Abstract
Objectives
To determine whether the impact of guided self-determination (GSD) applied in group training (GSD-GT) in people with chronically elevated HbA1c and type 1 diabetes mellitus (DM) was superior to ‘care as usual’ in improving HbA1c and psychological functioning.
Setting
An outpatient clinic at a university hospital in Western Norway.
Participants
A total of 178 adults (all Caucasian) aged 18–55 (mean age 36.7±10.7, 62% women) with type 1 DM for at least 1 year and HbA1c ≥64 mmol/mol (8.0%) were randomly assigned to participate in either GSD-GT or a control group (CG). Exclusion criteria were severe comorbidity, major psychiatric disorder, cognitive deficiency/language barriers and pregnancy.
Intervention
Intervention group met seven times for 2 hours over 14 weeks to promote patient autonomy and intrinsic motivation using reflection sheets and advanced professional communication in accordance with the GSD methodology.
Primary and secondary outcome measures
The primary outcome was HbA1c and secondary outcomes (all outcomes 9 months post intervention) were self-monitored blood glucose frequency, self-reported diabetes competence, autonomy support by healthcare providers (Health Care Climate Questionnaire), autonomous versus controlled diabetes motivation (Treatment Self-Regulation Questionnaire), diabetes distress (Problem Areas In Diabetes Scale (PAID) and Diabetes Distress Scale (DDS)), self-esteem (Rosenberg Self-Esteem Scale) and psychological well-being (World Health Organization five-item Well-Being Index scale).
Results
Among participants allocated to the GSD-GT (=90) 48 completed the study, whereas 83 completed in the CG (n=88). With 95% CIs GSD-GT did not have effect on HbA1c (B −0.18, CI (−0.48, 0.12), p=0.234). GSD-GT improved autonomy-motivated behaviour (B 0.51, CI (0.25, 0.77), p<0.001), diabetes distress (PAID, B −6.96, CI (−11.40, −2.52), p=0.002), total DDS (B −5.15, CI (−9.34, −0.96), p=0.016), DDS emotional burden (B −7.19, CI (−13.20, −1.19), p=0.019) and self-esteem (B 1.43, CI (0.34, 2.52), p=0.011).
Conclusions
Results from this behavioural intervention must be interpreted cautiously because of recruitment and attrition problems. Medical outcomes did not improve. Psychological outcomes improved, especially reduced diabetes distress.
Trial registration number
Clinical Trials.gov NCT 01317459.
Keywords: General diabetes, self-management, diabetes distress, educational method, psychological functioning, HbA1c
Strengths and limitations of this study.
This study evaluated the effect of a person-centred behavioural intervention among adults with type 1 diabetes mellitus (DM), scarcely evaluated in the literature compared with educational programmes among persons with type 2 DM.
Targeting persons with type 1 DM and chronically elevated HbA1c is a challenge because long-standing poor glycaemic control appears to be a complex and heterogeneous phenomenon.
The most obvious limitation of this study is that generalisability might be distorted due to recruitment problems, and attrition from the GSD-GT (guided self-determination applied in group training) programme.
Introduction
Diabetes is considered a demanding condition requiring complex self-management tasks for the individual. Effective self-management is a prerequisite for preventing long-term complications and the immediate risk of hypoglycaemia. Researchers underscore that diabetes self-management education is an ongoing process rather than a one-time event.1 American Diabetes Association (ADA) National Standards for Diabetes Self-Management Education and Support holds that self-management does not stop when a person with diabetes leaves the educator’s office.2 Consequently, motivation is a key concept, and one based on respect for the individual’s autonomy is essential as it is connected with success in reaching and maintaining goals.3 Therefore, the challenge for healthcare professionals (HCPs) is to implement an autonomy supportive approach instead of one based on control or disclaimed responsibility.4 Considerable barriers to such an approach exist5 and personal problems in living with the illness might remain unclarified and unresolved.6 This can contribute to the 37%-56% of people with type 1 diabetes mellitus (DM) who are living with blood sugar above target levels7 and at increased risk of late complications together with poor quality of life.8 The ADA states that diabetes care is often suboptimal; lacking are collaborative, multidisciplinary teams well suited to provide appropriate self-management education among persons with diabetes.9
One of the first structured diabetes treatment and education programmes for type 1 DM was developed between 1980 and 1990.10 While many trials have evaluated the effect of diabetes education programmes in type 2 DM, there is still a paucity of trials evaluating evidence-based self-management programmes promoting empowerment in adults with type 1 DM.11 Among persons with type 1 DM, the DAFNE (Dose Adjustment for Normal Eating) education programme showed significant improvements in HbA1c in the early stage of the trial12 but more modest longer term improvements.13 A self-management-oriented education programme (PRIMAS) showed effect on glycaemic control and reduced diabetes distress at 6 months of follow-up compared with an established education programme in a multicentre trial.14 In an uncontrolled evaluation of the DTTP (Diabetes Teaching and Treatment Programme) persons with moderately controlled type 1 DM improved their HbA1c and treatment satisfaction.15
Guided self-determination (GSD) is a theory-based and evidence-based problem-solving method to overcome barriers to collaborative care. It is based on life skills theory, dynamic judgement building and theories about behaviour change. GSD promotes patient autonomy, participation, skills building and intrinsic motivation.16 The method is applicable in group training (GSD-GT) or individual care for a variety of conditions. In a recent study, GSD improved physical well-being in women surgically treated for gynaecological cancer.17 For persons with type 1 DM, the GSD methodology has demonstrated some success. The first randomised controlled trial among adults showed significant improvement in glycaemic control and life skills.18 In later studies, GSD proved to be either borderline effective or not effective concerning glycaemic control among younger adults/adolescents; however, it reduced diabetes distress and lack of motivation and improved diabetes competence among young adult women.19 20 In addition, a case report on a young woman showed considerable reduction in HbA1c after a GSD intervention.21 In conclusion, GSD seems worthy of further research for several reasons. First, patient involvement and person-centred care are highly appreciated and recommended, but difficult to implement as part of clinical care.22 Second, GSD is one of few interventions which clinicians are able to facilitate in routine clinical care after rather short training. Third, persons with diabetes have a primary role in GSD, spending their time at home clarifying what is important for them to change and becoming able to express their thoughts in communication with HCPs. Consequently, efficiency of patient–provider communication increases without extra use of HCP resources. Last, GSD has the potential for improvement of HbA1c, as well as increased self-determination and decrease of diabetes-related burden.18 This randomised intervention study tests whether GSD-GT is superior to ‘care as usual’ (CU) in improving glycaemic control and psychological functioning among adults with suboptimally regulated type 1 DM.
