-spine fracture or markers of bone resorption in women at high risk of CVD. Results were also null among women with elevated baseline plasma homocysteine levels. Overall, results from randomized trials thus far do not support a preventive benefit of supplemental B vitamins on fracture risk.
Acknowledgments
This work was supported by grant funding through the National Institutes of Health, including the National Heart, Lung and Blood Institute (R01 HL47959) and the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR052817). Vitamin E and its placebo were supplied by Cognis Corporation (La-Grange, Illinois). All other agents and their placebos were supplied by BASF Corporation (Mount Olive, New Jersey). Pill packaging was provided by Cognis and BASF. We are indebted to the 5442 participants in the Women’s Antioxidant and Folic Acid Cardiovascular Study for their dedicated and conscientious collaboration; to the entire staff of the Women’s Antioxidant and Folic Acid Cardiovascular Study: including Marilyn Chown, BS, MPH, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, MA, Ara Sarkissian, MM, and Martin Van Denburgh, BA; and to the following individuals for their assistance in conducting this trial: Michelle Albert, MD, MPH, Tobias Kurth, MD, ScD, I-Min Lee, MBBS, ScD, Aruna Pradhan, MD, MPH, Paul Ridker, MD, MPH, and Jacqueline H. Suk, MD, MPH, Brigham and Women’s Hospital, Boston, Massachusetts; Gavin Blake, MBBS, Mater Misericordiae University Hospital, Dublin, Ireland; Claudia Chae, MD, MPH, Massachusetts General Hospital, Boston; Carlos Kase, MD, Boston University Medical Center, Boston, Massachusetts; and James O. Taylor, MD, East Boston Neighborhood Health Center, Boston, Massachusetts.
Footnotes
Authors’ roles:
Study design: KS, SC and JM. Study conduct: KS, SC and JM. Data collection: WC, DB, AMT and CS. Biochemical Assays: AMT. Data analysis: KS and LL. Data interpretation: KS, SC, JM, LL, WC, DK, and DB. Drafting manuscript: KS and LL. Revising manuscript content: SC, JM, LL, DB, DK, WC, AMT. Approving final version of manuscript: SC and JM. KS, LL and SC take responsibility for the integrity of the data analysis.
Bibliography
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Selhub J, Jacques PF, Rosenberg IH, Rogers G, Bowman BA, Gunter EW, Wright JD, Johnson CL. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): population reference ranges and contribution of vitamin status to high serum concentrations. Ann Intern Med. 1999;131(5):331–9. doi: 10.7326/0003-4819-131-5-199909070-00003. [DOI] [PubMed] [Google Scholar]
- 3.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward M, McNulty H, McPartlin J, Strain JJ, Weir DG, Scott JM. Plasma homocysteine, a risk factor for cardiovascular disease, is lowered by physiological doses of folic acid. QJM. 1997;90(8):519–24. doi: 10.1093/qjmed/90.8.519. [DOI] [PubMed] [Google Scholar]
- 5.Bostom AG, Selhub J, Jacques PF, Rosenberg IH. Power Shortage: clinical trials testing the “homocysteine hypothesis” against a background of folic acid-fortified cereal grain flour. Ann Intern Med. 2001;135(2):133–7. doi: 10.7326/0003-4819-135-2-200107170-00014. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Hu X, Zhang Q, Cao H, Wang J, Liu B. Homocysteine level and risk of fracture: A meta-analysis and systematic review. Bone. 2012;51(3):376–82. doi: 10.1016/j.bone.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 7.van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J, de Groot LC, Hofman A, Witteman JC, van Leeuwen JP, Breteler MM, Lips P, Pols HA, Uitterlinden AG. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350(20):2033–41. doi: 10.1056/NEJMoa032546. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda T, Tanaka S, Saito M, Shiraki Y, Shiraki M. Plasma level of homocysteine associated with severe vertebral fracture in postmenopausal women. Calcif Tissue Int. 2013;93(3):269–75. doi: 10.1007/s00223-013-9754-2. [DOI] [PubMed] [Google Scholar]
- 9.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med. 2004;350(20):2042–9. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson K, Gustafson L, Isaksson A, Hultberg B. Plasma homocysteine and markers of bone metabolism in psychogeriatric patients. Scand J Clin Lab Invest. 2005;65(8):671–80. doi: 10.1080/00365510500348153. [DOI] [PubMed] [Google Scholar]
- 11.Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Vaananen HK, Obrant KJ, Akesson K. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res. 2007;22(1):127–34. doi: 10.1359/jbmr.061003. [DOI] [PubMed] [Google Scholar]
- 12.Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20(6):921–9. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RL, Looker AC, Lu Z, Fan R, Eicher-Miller HA, Fakhouri TH, Gahche JJ, Weaver CM, Mills JL. B-vitamin status and bone mineral density and risk of lumbar osteoporosis in older females in the United States. Am J Clin Nutr. 2015;102(3):687–94. doi: 10.3945/ajcn.115.108787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Wijngaarden JP, Swart KM, Enneman AW, Dhonukshe-Rutten RA, van Dijk SC, Ham AC, Brouwer-Brolsma EM, van der Zwaluw NL, Sohl E, van Meurs JB, Zillikens MC, van Schoor NM, van der Velde N, Brug J, Uitterlinden AG, Lips P, de Groot LC. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100(6):1578–86. doi: 10.3945/ajcn.114.090043. [DOI] [PubMed] [Google Scholar]
- 15.Sawka AM, Ray JG, Yi Q, Josse RG, Lonn E. Randomized clinical trial of homocysteine level lowering therapy and fractures. Arch Intern Med. 2007;167(19):2136–9. doi: 10.1001/archinte.167.19.2136. [DOI] [PubMed] [Google Scholar]
