Abstract
Introduction: Kidney stone risk factors are understudied among Asians. Our study objective was to investigate associations of obesity and other chronic diseases with incident kidney stones among the urban Chinese.
Patients and Methods: Included in this study are two prospective cohorts: the Shanghai Women's Health Study (N = 69,166) and Shanghai Men's Health Study (N = 58,054). Incident kidney stones were determined by self-report in 2004 and 2008. Cox regression models were used to evaluate the associations of study variables with stone risk with adjustment of demographics, medical history, and dietary intakes.
Results: There were 2653 incident stones over 1,007,958 person-years of follow-up. Overall incidence rates (per 1000 person-years, 95% confidence interval [CI]) were 2.10 (1.99, 2.21) among women and 3.80 (3.59, 4.02) among men. Higher body mass index (BMI) was associated with risk (BMI ≥25 vs 18.5–24.9 kg/m2, women: hazard ratio [HR] = 1.14 [95% CI 1.01, 1.28]; men: HR = 1.17 [1.03, 1.32]). High waist–hip ratio (≥0.80 and ≥0.90 for women and men, respectively) was associated with risk (HR 1.13, 95% CI 1.01, 1.27 for women; HR 1.19, 95% CI 1.05, 1.35 for men). Coronary heart disease or stroke history was associated with risk in women only (HR 1.31, 95% CI 1.10, 1.56). Hypertension history was associated with risk in men only (HR 1.27, 95% CI 1.11, 1.45). No significant association with diabetes mellitus was observed.
Conclusions: Among the Chinese, kidney stone incidence in men is almost twice that of women. Obesity is a shared risk factor. Hypertension history is associated with risk in men, whereas history of coronary heart disease or stroke is associated with risk in women.
Keywords: : kidney calculi, diet, adult, risk factors, prospective studies
Introduction
Kidney stone disease is a global cause of morbidity and is associated with systemic conditions, including coronary heart disease, hypertension, and chronic kidney disease.1–4 Risk factors for incident kidney stones include dietary factors and chronic conditions such as obesity.5–7 As nonwhite populations have not been well represented in kidney stone epidemiologic research, whether these risk factors apply to other populations has been understudied.
The population in China represents a unique opportunity for kidney stone epidemiologic research because of the rapid urbanization and adoption of an increasingly Westernized diet over the last few decades.8 Urbanization in China has been accompanied by dramatic increases in prevalence rates of obesity, type 2 diabetes, hypertension, and cardiovascular disease.9–12 As in Western countries, stone disease in China affects predominantly men and is most prevalent between age 40 and 60.13 Approximately 80% of stones in China contain calcium, of which the majority is calcium oxalate composition.14 Kidney stone prevalence among the Chinese urban population is 4% to 6% compared to 5% in South Korea and almost 9% in the United States.13,15–17
However, kidney stone incidence rates and risk factors among the Chinese population have not been established. We investigated the associations of obesity, diabetes, hypertension, and cardiovascular diseases (coronary heart disease and stroke) with incident kidney stones in two population-based prospective cohorts of urban inhabitants of Shanghai, China: the Shanghai Women's Health Study (SWHS) and the Shanghai Men's Health Study (SMHS). These NIH-funded studies were designed to investigate the etiology and progression of cancer and other noncommunicable diseases, including nephrolithiasis. The investigation of these two population-based cohorts represents the first large-scale epidemiologic analysis of kidney stone disease in China.
Patients and Methods
Study population
Participants in the SWHS and the SMHS were included in the analysis. Study design and details of the two studies have been described previously.18,19 In short, a total of 74,941 women aged 40–70 years were recruited for the SWHS from 1996 to 2000, and a total of 61,480 men aged 40–74 years were recruited for the SMHS from 2002 to 2006 (overall study participation rate: women, 92.7%; men, 74.1%). All participants were recruited among the urban communities within Shanghai and followed by in-person surveys every 2–5 years and by linkage to the Shanghai Tumor Registry and Shanghai Vital Statistics database. Institutional review boards of all involved institutes approved the studies, and written informed consent was obtained from all participants before interview.
Data collection
At baseline survey, interviewers conducted in-person visits to participants' homes. Interviewers were retired nurses and other medical professionals who completed standardized training in survey administration. During the visit, a structured questionnaire was administered to assess demographic information, medical history, lifestyle and habits, physical activity, occupational history, dietary intake, and physical activity.