Patients and methods
Design
This study was a prospective, randomised trial with a control group (CG) and a treatment group. Participants were recruited from an outpatient setting at a university hospital in Western Norway. The hospital’s population is ethnically homogeneous, and includes both urban and rural populations. To test the effect of GSD, persons with type 1 DM and suboptimal metabolic control were invited to participate in an educational group treatment intervention (GSD-GT) or CU CG. The Regional Committee for Medical and Health Research Ethics approved the study (approval number 2010/1325), and gave access to the age, gender and HbA1c of non-responders. Participants gave written informed consent.
Recruitment
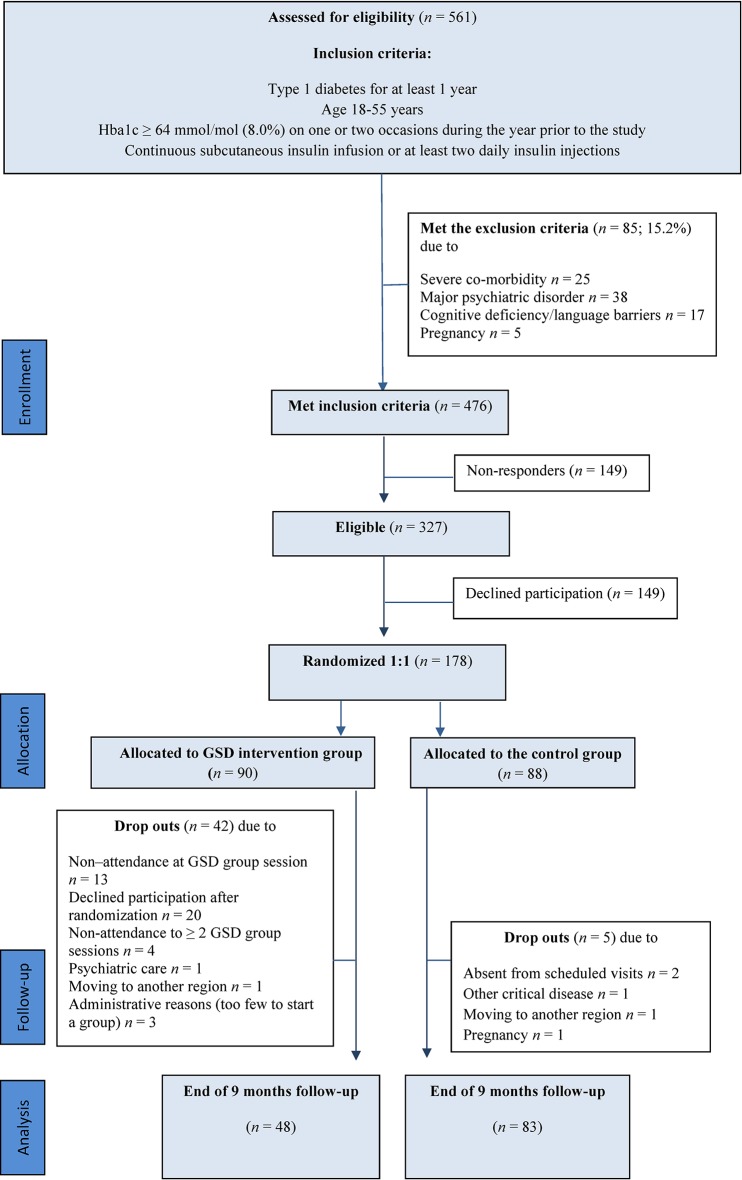
From March 2011 to March 2013, all persons with type 1 DM attending consultations at the university hospital (n=561) were assessed for eligibility according to the inclusion criteria (figure 1). Further details on inclusion and exclusion criteria are outlined elsewhere.23 A prestudy power calculation for a t-test assuming a 0.05 significance level and a power of 0.8 expecting 25% dropout and a difference of 0.6% change in HbA1c (SD 1.3) between groups led to 218 participants needed to include. Expected change in HbA1c was based on clinical relevance.
Figure 1.
Study flow diagram. GSD, guided self-determination.
After identifying 476 people who met study criteria, a request was sent by post 1–3 weeks in advance of their next clinical consultation, inviting them to take part in the study. To those who neither responded to the request nor attended at the clinic appointment, an additional letter was sent. If still no response nor attendance at the clinic, they were classified as non-responders (n=149). Another 149 persons actively declined participation. Their response was given when they were at the clinic, or by telephone if they were unable to meet for their scheduled appointment. Those willing to participate were consented when they were at the clinic (n=178). The participation rate in the study was 37.4% (178 of the 476 who met inclusion criteria). Participants received no monetary incentives. All sessions were free of charge.
Randomisation
The randomisation occurred externally and was stratified in computer-generated sequences unknown to the investigators. Blinding was considered impossible due to the nature of the intervention and was not attempted. Participants were randomised to either the usual care CG (n=88; M/W: n=40/48) or GSD-GT (n=90; M/W: n=26/64). The groups consisted of a minimum of two and a maximum of seven participants. Each GSD training group was balanced according to sex and age, preferably an equal number of men and women in each group and age ranging ±6 years.
In the CG and GSD-GT conditions, 5 and 42 participants were lost to follow-up, respectively; thus 131 participants (74%) completed the trial. The reasons for dropout in both study groups, and the total flow of participants through each stage of the trial are depicted in figure 1.
Intervention
The GSD intervention was based on a technique by which the HCPs encourage people with diabetes to reflect on different problem areas concerning their daily lives with diabetes and develop autonomous motivation for lifestyle changes. The technique was partly built on semistructured worksheets filled in before and between each appointment, making the people with diabetes prepared with enhanced self-insight and ability to talk about personal difficulties. The perceived obstacles and barriers were responded to by HCPs with specific communication skills who conducted each consultation consistent with the GSD methodology.18 Intervention group (IG) participants met seven times for 2-hour sessions over 14 weeks. Two GSD-trained diabetes specialist nurses supervised each session. Nurses were given 1 hour per week group-based feedback to secure fidelity to the protocol. No GSD-GT participants received additional treatment during the intervention period, except two participants who needed one extra consultation because of technical problems with their insulin pumps.
The CG received traditional outpatient consultations, ‘care as usual,’ consisting of individual counselling by nurses, physicians or dieticians, with measurements of HbA1c and advice on how to improve glycaemic control. No CG participants met with GSD-trained nurses during the intervention period.
