Abstract
Objective: This experimental work examined the healing effect and probable adverse impact of topical Prolavacid® solution (a polyhexamethylene biguanide-based wound cleanser) and topical Medihoney ointment in an animal model of cutaneous wound.
Approach: We randomly divided 22 adult Sprague-Dawley rats (all were male) in two groups (n = 11): (1) those for which Prolavacid solution was poured on the skin wound surface; and (2) those animals for which Medihoney® ointment was applied to the wounds. These two agents were applied daily throughout the study period (21 days). We photographically followed the wounds' contraction with imaging performed on days 0, 7, and 21 postwounding. The histopathologic features of the healing wounds were evaluated using skin biopsies taken on days 7 and 21 postwounding.
Results: The difference in mean wound surface area between two groups was not statistically significant on the examined days. Histopathological assessment indicated no statistically significant difference between the Prolavacid- and Medihoney-treated groups on days 7 and 21. We did not detect tissue necrosis following the topical application of Prolavacid solution.
Innovation: This was the first study to examine the efficacy and probable adverse consequences of topical Prolavacid on cutaneous wound healing process.
Conclusion: Our work showed no statistically significant difference between the efficacy of daily topical application of Prolavacid and Medihoney products on the healing process of fresh cutaneous wounds in our rat model.
Keywords: : anti-infective agents, honey, polyhexamethylene biguanide, wound healing

Introduction
Polyhexamethylene biguanide (PHMB), also called polyhexanide, is a cationic and highly water-soluble polymeric agent with broad-spectrum antimicrobial activity.1,2 This agent remains unaffected by sunlight and pH fluctuations.3 Because of its features, PHMB is used in a combination of different products, including skin disinfectant solutions, wound dressing materials, and contact lens cleaning solutions.1,2,4 In addition, PHMB can be used in the treatment of topical infections, such as Acanthamoeba keratitis in ophthalmologic cases.2 PHMB may be used in the management of both acute or chronic wounds types.1,5,6 One PHMB-based product that is commonly used in Iran, particularly for lower extremity wounds, is Prolavacid® solution (0.01% [w/v] PHMB-based wound cleanser; SUNMEDIC Co.7).
Medical-grade honey is a product with two main features, including acceleration of wound healing and antimicrobial activity.8–11 The U.S. Food and Drug Administration (FDA) agency approved the use of this product for wound care in diabetic ulcers, second-degree partial-thickness burns, traumatic wounds, and so on.12 Moreover, recent in vitro studies have affirmed the antibacterial properties of this product and provided evidence of its efficacy against multidrug-resistant organisms, which may infect wounds and lead to increased morbidity, including the methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa.9,10
Clinical Problem Addressed
According to Prolavacid solution producers, this product has minimal cell toxicity and no bacterial resistance. However, there is no strong documentation about this specific product. This experimental study histopathologically evaluated and compared the effects of Prolavacid solution and medical-grade honey (as an FDA-approved agent) on physiological processes of cutaneous wound healing in rats.
Materials and Methods
Ethical approval
The Animal Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (No. 9067), approved our study protocol. All of the required procedures were performed on animals after induction of general anesthesia. We made all efforts to minimize animals' suffering during the experiment.
Animals and excisional wound model
After consultation with a statistician for determination of sample size, 22 Sprague-Dawley rats (all were adult, male, and healthy; with a mean weight of 350 g) were selected. We kept these rats in separate and clean wire-bottomed cages. Their environment was temperature controlled (22°C ± 2°C) and humidity controlled (55% ± 15%), with 12-h light/dark photocycles. All rats had free access to equal amounts of water and standard animal food (Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran). They were allowed to adapt to their environment for 1 week before the study began. The rats were categorized into two groups using a simple randomization method (11 rats per each group): group 1: rats for which the PHMB-based solution Prolavacid (Paya Co., Iran) was applied to wound surfaces (called PHMB-treated group); and group 2: those for which medical-grade honey (Medihoney®; Comvita Ltd., New Zealand) was applied to their skin wounds (called Honey-treated group). Within the study time frame, the aforementioned products were applied daily (using a disposable applicator; each time a thin layer of product was created that fully covered the skin wound surface area). No wound dressing was used in this experiment. Both groups were followed for 21 days.