- 16.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Effect of folate and mecobalamin on hip fractures in patients with stroke: a randomized controlled trial. JAMA. 2005;293(9):1082–8. doi: 10.1001/jama.293.9.1082. [DOI] [PubMed] [Google Scholar]
- 17.Gommans J, Yi Q, Eikelboom JW, Hankey GJ, Chen C, Rodgers H, group Vts The effect of homocysteine-lowering with B-vitamins on osteoporotic fractures in patients with cerebrovascular disease: substudy of VITATOPS, a randomised placebo-controlled trial. BMC Geriatr. 2013;13:88. doi: 10.1186/1471-2318-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notice of Retraction: Sato Y, et al. Effect of Folate and Mecobalamin on Hip Fractures in Patients With Stroke: A Randomized Controlled Trial. JAMA. 2005;293(9):1082–1088. JAMA. 2016;315(22):2405. doi: 10.1001/jama.2016.7190. [DOI] [PubMed] [Google Scholar]
- 19.Green TJ, McMahon JA, Skeaff CM, Williams SM, Whiting SJ. Lowering homocysteine with B vitamins has no effect on biomarkers of bone turnover in older persons: a 2-y randomized controlled trial. Am J Clin Nutr. 2007;85(2):460–4. doi: 10.1093/ajcn/85.2.460. [DOI] [PubMed] [Google Scholar]
- 20.Keser I, Ilich JZ, Vrkic N, Giljevic Z, Colic Baric I. Folic acid and vitamin B(12) supplementation lowers plasma homocysteine but has no effect on serum bone turnover markers in elderly women: a randomized, double-blind, placebo-controlled trial. Nutr Res. 2013;33(3):211–9. doi: 10.1016/j.nutres.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Salari P, Abdollahi M, Heshmat R, Meybodi HA, Razi F. Effect of folic acid on bone metabolism: a randomized double blind clinical trial in postmenopausal osteoporotic women. Daru. 2014;22:62. doi: 10.1186/s40199-014-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahab-Ferdows S, Anaya-Loyola MA, Vergara-Castaneda H, Rosado JL, Keyes WR, Newman JW, Miller JW, Allen LH. Vitamin B-12 supplementation of rural Mexican women changes biochemical vitamin B-12 status indicators but does not affect hematology or a bone turnover marker. J Nutr. 2012;142(10):1881–7. doi: 10.3945/jn.112.165712. [DOI] [PubMed] [Google Scholar]
- 23.Bassuk SS, Albert CM, Cook NR, Zaharris E, MacFadyen JG, Danielson E, Van Denburgh M, Buring JE, Manson JE. The Women’s Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004;13(1):99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 24.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–8. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 26.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 27.Shin YS, Rasshofer R, Friedrich B, Endres W. Pyridoxal-5′-phosphate determination by a sensitive micromethod in human blood, urine and tissues; its relation to cystathioninuria in neuroblastoma and biliary atresia. Clin Chim Acta. 1983;127(1):77–85. doi: 10.1016/0009-8981(83)90077-3. [DOI] [PubMed] [Google Scholar]
- 28.Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Sleight P, Peto R, Collins R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303(24):2486–94. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann M, Stanger O, Paulweber B, Hufnagl C, Herrmann W. Folate supplementation does not affect biochemical markers of bone turnover. Clin Lab. 2006;52(3–4):131–6. [PubMed] [Google Scholar]
- 30.Herrmann M, Umanskaya N, Traber L, Schmidt-Gayk H, Menke W, Lanzer G, Lenhart M, Peter Schmidt J, Herrmann W. The effect of B-vitamins on biochemical bone turnover markers and bone mineral density in osteoporotic patients: a 1-year double blind placebo controlled trial. Clin Chem Lab Med. 2007;45(12):1785–92. doi: 10.1515/CCLM.2007.352. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann W, Kirsch SH, Kruse V, Eckert R, Graber S, Geisel J, Obeid R. One year B and D vitamins supplementation improves metabolic bone markers. Clin Chem Lab Med. 2013;51(3):639–47. doi: 10.1515/cclm-2012-0599. [DOI] [PubMed] [Google Scholar]
- 32.Enneman AW, Swart KM, Zillikens MC, van Dijk SC, van Wijngaarden JP, Brouwer-Brolsma EM, Dhonukshe-Rutten RA, Hofman A, Rivadeneira F, van der Cammen TJ, Lips P, de Groot CP, Uitterlinden AG, van Meurs JB, van Schoor NM, van der Velde N. The association between plasma homocysteine levels and bone quality and bone mineral density parameters in older persons. Bone. 2014;63:141–6. doi: 10.1016/j.bone.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Kang AH, Trelstad RL. A collagen defect in homocystinuria. J Clin Invest. 1973;52(10):2571–8. doi: 10.1172/JCI107449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH. Evidence for McKusick’s hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. 1996;1315(3):159–62. doi: 10.1016/0925-4439(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 35.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 36.Parhami F. Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids. 2003;68(6):373–8. doi: 10.1016/s0952-3278(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 37.Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez de Marticorena I, Hukkanen M, Huang P, MacIntyre I, Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001;158(1):247–57. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armour KE, Armour KJ, Gallagher ME, Godecke A, Helfrich MH, Reid DM, Ralston SH. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142(2):760–6. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300(17):2012–21. doi: 10.1001/jama.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey RL, van Wijngaarden JP. The Role of B-Vitamins in Bone Health and Disease in Older Adults. Curr Osteoporos Rep. 2015;13(4):256–61. doi: 10.1007/s11914-015-0273-0. [DOI] [PubMed] [Google Scholar]


