Abstract
Data suggests that individuals who binge eat are more responsive to food cues in the environment and less sensitive to satiety cues. The aim of this open trial was to evaluate the feasibility, acceptability, and initial effectiveness of a novel treatment grounded in Schachter’s externality theory targeting food cue reactivity and satiety responsiveness with obese adults who binge eat. Treatment was provided in groups, and utilized appetite monitoring, cue-exposure treatment, in vivo exercises, self-monitoring, and coping skills. Twenty-eight overweight and obese adults who binge eat (82% female; mean age = 47.5 years [SD = 12.8]; BMI = 38.9 [SD = 10.3]; 79% White non-Hispanic) participated in a 4-month group-based treatment program. Assessments were conducted at baseline, posttreatment, and 3-month follow-up time points. Results indicated that this treatment was well accepted and had high retention at posttreatment. Initial effectiveness showed significant decreases in BMI, and improvements in loss of control and overeating episodes, food responsiveness, and power of food. The majority of results were maintained at the 3-month follow-up time point. This open trial provides preliminary evidence for the feasibility, acceptability, and initial effectiveness of this treatment on both eating disorder symptoms and weight in obese adults who binge eat. Because these data are preliminary, further treatment development and randomized controlled studies are needed.
Keywords: binge eating, obesity, appetite, cue reactivity
Binge eating is characterized by feelings of loss of control and the consumption of a large amount of food, typically within a discrete amount of time (American Psychiatric Association, 2013). Binge eating disorder (BED) diagnosis includes having three or more of the following: eating more rapidly than normal, eating until feeling uncomfortably full, eating large amounts when sated, eating alone because of embarrassment, and feeling disgusted, depressed, and/or guilty after eating. Many individuals who have BED are also overweight or obese. Studies using interview-based assessments suggest that 23% to 46% of obese individuals report engaging in binge eating (Dymek-Valentine, Rienecke-Hoste, & Alverdy, 2004; Gormally, Black, Daston, & Rardin, 1982; Marcus, Wing, & Lamparski, 1985; Spitzer et al., 1992). However, evidence from ecological momentary assessment studies suggests that binge eating among overweight and obese individuals is more prevalent (66%–100%) than other data suggest (Greeno, Wing, & Shiffman, 2000; Le Grange, Gorin, Catley, & Stone, 2001). Both binge eating and obesity are associated with significant psychosocial consequences, medical comorbidities, co-occurring psychiatric disorders, and increased health care utilization (Dixon, 2010; Flegal, Kit, Orpana, & Graubard, 2013; Javaras et al., 2008; Kessler et al., 2013; Kessler et al., 2014; Striegel-Moore et al., 2004). Considering the negative psychosocial and physical comorbidities and the high prevalence of binge eating in overweight and obese populations, it is important to develop treatments that target both binge eating and weight loss.
Current treatments for BED targeting eating disorder symptoms include cognitive behavioral therapy (CBT), guided self-help CBT (CBTgsh), and interpersonal therapy (IPT; Kass, Kolko, & Wilfley, 2013). CBT and CBTgsh target eating patterns, dietary restraint, and self-evaluation of weight and shape that maintain binge eating using self-monitoring, psychoeducation, behavioral strategies, problem solving, and relapse prevention strategies. IPT, in comparison, emphasizes change in the interpersonal problem areas that maintain the binge eating behavior. IPT does not include any of the behavioral or cognitive skills that are core components of CBT. Randomized controlled studies have demonstrated that both CBT and IPT decrease binge eating and related psychopathology in the short and long term (Wilfley et al., 2002; Wilson, Grilo, & Vitousek, 2007; Wilson, Wilfley, Agras, & Bryson, 2010). However, these treatments do not produce clinically significant weight loss (Wilfley et al., 2002; Wilson et al., 2007; Wilson et al., 2010).
On the other hand, behavioral weight loss (BWL) is an efficacious treatment for overweight and obese adults (Diabetes Prevention Program Research Group, 2002; Espeland, 2007; Wadden, Butryn, & Wilson, 2007). BWL provides a treatment package consisting of dietary recommendations, physical activity guidelines, and behavioral techniques. BWL is typically provided over 1 year and overweight and obese participants in BWL lose an average of 8.6% of their total body weight at the end of 12 months (Diabetes Prevention Program Research Group, 2004). However, only a percentage of adults respond favorably to traditional BWL interventions, with one third to one half of participants failing to achieve meaningful weight loss (Wadden et al., 2009). Not surprisingly, overweight and obese adults who binge eat have more modest weight losses during BWL than participants who do not binge eat (Grilo, Masheb, Wilson, Gueorguieva, & White, 2011). While BWL has proven to be an efficacious weight loss program for overweight and obese adults who do not binge eat, and CBT and IPT have proven to be effective treatments for binge eating, none of these treatments have been able to address both the eating disorder symptoms and weight loss in overweight and obese adults who also binge eat. Thus, it is possible that new models for treatment are needed to address both binge eating and weight loss.
Schachter’s externality theory (Schachter, 1971; Schacter & Rodin, 1974) provides a model to develop alternative treatments targeting both binge eating and obesity. The externality theory suggests that overeating and/or binge eating results from high levels of reactivity to external cues to eat and reductions in sensitivity to internal hunger and satiety signals. The increased reactivity to cues to eat referred to in Schachter’s externality theory is typically labeled — food cue reactivity in the literature. Food cue reactivity originates and is maintained by learning and conditioning processes (Boutelle & Bouton, 2015; Bouton, 2010; Jansen, 1998; Wardle, 1990) and is considered one of the mechanisms that contributes to binge eating (Bouton & Sunsay, 2001; Franken & Muris, 2005; Sobik, Hutchison, & Craighead, 2005; Weingarten, 1983; Weinsier, Hunter, Heini, Goran, & Sell, 1998;). Food cue reactivity is initially developed through Pavlovian learning, where an innocuous cue (i.e., a recliner in front of the TV; unconditioned stimulus) is paired with a food, causing the unconditioned stimulus to acquire eliciting functions (e.g., cravings when sitting in the recliner). Food cue reactivity also develops and is maintained by operant learning, whereby the individual learns to associate eating or food-seeking responses with the reinforcing effects of eating (e.g., Bouton, 2011; Boutelle & Bouton, 2015). Over time, pairing of food cues with eating potentiates these formerly innocuous cues so that the food cue itself elicits physiological reactions and enhanced motivation for food consumption (Bouton & Sunsay, 2001; Weingarten, 1983; Weinsier et al., 1998). Calorically dense foods cues are compelling in capturing attention (Harrar, Toepel, Murray, & Spence, 2011) and certain individuals, such as those with BED, could have a hyperreactivity to the salient properties of food, coupled with motivations to engage in appetitive behaviors (Davis et al., 2009).