Assessments
Participant characteristics were assessed at baseline. Primary and secondary outcome measurements were planned to be performed at baseline and 9 months after the last session of group intervention (GSD-GT) or 9 months after inclusion (CG). Because of the need to get enough participants to start group sessions, there was a large variation in time from randomisation to start of intervention in the IG (mean=4.9 months, SD=3.6). There was also some variation in time from baseline to follow-up in the CG (mean=10.9 months, SD=2.4) and time from last session to follow-up in the IG (mean 9.3 months, SD=1.0) because many participants needed several reminders before they handed in the follow-up questionnaire.
Primary outcome measure
The primary endpoint was glycaemic control (HbA1c) which was assessed in connection with a regularly scheduled visit at the hospital. The blood samples were analysed using high-performance liquid chromatography (DCA Vantage/Siemens, DCA 2000 and DCA 2000+/Bayer), assays standardised and calibrated against the IFCC (International Federation of Clinical Chemists) standards.24
Secondary outcome measures
All participants reported the number of self-monitored blood glucose (SMBG) measurements completed in the past 2 weeks in the following six categories: ‘seven or more measurements per day’, ‘four to six measurements per day’, ‘one to three measurements per day’, ‘less than every day’, ‘less than every week’ and ‘no monitoring last 14 days’. Due to small size of some categories, we chose to collapse into the following three categories: ‘seven or more measurements per day’, ‘one to six measurements per day’ and ‘less than daily measurements’. Participants also completed seven self-report instruments assessing aspects of psychological functioning consistent with the theoretical framework of GSD.
The Problem Areas In Diabetes Scale (PAID) measures negative emotions related to living with diabetes (range=0–100 scale); higher scores represent greater distress.25
The Diabetes Distress Scale (DDS) measures the level of diabetes distress with an overall score and four subscales: emotional burden, regimen-related, interpersonal-related and medical care-related distress. The range is 1–6 for each item. Total score is calculated by transforming the mean score to a 0–100 range. Higher scores represent greater distress.26
The Perceived Competence for Diabetes Scale (PCDS) assesses the degree to which persons with diabetes feel they can manage the everyday aspects of diabetes care. The range is 1–7 for each item and the mean is used as a total score. Higher scores represent greater perceived competence.27
The Rosenberg Self-Esteem Scale (RSES) measures one’s overall self-esteem. The range is 1–4 for each item and total score is calculated as the mean of all items multiplied by 10. Higher scores represent better self-esteem.28
The World Health Organization five-item Well-Being Index (WHO-5) measures emotional well-being. The range for each item is 0–6 and a total score is calculated by transforming the sum to a 0–100 range. Higher scores represent better emotional well-being.29
The Health Care Climate Questionnaire (HCCQ) assesses patients’ perceptions of the degree to which healthcare providers are supportive of autonomy rather than controlling. The range is 1–7 for each item and total score is calculated as the mean of all items; higher scores represent greater perceived support for autonomy.30
The Treatment Self-Regulation Questionnaire (TSRQ) assesses the diabetes self-care practices and whether this behaviour is self-motivated (autonomous/internal) or controlled (external).31 Each item ranges from 1 to 7 and behaviour scores are calculated as the mean of items within the internal and external dimensions separately. A relative autonomy index (TSRQ Relative Autonomy Index, RAI) was also calculated.
The PCDS, the HCCQ and the TSRQ were translated into Norwegian and back-translated into English by professional translators, in accordance with the WHO guidelines.32
Statistical analysis
Mann-Whitney U tests and χ2 tests were used to assess randomisation efficacy by testing for differences in baseline measures. To assess differential attrition, members of GSD-GT who completed the study were compared with those who did not. We fitted a regression model for each outcome at 9 months to investigate the difference between IG and CG both unadjusted and adjusted for baseline outcome and sex. To take into account possible bias introduced by the high attrition rate in the IG, we additionally adjusted for variables showing unbalanced attrition. We used a linear regression model for all outcomes except SMBG where a multinomial logistic model was used. We used a linear regression model to test whether change in SMBG mediated change in HbA1c and whether psychological effects were mediated by increased autonomy.
There were no intermediate assessments of questionnaire data; therefore, it was not possible to do intention-to-treat analysis for persons who dropped out and per-protocol analyses were thus performed. HbA1c was assessed for those who did not complete the study because it could be obtained from medical records. Therefore, intention-to-treat analysis of HbA1c was performed. Because of the difference in follow-up time between GSD-GT and CG and because of the large variation within each group, we also did additional analyses where we estimated the association between change in outcome measures and length of follow-up within each group. Associations were tested using linear regression with change in outcome measure as dependent variable and follow-up time in months as independent variable with adjustment for baseline measurement of the outcome variable. For data analyses, the statistical software program SPSS Statistics V.22 (SPSS Inc.) was used. Significance level was set to 0.05. Missing values were handled by pairwise exclusion.
Results
Baseline characteristics
The mean age of all subjects in the study sample was 36.7 years (±10.7), the median disease duration was 19 years (range=1–46), 13.5% were unemployed, 96.6% were white and 31.5% had diabetes-related complications. A comparison of the baseline characteristics between the groups (without taking the attrition rate into account) suggests that the randomisation was successful for all parameters except sex, in that we found a significant difference in the number of women assigned to the study groups (M/W in the GSD-GT: n=26/64 (29/71%) vs M/W in the CG: n=37/46 (45/55%), p=0.022) (results not shown). Baseline characteristics of the sample are presented in table 1.
Table 1.