Interviewers measured body weight (kg), height (cm), and waist and hip circumference (cm) according to a standard protocol. Body mass index (BMI, kg/m2) was derived by dividing weight by height squared, and waist-hip ratio (WHR) was derived by dividing waist circumference by hip circumference.
Self-reported history of diabetes, hypertension, coronary heart disease, and stroke was collected from the baseline questionnaire by asking the subject whether he or she had ever been diagnosed by a physician. Hypertension in this study was defined by self-reported disease diagnosis and/or use of antihypertensive medication.
Dietary intake over the previous 12 months was assessed at baseline using a validated food frequency questionnaire, which covered ∼90% food items commonly consumed in urban Shanghai during the study period.20,21 During the interview, although drinking habits were assessed, overall water and fluid intake volume was not assessed. Sodium intake was not included in the analyses because of incomplete assessment and high within-individual variances.
Identification of incident kidney stone formers
A history of urinary tract stones was assessed by self-report at the third and fourth follow-up visits for SWHS (2004, 2008) and first and second follow-up visits for SMHS (2004, 2008). Study participants were asked whether they were diagnosed by a physician with a urinary tract stone (diagnosed, never diagnosed, suspected/probable, or unknown), followed by questions on date of first diagnosis (month and year), location of the stone (kidney, ureter, bladder, or unknown), and whether the diagnosis was made with ultrasonography and/or X-ray radiography. Incident kidney stone cases were defined by the first report of a urinary tract stone located in the kidney or ureter. Subjects whose first diagnosis occurred before baseline interview were excluded from the study as they may have changed their diet and other lifestyle after the initial stone event (n = 833 women, n = 1828 men), as were self-reported stone formers with missing diagnosis date (n = 14 women, n = 20 men).
The end of follow-up for this study was defined as the date of first stone diagnosis for cases and censored at the date of last assessment for controls. Prevalent and incident bladder stones, suspected/probable stones in the urinary tract, and unknown stone status were excluded (women: n = 120, men: n = 454). Of the 1451 women and 1202 men identified with incident kidney stones, 99% (n = 1436) and 99% (n = 1190), respectively, reported the stone was diagnosed by ultrasonography and/or X-ray radiography.
Statistical analysis
Participants who did not complete any follow-up surveys that collected the kidney stone information (n = 3046 women, n = 693 men) or had any malignancy diagnosis at or before baseline survey (women: n = 1654, men: n = 211) were excluded from this analysis. Additional exclusion criteria included extremes of reported total energy intake (women: <500 or >3500 kcal/day, n = 108; men: <800 or >4200 kcal/day, n = 220). Thus, a total of 69,166 women and 58,054 men were included in the final analytic set.
In descriptive analyses, baseline host characteristics of incident cases and controls were compared by univariable Cox proportional hazards regression model unless noted. BMI was categorized according to conventional WHO cutoffs (underweight: <18.5 kg/m2; normal: 18.5–24.9 kg/m2; overweight: 25–29.9 kg/m2; obese: ≥30 kg/m2). WHR was categorized based on sex-specific cutoffs (men: low <0.9, high ≥0.90; women: low <0.8, high ≥0.80).22 To compare nutrient consumptions between two groups, linear regression was employed with the inclusion of total energy intake (kcal/day) and age at interview as covariates in the model.
Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression models for incident stone risk, with age as the time scale. The exposures of interest included BMI, WHR, history of diabetes, hypertension, and coronary heart disease and/or stroke. Covariates were included as continuous variables unless otherwise noted: birth year, education level (<12th grade, high/vocational school, and college or above), family income per capita (low, middle, and high), pack-year smoking, cholelithiasis history (yes/no), regular exercise (MET-h/day/year, categorized as none, low, medium, and high), total energy intake (kcal), and intakes of dietary protein (g/day), fat (g/day), calcium (mg/day), potassium (mg/day), magnesium (mg/day), vitamin C (mg/day), calcium supplements (yes/no), and vitamin C supplements (yes/no). The proportional hazard assumption was examined by including time-varying covariates in the model. Tests for interaction were performed by creating and modeling the multiplicative interaction terms between each exposure of interest variable and gender. There was >80% of power to detect a significant association for each risk factor of interest in our study populations (estimated event rate in unexposed group: 2%, effect size >1.2 for each categorical variable).