Research suggests that food cue reactivity plays a significant role in binge eating, as obese binge eaters, compared to non–binge eaters, show decreases in skin conductance (Svaldi, Tuschen-Caffier, Peyk, & Blechert, 2010) and differential activity in both the prefrontal cortex (Karhunen et al., 2000) and the medial orbitofrontal cortex (Schienle, Schafer, Hermann, & Vaitl, 2009) when exposed to food cues. Overweight BED patients, compared to overweight non-BED patients, show differential attentional biases to food cues, in the orientation and the sustained attentional processes (Schag et al., 2013; Schmitz et al., 2014; Svaldi et al., 2010). Results of eye tracking and event-related potential studies suggest heightened and longer attentional processing of food stimuli in overweight women with BED compared to those without BED (Schag et al., 2013; Svaldi et al., 2010). Furthermore, one study using an antisaccade task suggests that participants with BED might be more — visually impulsive, with less control over their attention than both obese non-BED and healthy weight participants (Schag et al.).
The small body of literature on binge eating is complemented by additional data on food cue reactivity in overweight and obese individuals. However, it should be noted that the majority of these studies do not report on binge eating, so the effect of binge eating in overweight individuals cannot be delineated. Exposure to the sight and smell of food increases reported hunger (Ferriday & Brunstrom, 2008; Nederkoorn, Smulders, & Jansen, 2000; Oakes & Slotterback, 2000) and initiates “cephalic phase responses,” including release of insulin and changes in salivation, heart rate, gastric activity, and blood pressure (Nederkoorn, Smulders, Havermans, & Jansen, 2004; Nederkoorn et al., 2000; Nirenberg & Miller, 1982; Overduin, Jansen, & Eilkes, 1997). Studies utilizing innocuous cues, such as pairing a red square with administration of 1 ml of chocolate milkshake, have demonstrated a stronger conditioned salivary response for obese compared to lean participants (Meyer et al., 2015). While the attentional bias findings have largely been mixed, several studies have demonstrated that obese subjects display a greater attentional bias to food words and pictures than lean subjects (Castellanos et al., 2009; Nijs, Franken, & Muris, 2010; Nijs, Muris, Euser, & Franken, 2010; Nummenmaa, Hietanen, Calvo, & Hyona, 2011; Werthmann et al., 2011; Yokum, Ng, & Stice, 2011). Furthermore, evidence from the neuroimaging literature utilizing positron emission tomography and functional magnetic resonance imaging (fMRI) have shown that obese individuals exhibit a greater increase in fronto-striatal circuitry activation during anticipation of high-caloric foods as compared with lean control subjects (Stice, Spoor, Bohon, & Small, 2008; Stoeckel et al., 2008). In addition, studies with obese participants, compared to lean participants, suggest that food cues continue to activate corticolimbic responses to food cues, even when sated (Dimitropoulos, Tkach, Ho, & Kennedy, 2012; Martin et al., 2010). In sum, this body of research begins to support Schachter’s externality theory that obese individuals, compared to lean individuals, have differential physiological, neural, and self-reported responding to food cues.
There is very little data on satiety responsiveness in people who binge eat; however, the data that do exist suggest that people who binge eat have deficiencies in their ability to detect satiety. For example, overweight children who binge eat demonstrate shorter satiety duration after a meal, compared to overweight children who do not binge eat (Mirch et al., 2006). Animal data suggest that palatable — food seeking is associated with opioid activation, suggesting — reward-motivated behavior as opposed to — hunger-motivated behavior (Jarosz, Kessler, Sekhon, & Coscina, 2007; Jarosz, Sekhon, & Coscina, 2006). Animal data also indicate that repeated exposure to food in the context of the paired cues leads to a progressive increase in food intake, even in sated animals (Jarosz et al., 2007; Jarosz et al., 2006; Weingarten, 1983).
The studies from the obesity literature suggest that obese individuals do not compensate for calorically dense preloads, and consume more calories overall than their lean counterparts (Schachter, 1968; Spiegel, Shrager, & Stellar, 1989). While gastric motility and self-reported hunger ratings appear to be correlated in normal weight individuals, this same relationship does not seem to occur in obese individuals (Stunkard & Koch, 1964). A recent review suggests that responses on self-report scales of intuitive eating (which reflect listening to hunger and satiety feelings) are negatively associated with BMI (Van Dyke & Drinkwater, 2013). Other indications of differential satiety responding come from studies on gastric capacity, which show that obese individuals have increased gastric capacity compared to lean subjects, which may interfere with distention responses in the central nervous system and impact satiety (Geliebter, 1988; Geliebter & Hashim, 2001; Mejia-Rivas, Remes-Troche, Montano-Loza, Herrera, & Valdovinos-Diaz, 2009). In summary, data suggests that BMI is inversely related to sensitivity to hunger and satiety, and that obese individuals have differential gastric capacity and lack ability to compensate to caloric preloads, suggesting an opportunity to target increased sensitivity to internal cues of hunger and satiety.
There is very little data on interventions for the two factors identified in Schachter’s theory: decreased hunger sensitivity and increased food cue responsivity. To date, Appetite Awareness Training (AAT; which trains sensitivity to hunger signals) has been tested with adults who have BED in a small open label case series and a small randomized trial (Allen & Craighead, 1999; Craighead & Allen, 1996). In the randomized trial (Allen & Craighead, 1999), the AAT participants, compared to a waitlist control, reported significantly greater reductions in both binge eating and overeating episodes, but did not report changes in hunger or weight. AAT has also been tested in another small randomized study with overweight and obese children. In this study, children who attended a group-based ATT, compared to those in control, showed significant decreases in BMI immediately posttreatment; however, this difference was not retained at follow-up (Bloom, Sharpe, Mullan, & Zucker, 2013).
