Abstract
IN BRIEF Painful diabetic peripheral neuropathy (PDPN) has a large negative impact on patients’ physical and mental functioning, and pharmacological therapies rarely provide more than partial relief. Mindfulness-based stress reduction (MBSR) is a group psychosocial intervention that was developed for patients with chronic illness who were not responding to existing medical treatments. This study tested the effects of community-based MBSR courses for patients with PDPN. Among patients whose PDPN pharmacotherapy had been optimized in a chronic pain clinic, those randomly assigned to treatment with MBSR experienced improved function, better health-related quality of life, and reduced pain intensity, pain catastrophizing, and depression compared to those receiving usual care.
The prevalence of diabetes in North America is estimated to be 12.9% (1), and, of these patients, 50% develop diabetic peripheral neuropathy (DPN) (2). Up to 25% of patients with DPN develop neuropathic pain (3), defined as “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system” (4). Common descriptors include burning pain, “electrical shock” or shooting pain down the legs, and pain on contact with socks or bedclothes at night (allodynia). The pain is characteristically more severe at night and often disturbs sleep. Painful diabetic peripheral neuropathy (PDPN) can have a major impact on physical and mental functioning, thereby compromising the ability to work, attend to household responsibilities, and enjoy social relationships.
Findings from trials of PDPN treatments inform the management of other neuropathic pain conditions. Current evidence indicates that pharmacotherapy for neuropathic pain provides only partial pain relief, is not well tolerated (and therefore cannot be used) by many patients, and is associated with effects that can adversely affect patient safety and quality of life (QoL) (5,6). This is especially true with opiates, which are no longer recommended. Both the National Institute for Health and Care Excellence (7) and the American Diabetes Association’s Standards of Medical Care in Diabetes—2017 (8) endorse the essential role of psychosocial therapies in restoring physical and emotional functioning in patients with diabetes.
Mindfulness-based stress reduction (MBSR) is a group psychosocial intervention that was first developed by Kabat-Zinn (9) for patients with chronic illness who were not responding to existing medical treatments. Through mindfulness exercises, participants develop the ability to take the position of a witness to their experiences (meta-cognition), allowing a more objective assessment of stressors (such as pain) and improved self-regulation. This facilitates the choice of more adaptive responses rather than catastrophic ruminations or automatic reactions. The threat and sense of harm associated with pain, and even its intensity, can be diminished by developing a more open and accepting attitude toward this challenging experience.
The possibility of using mental training to change how pain is processed in the central nervous system and thereby diminish the pain experience is supported by recent research in neuroscience (10). Although MBSR has shown promise for a variety of painful conditions, there are few methodologically robust studies, and we have found no studies of cohorts with neuropathic pain.
The primary objective of this randomized, controlled trial was to evaluate the effectiveness of community-based MBSR courses to improve physical and mental functioning among patients with PDPN whose medical treatment has been optimized. Secondary objectives were to evaluate the effect of the intervention on pain severity, mood, and health-related QoL, as well as on diabetes self-care activities and glycemic control.
Research Design and Methods
Participants
With approval from the Ottawa Health Sciences Network ethics board and after obtaining informed written consent, we enrolled patients in this wait list–controlled, randomized trial between 5 July 2013 and 4 September 2015. The trial was registered with ClincalTrials.gov (NCT 02127762).
Most patients were recruited by telephone from a database of patients attending The Ottawa Hospital’s Endocrine and Diabetes Centre who had consented to be contacted regarding research participation. Others were referred from the community. Men and women who were ≥18 years of age, had type 1 or type 2 diabetes and symptoms of PDPN for >6 months, and could speak English or French and understand and complete our questionnaires were eligible to participate. Patients responding “yes” to ≥3 of the 7 subjective items on the Douleur Neuropathique 4 (DN4) (11) neuropathic pain scale were then asked to rate their pain on two visual analog scales rating from 0 to 10 their pain at rest and with activity) at the same time of day for 7 consecutive days. Patients were included if their mean score for either scale was ≥4. Patients were excluded if they had previously taken an MBSR or similar course.
Methods
At the first visit, patients were examined by a pain specialist who completed the DN4 and the clinical examination portion of the Michigan Neuropathy Screening Instrument (12) to confirm and document the severity of their neuropathy. All patients were given an explanation of the neurobiology of chronic pain and of how MBSR might improve their mental and physical functioning. Pharmacological treatment, usually pregabalin and duloxetine or a tricyclic antidepressant, was offered (5). Up to 5 months were allowed for optimization and stabilization of medication.
Randomization and Blinding
Patients were randomly allocated to a waiting list or MBSR using computer-generated random numbers in a permuted block design with randomly varying block lengths. Allocations were stratified by type 1 or type 2 diabetes and by pain severity as indicated by an average of the four pain-severity numeric rating scales of the Brief Pain Inventory (BPI; questions 3–6) (13) rated daily for the 7 days immediately before randomization. Ratings of 4–6 were classified as moderate pain and ratings of 7–10 as severe pain. Baseline pain intensity is known to be an important prognostic variable (14). Allocations were generated by an independent statistician and concealed from investigators and treating physicians. Treatment allocation occurred as close as possible to the start of the MBSR course when the next consecutively numbered opaque envelope for the appropriate strata was opened. After randomization, participants had had sufficient experience with the instruments that they were able to complete them without any contact from study staff who were aware of the treatment allocation.