Before wounding, the rats were anesthetized with intramuscular injection of xylazine (dose of 10 mg/kg; Alfasan International, Woerden, Netherlands) and thiopental sodium (dose of 40 mg/kg; Biochemie, GmbH, Austria); then, after the back (mid-dorsum of the rat) hair was shaved, the site of wound creation was disinfected using alcohol ethylic solution. In the next step, a full-thickness excisional and circular-shaped cutaneous wound (radius of 10 mm and estimated depth of 2 mm) was created on the shaved skin using scissors and forceps.
During the study time frame, each rat's wound was carefully examined every day for any possible complication, mainly the presence of any macroscopical signs of infection.
Photographic assessment of wound healing
The healing process was monitored photographically using images taken from each of the skin wounds during the study. This was done by a digital camera (PowerShot G9 Model, a 12.1 megapixel camera; Canon, Tokyo, Japan). We fixed the camera at a distance of 10 cm from the wound surface (vertical view) to calibrate the magnification of the photographs. In addition, a fine-line ruler was kept at wound level at the time of imaging. Photos were taken on days 0, 7, and 21 postwounding. Analysis was done using the Photoshop CS Program (Adobe Systems, San Jose, CA) (analysis menu; record measurements command). Analysis of the wound contraction was the second endpoint of the study.
Histopathological evaluation of wound healing
We took semicircular and full-thickness skin biopsy samples from the wound sites of the studied animals (both groups) on days 7 (half of the wound site with a margin of 2 mm) and 21 (the remaining part with a 2 mm margin) postwounding. To perform the skin biopsy, the rats were anesthetized by inhalation of ether on day 7 and euthanized with the same agent on day 21.
Tissue biopsy specimens were washed using sterile 0.9% saline immediately, fixed in 10% formalin (buffered formaldehyde), and sent for histopathological assessment using established techniques (hematoxylin and eosin and Masson's trichrome stainings, and light microscopic examination).
For histopathological evaluation of the wound healing process, we followed the scoring system introduced by Abramov et al. (Table 1).13
Table 1.
Histological scoring system for wound repair13
| Score | ||||
|---|---|---|---|---|
| Parameter | 0 | 1 | 2 | 3 |
| Acute and chronic inflammation | None | Scant | Moderate | Abundant |
| Amount of granulation tissue | None | Scant | Moderate | Abundant |
| Granulation tissue maturation | Immature | Mild maturation | Moderate maturation | Fully matured |
| Collagen deposition | None | Scant | Moderate | Abundant |
| Re-epithelialization | None | Partial | Complete but immature or thin | Complete and mature |
| Neovascularization | None | Up to five vessels per HPF | 6–10 vessels per HPF | More than 10 vessels per HPF |
HPF, high-power field; PHMB, polyhexamethylene biguanide.
The aforementioned scoring rule analyzes the following criteria: the degree of neovascularization, the degree of collagen deposition, the degree of re-epithelialization, amount of acute and chronic forms of inflammation, and amount and maturation of granulation tissue. In our study, all investigators who analyzed images or assessed tissue specimens were blinded to the agents given. Of note, analysis of the histopathologic changes was the first endpoint of the study.
Statistical analysis
The results are presented as mean ± standard deviation (SD). Statistical comparisons were made using the Mann–Whitney U-test (version 16; SPSS Statistics software, Chicago, IL). p-Values less than 0.05 were considered statistically significant.
Results
Wound contraction
All of the studied rats survived to the end of the experiment. The mean ± SD values of cutaneous wound surface area were calculated for the rats placed in each group using the photos taken on days 0, 7, and 21 postwounding (Table 2).
Table 2.
Skin wound surface area (mm2) in polyhexamethylene biguanide and Medihoney groups on different days postwounding
| Day 0 | Day 7 | Day 21 | |
|---|---|---|---|
| PHMB | 310.16 ± 13.22 | 48.54 ± 11.34 | 0.0 ± 0.00 |
| Medihoney | 316.87 ± 13.67 | 50.83 ± 9.49 | 0.26 ± 0.49 |
| p | 0.123 | 0.234 | 0.187 |
Values are mean ± SD.
