Abstract
Recent years have seen a renewed interest in studies of the lymphatic system. This review addresses the differences between in vivo and ex vivo methods for visualization and functional studies of lymphatic networks, with an emphasis on studies of collecting lymphatic vessels. We begin with a brief summary of the historical uses of both approaches. For the purpose of detailed comparisons, we subdivide in vivo methods into those visualizing lymphatic networks through the intact skin and those using surgically opened skin. We subdivide ex vivo methods into isobaric studies (using a pressure myograph) or isometric studies (using a wire myograph). For all four categories, we compile a comprehensive list of the advantages, disadvantages, and limitations of each preparation, with the goal of informing the research community as to the appropriate kinds of experiments best suited, and ill suited, for each.
Keywords: : imaging, NIRF, pressure, intravital
Article
Recent years have experienced a surge in the number of publications on the lymphatic system, with many studies employing new imaging modalities to visualize lymphatic vessels and/or networks. The development of transgenic mouse and zebrafish models expressing fluorescent lymphatic reporters has played a fundamental role in this increased interest, in many cases permitting the discovery of new lymphatic beds or uncovering transcriptional programs in other tissues that are shared by the lymphatic vasculature. Beyond these anatomical and developmental advances, the transgenic mouse is uniquely suited for imaging the function of single, contracting lymphatic collecting vessels, or even whole lymphatic networks, in certain tissue beds. A primary role of these vessels is to actively transport lymph and they do so through spontaneous active contractions of lymphatic smooth muscle cells.1
The purpose of this review is to address the differences between in vivo and ex vivo methods for visualizing and studying the lymphatic system, with an emphasis on studies of collecting lymphatic vessels. We expand on a brief comparison of methods to study lymphatic transport by Liao et al.,2 but do not include a discussion of radiographic methods, for example, lymphoscintigraphy, or clinical positron-emission tomography (PET) or magnetic resonance imaging (MRI). We do, however, address some aspects of whole-animal fluorescence microlymphangiography that have been covered elsewhere.3–6 We attempt to compile a comprehensive list of the advantages and limitations of in vivo and ex vivo methods with the goal of informing the research community, particularly new investigators, as to which method(s) may be best suited for addressing particular experimental questions.
The Historical Use of In Vivo Lymphatic Methods
In vivo studies of intact lymphatic vessels have been performed using intravital microscopy since the 1920s and earlier, although many of the early studies focused on anatomy and/or were largely descriptive. Examples include the observations of lymphatic contractions in testicular and inguinal lymphatics from rat and guinea pig by Pullinger and Florey7 and the cinematographic recordings of spontaneous lymphatic contractions in the rat mesentery by Webb.8 In 1949, Smith published important insights into modulators of spontaneous lymphatic contractions in rats, mice, and guinea pigs by using lymphatic or venous occlusions to broadly manipulate intralymphatic pressure in popliteal afferent lymphatics9; contractions were counted by eye, in lieu of diameter measurement, to assess the spontaneous contraction rate. As methods for online recording of microvascular diameter became widely available in the 1970s–1980s,10 quantitative analyses of lymphatic contractions were introduced in the rat, cat, sheep,11 and guinea pig mesenteries,12–16 as well as bat wing.17 Extensive in vivo studies in the rat mesentery by Benoit et al.14 and Zawieja et al.18 provided important insights as to how lymphatic contractions were modulated by pressure and coordinated among adjacent lymphangions. Later, when coupled with fast video microscopy, these techniques also allowed accurate recording of lymph flow within a single contracting vessel through particle velocity tracking.19 Previous estimates of lymph flow relied on cannulation of the thoracic duct or other large vessels to collect bulk lymph flow over a period of time, but, in doing so, disrupted the proper resistance and pressure gradients against which this lymph normally must traverse20,21; the earlier studies were limited to the estimation of total lymph flow from a given tissue, not necessarily a single vessel or network.
More recently, in vivo studies have used fluorescent dyes injected distally into the interstitium to visualize lymphatic vessel contraction/transport in the mouse to take advantage of the genetic tools available for that species. For example, injection of fluorescein isochiocyanate (FITC) dextran, combined with fluorescence recovery after photobleaching, allows measurement of fluid velocity in a superficial initial lymphatic network.22 These tracer techniques, when combined with computational models of transport in the tissue space, were used by Swartz et al.23 to measure the effects of particle size and interstitial fluid pressure on drainage at the network level as well as to separate the conductance properties of the vessel network from those of the interstitial tissue space. Hagendoorn et al.24 and Liao et al.25 injected FITC dextran into the mouse foot pad to visualize collecting vessels in the popliteal fossa of the lower leg; their method enabled accurate diameter measurement of the popliteal afferent lymphatics, but required removal of the overlying skin and superfusion of the preparation with artificial solutions. Sevick-Muraca and colleagues pioneered methods using indocyanine green (ICG), in conjunction with near-infrared (NIR) illumination for deeper light penetration, to study lymphatic collectors and networks through the intact mouse skin.26 The use of a fluorescent fatty acid (BODIPY®FL-C16) has allowed accurate visualization of the mesenteric lymphatic contractile activity in the rat under the unique, chylomicron-rich postprandial conditions of that network. This has enabled contractile activity, lymph flow, and lipid uptake to be assessed simultaneously after simple exteriorization of a loop of intestine.27–30 The ICG/NIR technique now has been adapted for imaging lymphatic networks in human limbs, where it is possible to characterize defects in lymph transport and/or hyperplasia in patients with primary lymphedemas.31–34 Additionally, integration of these lymphatic imaging techniques with a calibrated occlusion cuff to temporarily block lymph flow has allowed investigators to measure the effective pumping pressure of a lymphatic chain in both animal models and humans in the context of both normal function and disease.35,36
The Historical Use of Ex Vivo Lymphatic Methods
Ex vivo preparations of collecting lymphatic vessels can be classified broadly as isobaric or isometric. Both of these approaches will now be discussed briefly.