Sensitivity analyses were conducted restricted to never smokers in SMHS because of the high prevalence of smoking among urban Chinese men and the potential for confounding (70% of participants in SMHS compared to 3% in the SWHS). In addition, to minimize the potential metabolic, dietary, and lifestyle changes after cancer diagnosis on kidney stone risk, sensitivity analyses were conducted censoring incident cancer cases occurring within 6 months before stone diagnosis.
All analyses were carried out using SAS version 9.4 (SAS Institute, Inc.). All statistical tests were based on two-sided probability and p < 0.05 was considered statistically significant.
Results
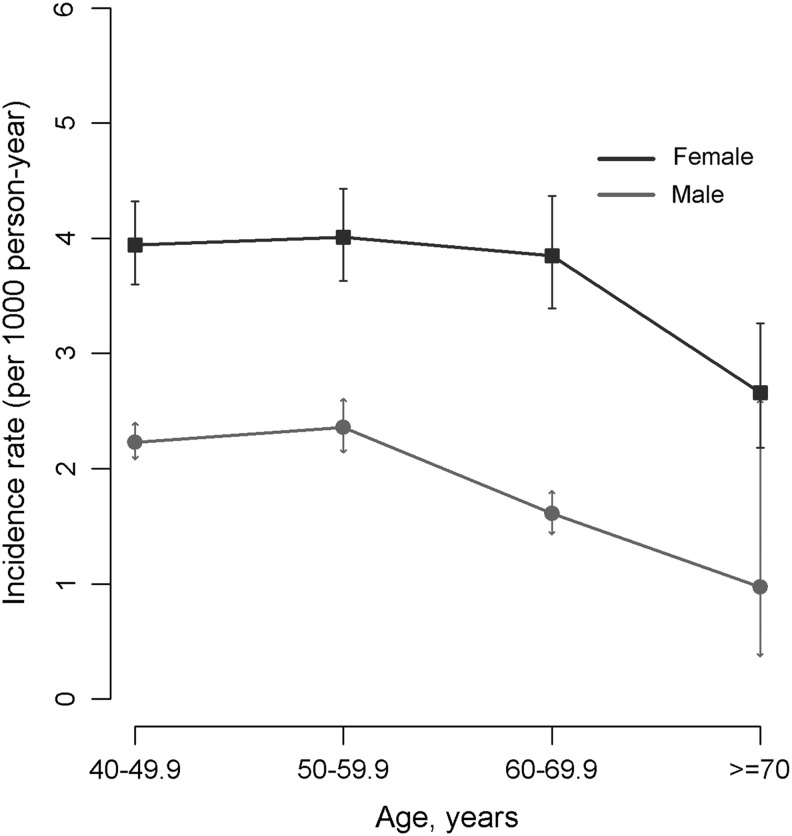
With an average 8.0 years of follow-up (range: 0.1–14.7, overall 1,007,958 person-years), overall kidney stone incidence rates were 3.80 (95% CI 3.59, 4.02) and 2.10 (95% CI 1.99, 2.21) per 1000 person-years for men and women, respectively (Fig. 1 and Table 1). The incidence rates in men were nearly twice the incidence in women, and the highest age-specific incidence rates were observed in the 50 to 59 age groups.
FIG. 1.
Incidence rates of kidney stones among urban Chinese men and women. Error bar represents 95% CI. CI = confidence interval.
Table 1.
Overall and Age-Specific Incidence Rates of Kidney Stones Among Urban Chinese Men and Women
| Women Cases/noncases | Men Cases/noncases | |
|---|---|---|
| Incident cases (n) | 1451/67,804 | 1202/56,895 |
| PY of follow-up | 691,836.17 | 316,122.06 |
| Incidence rate/1000PY (95% CI) | ||
| Crude | 2.10 (1.99, 2.21) | 3.80 (3.59, 4.02) |
| Age specific | ||
| 40–49.9 | 2.23 (2.08, 2.40) | 3.94 (3.60, 4.32) |
| 50–59.9 | 2.36 (2.14, 2.61) | 4.01 (3.63, 4.43) |
| 60–69.9 | 1.61 (1.43, 1.81) | 3.85 (3.39, 4.37) |
| ≥70 | 0.97 (0.37, 2.60) | 2.66 (2.18, 3.26) |
CI = confidence interval; PY = person-years.