Baseline characteristics of control group versus guided self-determination (GSD) intervention group (N=173)
| A. Control group (completers) | B. GSD intervention group | A versus B1 | B1 versus B2 | ||
| n=83 | B1 Follow-up n=48 |
B2 Lost to follow-up n=42 |
p | p | |
| Participant characteristics | |||||
| Sex, womena b | 46 (55.4) | 35 (72.9) | 29 (69.0) | 0.047 | 0.686 |
| Age, yearsc d | 37.2 (10.9) | 36.9 (9.4) | 36.3 (11.6) | 0.860 | 0.916 |
| Living alone, yes a b | 17 (20.5) | 5 (10.4) | 6 (14.3) | 0.138 | 0.576 |
| Education university, yes a b | 30 (36.1) | 23 (47.9) | 10 (23.8) | 0.186 | 0.018 |
| Employed a b | |||||
| Full-time | 54 (65.1) | 34 (70.8) | 28 (66.7) | 0.485 | 0.464 |
| Part-time | 16 (19.3) | 10 (20.8) | 7 (16.7) | ||
| Not working | 13 (15.7) | 4 (8.3) | 7 (16.7) | ||
| Diabetes duration, years c d | 20.6 (11.2) | 18.5 (10.6) | 18.0 (11.0) | 0.310 | 0.694 |
| Long-term complications, yes a b | 29 (34.9) | 11 (22.9) | 15 (35.7) | 0.150 | 0.181 |
| Treatment regimen, insulin pump, yes a b | 37 (44.6) | 16 (33.3) | 20 (47.6) | 0.206 | 0.168 |
| Severe hypoglycaemia, yes a b | 35 (42.7) | 24 (50.0) | 13 (31.7) | 0.419 | 0.081 |
| Body Mass Index (BMI) c d | 26.0 (4.1) | 25.0 (3.6) | 25.8 (4.0) | 0.145 | 0.333 |
| Outcomes | |||||
| HbA1c, mmol/mol c d | 78 (12.7) | 76 (10.4) | 81 (11.7) | ||
| HbA1c, % c d | 9.3 (1.2) | 9.1 (1.0) | 9.5 (1.1) | 0.320 | 0.018 |
| SMBGa b e | |||||
| ≥7 times per day | 8 (9.6) | 8 (16.7) | 5 (11.9) | 0.439 | 0.627 |
| 1–6 times per day | 51 (61.4) | 29 (60.4) | 24 (57.1) | ||
| <Every day | 24 (28.9) | 11 (22.9) | 13 (31.0) | ||
| PAID c d f | 35.3 (18.7) | 36.8 (19.3) | 41.6 (24.4) | 0.696 | 0.355 |
| DDSc d g(sum score) | 31.9 (16.7) | 33.1 (16.4) | 37.3 (20.9) | 0.643 | 0.340 |
| DDS emotional burden c d | 36.0 (22.5) | 36.9 (25.6) | 42.3 (26.0) | 0.985 | 0.297 |
| DDS physician distress c d | 17.7 (19.9) | 18.1 (18.2) | 19.7 (23.1) | 0.826 | 0.990 |
| DDS regimen distress c d | 45.8 (23.7) | 44.7 (21.8) | 53.9 (25.4) | 0.816 | 0.036 |
| DDS interpersonal distressc d | 21.0 (20.1) | 26.1 (19.3) | 24.9 (23.2) | 0.101 | 0.577 |
| PCDS c d h | 4.5 (1.6) | 4.4 (1.4) | 3.9 (1.6) | 0.734 | 0.088 |
| RSES c d i | 19.6 (5.5) | 19.5 (5.4) | 19.0 (6.2) | 0.876 | 0.624 |
| WHO-5 c d j | 57.6 (18.6) | 60.9 (19.8) | 57.1 (20.5) | 0.338 | 0.468 |
| HCCQ c d k | 5.1 (1.4) | 4.9 (1.6) | 4.8 (1.6) | 0.595 | 0.923 |
| TSRQ autonomyc d l | 5.2 (1.1) | 5.4 (1.0) | 4.9 (1.3) | 0.754 | 0.088 |
| TSRQ control c d m | 3.2 (1.3) | 3.5 (1.2) | 3.1 (1.2) | 0.073 | 0.061 |
| TSRQ RAI 14c d n | 2.0 (1.3) | 1.8 (1.5) | 1.8 (1.7) | 0.339 | 0.588 |
N (%), bChi-square (χ2), cMean (SD),dMann-Whitney, eSelf-Monitoring Blood Glucose, fProblem Areas in Diabetes scale (range 0-100), gDiabetes Distress Scale (range 0-100), hPerceived Competence in Diabetes Scale (range 1-7), iRosenberg Self-Esteem Scale (range 10-40), jWHO(5)Well-being Index (range 0-100),kHealth Care Climate Questionnaire (range 1-7), lTreatment Self-Regulation Questionnaire, Autonomous motivation (range 1-7), mTreatment Self-Regulation Questionnaire, Controlled motivation (range 1-7), nTreatment Self-Regulation Questionnaire, Relative Autonomy Index (range 0-6).
Due to the considerable number of dropouts in GSD-GT, participants were stratified into follow-up and lost to follow-up to analyse the effects of attrition. The only statistically significant difference at baseline between CG and GSD-GT follow-up was for sex (p=0.047). The GSD-GT lost to follow-up participants had poorer baseline glycaemic control and scored higher on diabetes distress (DDS, subscale 3, regimen distress) than GSD-GT follow-up participants. Similarly, there were significantly fewer persons with education at a university level in the GSD-GT lost to follow-up group than in the GSD-GT follow-up group.
Among participants allocated to the CG, there were no statistically significant baseline differences between those who fulfilled the trial (n=83) and those who were lost to follow-up (n=5, data not shown).
Primary outcome
As seen in table 2, HbA1c declined significantly within both groups (p<0.001) from baseline to follow-up, with no significant difference between the groups.
Table 2.