Cue-exposure therapy (CET), which targets extinction of food cue responsivity by exposing the participant to the cues predicting food intake without the conditioned response, has mainly been tested with patients who have bulimia nervosa (Jansen, 1998; Wardle, 1990). Case series indicate that CET results in decreases or elimination of binging and vomiting (Jansen, 1989; Martinez-Mallén et al., 2007; Scmidt, 2006; Toro et al, 2003). In a small randomized trial, 12 patients with bulimia nervosa were randomized to CET or Self-Control Treatment (Jansen, Broekmate, & Heymans, 1992). Results showed that all 6 participants in CET were abstinent from binge eating at posttreatment and at the 1-year follow-up, in contrast to only 33% of the self-control participants.
We have piloted these two interventions (AAT and CET) with overweight and obese children (Boutelle, Zucker, Peterson, Rydell, Cafri, Harnack, 2011). In this study, 36 overweight and obese children and their parents were randomized to either AAT or CET. Results showed that both AAT and CET reduced child binge eating while only CET reduced eating in the absence of hunger. Anecdotally, some families in the CET arm were confused about identifying and managing cravings without being able to detect hunger or satiety.
In response, we created a program called Regulation of Cues (ROC), which includes both AAT and CET. ROC is a group-based program that focuses on developing skills to address a lack of satiety sensitivity and increased food cue reactivity. ROC includes in vivo exercises to develop sensitivity to hunger and satiety cues and to reduce food cue reactivity, self-monitoring to train awareness of hunger/satiety and food cue responsivity, psychoeducation that describes the theory and current evidence supporting a treatment focusing on these two targets, and coping skills to address the urges to eat when not physically hungry. To test ROC, we conducted a pilot trial in which we randomly assigned 44 overweight and obese children and their parent to a 16-week ROC intervention or waitlist control (Boutelle, Zucker, Peterson, Rydell, Carlson, Harnack, 2014).
The ROC program was feasible and had high levels of acceptability. Furthermore, children in the ROC program lost weight; however, the weight loss was not significantly different than control (Boutelle, Zucker, Peterson, Rydell, Carlson, Harnack, 2014). The ROC program has not been tested in adults to date. We chose to test ROC with overweight adults who binge eat because they may be independently motivated and the binge eating, which is an extreme form of overeating, would show the greatest signal if there was one present. Thus, the goal of this open label pilot study was to evaluate the feasibility and acceptability of the ROC program with obese adults who binge eat. Our secondary goal was to evaluate the initial effectiveness of the ROC program on outcomes including BMI, overeating, and binge eating.
Methods
Participants
Twenty-eight overweight and obese adults who endorsed binge eating participated in the ROC program. The majority of participants were female and obese, with an average age of 48 years. See Table 1 for demographics of the sample. Participants were recruited via advertisements and referrals from physicians and mental health professionals.
Table 1.
Characteristics of study sample
| Full sample | Participants who completed the intervention | Participants who dropped out | |
|---|---|---|---|
| N=28 | N=22 | N=6 | |
| Mean (SD) BMI | 38.9 (10.3) | 38.8 (10.6) | 39.5 (10.4) |
| Mean (SD) age | 47.5 (12.8) | 51.1 (11.5) | 34.7 (9.1) * |
| Female | 82.1% | 77.3% | 100% |
| White non-Hispanic | 78.6% | 95.5% | 16.7% * |
| Married or living with partner | 60.7% | 59.1% | 66.7% |
| College degree | 71.4% | 72.7% | 66.7% |
| Working full time | 53.6% | 54.5% | 50.0% |
| Household Income | |||
| ≥ 100k | 28.6% | 33.3% | 33.3% |
| 50–99.9k | 35.7% | 33.3% | 66.7% |
| < 50k | 35.7% | 33.3% | 0% |
p<.05
Participants were screened by phone to determine initial eligibility, and then completed an assessment in person in the clinic. Inclusion criteria included: (a) overweight or obese (BMI > 25; measured in clinic), (b) binge eating at least one time per week (determined by EDE-Q; see measures section), (c) English speaking, and (d) willingness to attend the 4-month group program and all assessments. Exclusion criteria included: (a) major medical condition or other conditions that could either carry risk or prevent regular participation in treatment, (b) current diagnosis of bulimia nervosa, (c) current diagnosis of a significant psychiatric illness that would impact participation (i.e., severe depression, suicidality, posttraumatic stress disorder, schizophrenia), (d) significant cognitive impairment, or (e) prior weight loss surgery. All inclusion and exclusion criteria were self-reported except BMI.
Measures
Anthropometry
Height was measured using a standard stadiometer twice, and the average of the two values was used for analysis. Participants’ weight was measured twice on a calibrated slide scale without jackets, outerwear, or shoes. The average of the two weight values was used for analysis. Average height and weight was translated to BMI (kg/m2) for each participant.
The Eating Disorder Examination–Questionnaire (EDE-Q)
The EDE-Q (Fairburn & Beglin, 1994) was used to evaluate frequency data on key behavioral features of eating disorders in terms of the number of episodes of the behavior. The EDE-Q is a self-administered version of the Eating Disorder Examination interview (Fairburn & Cooper, 2008). The EDE-Q has excellent test-retest reliability and internal consistency for the subscales, and acceptable test-retest reliability for specific behaviors (Luce & Crowther, 1999). This study included the questions that evaluate the frequency of binge eating. Participants responded to the following questions: (a) Over the past 28 days, how many times have you eaten what other people would regard as an usually large amount of food? (b) On how many of these times did you have a sense of having lost control over your eating? Participants filled in the blank. From these questions, number of binge eating episodes (eaten a large amount of food and felt a sense of loss of control) over the past 28 days was calculated.
Binge Eating Scale (BES)
The BES (Gormally et al., 1982) was also used to provide a dimensional score of binge eating for this study. The BES consists of 16 items and alpha = .768 in the present study. Eight items describe binge eating behaviors and eight items describe feelings and cognitions associated with binge eating. Each item consists of four statements that reflect a range of severity (0 = none or very little; 3 = severe). Subjects choose the statement that best describes their perceptions and feelings about their eating behavior. This questionnaire yields a continuous measure of binge eating pathology (range = 0–46). Initial validity and consistency of the BES were established using two samples of overweight participants (Gormally et al., 1982). The BES has good test-retest reliability and moderate associations with binge eating severity as measured by food records (Timmerman, 1999).