Descriptive analyses of participant characteristics showed several differences between cases and noncases (Table 2). For both cohorts, incident stone cases were more likely to have had nonmanual labor occupation, greater BMI, and greater WHR measurements. Among women, incident stone formers were more likely to have a history of coronary heart disease or stroke, cholelithiasis, and calcium supplement intake. Among men, incident stone formers tended to have less cigarette smoking exposure, and a history of hypertension.
Table 2.
Participant Characteristics by Incident Kidney Stone Status
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Variables | Subjects without incident kidney stones (N = 67,715) | Subjects with incident kidney stones (N = 1451) | pa | Subjects without incident kidney stones (N = 56,852) | Subjects with incident kidney stones (N = 1202) | pa |
| Age (years), mean ± SD | 52.4 ± 9.0 | 51.4 ± 8.3 | <0.001 | 55.3 ± 9.7 | 54.4 ± 9.1 | 0.140 |
| Educational level (%) | 0.128 | <0.001 | ||||
| Less than 12th grade | 39,695 (57.4) | 798 (55.0) | 23,467 (41.3) | 427 (35.5) | ||
| High/vocational school | 24,955 (36.1) | 577 (39.8) | 27,050 (47.6) | 626 (52.1) | ||
| College or above | 3065 (4.5) | 76 (5.2) | 6335 (11.1) | 149 (12.4) | ||
| Income (%)b | 0.322 | 0.266 | ||||
| Low | 10,793 (15.9) | 197 (13.6) | 7186 (12.6) | 138 (11.5) | ||
| Middle | 50,490 (74.6) | 1102 (75.9) | 44,201 (77.8) | 940 (78.2) | ||
| High | 6432 (9.5) | 152 (10.5) | 5465 (9.6) | 124 (10.3) | ||
| Occupation (%) | 0.026 | 0.005 | ||||
| Professional | 19,167 (28.3) | 483 (33.3) | 14,937 (26.3) | 353 (29.4) | ||
| Clerical | 14,083 (20.8) | 288 (19.8) | 12,489 (22.0) | 279 (23.2) | ||
| Manual | 34,224 (50.5) | 676 (46.6) | 29,359 (51.7) | 570 (47.4) | ||
| Housewife (women only) | 241 (0.4) | 4 (0.3) | — | — | ||
| Cigarette smoking (%) | 0.770 | 0.013 | ||||
| Never | 65,872 (97.3) | 1413 (97.4) | 17,109 (30.1) | 392 (32.6) | ||
| Ever | 1843 (2.7) | 38 (2.6) | 39,742(69.9) | 810 (67.4) | ||
| Pack-year smoking, mean ± SD | 0.3 ± 3.0 | 0.2 ± 2.0 | 0.426 | 17.1 ± 17.6 | 15.9 ± 17.0 | 0.017 |
| Exercise (MET-h/day/year) (%) | 0.331 | 0.516 | ||||
| Rarely/none | 44,063 (65.1) | 974 (67.1) | 36,715 (64.6) | 795 (66.1) | ||
| Low, ≤1.28 (M); ≤0.8 (W) | 8653 (12.8) | 180 (12.4) | 6766 (11.9) | 149 (12.4) | ||
| Median, 1.29–2.99 (M); 0.9–1.99 (W) | 7654 (11.3) | 159 (11.0) | 7074 (12.4) | 150 (12.5) | ||
| High, >2.99 (M); >1.99 (W) | 7354 (10.9) | 138 (9.5) | 6297 (11.1) | 108 (9.0) | ||
| BMI (kg/m2) (%) | 0.086 | <0.001 | ||||
| <18.5 | 2286 (3.4) | 44 (3.0) | 2438 (4.3) | 34 (2.8) | ||