SD, standard deviation.
The results revealed no statistically significant difference in mean skin wound surface area between the two studied groups on days 0, 7, and 21 of the study. Of note, the rats' cutaneous wounds had no apparent sign of infection. However, it should be noted that we did not inoculate any infection in this study.
Histopathological examinations
The results (Fig. 1) are summarized in Table 3. No statistically significant difference was seen between the PHMB- and Honey-treated groups regarding the following factors: the degree of acute and chronic inflammation, the amount and maturation of granulation tissue, the degree of neovascularization, collagen deposition, or re-epithelialization. Moreover, no tissue necrosis was seen.
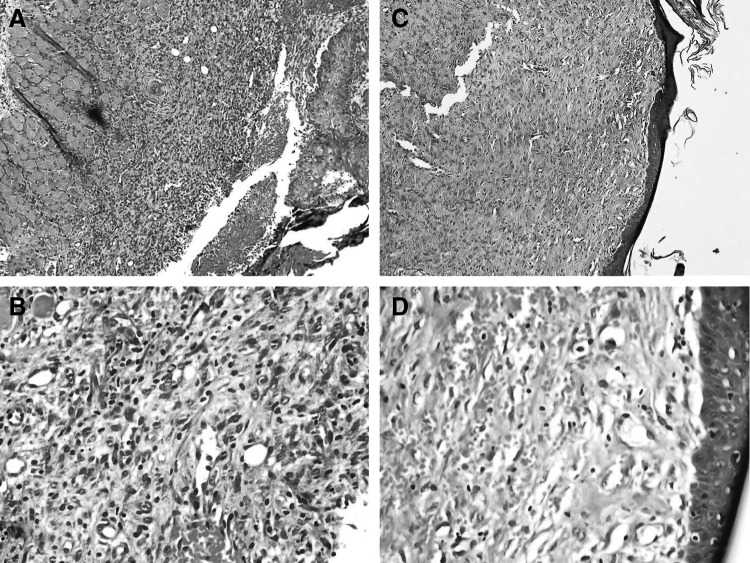
Figure 1.
(A, B) Histopathologic changes in the ulcers of polyhexamethylene biguanide group on day 7 showed ulceration, chronic inflammation (infiltration of lymphocytes), and granulation tissue formation. (C, D) Histopathologic changes in the lesions of the same group on day 21 showed full re-epithelialization and neovascularization. Magnification: (A, C) × 100; (B, D) × 400.
Table 3.
Histopathological scores of wound healing among two studied groups
| Groups | ||||||
|---|---|---|---|---|---|---|
| Day 7 | Day 21 | |||||
| Parameter | PHMB | Medihoney | p | PHMB | Medihoney | p |
| Acute and chronic inflammation | 2.00 ± 0.89 | 1.90 ± 0.94 | 1.00 | 2.54 ± 0.52 | 1.81 ± 0.87 | 0.069 |
| Amount of granulation tissue | 2.54 ± 0.52 | 2.63 ± 0.50 | 0.60 | 0.72 ± 1.00 | 0.81 ± 0.75 | 0.34 |
| Granulation tissue maturation | 2.45 ± 0.52 | 2.54 ± 0.52 | 0.91 | 1.36 ± 1.56 | 2.09 ± 1.37 | 0.28 |
| Collagen deposition | 1.63 ± 0.67 | 2.00 ± 0.44 | 0.08 | 1.54 ± 0.52 | 2.00 ± 0.89 | 0.23 |
| Reepithelialization | 0.45 ± 1.03 | 0.54 ± 1.03 | 0.51 | 2.72 ± 0.64 | 2.72 ± 0.64 | 0.83 |
| Neovascularization | 3.00 ± 0.00 | 3.00 ± 0.00 | 1.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 1.00 |
Values are mean ± SD.
Discussion
Cutaneous wounds, especially chronic types such as pressure ulcers, venous ulcers of the legs, and diabetic foot ulcers, are presently a worldwide health problem. These wounds provide a suitable environment for bacterial colonization and biofilm formation. Such local infections are now a prevalent therapeutic challenge in the area of wound management and result in extended duration of hospital stay, increase in the costs of treatment, and patients' morbidity and mortality.14,15 Based on these facts, wound dressings that accelerate the healing process and resolve the problem with local infections are highly desirable.