Intervention
Patients were enrolled in MBSR courses offered at multiple sites in the community by practitioners who had formal training in MBSR and ≥5 years of experience as workshop leaders. The methods and materials described in the teacher training course given by the Center for Mindfulness at the University of Massachusetts, where this method was developed, were used (15). The workshops consisted on nine sessions: eight weekly, 2.5-hour sessions and one 6-hour session on a weekend day midway through the course. Typically, 2–3 study patients would join a group of 12–20 MBSR participants with a variety of complaints such as pain, anxiety, or depression. There was no modification of the MBSR course for the purposes of this study. Patients in both the control and MBSR groups were discouraged from making any changes in medication from the time of randomization until after the final assessment. Patients in the control group were offered enrollment in an MBSR course after the study was complete.
Measures
Socio-demographic measures were collected at baseline. All outcomes were measured at baseline (visit 1, denoted as V1) when pharmacological treatment was offered, at the time of randomization (visit 2, denoted as V2), and at 2 weeks (visit 3, denoted as V3) and 3 months (visit 4, denoted as V4) after completion of the MBSR course. Control subjects had the same measures at the same intervals. A1C was measured at V1, V2, and V4.
Primary Outcome
Our primary outcome was a comparison of the prevalence in each group of response to the intervention, defined as a decrease of ≥1.0 on the mean of the seven interference scale items of the BPI (completed daily for 7 days) from the time of randomization (V2) to the 3-month follow-up (V4). The BPI is a measure of pain-related disability that is both recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) guidelines (16,17) and has been found by Jensen et al. (18) to be more sensitive to neuropathic pain than generic measures of health-related QoL. Zelman et al. (13) has validated the BPI for PDPN. The IMMPACT guidelines recommend a one-point change on the interference scale as a reasonable minimally clinically important change (16). The primary hypothesis was that participants in the study’s MBSR arm would have a 30% absolute greater prevalence of response than those in the control arm 3 months after completion of MBSR.
Secondary Outcomes
Secondary outcomes included:
IMMPACT-recommended measures (16,17), including the BPI for pain severity, the Patient Health Questionnaire-9 (PHQ-9) (19) for depression, the Patient Global Impression of Change (PGIC) (20) for QoL, the Profile of Mood States-2A (POMS-2A) for total mood disturbance (reported here), and the Perceived Stress Scale (PSS) (21), as well as adverse events records.
The Pain Catastrophizing Scale (PCS) (22), a well-validated 13-item instrument designed to evaluate the degree to which patients have negative self-statements and catastrophizing thoughts and ideations when in pain.
Short Form-12 Health Survey ver-sion 2 (SF-12) (23), a brief, 12-item generic measure of QoL. The SF-12 has been used in studies of back pain (24) and neuropathic pain (25). It includes eight subdomains: bodily pain, physical functioning, role physical, general health, vitality, social functioning, role emotional, and mental health. Two composite scores are calculated: Physical and Mental Health Composite Scales.
Neuropathy-Specific Quality of Life Questionnaire (NeuroQoL) (26), which was designed for and validated in patients with DPN and captures the key dimensions of patients’ experience. Factor analysis revealed three physical symptom measures and two psychosocial functioning measures with good reliability (α = 0.86–0.95). This instrument was more strongly associated with severity of DPN than the SF-12 and more fully mediated the relationship between DPN and overall QoL.
In addition, to address our interest in the possible benefits of MBSR on glycemic control, we included the following:
Summary of Diabetes Self-Care Activities (27), an 11-item self-report measure used to assess the diet, exercise, smoking, self-monitoring of blood glucose, and foot care habits of patients with diabetes. This scale has been found to be valid with moderate test-retest reliability.
Blood Sugar Reactions Question-naire. To assess adverse reactions patients may have experienced as a result of their glycemic control, we have used five questions from the Diabetes Care Profile of the Michigan Diabetes Research and Training Center (Section 6, questions 1–3, 5, and 6) (28).
A1C, which reflects blood glucose levels during the previous 12 weeks.
Sample Size
The sample size was determined to achieve 80% power to detect a minimally important absolute difference of 30% in the percentage of responders 3 months after intervention, assuming a control-arm percentage of 20%, an average of two study participants per MBSR group, an intracluster correlation coefficient (ICC) of 0.05, and a two-sided test at the 5% level of significance (29). The ICC accounts for the lack of statistical independence among responses from participants in the same therapy group; failing to account for the ICC risks underestimating the required sample size. We had no prior information regarding the ICC and therefore assumed a value of 0.05, which is commonly assumed in cluster-randomized trials (30). We chose a minimum detectable difference of 30% because that is the smallest difference that would encourage us to recommend this intervention. Based on these assumptions, we required a total of 80 patients. However, the study was stopped after 66 patients had been randomized due to exhaustion of funds.
Statistical Analysis
The primary outcome at 3 months (proportion of responders on the BPI) was analyzed using mixed-effects logistic regression accounting for the partially nested design and using random effects for therapy group. Degrees of freedom were calculated using the Kenward-Roger method. The analysis accounted for the stratification variables as fixed effects; it also adjusted for continuous BPI score at the time of randomization. The results of the binary outcomes were expressed as odds ratios (ORs) with 95% CIs rather than as an absolute risk difference because the binomial identity model did not converge, and we were therefore unable to calculate CIs around the absolute risk difference that appropriately accounted for clustering by therapy group. The ICC for the primary outcome was estimated using variance components from a one-way analysis of variance together with a 95% exact CI (31). The patient global impression of change was analyzed as a binary variable. Patients who reported “much improvement” or “very much improvement” at the 12-week follow-up were considered responders (20).