The Power of Food Scale (PFS)
The PFS (Lowe et al., 2009) was used to evaluate the psychological impact of the current food environment. The PFS consists of 21 items (alpha = .932 in the present study) that reflects three levels of food proximity: food readily available in the environment but not physically present, food present but not tasted, and food tasted but not consumed. An average of the 21 items was taken to derive the scale score, with higher scores reflecting greater impact by the food environment on psychological responding.
Eating Behavior Questionnaire (EBQ)
Two scales from the Children’s Eating Behavior Questionnaire (Wardle, Guthrie, Sanderson, & Rapoport, 2001) were adapted for adults for the purposes of this study, as there are no other measures that evaluate satiety responsivity and responsivity to food cues. The Food Responsiveness scale (EBQ-FR) includes 5 items that reflect eating in response to environmental food cues. The Satiety Responsiveness scale (EBQ-SR) includes 5 items and represents the ability of a participant to reduce food intake after eating. Internal consistency was moderate to high in the present sample (FR α = .778; SR α = .623).
Demographic Characteristics and Weight History (baseline only)
Each participant completed a demographic questionnaire that provided age, race/ethnicity, gender, education and income.
Treatment acceptability (posttreatment only)
The treatment acceptability questionnaire asked a number of questions regarding the acceptability of the ROC program, including: (a) How much did you like the ROC program? (responses were on a 5-point Likert scale that ranged from — didn’t like it to — loved it”); (b) The ROC program has taught me to have more control over my eating (responses were on a 5-point Likert scale that ranged from — strongly disagree to — strongly agree ); (c) I would recommend the ROC program to others (responses were on a 5-point Likert scale that ranged from — strongly disagree to — strongly agree ); (d) Did monitoring your hunger in session help you with your confidence in monitoring at home? (responses were yes or no); (e) Do you think exposure practices helped build your confidence to resist cravings at home? (responses were yes or no).
Design and Procedure
This study was approved by the Institutional Review Board at the University of Minnesota.. A single group quasi-experimental design was used. Participants who completed an initial phone screen and met inclusion criteria (see participant section) were scheduled for an in-person assessment meeting during which participants provided informed consent, completed questionnaires (see measures section), and had height and weight measured. Following the baseline assessment, participants attended a 4-month ROC treatment group, and completed assessments at posttreatment and at 3-month follow-up time points. Participants were compensated $25 at both the posttreatment and 3-month follow-up time points for time and effort.
Content of the Intervention
The ROC intervention was initially described for parents and children (Boutelle et al., 2014). For the current program, we adapted the parent group protocol to target changes in the adult participants (instead of the child) and we added additional information on binge eating and BED. The ROC program for the current project was administered in three waves of weekly 1.5-hour groups of 8–12 adults for 12 weeks, then biweekly for an additional two visits (total treatment = 14 visits over 4 months). The group sessions included psychoeducation and discussion, as well as in vivo learning at each session. Participants were provided with study-specific workbooks, handouts, and self-monitoring booklets. If a participant missed an intervention group meeting, the group leader called him/her and the missed materials were mailed/e-mailed to the participant. All of the groups were led by doctoral-level psychologists and assisted by master's-level co-therapists and an undergraduate volunteer. All therapists attended a 1-day training regarding the treatment and attended weekly supervision with the first author (KB). All sessions were audiotaped, reviewed by the first author for supervision, and any inconsistencies in provision of the treatment were addressed.
ROC Core Components
The ROC program includes four core components; in vivo exercises, self-monitoring, psychoeducation, and coping skills The in vivo exercises were chosen to directly address the two hypotheses identified in Schachter’s externality theory: reduced sensitivity to appetitive cues and increased sensitivity to external food cues. Self-monitoring focused on training awareness of hunger/satiety and food cue reactivity. The psychoeducation portion of the group focused on educating participants on the current research on how the neurobiological, physiological, and psychological food cue reactivity coupled with the current food environment can lead to overeating and binge eating, even when physically full. The coping skills were chosen as possible skills to offer participants to use when trying to resist overeating in today’s food environment.
In Vivo Learning
All group sessions included an in vivo learning exercise. The first five sessions focused on training sensitivity to hunger and satiety cues, and each group session began with participants eating dinner (which they brought from home) during which they self-monitored their hunger before, during, and after the meal (with prompting from the interventionist). The next six sessions focused on learning about responding to food cues when satiated. The urge to eat when physically satiated was described as a craving. Participants created a highly craved foods list and brought a different craved food to clinic each week for — exposure sessions. During the exposure, participants rated their cravings while looking at the food, holding the food, smelling the food, after taking two small bites of the food, and then put the food down and rated their cravings at 30-second intervals for the duration of the exposure. After the participant’s cravings habituated (craving = 2 or lower), the food was disposed of and the exposure ended. The last two group sessions included two in vivo exercises, and participants practiced hunger monitoring during dinner and completed an exposure exercise after dinner.
Self-monitoring of Hunger and Craving
During the first five sessions, participants learned how to detect and self-monitor hunger on a 5-point scale (1 = starving and 5 = stuffed). They were instructed to self-monitor hunger before, during, and immediately after each meal or snack, and 10 and 20 minutes after eating. Participants were encouraged to self-monitor their hunger at least two times per day.
Later in the program (Session 6), participants learned to detect and self-monitor their cravings. Cravings were defined as urges to eat when not physically hungry, and were rated on a 5-point scale (1 = no cravings and 5 = very high cravings). Participants were instructed to rate a minimum of one craving a day. Self-monitoring booklets are available upon request.
Psychoeducation
The overall goal of the psychoeducation portion of the group meetings were to increase participants’ awareness of the reasons why he/she may overeat, and to assist him/her in understanding how neurobiological, physiological, and psychological factors and the current food environment trigger overeating in vulnerable individuals. Participants learned about Schachter’s externality theory, basic learning theory and the development of food cue reactivity, current research on hunger/satiety cues and overeating and obesity, and current research on food cue reactivity and overeating and obesity.
Coping Skills
Participants learned a number of coping skills that could be used to manage urges to eat when not physiologically hungry, including: (a) changing the physical state of the body (e.g., deep breathing, relaxation); (b) increasing behavioral alternatives to eating (e.g., behavioral activation, delay, problem-solving); (c) changing the attentional focus (e.g., distraction, imagery, self-motivational statements); and (d) enhancing motivation to resist cues (e.g., decision balance, cost-benefit analyses). Each group session presented one coping skill, and participants were encouraged to use the coping skill during the week and to identify coping skills that helped them manage their urges to eat when they were not physically hungry.