Isobaric preparations of vessels harvested from the mesenteries of pigs, cows, or sheep37,38 were introduced in the 1970s–1980s. Notable among these is the pioneering study by McHale and Roddie in which bovine mesenteric lymphatics were isolated, cannulated, and held at defined pressures.38 The vessels were sufficiently large to allow direct measurement of lymph outflow using a drop counter. The results showed definitively that lymphatic vessels contract spontaneously ex vivo in the absence of external tethering forces and intact innervation and that pressure modulates both contractile strength and contraction frequency. McHale and colleagues subsequently investigated neural control of lymphatic pumping using such preparations.39–41
In the 1990s, the isolated, pressurized (isobaric) lymphatic vessel preparation was adapted for rat vessels42,43 and more recently for mouse vessels.44–46 The contractile function of these smaller vessels usually is assessed by measuring diameter changes, with output calculated from the product of contraction frequency and amplitude or ejection fraction; this indirect measurement is necessitated by the unavailability of flow meters that are accurate in the submicroliter/min range. A primary drawback of this technique is that it requires the assumption that the valves are 100% effective at preventing back flow and thus assumes that any change in volume leads to the positive displacement of fluid. However, speckle imaging techniques have recently been developed to allow quantitative flow measurements in small vessels, including lymphatics,47,48 in vivo and, although these require the presence of scattering elements, they could in principle be applied to ex vivo preparations. The primary focus of ex vivo isobaric studies to date has concerned the influence of hydrodynamic forces (pressure, flow), neural/neurotransmitter modulation, and cellular factors on contractile function and consequences of different pathological states on the associated signaling pathways.1 However, recent work has begun to investigate the impact of contractile function on immune cell trafficking as well as immune cell modulation of contractile function.49–53
Isometric studies of ex vivo lymphatic vessels were introduced in the 1970s by Orlov and colleagues, who used standard force transducers and organ bath chambers to study rings of excised segments of bovine and porcine lymphatics. Unfortunately, because most of those studies were published in Russian Journals,54–56 most remain untranslated and infrequently cited. The wire myograph technique, equipped with high-sensitivity force transducers for isometric studies of small arteries/arterioles,57 has been adapted to allow isometric studies of small lymphatic collectors from rat58–61 and mouse (M.J.D., unpublished). These preparations, whether intact or skinned (permeabilized plasma membranes), have provided insight into force production by these small vessels and their contractile machinery proteins, their calcium sensitivity, and the cellular signaling pathways that can retard or potentiate their contractile strength.58,61 Isometric preparations are particularly suited to assess pharmacologic responses and/or facilitate sharp electrode measurements of lymphatic muscle membrane potential since the latter technique requires a preparation with minimal movement. Similar measurements in pressurized vessels or lymphatic muscle strips require the use of myosin light chain kinase inhibitors that may have off-target or unintended effects. Telinius et al. have made extensive use of the wire myograph to study ion channels controlling the membrane potential of human lymphatics collected during surgery.62–65
Comparison of Methods
In vivo and ex vivo methods for studying lymphatic function each have their inherent advantages and limitations. Although several of the following issues have been discussed or debated previously in the context of the blood microvasculature,66–69 many points are worth reiterating relative to studies of the lymphatic system. Indeed, several historical warnings about the limitations of both approaches have been ignored in recent lymphatic publications. Table 1 summarizes both the advantages and disadvantages of the two approaches, with some of the most salient points highlighted in the following section.
Table 1.
Comparisons of Advantages and Disadvantages of Microscopic Methods Used to Study Lymphatic Function
| In vivoa | Ex vivo | |||||
|---|---|---|---|---|---|---|
| Intact | Exposed | Isobaric | Isometricb | |||
| Advantages | No change in Pext or Pintc | |||||
| No change in pO2, pH, etc. | ||||||
| Intact nerves, fat, adventitia, etc. | Adventitial or endothelial cell influences potentially can be removedd | |||||
| Studies of initial lymphatics possible1–3 | Accurate control of agonist/inhibitor concentrations | |||||
| Control of internal and externale pressure | Control of basal force (preload)4 | |||||
| Control of flow rate | ||||||
| Potentially wide field of view5 | Control of axial length | Direct measurement of force4 | ||||
| High-resolution ID/OD measurement | Limited movement (for Em, Ca2+)6,7 | |||||
| Longitudinal imaging studies possible | Control of inflow/outflow pressures | |||||
| Beads, cells, and labeled cells can be injected8,9 | ||||||
| Limitations | Uncertain agonist/inhibitor concentrations10 | Twisting is possible | LEC damage by wires | |||
| Anesthesia required; may affect reactivity | Unpressurized | |||||
| Pressures unknownf, uncontrollable | Possible issues with pipette resistance in small vessels11 | Unnatural geometry can alter D-R relationship12 | ||||
| Possible cytotoxicity of tracer dyes13 | Uncertainties in setting physiological axial length | |||||
| Possible ROS generation by tracer dyes/epi-illumination | Removal of external tethering forces | |||||
| Dye injection disturbs normal interstitial environment (external pressure, pO2, etc.) | Not truly isometric if thin wires used (e.g., for mouse vessels) | |||||
| Single vessel resolution is quite limited | Reduced resolution (epi-illumination) | Force transducers not sufficiently sensitive for mouse vessels | ||||
| Near-infrared optics needed | Pext is changed | Pext is changed | No flow14 | |||
| Must rely on indirect indices of flow (e.g., packet movement)g | Disruption of valves | |||||
| Potential issues with source/sink effects for charge transfer between cellsh | ||||||
| External pO2, pH, etc., determined by (artificial) superfusion solution | ||||||
| Fat can obscure visibility, particularly in older animals | Internal pO2 and pH determined by perfusion solution | |||||
| Possible movement from respiration, etc. | ||||||
| Limited to study of a few lymphangions | 2-mm length limitation4 | |||||
| Variable surgical traumai | Possible dissection/cannulation/cleaning trauma and effects of severed ends or sutures | |||||
Italic text indicates possible advantage or disadvantage depending on nature of protocol.
Assumes in vivo studies made under fluorescence illumination.
Assumes wire myograph used for rodent vessels as opposed to the classic isometric ring system.
Depending on the volume of tracer injected; Pext = external hydrostatic pressure; Pint = internal hydrostatic pressure (intraluminal pressure).
For example, for study designs testing responsiveness without the influence of periadipose fat/nerves, denudation.
External pressure can be controlled with an appropriate chamber.16
Unless measured using servo-nulling technique, in exposed preparations.
Recent methods have been developed to detect flow rates in vivo by speckle Doppler methods.15
Disconnection of a vessel from the rest of the network potentially affects conduction velocity and coordination of contraction waves.
D-R, dose-response; LEC, lymphatic endothelial cell; ROS, reactive oxygen species.
Table References
1. Hogan RD, Unthank JL. Mechanical control of initial lymphatic contractile behavior in bat's wing. Am J Physiol 1986; 251:H357–H363.
2. Swartz MA, Kaipainen A, Netti PA, Brekken C, Boucher Y, Grodzinsky AJ, Jain RK. Mechanics of interstitial-lymphatic fluid transport: Theoretical foundation and experimental validation. J Biomechanics 1999; 32:1297–1307.
3. Russo E, Teileira A, Vaahtomeri K, Willrodt AH, Bloch S, Nitschke M, Santambrogio L, Kerjaschki D, Sixt M, Halin C. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep 2016; 23:1723–1734.
4. Mulvany MJ. Procedures for Investigation of Small Vessels Using a Small Vessel Myograph. Aarhus, Denmark: Danish Myo Technology; 2003.
5. Kwon S, Sevick-Muraca EM. Mouse phenotyping with near-infrared fluorescence lymphatic imaging. Biomed Opt Express 2011; 2:1403–1411.
6. Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 2011. Dec 8; (58). pii: 3438.
7. Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol 2003; 285:H2573–H2577.
8. Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: A role for lymphatics in exchange. J Physiol 2010; 588:243–54.
9. Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 2011; 12:879–87.
10. Telinius N, Mohanakumar S, Majgaard J, Kim S, Pilegaard H, Pahle E, Nielsen J, de Leval M, Aalkjaer C, Hjortdal V, Boedtkjer DB. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol 2014; 592:4697–714.