Continuous secondary outcomes measured at the time of randomization and 2 weeks and 3 months after the intervention were analyzed using mixed-effects linear regression, accounting for the partially nested design and allowing for a heterogeneous variance structure (32). To take into account the partially nested design, a term accounting for variability between treatment clusters was added to the variance of the effect of treatment. This increased the standard error of the mean in the intervention arm, providing valid inferences about the effect of treatment. Had the clustering in the treatment arm not been taken into account, the resulting CIs would have been too narrow and P values would have been too small. Degrees of freedom were calculated using the Kenward-Roger method. The analysis accounted for the correlation in repeated measures on the same subject over time using a compound symmetric covariance structure.
Differences between the study arms over time were assessed by including visit (analyzed as a categorical variable), treatment arm, and the interaction between visit and treatment arm in the models. Analyses accounted for the stratification variables as fixed effects. To improve precision of the treatment effect estimator, our analysis also adjusted for prespecified baseline covariates anticipated to be strongly correlated with the response, namely the baseline measure of each outcome, education status, BPI interference score, and age in years. Least squares means were obtained from the model and used to calculate the mean change from the time of randomization to 2 weeks and 3 months after intervention in each group with 95% CIs. The effect of the intervention was expressed as between-arm least squares mean differences in change from baseline to 3 months with 95% CIs. All tests were evaluated at the two-sided 5% level of significance. SAS version 9.3 (SAS, Cary, N.C.) was used for all analyses.
Results
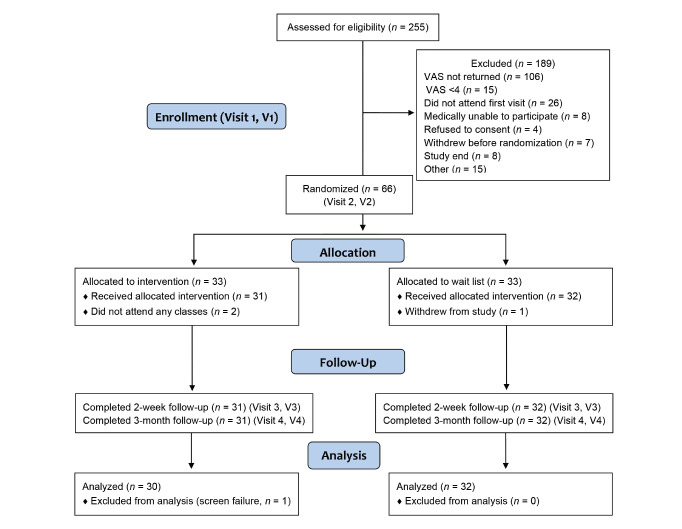
Two hundred and fifty-five subjects were screened for eligibility, of whom 66 were randomized. One hundred and eighty-nine were excluded for reasons shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 1). Of the 33 subjects allocated to MBSR, 31 received the intervention and completed follow-up, and 30 were included in the analysis. One was excluded from the analysis when it was discovered that the eligibility requirement had not been met (screening visual analog scale <4). Of the 33 patients allocated to the control group, one withdrew from the study, and 32 completed follow-up and analysis. Eighteen of the 30 subjects (60%) allocated to MBSR attended at least six of the nine sessions. There were 10 community groups, each attended by two to three study participants. No clinically important differences in patient characteristics at baseline were identified between the study arms (Table 1).
FIGURE 1.
CONSORT diagram of the study. VAS, visual analog scale.
TABLE 1.
Baseline Characteristics
| All (n = 62) | Control Group (n = 32) | MBSR Group (n = 30) | |
|---|---|---|---|
| Age (years; mean [SD]) | 59.7 (8.8) | 59.8 (8.7) | 59.7 (9.1) |
| Female (n [%]) | 35 (56) | 20 (62.5) | 15 (50) |
| Type 2 diabetes (n [%]) | 48 (77) | 24 (75) | 24 (80) |
| Severe pain (n [%]) | 17 (27) | 8 (25) | 9 (30) |
| Pain severity (mean [SD]) | 5.1 (1.8) | 4.9 (2.0) | 5.3 (1.6) |
| Pain interference (mean [SD]) | 4.8 (2.0) | 4.8 (2.2) | 4.9 (1.8) |
| Pain duration (years; mean [SD]) | 7.4 (6.0) | 8.0 (6.7) | 6.7 (5.2) |
| Post-secondary education (n [%]) | 46 (74) | 23 (71.9) | 23 (76.7) |
| Work status (n [%]) | |||
| Employed | 11(17.7) | 4 (12.5) | 7 (23.3) |
| Retired | 33 (53.2) | 18 (56.3) | 15 (50) |
| Disability | 15 (24.2) | 8 (25) | 7 (23.3) |
| Other | 3 (4.8) | 2 (6.3) | 1 (3.3) |
| (n = 59) | (n = 31) | (n = 28) | |
| A1C (% [SD]) | 8.28 (1.37) | 8.20 (1.37) | 8.38 (1.40) |
| A1C (mmol/mol [SD]) | 67 (15) | 66 (15) | 68 (15.3) |
Primary Outcome
In the MBSR group, 19 of 30 patients (63.3%) experienced a decrease in the mean BPI interference score of ≥1.0 from the time of randomization (V2) to 12 weeks after completion of the MBSR course (V4) compared to 7 of 32 control patients (21.9%) during the same interval (adjusted OR 9.9, 95% CI 1.5–63.8, P = 0.02). The absolute difference of 41.4% (number needed to treat [NNT] 2.4) exceeded our minimally important clinical difference of 30%.