Statistical Analysis
Treatment acceptability outcomes were assessed using descriptive statistics. Repeated measures ANOVA and MANOVA models with Bonferroni-adjusted comparisons were used to investigate changes in outcomes (a) from baseline to posttreatment and (b) baseline to 3-month follow-up. Outcomes were grouped together based on their conceptual content. ANOVA was used to evaluate BMI, while MANOVAs were used to evaluate disordered eating (binge eating, loss of control eating, overeating) and cognitive traits (power of food, food responsiveness, satiety responsiveness). An intent-to-treat approach was used, with baseline values on outcome scores being carried over for participants who dropped out. Effect sizes were computed by dividing the mean difference over time by the baseline standard deviation, but should be interpreted with caution given the potential unreliability of the standard deviations in this small sample.
Results
Completion Rates
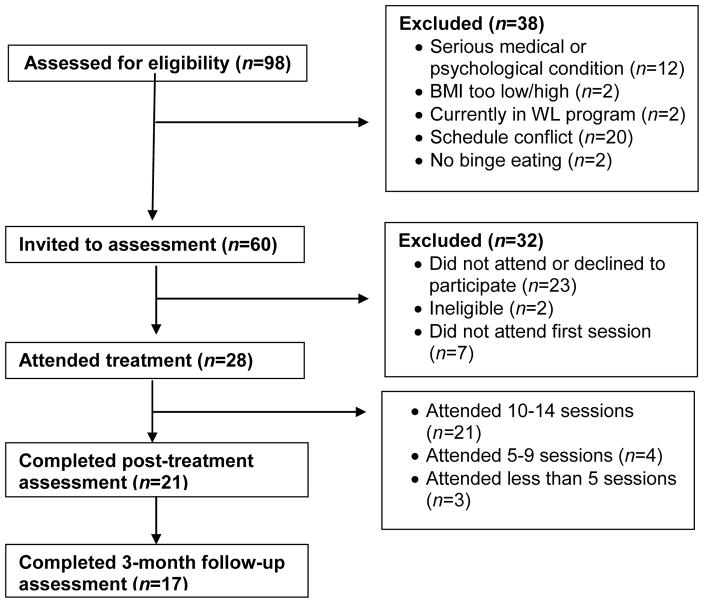
As can be seen in Figure 1, treatment completion rate was high for the ROC intervention. Twenty-eight participants enrolled in the study and 22 (78%) completed the intervention. One of the participants who completed the intervention missed the postintervention assessment but completed the 3-month follow-up. Four participants who completed the intervention missed the 3-month follow-up. Out of the 14 sessions, 75% (n = 21) attended 10–14 sessions, 14.2% (n = 4) attended 5–9 sessions, and 10.7% (n = 3) attended fewer than 5 sessions. All 5 male participants who enrolled in the treatment completed the intervention.
Figure 1.
Participant enrollment and retention in the ROC pilot trial
Treatment Evaluation
In terms of treatment acceptability, 83% percent of program completers — liked it a lot/loved it, 90% — agreed/strongly agreed that they would recommend the ROC program to others, and 87% — agreed/strongly agreed that the ROC program helped them gain more control over their eating. Eighty-three percent of participants who completed the program endorsed that the in-session hunger monitoring helped build their confidence to monitor hunger at home, and the same number (83%) endorsed that in-session exposure practices helped build their confidence to resist cravings at home.
Evaluation of Initial Effectiveness of ROC on Study Outcomes
The average weight loss from baseline to posttreatment was 4.3 lbs (SD = 7.4) and 1.8% (SD = 3.5%) of body weight, and from baseline to 3-month follow up was 5.2 lbs (SD = 10.3) and 2.2% (SD = 4.8%) of body weight (see Table 2). In those assessed posttreatment (i.e., treatment completers), 85.7% (17 of 21) lost weight, 66.6% (14 of 21) lost ≥ 5 lbs and ≥ 2% of their body weight, and 33.3% (7 of 21) lost ≥ 9 lbs and ≥ 4% of their body weight from baseline to posttreatment. Eleven of the 16 participants (68.8%) assessed at both posttreatment and 3-month follow-up continued to lose weight after the intervention ceased. The MANOVA for disordered eating was significant (F = 4.83; p = .009) and showed that scores on the BES, binge eating episodes, and binge eating days decreased at posttreatment and 3-month follow-up. Similarly, the MANOVA for cognitive traits was significant (F = 5.34; p = .005) and showed that power of food and food responsiveness decreased, and satiety responsiveness increased at posttreatment and 3-month follow-up.
Table 2.
Baseline, post-treatment, and 3-month follow-up scores on study outcome variables (N = 28).
| Observed Mean (SD) | Change from baseline to post-treatment | Change baseline to 3-month follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post treatment | 3-month follow-up | Mean difference (95% CI) | Effect size | p | Mean difference (95% CI) | Effect size | p | |
| Body composition | |||||||||
| BMI | 38.74 (10.20) | 38.01 (10.04) | 37.87 (10.09) | −0.71 (−1.36, −0.10) | .07 | .019 | −0.88 (−1.73, −0.03) | .09 | .042 |
| Disordered eating | |||||||||
| Binge eating scale | 18.86 (3.78) | 16.36 (6.68) | 17.36 (7.80) | −2.50 (−8.72, 3.72) | .66 | 1.00 | −1.50 (−7.72, 4.72) | .40 | 1.00 |
| Binge eating episodes* | 15.00 (13.53) | 9.39 (13.46) | 11.11 (13.62) | −5.61 (−11.83, 0.62) | .41 | .092 | −3.89 (−10.12, 2.33) | .29 | .399 |
| Cognitive traits | |||||||||
| Power of food scale | 3.63 (0.62) | 3.27 (0.82) | 3.23 (0.71) | −0.37 (−0.83, 0.09) | .60 | .168 | −0.41 (−0.86, 0.05) | .66 | .103 |
| Food responsiveness | 3.77 (0.76) | 3.36 (0.83) | 3.44 (0.75) | −0.41 (−0.87, 0.05) | .54 | .100 | −0.33 (−0.79, 0.13) | .43 | .257 |
| Satiety responsiveness | 2.27 (0.64) | 2.49 (0.61) | 2.39 (0.62) | 0.21 (−0.25, 0.67) | .33 | .784 | 0.12 (−0.34, 0.58) | .19 | 1.00 |
Over past 28 days
Note: 1 participant who completed the intervention missed the post-intervention assessment but completed the 3-month follow-up; 4 participants who completed the intervention missed the 3-month follow-up.