11. Bertram CD, Macaskill C, Moore JE Jr. Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Engin 2014; 17:1519–34.
12. Lew MJ, Angus JA. Wall thickness to lumen diameter ratios of arteries from SHR and WKY: Comparison of pressurized and wire-mounted preparations. J Vasc Res 1992; 29:435–442.
13. Gashev AA, Nagai T, Bridenbaugh EA. Indocyanine green and lymphatic imaging: Current problems. Lymphat Res Biol 2010; 8:127–130.
14. Garcia-Roldan JL, Bevan JA. Flow-induced constriction and dilation of cerebral resistance arteries. Circ Res 1990; 66:1445–1448.
Advantages of In Vivo Preparations
In vivo preparations can be classified broadly as intact, in which lymphatic vessels or networks are visualized through the intact skin (with no surgical intervention), or exposed, in which the skin is opened surgically and removed or retracted to expose the underlying vessels. Both variations offer the potential advantage of retaining relationships between the vessel, nerves, the parenchyma, and other extramural cells such as adipocytes, mast cells, and the appropriate influx/efflux of circulating immune cells. Critically, these preparations also maintain the appropriate pressure (assuming that the injection of contrast agents does not significantly alter the relevant tissue pressure—see “Limitations of InVivo Preparations”) and flow resistance relationships that exist within the lymphatic network. The effects of both intrinsic and extrinsic forces can be preserved, for example, extramural forces such as passive compression by contractions of adjacent smooth muscle or skeletal muscle70 or by venular and arteriolar vasomotion.71,72 A notable advantage of intact skin preparations is that artificial superfusion solutions can be avoided, thereby potentially maintaining the endogenous interstitial hydrostatic/osmotic pressure gradients, pO2/pCO2, and ion gradients, etc. However, these techniques are usually employed in immobilized animals under anesthesia, some forms of which may significantly impair the contribution of extrinsic forces such as skeletal muscle activity, respiration, and gastrointestinal motility or directly affect the intrinsic contractile function of lymphatic vessels.
Traditional intravital microscopic methods have utilized bright-field microscopy,14,18,71,73 but these methods are generally restricted to thin transparent tissues such as the mesentery, cremaster muscle, and bat wing that can be exteriorized and transilluminated and with little, if any, tissue overlaying the lymphatic vessel to be imaged. Such studies of lymphatic networks have the potential advantage of being able to measure volume flow rate by speckle microscopy, utilizing scattering effects from cells and large proteins that are present in lymph without injecting foreign particles.48,74 In recent years, in vivo studies of the lymphatic system have made increasing use of fluorescence microscopy in which appropriate tracers are injected distally to use as flow markers75 and/or to outline vessel edges for diameter measurement.25 Forays into the development of new NIR molecules with improved properties compared with ICG have increased the quantum yield of the fluorophore, reduced the associated toxicity, and enhanced intraluminal retention.76–79 For example, NIR fluorescence (NIRF) imaging methods utilize dyes such as the Food and Drug Administration-approved ICG,80 the PEG-coupled NIR dye P20D680,77 and the proprietary IR-Dye 800CW poly(ethylene glycol) (PEG) from LI-COR.
NIRF illumination allows greater light penetration through intact skin due to excitation and emission wavelengths occurring in the optical window, but this imaging method does suffer decreased resolution with increasing depth of penetration because light scattering though the overlying skin distorts vessel diameter measurements, producing potential errors of 2-fold at 2 mm and 10-fold at 5 mm of depth.81 The difference in resolution of single vessel edges is documented in a recent publication.82 As a result, NIRF studies through the intact skin are typically limited to a temporal window of a few contraction cycles and are able only to estimate contractile parameters (strength, frequency, flow) by indirect indices such as packet transfer of dye.83 However, over longer periods of time, these techniques have been used to measure more direct indices of lymphatic function such as average clearance rates84 or the effective pumping pressure.35,36 It is important to note that surgically opening the skin to enable increased resolution of valve structure and vessel edges obviates many of the other advantages of in vivo preparations mentioned above and in Table 1.
Limitations of In Vivo Preparations
In vivo lymphatic preparations may be affected negatively by the direct toxicity of some fluorescent tracers.85 For example, one report suggests that ICG, at certain concentrations in isolated vessels, has negative effects on lymphatic contractile function,85 although this result was disputed as different concentrations of ICG injection in vivo did not result in noticeable changes in acute lymphatic propulsion after injection.86 However, another study demonstrated that while ICG did not acutely alter lymphatic function in vivo, there were long-term reductions in lymphatic function due to dye retention at the injection site for up to 2 weeks after injection. This adverse effect was significantly reduced for PEG-coupled NIR dyes.81 Another potential concern is that prolonged illumination of fluorescent dyes, or even fluorescent reporter proteins, can result in local heating and/or the generation of reactive oxygen species that may impair endothelium-dependent responses and contractile function.87,88–90 Phototoxicity is a function of illumination intensity, wavelength, area, duration of exposure, and other factors, most of which must be adjusted for the sensitivity of the detection system used. On the other hand, phototoxicity can be utilized to the investigator's advantage, for example, to intentionally photodamage and impair the function of cells in one layer of the wall, as has been demonstrated for both the smooth muscle and endothelial layers of blood vessels.88 This property actually has been used with highly absorbent dyes to create new animal models of lymphatic damage.91,92
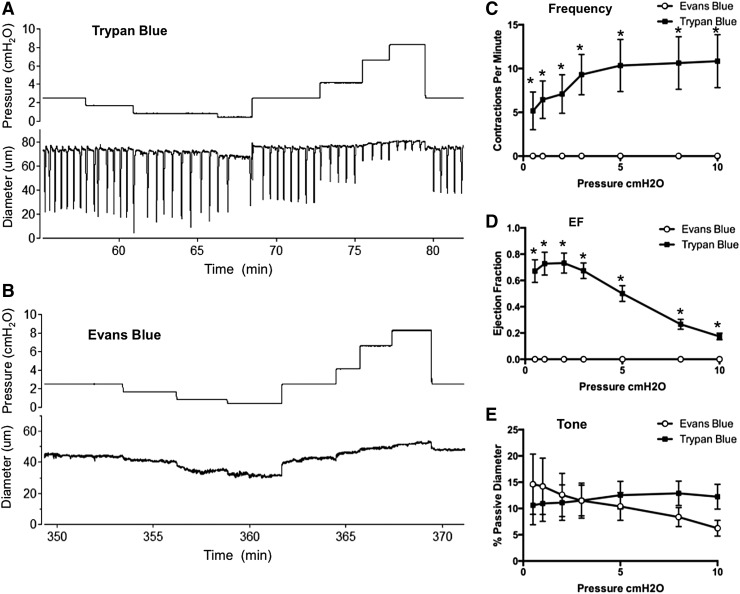