These findings are supported by the difference between groups in the PGIC score, with 14 of 30 in the MBSR group (46.7%) compared to 2 of 32 in the control group (6.2%) reporting that they were much or very much improved at the 12-week follow-up. The absolute difference was 40.5%, giving an NNT of 2.5 (adjusted OR 18.8, 95% CI 2.3–151.5, P = 0.007).
The estimated ICC for the primary outcome was 0.13 (95% CI –0.2 to 0.6).
Secondary Outcomes
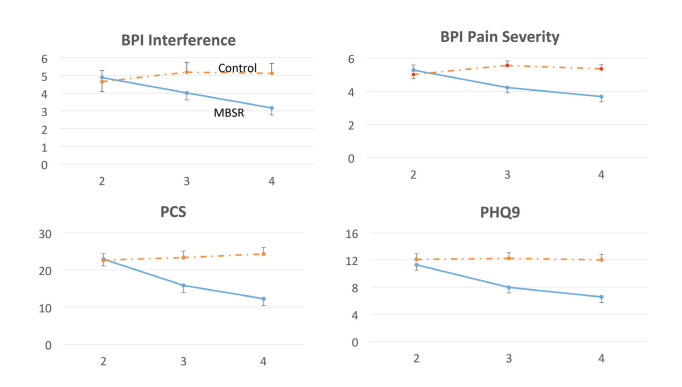
Adjusted mean scores at the time of randomization (V2), as well as adjusted mean change scores within groups and the between-group difference in change score between pre-randomization and the 12-week follow-up (V4 – V2) are given in Table 2. For the SF-12 variables, an increase in score indicates a benefit of MBSR; for all other variables, a decrease in score (negative value for V4 – V2) indicates a benefit or MBSR. Within the control group, measures either changed little or worsened over time. Within the MBSR group, all measures presented other than the SF-12 role emotion and A1C showed improvement 12 weeks after the course, with the 95% CI not including zero. There was also a general trend for progressive improvement between the 2- (V3) and 12-week (V4) follow-up visits (Figure 2). Some of the improvements within the MBSR group at 12 weeks were unexpectedly large, with a 46.5% decrease in pain catastrophizing, a 42.0% decrease in the PHQ-9 (depression assessment), a 30.1% decrease in pain severity, and a 52.3% increase (improvement) in the SF-12 bodily pain subscale. Many of the between-group differences in change scores between pre-randomization and the 12-week follow-up (V4 – V2, the prespecified comparison of interest) were statistically significant, showing a benefit of MBSR. Typically, the contrast did not reach significance when the 95% CI was wide or the control group showed some improvement.
TABLE 2.
Adjusted Mean Score at V2 (Pre-Randomization), Adjusted Mean Change Scores Within Groups, and Difference in Adjusted Mean Change Scores Between Groups
| Control Group (Mean [95% CI]) | MBSR Group (Mean [95% CI]) | MBSR (V4 – V2) – Control (V4 – V2) | ||
|---|---|---|---|---|
| Mean (95% CI) | P | |||
| BPI Interference | ||||
| V2 | 4.65 (3.56–5.74) | 4.89 (4.08–5.69) | ||
| V3 – V2 | 0.54 (–0.82 to 1.89) | –0.87 (–1.74 to 0.00) | ||
| V4 – V2 | 0.46 (–0.89 to 1.82) | –1.72 (–2.59 to –0.84) | –2.18 (–3.74 to –0.62) | 0.006 |
| BPI Pain Severity | ||||
| V2 | 5.01 (4.50–5.52) | 5.27 (4.63–5.90) | ||
| V3 – V2 | 0.55 (0.09–1.01) | –1.05 (–1.74 to –0.36) | ||
| V4 – V2 | 0.33 (–0.12 to 0.78) | –1.59 (–2.29 to –0.90) | –1.92 (–2.74 to –1.10) | <0.001 |
| Pain Catastrophizing | ||||
| V2 | 22.62 (19.15–26.09) | 22.94 (19.23–26.64) | ||
| V3 – V2 | 0.75 (–2.41 to 3.91) | –7.13 (–10.85 to –3.42) | ||
| V4 – V2 | 1.69 (–1.47 to 4.85) | –10.67 (–14.38 to –6.95) | –12.35 (–17.18 to –7.52) | <0.001 |
| PHQ-9 | ||||
| V2 | 12.06 (10.44–13.68) | 11.30 (9.65–12.95) | ||
| V3 – V2 | 0.19 (–1.41 to 1.78) | –3.34 (–5.14 to –1.55) | ||
| V4 – V2 | 0.06 (–1.66 to 1.53) | –4.75 (–6.55 to –2.96) | –4.69 (–6.96 to –2.43) | <0.001 |
| Perceived Stress | ||||
| V2 | 19.76 (17.68–21.85) | 19.20 (16.55–21.86) | ||
| V3 – V2 | 0.50 (–1.39 to 2.39) | –2.83 (–6.09 to 0.42) | ||
| V4 – V2 | 1.75 (–0.14 to 3.64) | –4.64 (–7.89 to –1.38) | –6.39 (–10.06 to –2.71) | 0.001 |
| SF-12 Mental Health Composite Scale | ||||
| V2 | 43.45 (40.78–46.12) | 43.20 (40.53–45.87) | ||
| V3 – V2 | –2.03 (–4.90 to 0.84) | 2.98 (–0.09 to 5.87) | ||
| V4 – V2 | –1.88 (–4.75 to 0.98) | 5.04 (2.18–7.90) | 6.93 (2.92–10.93) | <0.001 |
| SF-12 Physical Health Composite Scale | ||||
| V2 | 34.73 (31.91–37.55) | 31.22 (27.85–34.60) | ||
| V3 – V2 | –0.03 (–3.36 to 3.30) | 6.43 (3.12–9.74) | ||
| V4 – V2 | 0.93 (–2.39 to 4.26) | 5.82 (2.60–9.08) | 4.89 (0.36–9.41) | 0.04 |
