Abstract
IN BRIEF Type 2 diabetes has been labeled an epidemic in many American Indian communities. Thus, identifying factors that improve medication adherence for American Indian patients is crucial. We found significant and positive relationships among patient-centered care, medication adherence, and diabetes empowerment. In addition, diabetes empowerment partially mediated the relationship between patient-centered care and medication adherence.
Poor medication adherence is a primary cause of worse glycemic control outcomes in type 2 diabetes (1). Alternatively, consistent self-care activities can reduce longterm diabetes complications (2). A growing body of literature shows that patient-provider interactions have an influence on patient medication use behaviors (3,4). For example, patient dissatisfaction with physician communication is associated with worse medication adherence (5). Racial and ethnic minority patients are at greater risk than white patients for strained relationships and poorer communication with providers that can result in delayed or less-than-optimal care (6). An important question, then, is how health care providers’ approaches to relationships with patients might influence patients’ health behaviors outside the clinic, particularly for those from historically marginalized communities.
Patient-centered care (PCC) is a model of clinical practice aimed at shifting patient-provider relationships from authoritarianism to shared decision-making (7). PCC is described as “providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions” (8). Patient empowerment has emerged as a key element in the care of patients with chronic diseases and has been integrated into models of diabetes patient education, whereby patients have the “knowledge, skills, attitudes, and self-awareness necessary” to effectively self-manage their condition (9). The underlying tenet of PCC and empowerment approaches is that patients who see their own values, priorities, and constraints reflected in a treatment plan will be more motivated and able to follow that plan (7,9). Our research tests a conceptual model in which patient empowerment acts as a mediating factor in the relationship between PCC and medication adherence among patients with diabetes.
Exploring the impact of PCC on medication adherence is important because adherence is a serious and vexing problem in diabetes care. Diabetes medication adherence rates have been estimated to be between 36 and 85%, depending on the study population (10). Poor adherence to medication regimens is a root cause of substantial worsening of disease, death, and increased health care costs in the United States, whereas better adherence is correlated with better clinical outcomes (4). Interventions to improve adherence show mixed results; those that are successful are often complex and costly (11). Better understanding potential improvements in medication adherence that can be gained through physician interactions with patients, a comparatively cost-effective method, is a worthwhile endeavor.
Type 2 diabetes has been labeled an epidemic in many American Indian (AI) communities, with national AI prevalence rates more than double those observed in the general U.S. population (12). Diabetes is a major cause of mortality and a significant contributor to cardiovascular disease, the number one cause of death for AIs and Alaskan Natives (ANs) (13). Low medication adherence contributes to the prevalence of poor diabetes outcomes for AI/AN communities. A 2014 study of >300,000 diabetes patients revealed that AI/AN patients were significantly more likely to have poor glycemic control as indicated by high A1C levels and were significantly less likely to adhere to their oral diabetes medications compared to non-Hispanic whites (14). These disparities have complex causes rooted in the effects and process of colonization that contribute to current social determinants of health found in many AI communities in the United States (15).
Previous research on PCC and self-care behaviors for diabetes have largely controlled for patients’ race in their analyses rather than examining these factors within racial/ethnic groups. The benefits of PCC for racial and ethnic minority patients have been more closely examined in the realm of cancer treatment, where multiple studies have found a positive correlation with adherence, survival, and quality of life (6). To date, this study is the first of which we are aware to investigate the relationships between PCC, empowerment, and medication adherence in the treatment of AI patients with type 2 diabetes.
Methods
The Maawaji’ idi-oog Mino-ayaawin (Gathering for Health) study is a community-based participatory research collaboration between the University of Minnesota (UMN) and five Anishinabe (Ojibwe) communities in the upper Midwest region of the United States. The project is supported by resolutions from each tribal government. Community Research Councils composed of tribal members and health care providers at each site are active research partners. All study procedures were reviewed and approved by the UMN and national Indian Health Service institutional review boards.
Staff at the partnering tribal clinics generated simple random probability samples from medical records to form a recruitment list for the study. Patients who were ≥18 years of age, had a diagnosis of type 2 diabetes, lived on or near one of the five partnering reservations, and self-identified as AI were eligible for inclusion. Selected patients were mailed a study invitation letter with mail and call-in refusal options. A total of 194 participants completed a baseline computer-assisted personal interview (67% response rate) between 2013 and 2015. Participants received $50 for survey participation and a traditional gift of wild rice. Data for this report include responses from the 166 participants in the study sample who were taking medication(s) as part of their diabetes treatment regimen.