Discussion
In this study, we piloted the use of an intervention that targets reduced food-cue reactivity and improved satiety sensitivity for obese adults who binge eat in an open trial. The ROC program provides a comprehensive set of interventions that directly address these two targets, and includes psychoeducation, self-monitoring of hunger and cravings, in vivo skill building and coping skills. To date, programs for overweight and obese individuals who also struggle with binge eating typically target either binge eating or weight loss, but no currently available treatments have successfully addressed both (Grilo, et al., 2011). Thus, the ROC program has the potential to provide a novel intervention that could assist in the management of both eating disorder symptoms and weight.
Our first goal in this pilot trial was to evaluate the initial feasibility and acceptability of the ROC treatment with overweight and obese binge eating adults. Participants in the ROC program reported a high level of satisfaction with the program. Overall, a large percentage of the participants reported that they liked the program and that it helped them feel more in control of their eating, and almost all would recommend the ROC program to others. The majority of participants reported that in-session hunger monitoring and cue-exposure helped build their confidence outside of group.
In terms of initial effectiveness, participants in this pilot study reduced their BMI by an average of 0.73 BMI during the 4 months of treatment, which is remarkable because ROC does not include a diet or physical activity. This weight change, although small, suggests that targeting satiety responsiveness and food cue reactivity most likely led to small changes in overeating, which could have positive clinical implications if continued for a longer period of time. In addition to weight loss, ROC was associated with reductions in aspects of binge eating as well as overeating and loss-of-control eating episodes. The reductions of binge eating and weight loss support the potential impact of targeting basic behavioral mechanisms to develop novel interventions. Additionally, the majority of participants continued to show reductions in their BMI after the treatment ended, suggesting that targeting these mechanisms may be more long-lasting than diet and physical activity. However, because we did not follow the participants for more than 3 months after treatment, further research is needed to examine the enduring effects of targeting these basic mechanisms.
The ROC program also had effects on disordered eating and cognitions. The overall MANOVA for disordered eating showed significant changes over time; however, the separate measures (binge eating, loss of control eating, overeating) showed trends in the expected direction but were not statistically significant in this analysis. Additionally, the overall MANOVA for cognitive components also showed significant changes over time; however, the separate measures (power of food, food responsiveness, and satiety responsiveness) were in the expected direction but were not statistically significant in this analysis. Future studies should further examine these targets using additional measures, including the EDE interview to evaluate any changes in eating disorder cognitions and behaviors.
We piloted these ROC components without a diet or physical activity and included binge eaters to ensure acceptability of specific components to this population. It is possible that the components of ROC will be effective on their own, through targeting food cue reactivity and satiety sensitivity. It is also possible that the ROC components could strengthen existing BWL programs. These options will need to be evaluated in future trials, with control groups and longer interventions with follow-up assessment.
This study is the first to apply a novel model targeting basic behavioral mechanisms to the treatment of overweight and obese people with binge eating. We recruited a sample of overweight and obese binge eaters, and demonstrated feasibility, acceptability, and preliminary effectiveness in decreasing BMI and showed trends in reduction in eating disorder symptoms. This is notable, as no program to date effectively targets both weight and eating disorder symptoms for this population. However, as in all studies, there are a number of limitations that need to be noted. Most importantly, this study did not include a control group, which limits our ability to draw conclusions about the effectiveness of this model in comparison to a control or current standard of care for overweight and obese participants with binge eating.
However, our participants achieved a BMI reduction of 0.73 BMI in 4 months, which could be promising. This sample was also primarily White, highly educated, and predominantly female, which limits generalizability of our findings. The sample was also relatively small. In addition, much longer follow-up periods are needed to evaluate whether changes in these basic target mechanisms, binge eating and/or weight losses would be maintained. One of the measures, the EBQ, was not validated and changes in eating disorder symptoms trended in the expected direction from pre- to posttreatment, but the changes were not statistically significant. Finally, it is important to note that four participants were lost to follow-up at the 3-month follow-up time point, resulting in 57% of the starting sample completing the 3-month follow-up assessment. This was addressed in this pilot study by using intent-to-treat analyses, but further studies are needed to determine the long-term effects of this program.
Despite these limitations, our findings are the first to suggest that targeting the basic mechanisms of food cue reactivity and satiety sensitivity could provide alternative treatments for overweight and obese people who binge eat. Future studies are needed to compare the ROC program to a control condition, and to BWL, in longer treatments and with longer follow-up. Additionally, research is needed to develop measurements for food cue reactivity and satiety sensitivity. These putative mechanisms, as well as the ROC program, warrant further research and could launch a new generation of treatments targeting eating disorder symptoms and weight loss.
Highlights.
We tested a 4-month treatment targeting cue reactivity and satiety in binge eaters
There was high retention, completion and acceptability
Differences were seen on all study outcome pre- to post-treatment
This model may offer an alternative model for treating binge eating
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kerri N. Boutelle, University of California San Diego
Stephanie Knatz, University of California San Diego.
Jordan Carlson, University of California San Diego.
Kristie Bergmann, University of California San Diego.