| 18.5–24.9 | 46,180 (61.6) | 850 (58.6) | 35,745 (62.9) | 701 (58.3) | ||
| 25–29.9 | 20,322 (30.0) | 476 (32.8) | 17,204 (30.3) | 426 (35.4) | ||
| ≥30 | 3408 (5.0) | 81 (5.6) | 1431 (2.5) | 41 (3.4) | ||
| WHR (%)c | 0.027 | <0.001 | ||||
| <0.90 (M); <0.80 (W) | 27,539 (40.7) | 547 (37.7) | 26,309 (46.3) | 480 (40.0) | ||
| ≥0.90 (M); ≥0.80 (W) | 40,159 (59.3) | 904 (62.3) | 30,482 (53.7) | 721 (60.0) | ||
| History of coronary heart disease/stroke (%) | 0.003 | 0.969 | ||||
| No | 61,228 (90.4) | 1283 (88.4) | 51,744 (91.0) | 1106 (92.0) | ||
| Yes | 6487 (9.6) | 168 (11.6) | 5108 (9.0) | 96 (8.0) | ||
| History of hypertension (%) | 0.070 | <0.001 | ||||
| No | 51,592 (76.2) | 1077 (74.2) | 39,643 (69.7) | 778 (64.7) | ||
| Yes | 16,123 (23.8) | 374 (25.8) | 17,209 (30.3) | 424 (35.3) | ||
| History of type 2 diabetes (%) | 0.354 | 0.809 | ||||
| No | 64,940 (95.9) | 1387 (95.6) | 53,352 (93.8) | 1131 (94.1) | ||
| Yes | 2775 (4.1) | 64 (4.4) | 3500 (6.2) | 71 (5.9) | ||
| History of cholelithiasis (%) | 0.025 | 0.523 | ||||
| No | 60,316 (89.1) | 1266 (87.3) | 52,665 (92.6) | 1111 (92.4) | ||
| Yes | 7399 (10.9) | 185 (12.7) | 4187 (7.4) | 91 (7.6) | ||
| Energy intake (kcal/day), mean ± SD | 1675.2 ± 393.3 | 1673.7 ± 398.4 | 0.452 | 1910.5 ± 475.1 | 1934.3 ± 462.4 | 0.317 |
| Protein intake (g/day), mean ± SDc | 67.1 ± 20.6 | 67.5 ± 20.7 | 0.316 | 78.4 ± 23.6 | 79.6 ± 23.3 | 0.758 |
| Dietary calcium intake (mg/day), mean ± SDc | 464.7 ± 199.7 | 465.5 ± 199.7 | 0.978 | 586.6 ± 238.5 | 592.9 ± 240.5 | 0.921 |
| Calcium supplementation (%) | 0.063 | 0.367 | ||||
| No | 54,868 (81.0) | 1141 (78.6) | 54,195 (95.3) | 1140 (94.8) | ||
| Yes | 12,845 (19.0) | 310 (21.4) | 2657 (4.7) | 62 (5.2) | ||
| Dietary potassium intake (mg/day), mean ± SDc | 1778.1 ± 634.3 | 1795.3 ± 651.6 | 0.333 | 1926.1 ± 666.0 | 1960.2 ± 643.1 | 0.554 |
| Dietary magnesium intake (mg/day), mean ± SDc | 276.1 ± 79.7 | 276.5 ± 82.7 | 0.797 | 320.2 ± 92.1 | 324.4 ± 89.5 | 0.986 |
| Dietary vitamin C intake (mg/day), mean ± SDc | 90.9 ± 48.7 | 92.2 ± 49.3 | 0.481 | 96.2 ± 51.8 | 98.1 ± 52.0 | 0.761 |
| Vitamin C supplementation (%) | 0.784 | 0.087 | ||||
| No | 63,048 (93.1) | 1348 (92.9) | 53,718 (94.5) | 1124 (93.5) | ||
| Yes | 4667 (6.9) | 103 (7.1) | 3134 (5.5) | 78 (6.5) | ||
p-Values were obtained from Cox regression model.
Income (yuan per capita per year), for women, low: <4000; middle: 4000–8000; high: ≥8000; for men, low: <6000; middle: 6000–10,000; high: ≥10,000.
Energy and birth year adjusted.
BMI = body mass index; SD = standard deviation; WHR = waist–hip ratio.