Statistical Analysis
We used SPSS version 23 (IBM Corp., Armonk, N.Y.) to estimate descriptive statistics and bivariate associations among study variables. For multivariate analyses, we ran a series of ordinary least squares regressions in SPSS.
Measures
All survey measures were piloted for feedback with AI adults and further adapted by Community Research Council members.
PCC was measured with an adapted version of the Patient Assessment of Chronic Illness Care Short Form (16), including eight questions assessing a variety of PCC activities (Table 1). For these questions, participants were asked to think specifically about their primary care provider (PCP) who is responsible for diabetes care. Response options for the continuous measure used in multivariate analyses ranged from 1 = none of the time to 4 = all of the time, and we used an overall mean score, where higher values indicate greater endorsement of PCC items.
TABLE 1.
Participant Endorsement (%) of Diabetes Care Provider PCC Activities in the Past 6 Months
| None of the Time | Some of the Time | Most of the Time | All of the Time | |
|---|---|---|---|---|
| Asked for your ideas to make treatment plan? | 60.7 | 28.3 | 5.2 | 5.8 |
| Asked to talk about problems with medication? | 48.7 | 29.3 | 11.5 | 10.5 |
| Asked how visits with other providers were going? | 62.8 | 23.0 | 8.9 | 5.2 |
| Asked questions about your health habits? | 45.0 | 36.1 | 11.5 | 7.3 |
| Given a copy of your treatment plan? | 51.3 | 18.8 | 12.6 | 17.3 |
| Contacted after a visit to see how things were going? | 60.2 | 23.0 | 9.4 | 7.3 |
| Referred to a dietitian, health educator, or counselor? | 41.4 | 36.6 | 11.0 | 11.0 |
| Satisfied that your care was well organized? | 21.6 | 27.4 | 34.7 | 16.3 |
We measured medication adherence with the Morisky Medication Adherence Scale (MMAS) (17). The MMAS includes four items with yes-or-no response options summed so that higher scores indicate higher levels of adherence.
A sum score was created for diabetes empowerment assessed with the Diabetes Empowerment Scale-Short Form (18).
We also included several demographic variables in the analyses: self-reported sex (0 = male, 1 = female), age, reservation residential status (off = 0, on = 1), and educational status.
Results
Table 1 displays participant endorsement of various PCC activities. A majority of participants said they had been referred to other providers and discussed medication and health habits with their PCP at some, most, or all visits. More than half of respondents said they were never asked for ideas to make a treatment plan, given a copy of the treatment plan, asked about visits with other providers, or contacted after a visit.
Descriptive results and bivariate relationships among study variables are displayed in Table 2. PCC and diabetes empowerment were each positively and significantly (P <0.05) related to reports of medication adherence. Furthermore, men and older participants reported higher levels of adherence than women or younger participants.
TABLE 2.
Descriptive Statistics and Bivariate Correlations for All Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|
| 1. | Sex (female = 1) | 1.00 | ||||||
| 2. | Reservation residential status (on = 1) | 0.02 | 1.00 | |||||
| 3. | Educational attainment | 0.13 | –0.12 | 1.00 | ||||
| 4. | Age (years) | 0.03 | –0.13 | 0.11 | 1.00 | |||
| 5. | PCC | –0.06 | 0.10 | 0.00 | –0.01 | 1.00 | ||
| 6. | Medication adherence | –0.16* | 0.02 | 0.15 | 0.28** | 0.18* | 1.00 | |
| 7. | Diabetes empowerment | 0.01 | 0.05 | 0.12 | 0.02 | 0.16* | 0.20** | 1.00 |
| Percentage or mean (SD) | 55.73 | 78.65 | 1.55 (0.91) | 46.32 (12.2) | 0.84 (0.67) | 2.53 (1.26) | 16.03 (2.90) | |
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two-tailed).
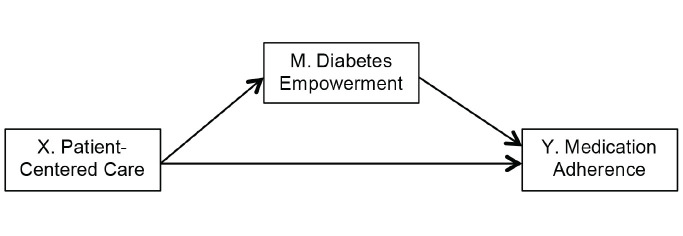
Figure 1 displays a conceptual model guiding our multivariate ordinary least squares regression analyses. We followed suggested steps for testing mediation among a set of three variables, X, Y, and M (19), with a series of regression models assuming X = PCC (independent variable), Y = medication adherence (outcome), and M = diabetes empowerment (the proposed mediator).