| SF-12 Bodily Pain | ||||
| V2 | 43.21 (34.05–52.37) | 33.11 (22.79–43.43) | ||
| V3 – V2 | –3.13 (–12.16 to 5.91) | 18.26 (6.12–30.41) | ||
| V4 – V2 | –2.34 (–11.37 to 6.69) | 17.30 (5.35–29.25) | 19.65 (5.05–34.24) | 0.01 |
| SF-12 Physical Function | ||||
| V2 | 30.35 (20.91–39.79) | 22.98 (12.98–32.99) | ||
| V3 – V2 | –2.34 (–11.83 to 7.14) | 17.55 (5.99–29.11) | ||
| V4 – V2 | 0.78 (–8.70 to 10.27) | 12.53 (0.97–24.09) | 11.75 (–2.16 to 25.66) | 0.09 |
| SF-12 Role Physical | ||||
| V2 | 37.14 (27.27–47.02) | 28.06 (17.43–38.70) | ||
| V3 – V2 | –2.34 (–10.53 to 5.84) | 11.79 (1.11–22.46) | ||
| V4 – V2 | –2.73 (–10.92 to 5.45) | 16.95 (6.28–27.63) | 19.69 (6.58–32.80) | 0.004 |
| SF-12 General Health | ||||
| V2 | 31.86 (24.66–39.05) | 27.85 (20.45–35.25) | ||
| V3 – V2 | 0.16 (–6.93 to 7.24) | 12.51 (5.71–19.31) | ||
| V4 – V2 | 3.44 (–3.65 to 10.52) | 16.17 (9.38–22.97) | 12.74 (3.02–22.46) | 0.01 |
| SF-12 Vitality | ||||
| V2 | 28.24 (20.94–35.54) | 25.49 (17.13–33.84) | ||
| V3 – V2 | 1.56 (–6.41 to 9.54) | 15.17 (4.38–25.96) | ||
| V4 – V2 | 7.03 (–0.94 to 15.01) | 17.44 (6.79–28.10) | 10.41 (–2.47 to 23.29) | 0.11 |
| SF-12 Social Function | ||||
| V2 | 52.48 (43.36–61.59) | 39.93 (30.48–49.38) | ||
| V3 – V2 | –6.25 (–14.58 to 2.08) | 18.00 (11.19–24.81) | ||
| V4 – V2 | –4.69 (–13.02 to 3.65) | 20.83 (14.11–27.56) | 25.52 (14.92–36.13) | <0.001 |
| SF-12 Role Emotion | ||||
| V2 | 56.76 (46.76–66.76) | 58.21 (49.20–67.22) | ||
| V3 – V2 | –9.38 (–18.92 to 0.17) | 6.04 (–3.11 to 15.19) | ||
| V4 – V2 | –5.47 (–15.01 to 4.08) | 7.02 (–1.98 to 16.01) | 12.49 (–0.28 to 25.26) | 0.06 |
| SF-12 Mental Health | ||||
| V2 | 54.97 (48.90–61.04) | 54.18 (48.20–60.17) | ||
| V3 – V2 | –1.17 (–7.74 to 5.40) | 5.71 (–0.96 to 12.39) | ||
| V4 – V2 | –5.47 (–12.04 to 1.10) | 10.83 (4.23–17.44) | 16.30 (7.08–25.52) | <0.001 |
| POMS2A – tmd rs | ||||
| V2 | 49.92 (41.43–58.40) | 52.33 (39.31–65.34) | ||
| V3 – V2 | –4.66 (–12.58 to 3.27) | –17.04 (–34.65 to 0.58) | ||
| V4 – V2 | –6.59 (–14.52 to 1.33) | –21.87 (–39.49 to –4.25) | –15.28 (–34.30 to 3.75) | 0.11 |
| NQ Pain | ||||
| V2 | 6.86 (5.57–8.16) | 6.39 (5.55–7.22) | ||
| V3 – V2 | 0.54 (–0.89 to 1.98) | –1.22 (–0.45 to –2.00) | ||
| V4 – V2 | 0.90 (–0.53 to 2.33) | –1.39 (–2.16 to –0.61) | –2.28 (–3.90 to –0.66) | 0.006 |
| NQ Feeling | ||||
| V2 | 7.74 (6.33–9.14) | 7.72 (6.23–9.21) | ||
| V3 – V2 | 1.00 (–0.48 to 2.48) | –1.26 (–2.47 to –0.06) | ||
| V4 – V2 | –0.09 (–1.57 to 1.40) | –1.62 (–2.82 to –0.42) | –1.53 (–3.42 to –0.36) | 0.11 |
| NQ Motor | ||||
| V2 | 8.00 (6.64–9.38) | 8.01 (6.68–9.33) | ||
| V3 – V2 | –0.17 (–1.39 to 1.05) | –1.93 (–3.32 to –0.54) | ||
| V4 – V2 | –0.86 (–2.08 to 0.36) | –2.16 (–3.55 to –0.77) | –1.30 (–3.12 to 0.51) | 0.16 |
| NQ Restrictions | ||||
| V2 | 8.47 (7.10–9.84) | 8.80 (7.42–10.19) | ||
| V3 – V2 | –0.47 (–1.50 to 0.57) | –2.08 (–3.53 to –0.63) | ||
| V4 – V2 | –0.44 (–1.47 to 0.60) | –2.25 (–3.70 to –0.80) | –1.81 (–3.55 to –0.07) | 0.04 |
| NQ Disruptions | ||||
| V2 | 7.11 (5.76–8.46) | 7.83 (6.52– 9.13) | ||
| V3 – V2 | 0.16 (–0.93 to 1.26) | –2.08 (–3.54 to –0.61) | ||
| V4 – V2 | 0.09 (–1.01 to 1.18) | –2.16 (–3.63 to –0.70) | –2.25 (–4.03 to –0.48) | 0.01 |
| NQ Emotional | ||||
| V2 | 7.41 (6.28–8.54) | 7.74 (6.53–8.95) | ||
| V3 – V2 | 0.73 (–0.40 to 1.86) | –1.71 (–3.04 to –0.38) | ||
| V4 – V2 | 0.38 (–0.74 to 1.50) | –2.72 (–4.05 to –1.39) | –3.10 (–4.79 to –1.41) | <0.001 |
| A1C (%) | ||||
| V2 | 8.09 (7.78–8.41) | 8.58 (8.14–9.03) | ||
| V3 – V2 | 0.16 (–0.68 to 0.99) | 0.14 (–0.74 to 1.01) | ||
| V4 – V2 | 0.07 (–0.14 to 0.27) | –0.31 (–0.80 to 0.19) | –0.37 (–0.90 to 0.57) | 0.16 |
| A1C (mmol/mol) | ||||
| V2 | 65.0 (62.0–68.0) | 70.0 (65.0–75.0) | ||
| V3 – V2 | 1.7 (–7.4 to 10.78) | 1.5 (–8.1 to 11.0) | ||
| V4 – V2 | 0.8 (–1.5 to 3.0) | –3.4 (–8.7 to 2.1) | –4.2 (– 9.8 to 6.2) | 0.16 |