Outcomes in control group and GSD intervention group (N=131)
| Within-group changea | Between-groups changeb | |||||||||||
| Control group Mean (SD) |
GSD group Mean (SD) |
Model 1 | Model 2 | |||||||||
| Baseline (n=83) |
Follow-up (n=83) |
p Value | Baseline (n=48) |
Follow-up (n=48) |
p Value | Effect sizec | Group differenced
(95% CI) |
p Value | Effect sized | Group differenced
(95% CI) |
p Value | |
| Primary outcome | ||||||||||||
| HbA1c %e | 9.3 (1.2) | 8.9 (1.3) | <0.001 | 9.1 (1.0) | 8.5 (1.1) | <0.001 | 0.005 | −0.15 (−0.45 to 0.15) | 0.316 | 0.007 | −0.18 (−0.48 to 0.12) | 0.234 |
| HbA1c mmol/mol | 78 (12.7) | 74 (14.1) | 76 (10.4) | 70 (11.7) | ||||||||
| Secondary outcomes medical | ||||||||||||
| PCDSf | 4.5 (1.6) | 4.7 (1.5) | 0.305 | 4.4 (1.4) | 4.7 (1.6) | 0.071 | 0.003 | 0.25 (−0.18 to 0.67) | 0.247 | 0.003 | 0.26 (−0.17 to 0.70) | 0.229 |
| HCCQg | 5.1 (1.4) | 5.0 (1.4) | 0.618 | 4.9 (1.6) | 5.0 (1.6) | 0.802 | 0.000 | 0.04 (−0.46 to 0.54) | 0.873 | 0.000 | 0.03 (−0.48 to 0.54) | 0.904 |
| Type of motivation | ||||||||||||
| TSRQh autonomy | 5.2 (1.1) | 5.1 (1.1) | 0.085 | 5.4 (1.0) | 5.6 (0.9) | 0.060 | 0.067 | 0.53 (0.28 to 0.79) | <0.001 | 0.061 | 0.51 (0.25 to 0.77) | <0.001 |
| TSRQ control | 3.2 (1.3) | 3.1 (1.3) | 0.532 | 3.5 (1.2) | 3.3 (1.1) | 0.072 | 0.000 | 0.13 (−0.13 to 0.40) | 0.321 | 0.000 | 0.16 (−0.11 to 0.43) | 0.237 |
| TSRQ index | 2.0 (1.3) | 1.9 (1.2) | 0.364 | 1.8 (1.5) | 2.3 (1.3) | 0.014 | 0.047 | 0.40 (0.06 to 0.73) | 0.020 | 0.038 | 0.35 (0.01 to 0.69) | 0.045 |
| Psychological | ||||||||||||
| PAIDi | 35.3 (18.7) | 34.2 (19.6) | 0.488 | 36.8 (19.3) | 29.8 (18.9) | 0.002 | 0.038 | −6.66 (−11.03 to −2.29) | 0.003 | 0.043 | −6.96 (−11.40 to −2.52) | 0.002 |
| DDSj overall | 31.9 (16.7) | 30.4 (17.5) | 0.323 | 33.1 (16.4) | 27.9 (16.8) | 0.012 | 0.022 | −4.45 (−8.62 to −0.27) | 0.037 | 0.033 | −5.15 (−9.34 to −0.96) | 0.016 |
| DDS emotional burden | 36.0 (22.5) | 35.7 (24.4) | 0.995 | 36.9 (25.6) | 35.7 (24.4) | 0.019 | 0.035 | −6.92 (−12.82 to −1.01) | 0.022 | 0.042 | −7.19 (−13.20 to −1.19) | 0.019 |
| DDS physician distress | 17.7 (19.9) | 17.8 (19.0) | 0.864 | 18.1 (18.2) | 19.3 (19.7) | 0.337 | 0.001 | 1.14 (−4.38 to 6.66) | 0.684 | 0.000 | −0.41 (−5.80 to 4.98) | 0.880 |
| DDS regimen distress | 45.8 (23.7) | 40.2 (22.1) | 0.005 | 44.7 (21.8) | 36.8 (22.8) | 0.001 | 0.015 | −4.96 (−10.55 to 0.64) | 0.082 | 0.018 | −5.38 (−11.07 to 0.32) | 0.064 |
| DDS interpersonal distress | 21.0 (20.1) | 22.2 (21.6) | 0.455 | 26.1 (19.3) | 22.8 (22.3) | 0.383 | 0.005 | −2.20 (−8.26 to 3.87) | 0.475 | 0.005 | −2.24 (−8.40 to 3.91) | 0.472 |
| RSESk | 19.6 (5.5) | 18.9 (5.5) | 0.027 | 19.5 (5.4) | 20.2 (4.8) | 0.267 | 0.041 | 1.30 (0.22 to 2.38) | 0.018 | 0.048 | 1.43 (0.34 to 2.52) | 0.011 |
| WHO-5l | 57.6 (18.6) | 56.3 (21.4) | 0.129 | 60.9 (19.8) | 60.6 (17.4) | 0.960 | 0.010 | 3.58 (−2.24 to 9.40) | 0.226 | 0.019 | 4.97 (−0.80 to 10.75) | 0.091 |
All within-group change values referred as t-tests, bModel 1: djusted for baseline value of outcome. Model 2: djusted for baseline value of outcome and sex, cPartial η2, dUnstandardised regression coefficient from linear regression adjusted for covariates, interpreted as difference in group means, eWithin-group or between-groups change equal for HbA1c in per cent or mmol/mol, fPerceived Competence in Diabetes Scale, gHealth Care Climate Questionnaire, hTreatment Self-Regulation Questionnaire, iProblem Areas In Diabetes Scale, jDiabetes Distress Scale, kRosenberg Self-Esteem Scale, lWorld Health Organization five-item Well-Being Index.
GSD, guided self-determination.
Secondary outcomes
The results for all secondary outcome measures are presented in table 2, except SMBG (table 3). Secondary outcomes are clustered into (A) medical measures, (B) type of motivation and (C) psychological measures.
Table 3.
Outcomes for SMBG in control group and GSD intervention group (N=131)
| Within-group changea | Between-groups changeb c | |||||||||
| Control group n (%) | GSD group n (%) | Model 1 | Model 2 | |||||||
| Baseline (n=83) |
Follow-up (n=82) |
p Value | Baseline (n=48) |
Follow-up (n=47) |
p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| SMBG | ||||||||||
| ≥7 times per day | 8 (9.6%) | 54 (65.9%) | <0.001 | 8 (16.7%) | 34 (72.3%) | <0.001 | 1.02 (0.35 to 2.95) | 0.971 | 0.92 (0.31 to 2.74) | 0.887 |
| 1–6 times per day | 51 (61.4%) | 10 (12.2%) | 29 (60.4%) | 8 (17.0%) | 1 | 1 | ||||
| <Daily | 24 (28.9%) | 18 (22.0%) | 11 (22.9%) | 5 (10.6%) | 3.38 (0.65 to 17.58) | 0.148 | 3.12 (0.57 to 16.97) | 0.189 | ||
All within-group change values referred as χ2, bModel 1: adjusted for baseline value of outcome. Model 2: adjusted for baseline value of outcome and sex, cOR from multinomial logistic regression.
GSD, guided self-determination; SMBG, self-monitored blood glucose.
Medical measures
The proportion of SMBG seven times per day or more increased significantly within both groups from baseline to follow-up. There was no significant difference between groups in change at follow-up. The change of HbA1c was not mediated by the change of SMBG (p=0.728, data not shown). Self-perceived diabetes competence (PCDS) and autonomy support from HCPs (HCCQ) showed no significant change, neither within nor between groups.
Type of motivation
The TSRQ Relative Autonomy Index showed a significant improvement within the GSD-GT group (p=0.014), and a significant difference between groups in change (B=0.35, p=0.045). This finding was due primarily to a significant improvement in TSRQ Autonomy for GSD-GT relative to CG (B=0.51, p<0.001). TSRQ control remained unchanged within both groups, with no significant difference between groups.