Selection of an appropriate dressing is a principal component in the field of wound care and management.16,17 Currently available dressings have a variety of properties that support the wound healing environment, such as optimizing the level of moisture at the skin wound surface, absorption of wound exudates, prevention of surrounding soft tissue maceration, and controlling the bacterial colonization.15 One main feature of some of these wound dressings is the use of antiseptic agents in their composition that contribute to the prevention or treatment of local wound infections.18
Of note, the use of systemic antibiotics for treating local wound infections may be unsuccessful because of difficulties such as their insufficient accumulation in soft tissue, increased bacterial resistance, and colonization of the wound by multiresistant microorganisms.19 Therefore, the use of systemic antibiotics in clinical practice as a single therapy remains controversial, especially in cases of infection that are complicated by the presence of a foreign body, biofilm, or reduced blood circulation and the presence of necrotic tissue.18 Because antiseptics are often topically applied to the wounded human skin for therapy, it is necessary to examine their efficacy in wound healing and their possible cytotoxic effects.
One of the most frequently used skin wound antiseptic agents is PHMB.18 It may be used separately, in the composition of wound dressings such as polyhexanide-containing biocellulose dressings or in the form of a gel or solution (like Prolavacid).20 PHMB kills bacteria by integrating into the cell membrane and reorganizing the membrane structure. The structural change prevents cells from pumping PHMB out of their membrane; thus, bactericidal concentrations of the agent are maintained in the cells.21
Although PHMB seems to be compatible with skin tissue, evidence shows that this product is cytotoxic in the peritoneal cavity and that the exposure of human endothelial cells and osteoblasts to this agent may result in severe cell damage.1,22 Moreover, there are reports of hypersensitivity reaction to this product.23 For this reason, some questions have arisen concerning the various effects of PHMB-based products on wound healing.
Although there is no strong documentation on Prolavacid, some previous studies have examined and compared the effects of PHMB solution on the skin wound healing process. Daeschlein et al. examined four cases suffering from poorly healing decubitus ulcers and found that PHMB was superior to silver nitrate and povidone/iodine regarding the improvement of wound healing, both clinically and histologically.24 The authors recommended this agent for treatment of second-degree burn wounds, which cannot primarily be covered by methods of plastic surgery. Another finding of the aforementioned study was that PHMB did not inhibit the re-epithelialization step of wound healing.24 In another research, Eberlein et al. compared the efficacy of polyhexanide-containing biocellulose dressings and silver dressings in the treatment of locally infected and painful wounds.25 According to their results, both dressings were effective in reducing pain (a common problem associated with chronic wounds with concomitant local infection) and the bacterial burden; however, the polyhexanide-containing biocellulose dressing decreased the critical bacterial load of the wound in a shorter time.25 Other studies also confirmed the role of PHMB dressings in reducing the pain of infected chronic wounds.14 Fabry et al. demonstrated a significantly better antiseptic, anti-inflammatory effect and tissue compatibility for the PHMB-based solution Lavasept® (B. Braun, GmbH, Germany) compared to Ringer solution. These authors reported no evidence of impaired wound healing.26 Results of the current work (in accordance with previous studies) show that 0.01% (w/v) PHMB-based solution Prolavacid had no negative effect on the healing process of superficial cutaneous wounds in this rat model. Moreover, the current study shows that Prolavacid had no statistically significant difference with medical-grade honey (an FDA-approved agent) regarding the wound healing process.
This study had a main limitation that should be considered in interpreting the findings. For each rat, biopsies on days 7 and 21 were taken from one wound (half of the wound site was biopsied at each day). This limitation affected only the wound size variable on the 21-day follow-up (wound size on day 7 postwounding was examined before the biopsy was taken). However, rectifying this matter in future works could provide a better interpretation of wound surface area and the wound contraction process. The second limitation was that Prolavacid is a solution, whereas the medical-grade honey has a relatively thick liquid form. Thus, the water loss and the barrier function could be greater in the Prolavacid group. Next, this study included no nontreated group as a control. This will make some difficulties in interpreting negative effects of the examined agents on wound healing. Additional limitations include the following:
• This work was not a human clinical trial, and there may be different responses in human versus rat wound models.