Table 3 presents multivariable-adjusted HRs and 95% CIs for sex-specific associations of anthropometric and medical characteristics with incident kidney stones. Higher BMI was associated with incident stone risk for both men and women (BMI ≥25 kg/m2 vs 18.5–24.9 kg/m2, women: HR = 1.14 [95% CI 1.01, 1.28], p = 0.032; men: HR = 1.17 [1.03, 1.32], p = 0.017). WHR was associated with an increased risk (women: HR = 1.13 [1.01–1.27], p = 0.037; men: HR = 1.19 [1.05, 1.35], p = 0.006). History of coronary heart disease or stroke was associated with risk in women (HR = 1.31 [1.10, 1.56], p = 0.002), but not in men. Hypertension was associated with risk in men only (HR = 1.27 [1.12, 1.45], p < 0.001). No significant associations were observed for history of type 2 diabetes in either cohort. The association between a history of coronary heart disease or stroke and incident stones may be modified by gender, although only a marginally significant interaction was observed (pinteraction = 0.089, in text only).
Table 3.
Multivariable Cox Models for Associations Between Risk of Incident Kidney Stones and Comorbid Diseases Among Urban Chinese Inhabitants
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Variables | Cases/noncases | HR (95% CI) | p | Cases/noncases | HR (95% CI) | p |
| BMI (kg/m2) (%) | ||||||
| <18.5 | 44/2286 | 1.01 (0.75, 1.37) | 0.944 | 34/2438 | 0.82 (0.58, 1.16) | 0.252 |
| 18.5–24.9 | 850/41,680 | Ref. | 701/35,745 | Ref. | ||
| 25–29.9 | 476/20,322 | 1.14 (1.01, 1.28) | 0.036 | 426/17,204 | 1.15 (1.01, 1.31) | 0.032 |
| ≥30 | 81/3408 | 1.17 (0.92, 1.49) | 0.194 | 41/1431 | 1.30 (0.94, 1.80) | 0.111 |
| ≥25 | 557/23,730 | 1.14 (1.01, 1.28) | 0.032 | 467/18,635 | 1.17 (1.03, 1.32) | 0.017 |
| WHR (%) | ||||||
| <0.90 (M); <0.80 (W) | 547/27,539 | Ref. | 480/26,309 | Ref. | ||
| ≥0.90 (M); ≥0.80 (W) | 904/40,159 | 1.13 (1.01, 1.27) | 0.037 | 721/30,482 | 1.19 (1.05, 1.35) | 0.006 |
| History of coronary heart disease/stroke | ||||||
| No | 1283/61,228 | Ref. | 1106/51,744 | Ref. | ||
| Yes | 168/6487 | 1.31 (1.10, 1.56) | 0.002 | 96/5108 | 0.92 (0.74, 1.16) | 0.491 |
| History of hypertension | ||||||
| No | 1077/51,592 | Ref. | 778/39,643 | Ref. | ||
| Yes | 374/16,123 | 1.09 (0.96, 1.25) | 0.193 | 424/17,209 | 1.27 (1.11, 1.45) | <0.001 |
| History of type 2 diabetes | ||||||
| No | 1387/64,940 | Ref. | 1131/53,352 | Ref. | ||
| Yes | 64/2775 | 1.11 (0.85, 1.44) | 0.444 | 71/3500 | 0.98 (0.77, 1.26) | 0.875 |
Cox regression models included birth year, pack-year smoking, education, income, energy intake (kcal), dietary protein intake, dietary calcium intake, calcium supplement (yes/no), dietary vitamin C intake, vitamin C supplement (yes/no), dietary potassium intake, dietary magnesium intake, physical activity (MET-h/day/year), BMI at baseline (kg/m2), WHR, history of coronary heart disease/stroke (yes/no), history of type 2 diabetes, history of hypertension (yes/no), and history of cholelithiasis (yes/no).
HR = hazard ratio.
Sensitivity analysis conducted among never smokers in men showed nonsignificant associations for BMI and WHR, whereas only a history of hypertension maintained an association (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/end). Sensitivity analysis censoring follow-up 6 months before cancer diagnosis showed that all significant/borderline significant associations observed in the main analysis remained largely unchanged (Supplementary Table S2).
Discussion
This first large prospective cohort study revealed several important findings related to incident kidney stone risk among middle aged and elderly urban Chinese inhabitants. Incidence rates are higher among men and decline after age 50–59 in both men and women, similar to what has been observed in other population-based studies in South Korea and Japan.17,23 As a comparison, in a prospective study of predominantly white males in the United States, incidence rates were highest among age 40–59 (3.09–3.77/1000/year), were lower among those 60–69 (2.61–2.73/1000/year), and declined after age 70 (1.36/1000/year).24 Incidence rates among women are higher among younger women between age 20 and 40 (1.83/1000/year) and plateau to <1 per 1000 person-years among older women.5,25 In this study, the reasons for the incidence rates for both urban-dwelling Chinese men and women being slightly higher are unclear, but may be attributable to dietary and lifestyle factors, differences in the racial susceptibility toward risk, or methodological differences.