Prespecified comparison of interest was between-group difference in change from V2 to V4 (column 4). For SF-12 variables, a higher score and a positive value in column 4 indicates benefit of MBSR. For all other variables, a lower score and a negative value in column 4 indicates benefit of MBSR.
BPI Interference, mean of the seven interference scales of the BPI; BPI Pain Severity, mean of the four pain severity scales of the BPI; Pain Catastrophizing, total score of the PCS; POMS2A – tmd rs, POMS2A total mood disturbance raw score. NQ, NeuroQoL; NQ Pain, pain severity; NQ feeling, sensory changes; NQ motor, motor changes; NQ restrictions, interference with daily activities; NQ Disruptions, interference with emotional and physical roles; NQ Emotional, effect of neuropathy on mood.
FIGURE 2.
Increasing improvements in pain interference, pain severity, pain catastrophizing, and depression from 2 to 12 weeks after MBSR. The numerals 2, 3, and 4 on the x-axis denote V2 (pre-randomization), V3 (2 weeks after the MBSR course), and V4 (12 weeks after the MBSR course). Bars indicate 1 SE.
A1C showed little change in either group. There were no significant between-group differences (V4 – V2) on any subscales of the Summary of Diabetes Self-Care Activities or the blood glucose reactions questionnaire (not shown).
Discussion
Participation in an MBSR course improved function and reduced pain intensity, pain catastrophizing, depression, and perceived stress while improving health-related QoL. Many of these measures showed continued improvement from 2 to 12 weeks after the course (Figure 2). Our experimental hypothesis, that the proportion of patients experiencing a decrease of ≥1 in mean BPI pain interference score would be ≥30% in the MBSR group, was confirmed (63.3 and 21.9% in the MBSR and control groups, respectively). The clinical meaningfulness of this finding is supported by the results of the PGIC scale, in which the proportion of patients endorsing much or very much improvement in their condition at the 12-week follow-up was 40.5% greater in the MBSR group than in the control group (46.7 vs. 6.2%). We did not demonstrate differences between groups in measures of diabetes self-care, blood glucose reactions, or A1C.
Our methods were guided by the IMMPACT recommendations (16,17). This study is unique in selecting a homogeneous group of subjects all experiencing peripheral neuropathic pain, a feature that facilitates interpretation of the results. All patients were assessed by the principal investigator, and the diagnosis of PDPN was confirmed. At the first visit, each patient received a detailed explanation of the neurobiology of chronic pain and how MBSR might be beneficial. All patients had the opportunity to have drug treatment of their pain optimized during a period of up to 5 months before randomization. Our intention was that this would increase clinical relevance by demonstrating the added value of MBSR to best medical practice.
Multiple outcome domains were assessed to fully describe the clinical impact of treatment on patients’ well-being. The primary outcome measure, BPI interference, as well as pain intensity, were assessed daily for 7 days at each visit to improve the reliability of self-reporting. The primary outcome (a decrease of ≥1 in mean BPI interference score) was recommended by IMMPACT because it correlates with patient perception of significant improvement (the PGIC).
Patient retention was excellent, with 94% of those randomized included in the analysis. A pragmatic aspect of the trial was the referral of subjects to community MBSR groups that included patients with differing symptoms. The intervention is therefore similar to the service available to patients in the community, in contrast to a specially designed modification of MBSR with groups consisting only of patients with PDPN.