Psychological measures
Participants in the GSD-GT group exhibited a significant reduction in diabetes-related distress relative to the CG as measured by the PAID, the DDS overall score and the DDS emotional burden subscale (B=−6.96, p=0.002; B=−5.15, p=0.016; and B=−7.19, p=0.019 respectively). In addition, a reduction in DDS regimen distress was reported within the GSD-GT group as well as the CG; the difference in group improvement did not reach significance. The GSD-GT group showed an increase in self-esteem (RSES) relative to the CG (B=1.43, p=0.011); the CG experienced a decrease in self-esteem (p=0.027). The level of overall well-being (WHO-5) showed no significant change, neither within nor between the study groups. Neither PAID, DDS total nor RSES was significantly mediated by TSRQ autonomy (p>0.070, data not shown).
Length of follow-up
Results from linear regression analyses for the association between outcome measures and length of follow-up in the IG are shown in table 4. There were no significant associations, that is, the change was not larger for patients with longer follow-up. Corresponding analyses for the CG also showed no significant associations with length of follow-up (results not shown).
Table 4.
Associations between length of follow-up and change in primary outcome and significant secondary outcomes in the GSD intervention group
| n | Time to follow-upa
Mean (SD) |
Change Mean (SD) |
Bb | p Value | |
| HbA1c, % | 87 | 16.1 (5.6) | −0.51 (1.21) | −0.03 | 0.154 |
| PAIDc | 47 | 17.6 (3.6) | −6.7 (13.8) | −0.2 | 0.764 |
| DDSdoverall | 47 | 17.6 (3.6) | −5.4 (14.0) | −0.4 | 0.542 |
| DDS emotional burden | 48 | 17.6 (3.6) | −7.1 (20.1) | 0.1 | 0.945 |
| RSESe | 47 | 17.6 (3.6) | 0.6 (3.5) | −0.1 | 0.435 |
| TSRQ autonomyf | 49 | 17.6 (3.6) | 0.2 (0.9) | 0.03 | 0.388 |
Total follow-up time from date of randomization measured in months, bB=unstandardized regression coefficients, cProblem Areas in Diabetes scale, dDiabetes Distress Scale, eRosenberg Self-Esteem Scale, fTreatment SelfRegulation Questionnaire, Autonomous motivation.
GSD, guided self-determination;
Discussion
Statement of the principal findings and possible explanations
Contrary to the study hypothesis, a group-based GSD programme among persons with type 1 DM and chronically elevated HbA1c did not improve the medical outcomes (glycaemic control, frequency of SMBG, perceived competence for diabetes management, perception of HCPs being supportive of patient autonomy). The hypothesis regarding improvement of psychological functioning was partly confirmed, with improvement in the level of autonomy-motivated behaviour, diabetes distress and self-esteem, but not the level of general psychological well-being.
Long-standing elevated HbA1c appears to be a complex and heterogeneous phenomenon33 and educational programmes have been evaluated for their effectiveness for improving glycaemic control.13 34 35 Some researchers indicate that evaluation studies need to better understand the complexity of variance in HbA1c. 36 The present study revealed a substantial decrease in HbA1c in both groups, with no difference between groups. It is likely that the change in CG HbA1c was driven by the ‘observer effect,’ that is, that individuals modify or improve their behaviour in response to their awareness of being observed.37 Additional measures of quality of metabolic control would have been follow-up data on mild or severe hypoglycaemia, but these were not assessed.
Strengths of the study in relation to other studies
The GSD-GT intervention demonstrated a significant improvement in diabetes distress. There were no between-group differences in diabetes distress at baseline among those completing the study. The GSD-GT study group consisted of persons with a rather substantial level of self-reported diabetes distress (43% scoring ≥40 on the PAID scale) at baseline, comparable to previous research.19 Our results confirm that although a behavioural intervention may not improve medical outcomes among persons with persistently elevated HbA1c psychological outcomes may improve. This raises the possibility that if interventions resulting in behaviour change also reduce diabetes distress, the gains in diabetes self-management might be sustained over the long term, as argued by Zagarins et al. 38 One of the key elements of GSD is to support persons with diabetes to clarify and express their unique difficulties and barriers to healthy coping. This is done by mobilising their own potential for change in interactions with autonomy supportive HCPs and by using semistructured reflection sheets. Instead of being instructed by HCPs, the core principle in GSD is individualised goal setting, a treatment strategy comparable to other behavioural interventions.14 In a cross-sectional study, HCPs being autonomy supportive were associated with the perceived level of diabetes distress mediated through the perceived level of diabetes competence.23 In the current study, and incongruent with prior research on the GSD approach,18 19 there was no change in HCP autonomy support (HCCQ) and level of diabetes competence (PCDS). However, GSD increased autonomous motivation (TSRQ autonomy) and reduced diabetes distress. In terms of patient outcomes, improvement of autonomous motivation for change is hypothesised to be the key mechanism; autonomy support from HCPs is merely one strategy for activating that mechanism. Although the mediation analysis in the current study did not confirm that increasing autonomy mediates mental health benefits (PAID, DDS total and RSES), the individual’s use of reflection sheets may play a more important role in GSD. This interpretation is consistent with a recently published qualitative paper evaluating how a GSD approach could bring about a dramatic change in a young woman’ s perception of her diabetes.21
Weaknesses of the study and future research
The most obvious limitation of this study is that generalisability might be distorted due to recruitment problems and attrition from the GSD-GT programme. As the power calculation premise was violated, the interpretation of the results is challenging. It remains to a certain extent unclear whether the results are attributable to insufficient power, differential attrition or the effect of the intervention. However, there was no significant difference in change in HbA1c between GSD-GT participants who completed the study versus those who did not (p value from t-test 0.71), indicating that it was not the participants with the poorest effect of the intervention who dropped out. Because of lacking follow-up questionnaires we were not able to do the same test for psychosocial measurements, but it is reasonable to assume that differences between dropouts and completers would mirror what we found for HbA1c.
Nevertheless, it is worthwhile to consider potential reasons for high rates of attrition in the IG. Only four participants dropped out after attending a GSD session, comparable to the two participants in the CG who did not make their follow-up medical visits. Most of the dropouts in the GSD-GT group did not attend a single session (33/39), suggesting some possible interpretations for this postrandomisation/preparticipation attrition:
The time from randomisation to start of GSD-GT (mean 4.9 months, SD=3.6) could perhaps discourage individuals who were highly motivated by time of randomisation but considered the waiting time to be too long until the GSD-GT started. Another aspect might be that some individuals found the intervention too comprehensive and demanding after more detailed information was given with regard to the preintervention worksheets that participants were encouraged to fill in before each group session.