In this context, intradermal or subcutaneous injection of Evans Blue dye is a common tool to aid in the identification of lymphatic vessels and mapping of lymphatic networks.23,93,94 The dye has also been used recently to assess backflow through normal and defective lymphatic valves in vivo.95–97 In the course of a recent study, we noted that peripheral lymphatic vessels in the mouse, which show robust spontaneous contractions after cannulation/pressurization ex vivo, failed to show any such spontaneous activity after prior injection of Evan's Blue dye into a distal portion of the network from which the vessel was dissected. We therefore tested the possible toxic effect of Evans Blue dye, in quantities and concentrations typical of those in the literature (5–20 μL, 0.5%), on lymphatic contractions using popliteal lymphatics studied ex vivo. All experiments were approved by the University of Missouri Animal Care and Use Committee and conformed to the U.S. Public Health Service policy for the humane care and use of laboratory animals (PHS Policy, 1996). Mice were anesthetized with pentobarbital sodium (Nembutal; 60 mg/kg, i.p.). A solution of Evan's Blue (∼10 μL, 0.5% in Krebs; Fisher Scientific #23860) was injected into the dorsal surface of one foot 5–10 minutes before dissection and isolation of popliteal lymphatic vessels. A similar volume of Trypan Blue dye (0.4% in sterile saline; VWR BioWhittaker, Atlanta, GA), a vital dye that does not cross the cell membrane, was injected into the other foot. Trypan Blue was used as a control dye since spontaneous contractile activity was reported previously to persist after its use.9 Popliteal afferent lymphatics from both legs were then dissected and prepared for ex vivo assessment of contractile activity as described previously.44 Vessels from hind paws injected with Evans Blue exhibited complete loss of spontaneous contractions; in contrast, vessels draining hind paws injected with Trypan Blue exhibited spontaneous contractions with amplitudes and frequencies similar to those reported previously for popliteal afferent lymphatics dissected from noninjected mice.44 Typical diameter recordings from vessels treated with each dye are shown in Figure 1 panels A and B, with the contraction patterns tested at seven different pressure levels between 0.5 and 10 cm H2O in each case. All vessels exposed to Evans Blue failed to develop spontaneous contractions throughout the entire experimental protocol, a period of over 3 hours. The summary data from six to seven vessels exposed to each dye are shown in panels C–F, where it is obvious that vessels exposed to Evans Blue dye showed complete loss of spontaneous contractile activity, but with little effect on tone. We therefore conclude that caution be used when using Evans Blue dye in longitudinal studies, when active lymph uptake or transport is being measured, or when valve backflow is assessed under conditions where it can be affected by the loss of contractile function.98
FIG. 1.
Evans Blue, but not Trypan Blue, critically impairs murine collecting vessel contractions. Trypan Blue (10 μL) and Evans Blue (10 μL) were injected into opposite hind paws and popliteal collecting vessels were dissected from the respective hind limbs and cannulated for ex vivo isobaric myograph studies. Raw diameter traces are shown in (A, B). frequency (C), ejection fraction (EF) (D), and vessel tone (E) were calculated for popliteal vessels exposed to Trypan Blue (n = 6) or Evans Blue (n = 7). *indicates significantly different at p < 0.05 using 2-way analysis of variance with Tukey's post-hoc test.
Apart from toxicity concerns, a seldom discussed limitation of using tracers is that dye injection, even at a remote site, will almost inevitably alter both the normal filling pressure of the local lymphatic capillaries and the intraluminal pressure in the downstream collecting vessel(s). In addition to the rapid change in interstitial volume and pressure during injection and the associated distortion or damage of the interstitial matrix, there is likely to be a local effect on the interstitial oncotic pressure that may acutely increase capillary filtration and enhance lymph production over the physiological baseline levels. This is supported by a recent publication that showed enhanced lymphatic function after injection of a tracer that did not reach a steady state until around 10 minutes after injection.78 Moreover, such changes will not be known and may be variable from day to day depending on the site and volume of injection and the retention of the injected molecule within the tissue. The consistency of injecting a very small dye volume (<5 μL) at a controlled depth (e.g., intradermal rather than subcutaneous) can be improved with significant practice and care. Using microneedles to deliver the contrast agent could minimize some of the variability.99
In this context, interstitial and intralymphatic pressure gradients100 and/or flow changes19 are seldom measured in in vivo lymphatic preparations even though methods for doing so have been developed and even though interventions are often imposed that likely change one or both variables. For example, acute injection of a tracer will likely produce a transient elevation in the driving pressure for lymph flow that will override intrinsic propulsive mechanisms, initially producing misleading measurements of tracer clearance and/or dye movement. Furthermore, the time course of the decay of this artificial pressure head will vary with a number of factors, including the injection volume, the degree of tissue displacement, compliance of the injection target (intradermal, subcutaneous, and/or intralymphatic compartment), and the extent to which the injection device (i.e., needle) is withdrawn.
The scenarios just described highlight the need to know the hydrostatic pressures in and around initial and collecting lymphatic vessels under the conditions used in many in vivo experiments. Unfortunately, no measurements of intraluminal pressures in any lymphatic network of the mouse have been made in vivo, either in wild-type mice or any of the transgenic mouse strains used increasingly for lymphatic studies. Indeed, only a few studies have measured lymphatic intraluminal pressure in any species in vivo.14,17,70,73,100–102 For example, Hogan and Unthank73 and Clough and Smaje103 investigated the hydrostatic driving forces controlling the filling of initial lymphatics in a bat and cat, respectively, by measuring interstitial and intraluminal pressures simultaneously. Zweifach and colleagues measured the pressure profile through an entire lymphatic network (in rat and cat mesenteries12,100), thereby establishing the concept that collecting vessels pump against an adverse pressure gradient. However, the hydrostatic pressure profile has not been verified for any lymphatic network other than the mesentery, with the possible exception of the diaphragm.102 Alterations in the normal hydrostatic pressures may well occur in transgenic mice with elevated blood pressure (e.g., eNOS−/− mice), which may have increased net filtration across blood capillaries, leading to changes in interstitial fluid pressure and/or lymphatic capillary pressure that are unknown. Likewise, mice with engineered lymphatic valve or lympho-venous valve defects will likely experience changes in lymphatic capillary pressure secondary to elevated pressures in their central lymphatic trunks, and these animals may be particularly susceptible to the slightest changes in body position if nonfunctional valves allow backflow. Any mice with edema, lymphedema or inflammation will likely have elevated pressures in some portions of their lymphatic trunks that can indirectly influence contractile activity. Additional information regarding baseline hydrostatic pressures inside and outside of lymphatic capillaries and collecting vessels, particularly from the mouse, would not only inform in vivo studies but would also aid investigators using ex vivo methods to gauge the physiologically relevant range of pressures over which to perform studies.