• The study was conducted on noninfected wounds, whereas the intended use for human chronic wounds often, and typically, involves infection and/or bacterial colonization.
• No untreated control groups were included in the study design, leaving us uncertain as to whether either agent had any effect on the expected rate of wound healing for this type of wound in a rat in the absence of treatment.
Key Findings.
• Prolavacid solution caused no significant negative impact on the microscopic processes involved in the skin wound healing process, including acute and chronic inflammation, the degree of granulation tissue formation and maturation, neovascularization, the amount of collagen bundle deposition, and wound surface re-epithelialization.
• The use of Prolavacid solution on fresh wounds does not lead to significant tissue necrosis.
• The healing effects of the PHMB-based solution did not significantly differ from those of medical-grade honey, for noninfected or noncontaminated wounds.
Innovation
Due to the widespread use of Prolavacid solution as a skin disinfectant in Iran, this work evaluated this agent's efficacy and probable adverse effects following local use on fresh cutaneous wounds.
Conclusion
According to this study, the efficacy of a daily topical application of 0.01% (w/v) PHMB-based solution Prolavacid to fresh skin wounds in rats is similar to that of medical-grade honey for the healing process. No significant negative impact on the physiologic processes involved in the cutaneous wound healing process occurred following the usage of this PHMB-based product. However, further studies on human cases and on other types of wounds are recommended to thoroughly evaluate different aspects of this solution and compare it with other approved products.
Abbreviations and Acronyms
- FDA
U.S. Food and Drug Administration
- HPF
high-power field
- PHMB
polyhexamethylene biguanide
- SD
standard deviation
Acknowledgments and Funding Sources
The authors declare that there are no conflicts of interest. Only institutional funds were used to perform this research.
Author Disclosure and Ghostwriting
None declared.
About the Authors
Shahram Paydar, MD, a trauma surgeon and Associate Professor of Surgery, and Amirreza Dehghanian, MD, a fellow in surgical pathology and Assistant Professor of Clinical and Surgical Pathology, both work at the Shahid Rajaee Trauma Hospital, Shiraz, Iran. Bijan Ziaeian, MD, a fellow in thoracic surgery and Professor of Surgery, works at the Namazee Hospital, Shiraz, Iran. Mohsen Heidarpour, MD, and Roshanak Alavi Moghadam, MD, are two general surgeons and researchers working in affiliation with the Trauma Research Center, Shahid Rajaee Trauma Hospital, Shiraz University of Medical Sciences, Shiraz, Iran. Behnam Dalfardi, MD, is an internal medicine resident at Shiraz University of Medical Sciences, Shiraz, Iran. Abbas Hallaj Karladani, PhD, is an Assistant Professor of Orthopedics at the University of Gothenburg, Sweden.