Another limitation is the difference in length of follow-up between the GSD-GT group and the CG. We did however not find any association between the changes in outcomes and length of follow-up in the two groups. Thus, we do not think that the longer follow-up in the GSD-GT group can explain the reported effects of the intervention. In addition, frequency of blood sugar measurement was measured using a rather crude categorisation which might cause loss of important information. Using SMBG as a continuous variable would have been optimal as that would have offered a between-group comparison of the absolute number of measurements per day. However, when designing this study we considered the variation in demands of the disease to differ too much from one day to another, making it difficult to give a valid estimate of the number of measurements per day. We thus choose to use a variable with six categories, but had to collapse this into three categories because of few patients in each category. We tried different categorisations but did not find any significant intervention effect.
Consistent with previous studies, targeting distressed persons with diabetes39 can be difficult because those with the greatest need for psychological support are most likely to drop out of the psychological intervention. Conversely, those who completed the study had lower levels of diabetes distress and HbA1c at baseline, reflecting less need for improvement of competence to manage the everyday aspects of their diabetes care. Perhaps future research on behavioural interventions, especially GSD, should assess patient interest in making changes prior to randomisation so that study participants are appropriate for the intervention.
In the present study, the failure to achieve the primary study goal could possibly be explained by incongruence between the research focus on medical outcomes and participants’ possibly prioritising aspects of life other than clinical improvements. Consequently, it is important to assess individual goals in future research using personalised approaches to improving outcomes. In line with the DAWN2 study, there are still unmet needs of people with diabetes and those who care for them, and promotion of innovative efforts to improve self-management and life skills performance should be facilitated.40 Efforts to manage emotional distress have been suggested as an integral part of diabetes care.41 Improving psychosocial outcomes requires a shift away from a purely medical model to a person-centred model with greater emphasis on psychosocial aspects. Barnard and colleagues have advocated a holistic model of diabetes care aiming at enhanced diabetes self-management and improved outcomes by considering intrinsic thoughts, as well as the environment and therapy regimen.42
Implications for clinicians
The present study exemplifies a complex behavioural intervention with feasibility challenges and does not confirm improvements in HbA1c and other medical outcomes. However, autonomous motivation improved and might be one mechanism for reducing diabetes distress.
Supplementary Material
Acknowledgments
The authors wish to thank all of the diabetes specialist nurses at the outpatient clinic for their contribution to the data collection and the GSD-trained nurses for participating. We also wish to thank all study participants for their valuable contributions and psychiatrist Jorunn Torgauten, Haukeland University Hospital, for her appreciated assistance to secure fidelity to the methodology and appropriate treatment to persons revealing profound psychological issues. We also wish to thank Jannicke Igland for her valuable contribution in revising the statistical analyses.
Footnotes
Contributors: JM, MG, BR, VZ and HT contributed to conception and design of the study. JM collected the data. JM, JA and MP gave substantial contribution to the analysis and interpretation of data. JM wrote the first draft of this manuscript and MG, BR and MP gave substantial contributions to the interpretation of data and revised the manuscript critically. VZ and HT gave substantial contributions to the intellectual content of the manuscript and revised the manuscript critically. All authors read and contributed to the final draft of the paper.
Competing interests: Author MP declared the following potential conflict of interest: Consulting fees from Astra Zeneca, Calibra, Lilly, and Novo Nordisk; advisory panel of GlaxcoSmithCline, Lilly, and Novo Nordisk; research grants from Novo Nordisk; Speaker for Novo Nordisk. The remaining authors declare that they have no conflict of interest.
Patient consent: Obtained.
Ethics approval: The Regional Committee for Medical and Health Research Ethics (approval number 2010/1325).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
REFERENCES
- 1. Clark M. Diabetes self-management education: a review of published studies. Prim Care Diabetes 2008;2:113–20. 10.1016/j.pcd.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 2. Haas L, Maryniuk M, Beck J, et al. . National standards for diabetes self-management education and support. Diabetes Care 2013;36 Suppl 1:S100–S108. 10.2337/dc13-S100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55:68–78. 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- 4. Peyrot M, Rubin RR, Funnell MM, et al. . Access to diabetes self-management education: results of national surveys of patients, educators, and physicians. Diabetes Educ 2009;35:258–63. 10.1177/0145721708329546 [DOI] [PubMed] [Google Scholar]
- 5. Peyrot M, Rubin RR, Lauritzen T, et al. . Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005;22:1379–85. 10.1111/j.1464-5491.2005.01644.x [DOI] [PubMed] [Google Scholar]
- 6. Zoffmann V, Kirkevold M. Life versus disease in difficult diabetes care: conflicting perspectives disempower patients and professionals in problem solving. Qual Health Res 2005;15:750–65. 10.1177/1049732304273888 [DOI] [PubMed] [Google Scholar]
- 7. Livingstone SJ, Looker HC, Hothersall EJ, et al. . Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med 2012;9:e1001321 10.1371/journal.pmed.1001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Achhab Y, Nejjari C, Chikri M, et al. . Disease-specific health-related quality of life instruments among adults diabetic: A systematic review. Diabetes Res Clin Pract 2008;80:171–84. 10.1016/j.diabres.2007.12.020 [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes Care 2007;30:S4 10.2337/dc07-S004 [DOI] [PubMed] [Google Scholar]
- 10. Mühlhauser I, Bruckner I, Berger M, et al. . Evaluation of an intensified insulin treatment and teaching programme as routine management of type 1 (insulin-dependent) diabetes. The Bucharest-Düsseldorf Study. Diabetologia 1987;30:681–90. 10.1007/BF00296989 [DOI] [PubMed] [Google Scholar]