FIGURE 1.
Conceptual model.
First, we examined associations between X and Y after adjusting for control variables (Table 3, Step 1) and found that PCC was significantly and positively associated with medication adherence. Second, we determined whether X was associated with the mediator M (Table 3, Step 2). Results show that PCC was significantly associated with diabetes empowerment after accounting for the effects of control variables. Third, we investigated a model (Table 3, Step 3) in which both X and M are regressed on the outcome Y. After including diabetes empowerment in the model, the standardized coefficient for the relationship between PCC and medication adherence was reduced slightly (Step 1 compared to Step 3) from β = 0.16 to β = 0.12 and dropped from statistical significance. Those reporting higher levels of diabetes empowerment were also more likely to report better medication adherence, even after accounting for all control variables and PCC. Taken together, these analyses suggest that the effects of PCC on medication adherence are partially mediated by increases in diabetes empowerment associated with PCC.
TABLE 3.
Results of Ordinary Least Squares Regression Analyses
| Model A (Step 1) | Model B (Step 2) | Model C (Step 3) | ||||
|---|---|---|---|---|---|---|
| Outcome Variable: | Medication Adherence | Diabetes Empowerment | Medication Adherence | |||
| b (SE) | β | b (SE) | β | b (SE) | β | |
| Reservation residential status (on = 1) | 0.20 (0.23) | 0.06 | 0.40 (0.52) | 0.06 | 0.09 (0.23) | 0.03 |
| Sex (female = 1) | –0.47 (0.19) | –0.19* | 0.01 (0.43) | 0.002 | –0.48 (0.18) | –0.19* |
| Age | 0.03 (0.01) | 0.27*** | 0.004 (0.02) | 0.02 | 0.03 (0.01) | 0.25** |
| Educational attainment | 0.21 (0.10) | 0.15* | 0.40 (0.23) | 0.13 | 0.19 (0.10) | 0.14 |
| PCC | 0.30 (0.13) | 0.16* | 0.68 (0.32) | 0.16* | 0.22 (0.14) | 0.12 |
| Diabetes empowerment | 0.06 (0.03) | 0.15* | ||||
Bold type indicates statistical significance.
P <0.05;
P <0.01;
P <0.001.
Discussion
Our findings include the first reports of which we are aware of AI patient endorsement of PCC activities. Diabetes-related PCC during at least some clinical visits was reported by <60% of patients for seven of eight items (Table 1) in the domains of patient activation, goal-setting, and follow-up (16). A minority of study participants said that PCC activities happened all of the time. Nearly 40% of participants reported that their PCP asked them for ideas when making a treatment plan, which is comparatively higher than the 29.2% endorsement made by diabetes patients in a separate, non-AI–specific study sample (20). Still, given the prevalence of type 2 diabetes among AI patient populations, as well as the documented value of PCC in decreasing complications, these data highlight opportunities for improvement in the delivery of diabetes care through the adoption of PCC strategies.
Our results show that PCC and patient empowerment were each significantly and positively correlated with diabetes medication adherence. In addition, PCC was correlated with patient empowerment. Multivariate regression analyses suggest that patient empowerment partially mediates effects between PCC and medication adherence. This work supports previous research reporting positive correlations between multiple aspects of PCC and medication adherence (2–4) and provides new evidence that patient empowerment factors may mediate this relationship.
Attention to medication adherence is generally focused on factors that are assumed to be under individual patient control (11). Framing medication adherence in this way prevents deep consideration of the broader contexts that directly affect behavior. For many AI patients, exposure to high rates of physical and mental health comorbidities, poverty, and violence (15) are systemic, historically anchored, and intergenerational. This helps to explain the disparity in diabetes medication adherence between Caucasian and AI/AN patients documented in earlier work. Our findings suggest that PCC and patient empowerment may be methods of engaging with AI diabetes patients in a clinical context to understand and overcome these challenges to promote better medication adherence.
These findings are observational in nature, and our cross-sectional study design limits strong conclusions about the causal ordering of study variables. We operationalized PCC as a summation of endorsements of a range of patient-centered constructs; future research is needed to elucidate the specific mechanisms of PCC that may be more or less impactful on health outcomes and patient behavior. Our findings, in concert with results from previous studies, bolster the evidence that physicians’ efforts to incorporate individual patients’ cultural values, needs, and preferences strengthen the patient-provider relationship and could have a positive influence on medication adherence behavior (4–7). Future work should investigate models of diabetes care that incorporate AI cultural values, including those that involve stakeholders outside of the patient-physician dyad.