Carol B. Peterson, University of Minnesota
References
- Allen HN, Craighead LW. Appetite monitoring in the treatment of binge eating disorder. Behavior Therapy. 1999;30(2):253–272. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: Author; 2013. [Google Scholar]
- Bloom T, Sharpe L, Mullan B, Zucker N. A pilot evaluation of appetite- awareness training in the treatment of childhood overweight and obesity: A preliminary investigation. International Journal of Eating Disorders. 2013;46(1):47–51. doi: 10.1002/eat.22041. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Bouton ME. Implications of learning theory for developing programs to decrease overeating. Appetite. 2015 doi: 10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2011;79:759–771. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Zucker N, Peterson CB, Rydell S, Carlson J, Harnack LJ. An intervention based on Schachter's externality theory for overweight children: The regulation of cues pilot. Journal of Pediatric Psychology. 2014;39(4):405–417. doi: 10.1093/jpepsy/jst142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Learning and the persistence of appetite: Extinction and the motivation to eat and overeat. Physiology & Behavior. 2011;103(1):51–8. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Sunsay C. Contextual control of appetitive conditioning: influence of a contextual stimulus generated by a partial reinforcement procedure. Quarterly Journal of Experiment Psychology. 2001;54:109–125. doi: 10.1080/713932752. [DOI] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, Cowan RL. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. International Journal of Obesity. 2009;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Craighead LW, Allen HN. Appetite awareness training: A cognitive behavioral intervention for binge eating. Cognitive and Behavioral Practice. 1996;2(2):249–270. [Google Scholar]
- Davis CA, Levitan RD, Reid C, Carter JC, Kaplan AS, Patte KA, … Kennedy JL. Dopamine for “wanting” and opioids for “liking”: a comparison of obese adults with and without binge eating. Obesity. 2009;17(6):1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obesity Research. 2004;12:1426–3. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58(1):303–312. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB. The effect of obesity on health outcomes. Molecular & Cellular Endocrinology. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Dymek-Valentine M, Rienecke-Hoste R, Alverdy J. Assessment of binge eating disorder in morbidly obese patients evaluated for gastric bypass: SCID versus QEWP-R. Eating & Weight Disorders. 2004;9:211–216. doi: 10.1007/BF03325069. [DOI] [PubMed] [Google Scholar]
- Espeland M. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. New York: Guilford; 2008. [Google Scholar]
- Fairburn CG, Cooper Z. Eating Disorder Examination (16.0D) C G. 2008 doi: 10.1192/bjp.154.6.807. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45(2):198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ferriday D, Brunstrom JM. How does food-cue exposure lead to larger meal sizes? British Journal of Nutrition. 2008;100(6):1325–1332. doi: 10.1017/S0007114508978296. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Journal of the American Medical Association. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiology & Behavior. 1988;44(4–5):665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiology & Behavior. 2001;74(4–5):743–746. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behavior. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR, Shiffman S. Binge antecedents in obese women with and without binge eating disorder. Journal of Consulting & Clinical Psychology. 2000;68:95–102. [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT, Gueorguieva R, White MA. Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: A randomized controlled trial. Journal of Consulting & Clinical Psychology. 2011;79:675–685. doi: 10.1037/a0025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrar V, Toepel U, Murray MM, Spence C. Food's visually perceived fat content affects discrimination speed in an orthogonal spatial task. Experimental Brain Research. 2011;214(3):351–356. doi: 10.1007/s00221-011-2833-6. [DOI] [PubMed] [Google Scholar]
- Jansen A. A learning model of binge eating: Cue reactivity and cue exposure. Behavior Research & Therapy. 1998;36:257–272. doi: 10.1016/s0005-7967(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Jansen A, Broekmate J, Heymans M. Cue-exposure vs self-control in the treatment of binge eating: a pilot study. Behavior Research & Therapy. 1992;30(3):235–241. doi: 10.1016/0005-7967(92)90069-s. [DOI] [PubMed] [Google Scholar]
- Jansen A, Van den Hout MA, De Loof C, Zandbergen J, Griez E. A case of bulimia successfully treated by cue exposure. Journal of Behavior Therapy & Experimental Psychiatry. 1989;20(4):327–332. doi: 10.1016/0005-7916(89)90064-5. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Kessler JT, Sekhon P, Coscina DV. Conditioned place preferences (CPPs) to high-caloric “snack foods” in rat strains genetically prone vs. resistant to diet-induced obesity: resistance to naltrexone blockade. Pharmacology Biochemistry & Behavior. 2007;86:699–704. doi: 10.1016/j.pbb.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Jarosz PA, Sekhon P, Coscina DV. Effect of opioid antagonism on conditioned place preferences to snack foods. Pharmacology Biochemistry & Behavior. 2006;83:257–264. doi: 10.1016/j.pbb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Javaras KN, Pope HG, Lalonde JK, Roberts JL, Nillni YI, Laird NM, Bulik CM, Crow SJ, McElroy SL, Walsh BT, Tsuang MT, Rosenthal NR, Hudson JI. Co-occurrence of binge eating disorder with psychiatric and medical disorders. Journal of Clinical Psychiatry. 2008;69:266–273. doi: 10.4088/jcp.v69n0213. [DOI] [PubMed] [Google Scholar]
- Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI. Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Research. 2000;99:29–42. doi: 10.1016/s0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- Kass AE, Kolko RP, Wilfley DE. Psychological treatments for eating disorders. Current Opinions in Psychiatry. 2013;26:549–555. doi: 10.1097/YCO.0b013e328365a30e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Benjet C, Bruffaerts R, de Girolamo G, de Graaf R, Maria Haro J, Kovess-Masfety V, O'Neill S, Posada-Villa J, Sasu C, Scott K, Viana MC, Xavier M. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biological Psychiatry. 2013;73:904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Shahly V, Hudson JI, Supina D, Berglund PA, Chiu WT, Gruber M, Aguilar-Gaxiola S, Alonso J, Andrade LH, Benjet C, Bruffaerts R, de Girolamo G, de Graaf R, Florescu SE, Haro JM, Murphy SD, Posada-Villa J, Scott K, Xavier M. A comparative analysis of role attainment and impairment in binge-eating disorder and bulimia nervosa: results from the WHO World Mental Health Surveys. Epidemiological & Psychiatric Science. 2014;23:27–41. doi: 10.1017/S2045796013000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grange D, Gorin A, Catley D, Stone AA. Does momentary assessment detect binge eating in overweight women that is denied at interview? European Eaing Disorder Review. 2001;9:309–324. [Google Scholar]
- Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, Ochner CN, Coletta MC, Bellace D, Wallaert M, Halford J. The Power of Food Scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–118. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Luce KH, Crowther JH. The reliability of the eating disorder examination—Self-report questionnaire version (EDE-Q) International Journal of Eating Disorders. 1999;25(3):349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Wing RR, Lamparski DM. Binge eating and dietary restraint in obese patients. Addictive Behavior. 1985;10:163–168. doi: 10.1016/0306-4603(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Martinez-Mallén E, Castro-Fornieles J, Lázaro L, Moreno E, Morer A, Font E, Julien J, Vila M, Toro J. Cue exposure in the treatment of resistant adolescent bulimia nervosa. International Journal of Eating Disorders. 2007;40(7):596–601. doi: 10.1002/eat.20423. [DOI] [PubMed] [Google Scholar]
- Mejia-Rivas M, Remes-Troche J, Montano-Loza A, Herrera M, Valdovinos-Diaz MA. Gastric capacity is related to body mass index in obese patients. A study using the water load test. Revista de Gastroenterología de México. 2009;74(1):71–73. [PubMed] [Google Scholar]
- Meyer MD, Risbrough VB, Liang J, Boutelle KN. Pavlovian conditioning to hedonic food cues in overweight and lean individuals. Appetite. 2015;87:56–61. doi: 10.1016/j.appet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, Krakoff J, Yanovski JA. Effects of binge eating on satiation, satiety, and energy intake of overweight children. American Journal of Clinical Nutrition. 2006;84:732–738. doi: 10.1093/ajcn/84.4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders F, Havermans R, Jansen A. Exposure to bingefood in bulimia nervosa: finger pulse amplitude as a potential measure of urge to eat and predictor of food intake. Appetite. 2004;42(2):125–130. doi: 10.1016/j.appet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35(1):45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- Nijs IM, Franken IH, Muris P. Food-related Stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eating Behavior. 2010;11(4):258–265. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54(2):243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nirenberg TD, Miller PM. Salivation: An assessment of food craving. Behavior Research & Therapy. 1982;20(4):405–407. doi: 10.1016/0005-7967(82)90102-4. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Hietanen JK, Calvo MG, Hyona J. Food catches the eye but not for everyone: A BMI-contingent attentional bias in rapid detection of nutriments. PLoS One. 2011;6(5):e19215. doi: 10.1371/journal.pone.0019215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes ME, Slotterback CS. Self-reported measures of appetite in relation to verbal cues about many foods. Current Psychology. 2000;19(2):137–142. [Google Scholar]
- Overduin J, Jansen A, Eilkes H. Cue reactivity to food- and body-related stimuli in restrained and unrestrained eaters. Addictive Behavior. 1997;22(3):395–404. doi: 10.1016/s0306-4603(97)80002-0. [DOI] [PubMed] [Google Scholar]
- Schachter S. Obesity and eating. Internal and external cues differentially affect the eating behavior of obese and normal subjects. Science. 1968;161(3843):751–756. doi: 10.1126/science.161.3843.751. [DOI] [PubMed] [Google Scholar]
- Schachter S. Some extraordinary facts about obese humans and rats. American Psychologist. 1971;26:129–144. doi: 10.1037/h0030817. [DOI] [PubMed] [Google Scholar]
- Schacter S, Rodin J. Obese humans and rats. Washington, DC: Erlbaum/Halstead; 1974. [Google Scholar]
- Schag K, Teufel M, Junne F, Preissl H, Hautzinger M, Zipfel S, Giel KE. Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PLoS One. 2013;8(10):e76542. doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: Reward sensitivity and brain activation to images of food. Biological Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Marks I. Cue exposure to food plus response prevention of binges for bulimia: A pilot study. International Journal of Eating Disorders. 1988;7(5):663–672. [Google Scholar]
- Schmitz F, Naumann E, Trentowska M, Svaldi J. Attentional bias for food cues in binge eating disorder. Appetite. 2014;80C:70–80. doi: 10.1016/j.appet.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Spiegel TA, Shrager EE, Stellar E. Responses of lean and obese subjects to preloads, deprivation, and palatability. Appetite. 1989;13(1):45–69. doi: 10.1016/0195-6663(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Devlin M, Walsh BT, Hasin D, Wing R, Marcus M, Stunkard A, Wadden T, Yanovski S, Agras S, Mitchell J, Nonas C. Binge eating disorder: A multisite field trial of the diagnostic criteria. International Journal of Eating Disorders. 1992;11:191–203. [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Striegel-Moore RH, Dohm FA, Wilfley DE, Pike KM, Bray NL, Kraemer HC, Fairburn CG. Toward an understanding of health services use in women with binge eating disorder. Obesity Research. 2004;12:799–806. doi: 10.1038/oby.2004.96. [DOI] [PubMed] [Google Scholar]
- Stunkard A, Koch C. The Interpretation of Gastric Motility. I. Apparent Bias in the Reports of Hunger by Obese Persons. Archives General Psychiatry. 1964;11:74–82. doi: 10.1001/archpsyc.1964.01720250076010. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;55:685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Timmerman GM. Binge Eating Scale: Further assessment of validity and reliability. Journal of Applied Biobehavioral Research. 1999:4. [Google Scholar]
- Toro J, Cervera M, Feliu MH, Garriga N, Jou M, Martinez E, Toro E. Cue exposure in the treatment of resistant bulimia nervosa. International Journal of Eating Disorders. 2003;34(2):227–234. doi: 10.1002/eat.10186. [DOI] [PubMed] [Google Scholar]
- Van Dyke N, Drinkwater EJ. Relationships between intuitive eating and health indicators: literature review. Public Health Nutitrition. 2013;21:10. doi: 10.1017/S1368980013002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132(6):2226–2238. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, Foreyt JP, Hill JO, Trence DL, Vitolins MZ Look AHEAD Research Group. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity. 2009;17(4):713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle J. Conditioning processes and cue exposure in the modification of excessive eating. Addictive Behavior. 1990;15:387–393. doi: 10.1016/0306-4603(90)90047-2. [DOI] [PubMed] [Google Scholar]
- Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children's Eating Behaviour Questionnaire. Journal of Child Psychology & Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Weinsier RL, Hunter GR, Heini AF, Goran MI, Sell SM. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. American Journal of Medicine. 1998;105:145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A. Can(not) take my eyes off it: Attention bias for food in overweight participants. Health Psychology. 2011;30(5):561–569. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Welch RR, Stein RI, Spurrell EB, Cohen LR, Saelens BE, Dounchis JZ, Frank MA, Wiseman CV, Matt GE. A randomized comparison of group cognitive-behavioral therapy and group interpersonal psychotherapy for the treatment of overweight individuals with binge-eating disorder. Archives of General Psychiatry. 2002;59:713–721. doi: 10.1001/archpsyc.59.8.713. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Grilo CM, Vitousek KM. Psychological treatment of eating disorders. American Psychologist. 2007;62:199–216. doi: 10.1037/0003-066X.62.3.199. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Wilfley DE, Agras WS, Bryson SW. Psychological treatments of binge eating disorder. Archives of General Psychiatry. 2010;67:94–101. doi: 10.1001/archgenpsychiatry.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19(9):1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]