- 11. Fitzpatrick SL, Schumann KP, Hill-Briggs F. Problem solving interventions for diabetes self-management and control: a systematic review of the literature. Diabetes Res Clin Pract 2013;100:145–61. 10.1016/j.diabres.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hopkins D, Lawrence I, Mansell P, et al. . Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care 2012;35:1638–42. 10.2337/dc11-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hermanns N, Kulzer B, Ehrmann D, et al. . The effect of a diabetes education programme (PRIMAS) for people with type 1 diabetes: results of a randomized trial. Diabetes Res Clin Pract 2013;102:149–57. 10.1016/j.diabres.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 15. Müller N, Kloos C, Sämann A, et al. . Evaluation of a treatment and teaching refresher programme for the optimization of intensified insulin therapy in type 1 diabetes. Patient Educ Couns 2013;93:108–13. 10.1016/j.pec.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 16. Zoffmann V, Kirkevold M. Realizing empowerment in difficult diabetes care: a guided self-determination intervention. Qual Health Res 2012;22:103–18. 10.1177/1049732311420735 [DOI] [PubMed] [Google Scholar]
- 17. Olesen ML, Duun-Henriksen AK, Hansson H, et al. . A person-centered intervention targeting the psychosocial needs of gynecological cancer survivors: a randomized clinical trial. J Cancer Surviv 2016;10:832–41. 10.1007/s11764-016-0528-5 [DOI] [PubMed] [Google Scholar]
- 18. Zoffmann V, Lauritzen T. Guided self-determination improves life skills with type 1 diabetes and A1C in randomized controlled trial. Patient Educ Couns 2006;64:78–86. 10.1016/j.pec.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 19. Zoffmann V, Vistisen D, Due-Christensen M. Flexible guided self-determination intervention for younger adults with poorly controlled Type 1 diabetes, decreased HbA1c and psychosocial distress in women but not in men: a real-life RCT. Diabet Med 2015;32:1239–46. 10.1111/dme.12698 [DOI] [PubMed] [Google Scholar]
- 20. Husted GR, Thorsteinsson B, Esbensen BA, et al. . Effect of guided self-determination youth intervention integrated into outpatient visits versus treatment as usual on glycemic control and life skills: a randomized clinical trial in adolescents with type 1 diabetes. Trials 2014;15:321 10.1186/1745-6215-15-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zoffmann V, Prip A, Christiansen AW. Dramatic change in a young woman's perception of her diabetes and remarkable reduction in HbA1c after an individual course of Guided Self-Determination. BMJ Case Rep 2015;2015 10.1136/bcr-2015-209906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zoffmann V, Harder I, Kirkevold M. A person-centered communication and reflection model: sharing decision-making in chronic care. Qual Health Res 2008;18:670–85. 10.1177/1049732307311008 [DOI] [PubMed] [Google Scholar]
- 23. Mohn J, Graue M, Assmus J, et al. . Self-reported diabetes self-management competence and support from healthcare providers in achieving autonomy are negatively associated with diabetes distress in adults with Type 1 diabetes. Diabet Med 2015;32:1513–9. 10.1111/dme.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoelzel W, Weykamp C, Jeppsson JO, et al. . IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem 2004;50:166–74. 10.1373/clinchem.2003.024802 [DOI] [PubMed] [Google Scholar]
- 25. Hermanns N, Kulzer B, Krichbaum M, et al. . How to screen for depression and emotional problems in patients with diabetes: comparison of screening characteristics of depression questionnaires, measurement of diabetes-specific emotional problems and standard clinical assessment. Diabetologia 2006;49:469–77. 10.1007/s00125-005-0094-2 [DOI] [PubMed] [Google Scholar]
- 26. Polonsky WH, Fisher L, Earles J, et al. . Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–31. 10.2337/diacare.28.3.626 [DOI] [PubMed] [Google Scholar]
- 27. Williams GC, McGregor HA, King D, et al. . Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Couns 2005;57:39–45. 10.1016/j.pec.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 28. Alessandri G, Vecchione M, Eisenberg N, et al. . On the factor structure of the Rosenberg (1965) General Self-Esteem Scale. Psychol Assess 2015;27:621–35. 10.1037/pas0000073 [DOI] [PubMed] [Google Scholar]
- 29. Hajos TR, Pouwer F, Skovlund SE, et al. . Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with Type 1 or Type 2 diabetes mellitus. Diabet Med 2013;30:e63–e69. 10.1111/dme.12040 [DOI] [PubMed] [Google Scholar]
- 30. Williams GC, McGregor HA, Zeldman A, et al. . Testing a self-determination theory process model for promoting glycemic control through diabetes self-management. Health Psychol 2004;23:58–66. 10.1037/0278-6133.23.1.58 [DOI] [PubMed] [Google Scholar]
- 31. Levesque CS, Williams GC, Elliot D, et al. . Validating the theoretical structure of the Treatment Self-Regulation Questionnaire (TSRQ) across three different health behaviors. Health Educ Res 2007;22:691–702. 10.1093/her/cyl148 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Process of translation and adaptation of instruments. 2015.
- 33. Devries JH, Snoek FJ, Heine RJ. Persistent poor glycaemic control in adult Type 1 diabetes. A closer look at the problem. Diabet Med 2004;21:1263–8. 10.1111/j.1464-5491.2004.01386.x [DOI] [PubMed] [Google Scholar]
- 34. Hill-Briggs F, Gemmell L. Problem solving in diabetes self-management and control: a systematic review of the literature. Diabetes Educ 2007;33:1032–50. 10.1177/0145721707308412 [DOI] [PubMed] [Google Scholar]
- 35. Bott U, Bott S, Hemmann D, et al. . Evaluation of a holistic treatment and teaching programme for patients with Type 1 diabetes who failed to achieve their therapeutic goals under intensified insulin therapy. Diabet Med 2000;17:635–43. 10.1046/j.1464-5491.2000.00345.x [DOI] [PubMed] [Google Scholar]
- 36. Rogvi S, Tapager I, Almdal TP, et al. . Patient factors and glycaemic control--associations and explanatory power. Diabet Med 2012;29:e382–e389. 10.1111/j.1464-5491.2012.03703.x [DOI] [PubMed] [Google Scholar]
- 37. Shadish WR, Cook TD, Campbell DT. Experimental and quasi-experimental designs for generalized causal inference. New York: Houghton Mifflin Company, 2002. [Google Scholar]
- 38. Zagarins SE, Allen NA, Garb JL, et al. . Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. J Behav Med 2012;35:299–304. 10.1007/s10865-011-9359-z [DOI] [PubMed] [Google Scholar]
- 39. van Son J, Nyklícek I, Pop VJ, et al. . The effects of a mindfulness-based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind): a randomized controlled trial. Diabetes Care 2013;36:823–30. 10.2337/dc12-1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nicolucci A, Kovacs Burns K, Holt RI, et al. . Diabetes Attitudes, Wishes and Needs second study (DAWN2TM): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. [Erratum appears in Diabet Med. 2013 Oct;30(10):1266]. Diabet Med 2013;30::767-777.. [DOI] [PubMed] [Google Scholar]
- 41. Gonzales R, Handley MA. Improving glycemic control when "usual" diabetes care is not enough. Arch Intern Med 2011;171:1999–2000. 10.1001/archinternmed.2011.496 [DOI] [PubMed] [Google Scholar]
- 42. Barnard KD, Lloyd CE, Dyson PA, et al. . Kaleidoscope model of diabetes care: time for a rethink? Diabet Med 2014;31:522–30. 10.1111/dme.12400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.