Advantages of Ex Vivo Preparations
For purposes of comparison, ex vivo methods can be subdivided into isobaric preparations and isometric (wire myograph) preparations. Isobaric preparations allow lymphatic vessels to be studied under defined hydrodynamic conditions so that pressure and flow, to a lesser extent, can be controlled. Pressure control is the primary advantage of the isolated, cannulated vessel method (termed pressure myography) because lymphatic vessels are exquisitely sensitive to changes in transmural pressure and can double their contraction frequency in response to as little as a 0.5 cm H2O increase in pressure.104 With a cannulated lymphatic vessel containing at least one valve, setting both input (upstream) pressure and output (downstream) pressure to equal levels allows determination of the effect of changing baseline pressure and preservation of pulsatile flow associated with spontaneous contractions, while eliminating continuous forward flow.105 Alternatively, elevating input pressure while simultaneously reducing output pressure by an equivalent amount (relative to baseline) allows the maintenance of a constant midpoint pressure to assess the effect of net forward flow42,106,107 (although the contraction wave typically initiates from one of the severed ends rather than the middle of the cannulated vessel). Other combinations of pressure and flow changes are also possible. One limitation of these preparations is that flow changes are induced by changing pressure at some point significantly upstream and/or downstream of the vessel itself and thus actual volume flow rates (and the imposed wall shear stress) remain unknown. Additionally, theses flow studies require pipettes with similar hydraulic resistances and that also are of comparable size to the vessel being studied. However, a recently developed system using feedback controlled displacement pumps has overcome this limitation, as the actual imposed flow on the vessel is continuously recorded through the position of syringe pistons that displace the fluid through the vessel.108,109
Lymphatic vessels also can be excised and studied under isometric conditions. For larger vessels, such as those from bovine and sheep, the segments are cut into rings and mounted on standard force transducer systems in organ baths.54,110,111 For smaller vessels (<200 μm in diameter), wire myograph systems112 are required. In the latter, the vessel is threaded by two 40-μm stainless steel wires, with one wire attached to a force transducer capable of detecting force transients as small as 1 mN60,61,113 and the other attached to a movable jaw to stretch the vessel and set the level of basal force (i.e., preload). For murine vessels (e.g., popliteal lymphaties), wires thinner than 40 μm must be used because the inner diameter can be as small as 50 μm, leading to potential damage of the endothelium during mounting. Although thinner wires, such as 17 and 25-μm wires, are commercially available, they cannot be stretched as tightly and undergo more deflection, leading to substantial deviation from a truly isometric state, which may or may not be a critical issue for a particular experiment. Some advantages and limitations of isometric preparations, in comparison with isobaric preparations, are listed in Table 1.
Two additional points are worth mentioning. First, a selective advantage of isobaric over isometric preparations is that in the former composition of intraluminal as well as extramural solutions can be controlled and inhibitors/activators can be added inside114 or outside, as appropriate. However, in either preparation, matching the solution compositions to those found in vivo can be problematic.115 Second, both isobaric and isometric vessel preparations are well suited for high-resolution fluorescence imaging under an inverted microscope because the use of thin coverslips as chamber bottoms in conjunction with high-magnification objectives allows light collection from a narrow focal plane for improved optical sectioning and resolution.116–119
Limitations of Ex Vivo Preparations
The most common criticism of ex vivo preparations is that the removal of extrinsic regulatory mechanisms can substantially alter lymphatic vessel tone and/or contractile properties. The simple excision of the vessel inevitably results in damaged cells at the two ends, where the vessel has been both cut and subsequently tied to glass pipettes. This damage is unavoidable for vessel excision but may alter calcium dynamics and charge accumulation, and may explain the fact that otherwise undamaged isobaric lymphatic preparations almost always initiate contraction from one of the two cut ends. Additionally, once cannulated, the vessel is typically stretched lengthwise to remove slack, but unless the in vivo length was measured before excision and used as a reference point, the ex vivo length and axial tension will be approximate at best. This limitation will be further compounded for long segments where the lack of an extracellular matrix tethering the vessel may result in a gradient of axial strain (diminishing toward the center) that can directly affect the recorded diameters, amplitudes, vessel tone, and compliance measurements based on the region analyzed. Thus, significant variability from preparation to preparation could be reduced by having standardized protocols for setting axial length.
Other drawbacks of ex vivo preparations include the disruption of acute and tonic neural input and the influence of parenchymal cells, such as adipocytes and immune cells, which potentially release vasoactive products.42,120 On the other hand, the removal of such neural and parenchymal cell influences might be desirable for testing certain hypotheses. Despite excision of the collecting vessel from the tissue, ex vivo lymphatic preparations may still have significant populations of immune cells (including neutrophils, macrophages, monocytes, dendritic cells, fibroblasts, and mast cells) that reside on, nearby, or within the vessel wall and can modulate contraction121–123; thus, it may be difficult to completely eliminate the influence of such factors, both in healthy vessels and when assessing contractile function (or permeability) in disease states. In other contexts, it may be advantageous to preserve cross talk between immune cells and other cells in the lymphatic wall and/or to target local immune cells for mechanistic studies. These factors should be weighed carefully when assessing the advantages and disadvantages of using an ex vivo preparation. Of course, alternative and intermediate strategies are also possible, for example, cleaning only a small section of a collecting lymphatic vessel wall to accurately measure the diameter in that region ex vivo, while leaving intact the adventitial/adipose cells along the rest of the vessel.
The microdissection techniques used for isolating lymphatic vessels in preparation for ex vivo studies can be (but are not necessarily) highly traumatic, depending on the location of the vessel, its size, and the amount/type of parenchymal tissue, fat, or connective tissue surrounding it. Typically, there is a steep learning curve for successful dissection/cannulation, particularly for small vessels (<80 μm I.D.) such as those from the mouse. Additional precautions are warranted for wire myograph-mounted vessels, where extra care must be exercised to prevent damage to the endothelium, with some damage being inevitable along the inner surface of the vessel in contact with the wires. On the other hand, it may be advantageous to intentionally denude the vessel of its endothelium using a larger wire, a thread, or an air bolus.124 Isometric preparations are particularly suited for membrane potential118,125,126 and/or intracellular Ca2+ measurements117 because movement is minimized, allowing sharp electrode impalements and/or focus to be maintained. However, a potential confounding factor is the lack of oscillations in stretch that the smooth muscle cells normally undergo during the contraction/dilation cycle, which may in turn alter calcium and/or membrane potential dynamics. Finally, the limitations regarding dye toxicity and phototoxicity discussed above for in vivo methods also apply to ex vivo methods if fluorescence imaging is used in place of, or in conjunction with, bright-field illumination.
Conclusions
In summary, the relative advantages/disadvantages of in vivo and ex vivo preparations to study lymphatic function must be evaluated in light of the particular goals of a study; for example: Is pressure control the most important factor? Is having an intact parenchymal cell coverage required? Is accurate diameter measurement critical? Is maintaining the influence of iNOS generation from inflammatory cells? In some cases, comparing results from ex vivo vs in vivo studies can lead to conflicting conclusions.62,127,128 In other cases, there may be close agreement between the two. Examples in support of the latter statement come from comparisons between the in vivo14 and ex vivo behavior of rat mesenteric collecting lymphatics,14 where striking similarities have been found in the optimal pressure for contractile function, in the effects of elevating pressure on contractile strength and frequency,104,129 and in the maximum shortening velocity of lymphatic smooth muscle.130
Ultimately, information obtained using both in vivo and ex vivo preparations must be weighed and integrated to produce an accurate understanding of lymphatic vessel and network behavior, with the goal of translating those findings to human disease and helping patients who suffer from lymphedema and other lymphatic disorders.