We have found no published studies of a mindfulness intervention for a cohort of patients with chronic neuropathic pain. Cherkin et al. (33) recently reported the results of a large study comparing treatment as usual (TAU) to cognitive behavioral therapy (CBT) and MBSR for patients with low back pain, with follow-up of nearly 300 patients at 52 weeks after starting the intervention. Their primary outcomes were also binary, with clinically meaningful improvement defined as a 30% improvement from baseline on the modified Roland Disability Questionnaire (RDQ) or a back pain bothersomeness scale. At 26 weeks (14 weeks after course completion) in the MBSR group, 60.5% responded on the RDQ (vs. 44.1% in the TAU group); 43.6% responded on the bothersomeness scale (vs. 26.6% in the TAU group).
These response rates are similar to our study, although our control group showed less improvement, which may reflect a difference between the course of back pain compared to neuropathic pain, which is persistent. The magnitudes of change in their MBSR group at 26 weeks in measures of depression, pain intensity, and SF-12 Physical Health Composite Scale were similar to those found in the present study. We found significant improvement in the SF-12 Mental Health Composite Scale, whereas they did not.
Veehof et al. (34) published a meta-analysis of acceptance- and mindfulness-based interventions for the treatment of chronic pain, including 25 randomized, controlled trials with 1,285 patients. At follow-up (2–6 months after completing treatment), small effects (as measured with Cohen’s standardized mean difference) were found on pain intensity (0.41) and disability (0.39); moderate effects on QoL (0.66), anxiety (0.59), and depression (0.3); and a large effect on pain interference (1.05). The authors observed that, as in the present study, effect sizes generally were larger several months after the intervention than during the first weeks after the course, suggesting long-term application of the course content rather than a nonspecific effect of the intervention.
In contrast, a Cochrane review by Williams et al. (35) of CBT for chronic pain found small post-treatment effects on pain intensity, mood, and disability that generally were not maintained at follow-up. Cherkin et al. (33), however, showed no such differences between CBT and MBSR for chronic back pain.
Both the pain subscale of the DPN-specific NeuroQoL and the BPI pain intensity scale, which interrogate about patients’ experience of pain, showed reductions of 21.8 and 30.1%, respectively, 12 weeks after the MBSR course. It may seem surprising that a psychosocial intervention could result in a significant decrease in pain intensity, but this has been found in other studies (36). This finding supports the biopsychosocial model of pain (37), which postulates that the pain experienced by a patient depends on affective-motivational and cognitive-evaluative processes in the central nervous system and not only on the degree of nociceptor activation. Thus, MBSR may modulate the pain experience itself in addition to reducing the physical and emotional consequences of living with chronic pain.
van Son et al. (38) randomized patients with diabetes and emotional distress to a mindfulness course compared to a TAU group and found improvements in mood and health-related QoL similar to those found in the present study and likewise failed to demonstrate an effect on A1C. Meta-analyses of psychological interventions to improve glycemic control have yielded mixed results (39–41).
Strengths and Limitations
There are few large trials of mindfulness-based interventions. In their meta-analysis of acceptance- and mindfulness-based interventions for the treatment of chronic pain, Veehof et al. (34) assigned quality points for an n ≥50 because that is deemed a sufficient number of participants to show standardized effect sizes ≥0.80, assuming statistical power of 0.80 and an alpha of 0.05. The consistency of our results across multiple domains affected by pain and the very low P values make it highly unlikely that the difference between the treatment and control groups occurred by chance.
The CI of some measures in our study are wide, and therefore the mean changes lack the precision that a future meta-analysis may provide. The choice of optimized treatment as usual care for the control group does not control for nonspecific effects and does not allow discovery of the mechanism of effect of the intervention. Subgroup analysis in Veehof et al.’s meta-analysis (34) comparing studies with TAU control groups to support group or education control groups showed a difference only for anxiety. Our priority was external validity with the intention to inform clinicians of the added value of referring their medically optimized patients with PDPN to a community MBSR course. As accumulating evidence establishes the efficacy of MBSR for neuropathic pain, attention may then be best directed to experiments with active control conditions that are designed to improve efficiency and efficacy by revealing the essential elements of the therapeutic effect (42).
Conclusion and Clinical Relevance
Among patients with PDPN whose pharmacotherapy had been optimized in a chronic pain clinic, treatment with MBSR, compared with usual care, resulted in improved function; reduced pain intensity, pain catastrophizing, depression, and perceived stress; and better health-related QoL. Our results suggest that clinicians can expect their patients with PDPN to benefit from referral to community MBSR courses led by qualified teachers.
Acknowledgments
The authors thank the patients who participated in the study and the MBSR teachers who accepted these patients into their groups.
Funding
This study was supported by grants from the Canadian Diabetes Association (CDA OG-2-12-3722-HN) and the University of Ottawa Anesthesiology Research Fund.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
All authors reviewed the manuscript. H.J.N. designed the study, obtained funding, examined the patients, supervised all phases of the research, and wrote the manuscript. P.P., D.W., M.T., C.S., I.G., A.S., and H.L. contributed to the experimental design. M.T. supervised the data analysis. D.W. and Y.S. participated in all phases of the research. H.J.N. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
Parts of this study were presented in poster form at the 16th World Congress on Pain of the International Society for the Study of Pain, Yokohama, Japan, 26–30 September 2016.