Obesity is an independent risk factor for incident kidney stones among both Chinese men and women. These trends are observed, although modeling both BMI and WHR together, suggesting both overall adiposity as measured by BMI and central fat distribution as measured by WHR relate to kidney stone risk. Because of a small sample size in the obesity category (≥30 kg/m2), we did not observe significant associations in this subgroup for either gender. However, the effect size of association is similar or even larger for obese subjects when compared to those who are overweight. As overweight and obesity are emerging public health problems in China, there is an urgent need for interventions to prevent excess weight gain.
Differences in urinary composition may, in part, explain differences in kidney stone risk by race. A previous study reported that Asian American/Pacific Islander populations excrete significantly less urinary citrate than whites.26 Citrate inhibits calcium stone formation by complexing with free calcium and directly inhibiting the precipitation and aggregation of early stone crystals. In a single-center study of 507 stone formers in Southern China, of which 78% had majority calcium oxalate stone composition, the vast majority of those (94%) had hypocitraturia. The rates of hyperoxaluria, hypercalciuria, and hyperuricosuria were comparably low (all <35%).27 A study of calcium stone formers in Eastern China similarly found a high prevalence of hypocitraturia (80%).28
In this study, a history of coronary heart disease or stroke was independently associated with incident kidney stone disease in women, but not in men. The reasons for differences by sex are unclear, but are more likely attributable to intrinsic differences in stone risk by gender rather than a difference in the two cohorts studied, since the methodology of SWHS and SMHS is mirrored. Similarly, in this study, hypertension history was associated with kidney stone risk in Chinese men, but not in women. Prior studies of nephrolithiasis relating to coronary heart disease observed an association in women, but not in men,1,29 adding further evidence that gender is an important effect modifier in kidney stone risk. We also found a marginally significant interaction in this study, which may be caused by insufficient statistical power.
Diabetes history is a well-established risk factor for kidney stone disease.6 In this study, the absence of an observed association could be because of two possibilities. First, diabetes in China is underdiagnosed,10,30 and this nondifferentiated misclassification may have biased the association toward the null. The self-reported prevalence at baseline of diabetes were 4% and 6% in the SWHS and SMHS, respectively, compared to 9% and 11% among women and men, respectively, published in a national diabetes screening study.30 Associations may be observed over the next few decades within the context of the changing dietary and lifestyle patterns. Second, it is possible that effect modification by race impacts the magnitude of associations between diabetes and stone risk. This would need to be evaluated in a prospective multiethnic cohort.
The findings of this study should be interpreted within the context of the limitations of the study design. Self-reported kidney stones were not adjudicated with the medical record. However, follow-up data were collected in person and obtained from a trained interviewer with a medical background. Furthermore, a high proportion of kidney stones diagnoses were reported to have been confirmed by imaging (99%). The use of self-reported baseline measures, such as hypertension, could have resulted in underestimation of the associations with stone risk. Data on family history, stone composition, and 24-hour urine constituents were not collected in this study, which could have further elucidated inherited and metabolic causes for stone formation. The population in Shanghai has relatively high socioeconomic status in China, and the findings may not be generalizable to other Chinese populations. Despite these limitations, missing data were very minimal (<1%), and the use of detailed medical and dietary instruments in this prospective cohort is among the strengths of the study design.
Conclusions
In conclusion, the incident rates of kidney stones among middle aged and elderly Chinese are slightly higher than those in Western populations, and incident kidney stones are more common in men than women. Obesity remains a common risk factor for both Chinese men and women. Risk associations with cardiovascular disease and hypertension appear to vary by gender, whereas no association was seen with a history of diabetes mellitus.