Acknowledgments
The authors thank Shanyu Ho and Min Li for their excellent technical assistance. This work was supported by an NIH grant HL-120867 to M.J.D.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: Mechanics, mechanisms and malfunction. J Physiol 2016; 594:5749–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao S, Jones D, Cheng G, Padera TP. Method for the quantitative measurement of collecting lymphatic vessel contraction in mice. J Biol Methods 2014; 1:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szuba A, Shin WS, Strauss HW, Rockson SG. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 2003; 44:43–57 [PubMed] [Google Scholar]
- 4.Stewart KC, Lyster DM. Interstitial lymphoscintigraphy for lymphatic mapping in surgical practice and research. J Invest Surg 1997; 10:249–262 [DOI] [PubMed] [Google Scholar]
- 5.Szuba A, Rockson SG. Lymphedema: Classification, diagnosis and therapy. Vasc Med 1998; 3:145–156 [DOI] [PubMed] [Google Scholar]
- 6.Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. J Clin Invest 2014; 124:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullinger BD, Florey HW. Some observations on the structure and functions of lymphatics: Their behavior in local oedema. Br J Pathol 1935; 16:49–61 [Google Scholar]
- 8.Webb RL. Observations on the propulsion of lymph through the mesenteric lymphatic vessels of the living rat. Anat Rec 1933; 57:345–350 [Google Scholar]
- 9.Smith RO. Lymphatic contractility; a possible intrinsic mechanism of lymphatic vessels for the transport of lymph. J Exp Med 1949; 90:497–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intaglietta M, Hammersen F. Measurement of diameter in microvascular studies. In: Baker CH, Nastuk WL, eds. Microcirculatory Technology. New York: Academic Press; 1986:137–148 [Google Scholar]
- 11.Johnston MG, Gordon JL. Regulation of lymphatic contractility by arachidonate metabolites. Nature 1981; 293:294–297 [DOI] [PubMed] [Google Scholar]
- 12.Hargens AR, Zweifach BW. Transport between blood and peripheral lymph in intestine. Microvasc Res 1976; 11:89–101 [DOI] [PubMed] [Google Scholar]
- 13.Hargens AR, Zweifach BW. Contractile stimuli in collecting lymph vessels. Am J Physiol 1977; 233:H57–H65 [DOI] [PubMed] [Google Scholar]
- 14.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol 1989; 257:H2059–H2069 [DOI] [PubMed] [Google Scholar]
- 15.Zawieja DC, Greiner ST, Davis KL, Hinds WM, Granger HJ. Reactive oxygen metabolites inhibit spontaneous lymphatic contractions. Am J Physiol 1991; 260:H1935–H1943 [DOI] [PubMed] [Google Scholar]
- 16.van Helden DF. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol 1993; 471:465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicoll PA, Hogan RD. Pressures associated with lymphatic capillary contraction. Microvasc Res 1978; 15:257–258 [DOI] [PubMed] [Google Scholar]
- 18.Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol. 1993; 264:H1283–H1291 [DOI] [PubMed] [Google Scholar]
- 19.Dixon JB, Zawieja DC, Gashev AA, Cote GL. Measuring microlymphatic flow using fast video microscopy. J Biomed Opt 2005; 10:64016. [DOI] [PubMed] [Google Scholar]
- 20.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med 1948; 33:1349–1352 [PubMed] [Google Scholar]
- 21.Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Outflow pressure reduces lymph flow rate from various tissues. Microvasc Res 1987; 33:135–142 [DOI] [PubMed] [Google Scholar]
- 22.Berk DA, Swartz MA, Leu AJ, Jain RK. Transport in lymphatic capillaries. II. Microscopic velocity measurement with fluorescence photobleaching. Am J Physiol 1996; 270:H330–H337 [DOI] [PubMed] [Google Scholar]
- 23.Swartz MA, Berk DA, Jain RK. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am J Physiol 1996; 270:H324–H329 [DOI] [PubMed] [Google Scholar]
- 24.Hagendoorn J, Padera TP, Kashiwagi S, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ Res 2004; 95:204–209 [DOI] [PubMed] [Google Scholar]
- 25.Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci U S A 2011; 108:18784–18789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson HA, Kwon S, Hall MA, Rasmussen JC, Aldrich MB, Sevick-Muraca EM. Non-invasive optical imaging of the lymphatic vasculature of a mouse. J Vis Exp 2013. March 8; (73):e4326. [DOI] [PMC free article] [PubMed]
- 27.Kassis T, Kohan AB, Weiler MJ, et al. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt 2012; 17:086005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassis T, Yarlagadda SC, Kohan AB, Tso P, Breedveld V, Dixon JB. Postprandial lymphatic pump function after a high-fat meal: A characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol 2016; 310:G776–G789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci Rep 2016; 6:24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choe K, Jang JY, Park I, et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest 2015; 125:4042–4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Garay ML, Aldrich MB, Rasmussen JC, et al. A novel mutation in CELSR1 is associated with hereditary lymphedema. Vasc Cell 2016; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrows PE, Gonzalez-Garay ML, Rasmussen JC, et al. Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proc Natl Acad Sci U S A 2013; 110:8621–8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapinski PE, Kwon S, Lubeck BA, et al. RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. J Clin Invest 2012; 122:733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu B, Sevick-Muraca EM. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br J Radiol 2015; 88:20140547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT, Schwarz EM. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J Physiol 2014; 592:1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson TS, Akin RE, Weiler MJ, Kassis T, Kornuta JA, Dixon JB. Minimally invasive method for determining the effective lymphatic pumping pressure in rats using near-infrared imaging. Am J Physiol Regul Integr Comp Physiol 2014; 306:R281–R290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGeown JG, McHale NG, Roddie IC, Thornbury K. Peripheral lymphatic responses to outflow pressure in anaesthetized sheep. J Physiol 1987; 383:527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 1976; 261:255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen JM, McHale NG, Rooney BM. Effect of norepinephrine on contractility of isolated mesenteric lymphatics. Am J Physiol 1983; 244:H479–H486 [DOI] [PubMed] [Google Scholar]
- 40.Allen JM, McHale NG. Neuromuscular transmission in bovine mesenteric lymphatics. Microvasc Res 1986; 31:77–83 [DOI] [PubMed] [Google Scholar]
- 41.Allen JM, Iggulden HL, McHale NG. Beta-adrenergic inhibition of bovine mesenteric lymphatics. J Physiol 1986; 374:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 2002; 450:1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuno R, Dornyei G, Koller A, Kaley G. Myogenic responses of isolated lymphatics: Modulation by endothelium. Microcirculation 1997; 4:413–420 [DOI] [PubMed] [Google Scholar]
- 44.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol 2013; 591:2139–2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno R, Ono N, Ohhashi T. Parathyroid hormone-related protein-(1–34) inhibits intrinsic pump activity of isolated murine lymph vessels. Am J Physiol Heart Circ Physiol 2001; 281:H60–H66 [DOI] [PubMed] [Google Scholar]
- 46.Maejima D, Kawai Y, Ajima K, Ohhashi T. Platelet-derived growth factor (PDGF)-BB produces NO-mediated relaxation and PDGF receptor b-dependent tonic contraction in murine iliac lymph vessels. Microcirculation 2011; 18:474–486 [DOI] [PubMed] [Google Scholar]