Many AI communities face a type 2 diabetes crisis. Practical strategies are needed to help improve the management of this disease. This study demonstrates that providers can use PCC to promote empowerment among AI diabetes patients and potentially improve medication adherence rates. As Dr. William Osler once said, “The good physician treats the disease; the great physician treats the patient who has the disease.”
Acknowledgments
The authors gratefully acknowledge the commitment and contributions to this project provided by Community Research Council members, interviewers, and clinic staff: Alexis Manson, Charity Prentice-Pemberton, Romona Nelson, Eileen Miller, Geraldine Brun, Murphy Thomas, Doris Isham, Stan Day, Jane Villebrun, Beverly Steel, Muriel Deegan, Peggy Connor, Michael Connor, Ray Villebrun, Pam Huges, Cindy McDougall, Melanie McMichael, Robert Thompson, Sandra Kier, Sidney Kellar, Lisa Perry, Robert Miller, Tweed Shuman, Lorraine Smith, Rose Barber, Mary Sikora-Petersen, Tina Handeland, Philip Chapman, GayeAnn Allen, Frances Whitefield, Tracy Martin, Geraldine Whiteman, Hope Williams, Daniel Chapman, and Betty Graveen.
Funding
Research reported here was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (DK091250), to M.L.W., principal investigator. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
N.L.R., E.B.D., and L.L.L. shared responsibility for writing the literature review and discussion. S.M.W. assisted with data analysis and prepared tables. M.L.W. led data collection and analysis. All authors edited the manuscript. M.L.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Grant R, Adams AS, Trinacty CM, et al. . Relationship between patient medication adherence and subsequent clinical inertia in type 2 diabetes glycemic management. Diabetes Care 2007;30:807–812 [DOI] [PubMed] [Google Scholar]
- 2.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord 2013;12:12–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zolnierek KBH, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care 2009;47:826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med 2010;8:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linetzky B, Jiang D, Funnell MM, Curtis BH, Polonsky WH. Exploring the role of the patient-physician relationship on insulin adherence and clinical outcomes in type 2 diabetes: insights from the MOSAIc study. J Diabetes 2017;9:596–605 [DOI] [PubMed] [Google Scholar]
- 6.Mead EL, Doorenbos AZ, Javid SH, et al. . Shared decision-making for cancer care among racial and ethnic minorities: a systematic review. Am J Public Health 2013;103:e15–e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barry MJ, Edgman-Levitan S. Shared decision making: the pinnacle of patient-centered care. N Engl J Med 2012;366:780–781 [DOI] [PubMed] [Google Scholar]
- 8.Baker A. Crossing the quality chasm: a new health system for the 21st century. BMJ 2001;323:1192. [PubMed] [Google Scholar]
- 9.Funnell MM, Anderson RM, Arnold MS, et al. . Empowerment: an idea whose time has come in diabetes education. Diabetes Educ 1991;17:37–41 [DOI] [PubMed] [Google Scholar]
- 10.Rubin R. Adherence to pharmacologic therapy patients with type 2 diabetes mellitus. Am J Med 2005;118(Suppl. 5A):27S–34S [DOI] [PubMed] [Google Scholar]
- 11.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Summary health statistics: national health interview survey, 2014, Table A-4 [Internet]. Available from https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2014_SHS_Table_A-4.pdf. Accessed 13 September 2017
- 13.U.S. Department of Health and Human Services , Indian Health Service, Office of Public Health Support, Division of Program Statistics. Trends in Indian Health 2014 Edition [Internet]. Available from https://www.ihs.gov/dps/includes/themes/newihstheme/display_objects/documents/Trends2014Book508.pdf. Accessed 13 September 2017 [Google Scholar]
- 14.Schmittdiel JA, Steiner JF, Adams AS, et al. . Diabetes care and outcomes for American Indians and Alaska Natives in commercial integrated delivery systems: a SUrveillance, PREvention, and ManagEment of diabetes mellitus (SUPREME-DM) study. BMJ Open Diabetes Res Care 2014;2:e000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarche M, Spicer P. Poverty and health disparities for American Indian and Alaska Native children. Ann N Y Acad Sci 2008;1136:126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005;43:436–444 [DOI] [PubMed] [Google Scholar]
- 17.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74 [DOI] [PubMed] [Google Scholar]
- 18.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF). Diabetes Care 2003;26:1641–1642 [DOI] [PubMed] [Google Scholar]
- 19.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182 [DOI] [PubMed] [Google Scholar]
- 20.Willaing I, Peyrot M. Active involvement of people with diabetes and support for self-management. Poster presented at the International Diabetes Federation 2013 World Diabetes Congress, Melbourne, Australia, 5 December 2013 [Google Scholar]