Supplementary Material
Abbreviations Used
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- SMHS
Shanghai Men's Health Study
- SWHS
Shanghai Women's Health Study
- WHR
waist–hip ratio
Acknowledgments
This study was supported by grants from US National Institutes of Health (R37 CA070867 and UM1 CA182910, R01 CA082729 and UM1 CA173640).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ferraro PM, Taylor EN, Eisner BH, et al. . History of kidney stones and the risk of coronary heart disease. JAMA 2013;310:408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rule AD, Roger VL, Melton LJ 3rd, et al. . Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 2010;21:1641–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Madore F, Stampfer MJ, Rimm EB, Curhan GC. Nephrolithiasis and risk of hypertension. Am J Hypertens 1998;11:46–53 [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 2009;4:804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005;293:455–462 [DOI] [PubMed] [Google Scholar]
- 6.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005;68:1230–1235 [DOI] [PubMed] [Google Scholar]
- 7.Taylor EN, Chan AT, Giovannucci EL, Curhan GC. Cholelithiasis and the risk of nephrolithiasis. J Urol 2011;186:1882–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong P, Liang S, Carlton EJ, et al. . Urbanisation and health in China. Lancet 2012;379:843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: A systematic review and meta-analysis. Diabetes Res Clin Pract 2014;104:63–72 [DOI] [PubMed] [Google Scholar]
- 10.Bragg F, Holmes MV, Iona A, et al. . Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA 2017;317:280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bundy JD, He J. Hypertension and related cardiovascular disease burden in China. Ann Glob Health 2016;82:227–233 [DOI] [PubMed] [Google Scholar]
- 12.Xi B, Liang Y, He T, et al. . Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev 2012;13:287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng Q, He Y. Age-specific prevalence of kidney stones in Chinese urban inhabitants. Urolithiasis 2013;41:91–93 [DOI] [PubMed] [Google Scholar]
- 14.Sun X, Shen L, Cong X, Zhu H, He L, Lu J. Infrared spectroscopic analysis of 5,248 urinary stones from Chinese patients presenting with the first stone episode. Urol Res 2011;39:339–343 [DOI] [PubMed] [Google Scholar]
- 15.Scales CD, Jr., Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol 2012;62:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng G, Mai Z, Xia S, et al. . Prevalence of kidney stones in China: An ultrasonography based cross-sectional study. BJU Int 2017;120:109–116 [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Jo MK, Kwak C, et al. . Prevalence and epidemiologic characteristics of urolithiasis in Seoul, Korea. Urology 2002;59:517–521 [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, Chow WH, Yang G, et al. . The Shanghai Women's Health Study: Rationale, study design, and baseline characteristics. Am J Epidemiol 2005;162:1123–1131 [DOI] [PubMed] [Google Scholar]
- 19.Shu XO, Li H, Yang G, et al. . Cohort profile: The Shanghai Men's Health Study. Int J Epidemiol 2015;44:810–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu XO, Yang G, Jin F, et al. . Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. Eur J Clin Nutr 2004;58:17–23 [DOI] [PubMed] [Google Scholar]
- 21.Villegas R, Yang G, Liu D, et al. . Validity and reproducibility of the food-frequency questionnaire used in the Shanghai Men's Health Study. Br J Nutr 2007;97:993–1000 [DOI] [PubMed] [Google Scholar]
- 22.WHO. Waist circumference and waist-hip ratio: Report of a WHO expert consultation, Geneva, December 8–11, 2008 [Google Scholar]
- 23.Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: National trends between 1965 and 2005. Urology 2008;71:209–213 [DOI] [PubMed] [Google Scholar]
- 24.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328:833–838 [DOI] [PubMed] [Google Scholar]
- 25.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997;126:497–504 [DOI] [PubMed] [Google Scholar]
- 26.Eisner BH, Porten SP, Bechis SK, Stoller ML. The role of race in determining 24-hour urine composition in white and Asian/Pacific Islander stone formers. J Urol 2010;183:1407–1411 [DOI] [PubMed] [Google Scholar]
- 27.Wu W, Yang D, Tiselius HG, et al. . The characteristics of the stone and urine composition in Chinese stone formers: Primary report of a single-center results. Urology 2014;83:732–737 [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Sun X, Zhu H, Cong X, Ning B. Comparison of renal function and metabolic abnormalities of cystine stone patients and calcium oxalate stone patients in China. World J Urol 2013;31:1219–1223 [DOI] [PubMed] [Google Scholar]
- 29.Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 2011;26:864–868 [DOI] [PubMed] [Google Scholar]
- 30.Yang W, Lu J, Weng J, et al. . Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.