- 47.Galanzha EI, Tuchin VV, Zharov VP. In vivo integrated flow image cytometry and lymph/blood vessels dynamic microscopy. J Biomed Opt 2005; 10:054018. [DOI] [PubMed] [Google Scholar]
- 48.Kalchenko V, Kuznetsov Y, Meglinski I, Harmelin A. Label free in vivo laser speckle imaging of blood and lymph vessels. J Biomed Opt 2012; 17:050502. [DOI] [PubMed] [Google Scholar]
- 49.Kuan EL, Ivanov S, Bridenbaugh EA, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J Immunol 2015; 194:5200–5210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 2012; 302:H643–H653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao S, von der Weid PY. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis 2014; 17:325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov S, Scallan JP, Kim KW, et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J Clin Invest 2016; 126:1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zawieja SD, Gasheva O, Zawieja DC, Muthuchamy M. Blunted flow-mediated responses and diminished nitric oxide synthase expression in lymphatic thoracic ducts of a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol 2016; 310:H385–H393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orlov RS, Borisova RP. Spontaneous and evoked contractile activity of lymphatic vessel smooth muscle. Dokl Akad Nauk SSSR 1974; 215:1013–1015 [PubMed] [Google Scholar]
- 55.Orlov RS, Lobacheva TA. Intravascular pressure and spontaneous lymph node contractions. Biull Eksp Biol Med (Russian) 1977; 83:392–394 [PubMed] [Google Scholar]
- 56.Orlov RS, Borisov AV, Borisova RP. Lymphatic vessels: Structure and mechanisms of contractile activity. Leningrad, USSR: Nauka; 1983. Lymphatic vessels; p. 253 (In Russian) [Google Scholar]
- 57.Halpern W, Mulvany MJ, Warshaw DM. Mechanical properties of smooth muscle cells in the walls of arterial resistance vessels. J Physiol 1978; 275:85–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dougherty PJ, Davis MJ, Zawieja DC, Muthuchamy M. Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics. Am J Physiol Regul Integr Comp Physiol 2008; 294:R1524–R1532 [DOI] [PubMed] [Google Scholar]
- 59.Wang W, Nepiyushchikh Z, Chakraborty S, et al. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol 2009; 297:H726–H734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol 2011; 589:5415–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dougherty PJ, Nepiyushchikh ZV, Chakraborty S, et al. PKC activation increases Ca(2)(+) sensitivity of permeabilized lymphatic muscle via myosin light chain 20 phosphorylation-dependent and -independent mechanisms. Am J Physiol Heart Circ Physiol 2014; 306:H674–H683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Telinius N, Drewsen N, Pilegaard H, et al. Human thoracic duct in vitro: Diameter-tension properties, spontaneous and evoked contractile activity. Am J Physiol Heart Circ Physiol 2010; 299:H811–H818 [DOI] [PubMed] [Google Scholar]
- 63.Telinius N, Baandrup U, Rumessen J, et al. The human thoracic duct is functionally innervated by adrenergic nerves. Am J Physiol Heart Circ Physiol 2014; 306:H206–H213 [DOI] [PubMed] [Google Scholar]
- 64.Telinius N, Mohanakumar S, Majgaard J, et al. : Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol. Nov 1 2014;592(21):4697–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Telinius N, Majgaard J, Kim S, et al. Voltage-gated sodium channels contribute to action potentials and spontaneous contractility in isolated human lymphatic vessels. J Physiol 2015; 593:3109–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halpern W, Chilian WM. Methodology. In: Mulvany MJ, Aalkjaer C, Heagerty AM, Nyborg NCB, Strandgaard S, eds. Resistance Arteries: Structure and Function. Amsterdam: Excerpta Medica; 1991:26–29 [Google Scholar]
- 67.Lew MJ. Wall stress and wall to lumen ratios differ between pressurized and myograph-mounted arteries. In: Mulvany MJ, Aalkjaer C, Heagerty AM, Nyborg NCB, Strandgaard S, eds. Resistance Arteries: Structure and Function. Amsterdam: Excerpta Medica; 1991:353–356 [Google Scholar]
- 68.Davis MJ, Kuo L, Chilian WM, Muller JM. Isolated, perfused microvessels. In: Barker JH, Anderson GL, Menger MD, eds. Clinically Applied Microcirculation Research. Boca Raton, Florida: CRC Press; 1995:435–456 [Google Scholar]
- 69.Duling BR, Rivers RJ. Isolation, cannulation, and perfusion of microvessels. In: Baker CH, Nastuk WL, eds. Microcirculatory Technology. New York: Academic Press; 1986:265–280 [Google Scholar]
- 70.Ikomi E, Zweifach BW, Schmid-Schonbein GW. Fluid pressures in the rabbit popliteal afferent lymphatics during passive tissue motion. Lymphology 1997; 30:13–23 [PubMed] [Google Scholar]
- 71.Skalak TC, Schmid-Schönbein GW, Zweifach BW. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res 1984; 28:95–112 [DOI] [PubMed] [Google Scholar]
- 72.Dongaonkar RM, Stewart RH, Laine GA, Davis MJ, Zawieja DC, Quick CM. Venomotion modulates lymphatic pumping in the bat wing. Am J Physiol Heart Circ Physiol 2009; 296:H2015–H2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogan RD, Unthank JL. The initial lymphatics as sensors of interstitial fluid volume. Microvasc Res 1986; 31:317–324 [DOI] [PubMed] [Google Scholar]
- 74.Blatter C, Meijer EF, Nam AS, et al. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci Rep 2016; 6:29035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res 2010; 106:920–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Proulx ST, Luciani P, Derzsi S, et al. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res 2010; 70:7053–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proulx ST, Luciani P, Christiansen A, et al. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials 2013; 34:5128–5137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiler M, Dixon JB. Differential transport function of lymphatic vessels in the rat tail model and the long-term effects of Indocyanine Green as assessed with near-infrared imaging. Front Physiol 2013; 4:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies-Venn CA, Angermiller B, Wilganowski N, et al. Albumin-binding domain conjugate for near-infrared fluorescence lymphatic imaging. Mol Imaging Biol 2012; 14:301–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kwon S, Sevick-Muraca EM. Mouse phenotyping with near-infrared fluorescence lymphatic imaging. Biomed Opt Express 2011; 2:1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiler M, Kassis T, Dixon JB. Sensitivity analysis of near-infrared functional lymphatic imaging. J Biomed Opt 2012; 17:066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chong C, Scholkmann F, Bachmann SB, et al. In vivo visualization and quantification of collecting lymphatic vessel contractility using near-infrared imaging. Sci Rep 2016; 6:22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwon S, Agollah GD, Wu G, Sevick-Muraca EM. Spatio-temporal changes of lymphatic contractility and drainage patterns following lymphadenectomy in mice. PLoS One 2014; 9:e106034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karlsen TV, McCormack E, Mujic M, Tenstad O, Wiig H. Minimally invasive quantification of lymph flow in mice and rats by imaging depot clearance of near-infrared albumin. Am J Physiol Heart Circ Physiol 2012; 302:H391–H401 [DOI] [PubMed] [Google Scholar]