References
- 1.International Diabetes Federation Diabetes Atlas. 7th ed. [Internet]. Brussels, Belgium, International Diabetes Federation, 2015. Available from http://www.diabetesatlas.org. Accessed 14 March 2017
- 2.Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig 2011;2:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–662 [DOI] [PubMed] [Google Scholar]
- 4.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157:1599–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulin D, Boulanger A, Clark A, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014;19:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence Neuropathic pain in adults: pharmacological management in non-specialist settings (NICE clinical guideline 173). Available from https://www.nice.org.uk/guidance/cg173/chapter/1-recommendations?unlid=773239836201724223722. Accessed 5 February 2017 [PubMed]
- 8.American Diabetes Association Comprehensive medical evaluation and assessment of comorbidities. Sec. 3 in Standards of Medical Care in Diabetes—2017. Diabetes Care 40(Suppl. 1):S25–S32 [DOI] [PubMed] [Google Scholar]
- 9.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry 1982;4:33–47 [DOI] [PubMed] [Google Scholar]
- 10.deCharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A 2005;102:18626–18631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spallone V, Morganti R, D’Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012;29:578–585 [DOI] [PubMed] [Google Scholar]
- 12.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan Neuropathy Screening Instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
- 13.Zelman DC, Gore M, Dukes E, Tai KS, Brandenburg N. Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J Pain Symptom Manage 2005;29:401–410 [DOI] [PubMed] [Google Scholar]
- 14.Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149:177–193 [DOI] [PubMed] [Google Scholar]
- 15.Blacker M, Meleo-Meyer F, Kabat-Zinn J, Santorelli SF. Mindfulness-Based Stress Reduction (MBSR) Curriculum Guide. Worcester, Mass., Center for Mindfulness in Medicine, Health Care, and Society, University of Massachusetts Medical School, 2009 [Google Scholar]
- 16.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105–121 [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146:238–244 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain 1999;83:157–162 [DOI] [PubMed] [Google Scholar]
- 19.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9). J Affect Disord 2004;81:61–66 [DOI] [PubMed] [Google Scholar]
- 20.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149–158 [DOI] [PubMed] [Google Scholar]
- 21.Ezzati A, Jiang J, Katz MJ, Sliwinski MJ, Zimmerman ME, Lipton RB. Validation of the Perceived Stress Scale in a community sample of older adults. Int J Geriatr Psychiatry 201429:645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524–532 [Google Scholar]
- 23.Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233 [DOI] [PubMed] [Google Scholar]
- 24.Luo X, George ML, Kakouras I, et al. Reliability, validity, and responsiveness of the Short Form 12-Item Survey (SF-12) in patients with back pain. Spine 2003;28:1739–1745 [DOI] [PubMed] [Google Scholar]
- 25.Galvez R, Marsal C, Vidal J, Ruiz M, Rejas J. Cross-sectional evaluation of patient functioning and health-related quality of life in patients with neuropathic pain under standard care conditions. Eur J Pain 2007;11:244–255 [DOI] [PubMed] [Google Scholar]
- 26.Vileikyte L, Peyrot M, Bundy C, et al. The development and validation of a neuropathy- and foot ulcer-specific quality of life instrument. Diabetes Care 2003;26:2549–2555 [DOI] [PubMed] [Google Scholar]
- 27.Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 28.Michigan Diabetes Research and Training Center Diabetes Care Profile [Internet]. Available from http://diabetesresearch.med.umich.edu/peripherals/profs/documents/svi/dcp.pdf. Accessed 20 March 2017
- 29.Moerbeek M, Wong WK. Sample size formulae for trials comparing group and individual treatments in a multilevel model. Stat Med 2008;27:2850–2864 [DOI] [PubMed] [Google Scholar]
- 30.Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and statistics in medicine. Stat Med 2007;26:2–19 [DOI] [PubMed] [Google Scholar]
- 31.Searle S. Linear Models. New York, Wiley, 2012 [Google Scholar]
- 32.Lohr S, Schochet P, Sanders E. Partially Nested Randomized Controlled Trials in Education Research: A Guide to Design and Analysis [Internet]. Available from https://ies.ed.gov/ncer/pubs/20142000/pdf/20142000.pdf. Accessed 14 March 2017
- 33.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA 2016;315:1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KMG. Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Cogn Behav Ther 2016;45:5–31 [DOI] [PubMed] [Google Scholar]
- 35.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiner K, Tibi L, Lipsitz JD. Do mindfulness-based interventions reduce pain intensity? A critical review of the literature. Pain Med 2013;14:230–242 [DOI] [PubMed] [Google Scholar]
- 37.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624 [DOI] [PubMed] [Google Scholar]
- 38.van Son J, Nyklicek I, Pop VJ, et al. The effects of a mindfulness-based intervention on emotional distress, quality-of-life, and HbA1c in outpatients with diabetes (DiaMind): a randomized controlled trial. Diabetes Care 2013;36:823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–1597 [DOI] [PubMed] [Google Scholar]
- 40.Wang MY, Tsai PS, Chou KR, Chen CM. A systematic review of the efficacy of non-pharmacological treatments for depression on glycaemic control in type 2 diabetics. J Clin Nurs 2008;17:2524–2530 [DOI] [PubMed] [Google Scholar]
- 41.Winkley K, Ismail K, Landau S, Eisler I. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ 2006;333:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Street LL. Control groups in psychosocial intervention research: ethical and methodological issues. Ethics Behav 2002;12:1–30 [DOI] [PubMed] [Google Scholar]