References
- 1.Müller G, Kramer A, Schmitt J, et al. . Reduced cytotoxicity of polyhexamethylene biguanide hydrochloride (PHMB) by egg phosphatidylcholine while maintaining antimicrobial efficacy. Chem Biol Interact 2011;190:171–178 [DOI] [PubMed] [Google Scholar]
- 2.Yanai R, Ueda K, Nishida T, et al. . Effects of ionic and surfactant agents on the antimicrobial activity of polyhexamethylene biguanide. Eye Contact Lens 2011;37:85–89 [DOI] [PubMed] [Google Scholar]
- 3.Romanowski EG, Yates KA, O'Connor KE, et al. . Evaluation of polyhexamethylene biguanide (PHMB) as a disinfectant for adenovirus. JAMA Ophthalmol 2013;131:495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirker KR, Fisher ST, James GA, et al. . Efficacy of polyhexamethylene biguanide-containing antimicrobial foam dressing against MRSA relative to standard foam dressing. Wounds 2009;21:229–233 [PubMed] [Google Scholar]
- 5.Fleck CA. Fighting infection in chronic wounds. Adv Skin Wound Care 2006;19:184., 186, 188. [DOI] [PubMed] [Google Scholar]
- 6.Roth C, Beule AG, Kramer A, et al. . Response analysis of stimulating efficacy of polihexanide in an in vitro wound model with respiratory ciliary epithelial cells. Skin Pharmacol Physiol 2010;23 Suppl:35–40 [DOI] [PubMed] [Google Scholar]
- 7.Paya Co. Prolavacid®. http://prolavacid.com (last accessed July17, 2016)
- 8.Simon A, Traynor K, Santos K, et al. . Medical honey for wound care—still the ‘latest resort’?. Evid Based Complement Alternat Med 2009;6:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper R, Jenkins L, Hooper S. Inhibition of biofilms of Pseudomonas aeruginosa by Medihoney in vitro. J Wound Care 2014;23:93–96, 98–100, 102 passim. [DOI] [PubMed] [Google Scholar]
- 10.Müller P, Alber DG, Turnbull L, et al. . Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS One 2013;8:e57679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biglari B, Moghaddam A, Santos K, et al. . Multicentre prospective observational study on professional wound care using honey (Medihoney™). Int Wound J 2013;10:252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DS, Sinno S, Khachemoune A. Honey and wound healing: an overview. Am J Clin Dermatol 2011;12:181–190 [DOI] [PubMed] [Google Scholar]
- 13.Abramov Y, Golden B, Sullivan M, et al. . Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen 2007;15:80–86 [DOI] [PubMed] [Google Scholar]
- 14.Sibbald RG, Coutts P, Woo KY. Reduction of bacterial burden and pain in chronic wounds using a new polyhexamethylene biguanide antimicrobial foam dressing-clinical trial results. Adv Skin Wound Care 2011;24:78–84 [DOI] [PubMed] [Google Scholar]
- 15.Lipp C, Kirker K, Agostinho A, et al. . Testing wound dressings using an in vitro wound model. J Wound Care 2010;19:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelrahman T, Newton H. Wound dressings: principles and practice. Surgery (Oxford) 2011;29:491–495 [Google Scholar]
- 17.Lionelli GT, Lawrence WT. Wound dressings. Surg Clin North Am 2003;83:617–638 [DOI] [PubMed] [Google Scholar]
- 18.Atiyeh BS, Dibo SA, Hayek SN. Wound cleansing, topical antiseptics and wound healing. Int Wound J 2009;6:420–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch T, Seipp HM, Jacobsen F, et al. . Antiseptics in surgery. Eplasty 2010;10:e39. [PMC free article] [PubMed] [Google Scholar]
- 20.Piatkowski A, Drummer N, Andriessen A, et al. . Randomized controlled single center study comparing a polyhexanide containing bio-cellulose dressing with silver sulfadiazine cream in partial-thickness dermal burns. Burns 2011;37:800–804 [DOI] [PubMed] [Google Scholar]
- 21.Moore K, Gray D. Using PHMB antimicrobial to prevent wound infection. Wounds UK 2007;3:96–102 [Google Scholar]
- 22.Vörös P, Dobrindt O, Perka C, et al. . Human osteoblast damage after antiseptic treatment. Int Orthop 2014;38:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leysen J, Goossens A, Lambert J, et al. . Polyhexamethylene biguanide is a relevant sensitizer in wet wipes. Contact Dermatitis 2014;70:323–325 [DOI] [PubMed] [Google Scholar]
- 24.Daeschlein G, Assadian O, Bruck JC, et al. . Feasibility and clinical applicability of polihexanide for treatment of second-degree burn wounds. Skin Pharmacol Physiol 2007;20:292–296 [DOI] [PubMed] [Google Scholar]
- 25.Eberlein T, Haemmerle G, Signer M, et al. . Comparison of PHMB-containing dressing and silver dressings in patients with critically colonised or locally infected wounds. J Wound Care 2012;21:12, 14,–16, 18–20 [DOI] [PubMed] [Google Scholar]
- 26.Fabry W, Trampenau C, Bettag C, et al. . Bacterial decontamination of surgical wounds treated with Lavasept. Int J Hyg Environ Health 2006;209:567–573 [DOI] [PubMed] [Google Scholar]