- 85.Gashev AA, Nagai T, Bridenbaugh EA. Indocyanine green and lymphatic imaging: Current problems. Lymphat Res Biol 2010; 8:127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aldrich MB, Davies-Venn C, Angermiller B, et al. Concentration of indocyanine green does not significantly influence lymphatic function as assessed by near-infrared imaging. Lymphat Res Biol 2012; 10:20–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller FN, Sims DE, Schuschke DA, Abney DL. Differentiation of light-dye effects in the microcirculation. Microvasc Res 1992; 44:166–184 [DOI] [PubMed] [Google Scholar]
- 88.Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol 2000; 278:H604–H612 [DOI] [PubMed] [Google Scholar]
- 89.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 2000; 86:94–100 [DOI] [PubMed] [Google Scholar]
- 90.Koller A, J.M. R, Wolin MS, Messina EJ, Kaley G. Modified arteriolar responses to ATP after impairment of endothelium by light-dye techniques in vivo. Microvasc Res 1991; 41:63–72 [DOI] [PubMed] [Google Scholar]
- 91.Kilarski WW, Guc E, Teo JC, Oliver SR, Lund AW, Swartz MA. Intravital immunofluorescence for visualizing the microcirculatory and immune microenvironments in the mouse ear dermis. PLoS One 2013; 8:e57135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tammela T, Saaristo A, Holopainen T, et al. Photodynamic ablation of lymphatic vessels and intralymphatic cancer cells prevents metastasis. Sci Transl Med 2011; 3:69ra11. [DOI] [PubMed] [Google Scholar]
- 93.Gale NW, Prevo R, Espinosa J, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol Cell Biol 2007; 27:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petrova TV, Karpanen T, Norrmen C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 2004; 10:974–981 [DOI] [PubMed] [Google Scholar]
- 96.Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol 2011; 354:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kazenwadel J, Betterman KL, Chong CE, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J Clin Invest 2015; 125:2979–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE., Jr. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 2011; 301:H48–H60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brambilla D, Proulx ST, Marschalkova P, Detmar M, Leroux JC. Microneedles for the noninvasive structural and functional assessment of dermal lymphatic vessels. Small 2016; 12:1053–1061 [DOI] [PubMed] [Google Scholar]
- 100.Zweifach BW, Lipowsky HH. Pressure-flow relations in blood and lymph microcirculation. In: Renkin EM, Michel CC, eds. Handbook of Physiology Section 2: The Cardiovascular System, Vol. IV, Chp. 7, p.297. Bethesda, Maryland: American Physiological Society; 1984:251–307 [Google Scholar]
- 101.Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol 1980; 239:H775–H783 [DOI] [PubMed] [Google Scholar]
- 102.Negrini D, Gonano C, Miserocchi G. Microvascular pressure profile in intact in situ lung. J Appl Physiol 1992; 72:332–339 [DOI] [PubMed] [Google Scholar]
- 103.Clough G, Smaje LH. Simultaneous measurement of pressure in the interstitium and the terminal lymphatics of the cat mesentery. J Physiol 1978; 283:457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the lymphatic pump. Am J Physiol 2012; 303:H809–H824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol 1990; 259:H1063–H1070 [DOI] [PubMed] [Google Scholar]
- 106.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: A self-regulatory mechanism in rat thoracic duct. J Physiol 2006;575:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 2011; 18:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kornuta JA, Dixon JB. Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann Biomed Eng 2014; 42:1691–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kornuta JA, Nipper ME, Dixon JB. Low-cost microcontroller platform for studying lymphatic biomechanics in vitro. J Biomech 2013; 46:183–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lobov GI, Orlov RS, Kostikova MA. Active and passive mechanical properties of the wall of the lymphangion. Fiziol Zh SSSR Im I M Sechenova 1989; 75:218–226 [PubMed] [Google Scholar]
- 111.Allen JM, McCarron JG, McHale NG, Thornbury KD. Release of [3H]-noradrenaline from the sympathetic nerves to bovine mesenteric lymphatic vessels and its modification by alpha-agonists and antagonists. Br J Pharmacol 1988; 94:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mulvany MJ. Procedures for Investigation of Small Vessels Using a Small Vessel Myograph. Aarhus, Denmark: Danish Myo Technology; 2003 [Google Scholar]
- 113.Zhang R-Z, Gashev AA, Zawieja DC, Lane MM, Davis MJ. Length-dependence of lymphatic phasic contractile activity under isometric and isobaric conditions. Microcirculation 2007; 14:613–625 [DOI] [PubMed] [Google Scholar]
- 114.Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol 2013; 591:443–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stechman MJ, Ahmad BN, Loh NY, et al. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab Anim 2010; 44:218–225 [DOI] [PubMed] [Google Scholar]
- 116.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp 2011. December 8;(58). pii: [DOI] [PMC free article] [PubMed]
- 117.Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol 2003; 285:H2573–H2577 [DOI] [PubMed] [Google Scholar]
- 118.Imtiaz MS, Zhao J, Hosaka K, von der Weid PY, Crowe M, van Helden DF. Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization. Biophys J 2007; 92:3843–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Imtiaz MS, von der Weid PY, van Helden DF. Synchronization of Ca2+ oscillations: A coupled oscillator-based mechanism in smooth muscle. Febs J 2010; 277:278–285 [DOI] [PubMed] [Google Scholar]
- 120.Nizamutdinova IT, Maejima D, Nagai T, et al. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 2014; 21:640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chakraborty S, Zawieja SD, Wang W, et al. Lipopolysaccharide modulates neutrophil recruitment and macrophage polarization on lymphatic vessels and impairs lymphatic function in rat mesentery. Am J Physiol Heart Circ Physiol 2015; 309:H2042–H2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 2012; 303:H693–H702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chatterjee V, Gashev AA. Mast cell-directed recruitment of MHC class II positive cells and eosinophils towards mesenteric lymphatic vessels in adulthood and elderly. Lymphat Res Biol 2014; 12:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chan AK, von der Weid PY. 5-HT decreases contractile and electrical activities in lymphatic vessels of the guinea-pig mesentery: Role of 5-HT 7-receptors. Br J Pharmacol 2003; 139:243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol 2013; 304:G623–G634 [DOI] [PubMed] [Google Scholar]
- 126.von der Weid PY, Lee SY, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol 2014; 12:66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunert C, Baish JW, Liao S, Padera TP, Munn LL. Reply to Davis: Nitric oxide regulates lymphatic contractions. Proc Natl Acad Sci U S A 2016; 113:E106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davis MJ. Is nitric oxide important for the diastolic phase of the lymphatic contraction/relaxation cycle? Proc Natl Acad Sci U S A 2016; 113:E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol 2012; 303:H795–H808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang R, Taucer AI, Gashev AA, Muthuchamy M, Zawieja DC, Davis MJ. Maximum shortening velocity of lymphatic muscle approaches that of striated muscle. Am J Physiol Heart Circ Physiol 2013; 305:H1494–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]