Abstract
Diabetic ketoacidosis (DKA) remains a common medical emergency. Over the last few years, new national guidelines have changed the focus in managing the condition from being glucose-centered to ketone-centered. With the advent of advancing technology and the increasing use of hand-held, point-of-care ketone meters, greater emphasis is placed on making treatment decisions based on these readings. Furthermore, recent warnings about euglycemic DKA occurring in people with diabetes using sodium-glucose co-transporter 2 (SGLT-2) inhibitors urge clinicians to inform their patients of this condition and possible testing options. This review describes the reasons for a change in treating DKA, and outlines the benefits and limitations of using ketone readings, in particular highlighting the difference between urine and capillary readings.
Keywords: diabetes, diabetic ketoacidosis, ketones, measurement, SGLT-2, glucose uptake
Abbreviations: CoA - coenzyme A, DKA - diabetic ketoacidosis, SGLT-2 - sodium-glucose co-transporter 2, TCA - tricarboxylic acid
1. Introduction
Diabetic ketoacidosis (DKA) is the hallmark of absolute insulin deficiency. It occurs predominantly in people with type 1 diabetes, although it is occasionally seen in type 2 diabetes or gestational diabetes as well. Insulin has several actions, depending on the concentration present in the circulation. At a relatively high concentration it is responsible for cellular uptake of glucose, inhibition of glycogenolysis, and stimulation of glycogen synthesis. At a very low concentration, insulin switches off lipolysis and ketogenesis. However, in situations of absolute insulin deficiency or when concentration of counter-regulatory hormones such as glucagon, cortisol, or catecholamines is high (e.g. acute illness), there is little or no insulin-mediated cellular glucose uptake, resulting in the need for an alternative energy substrate.
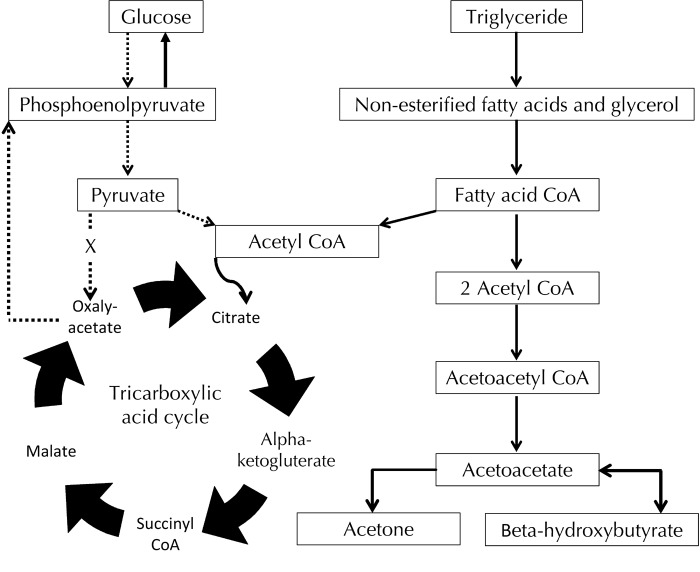
The metabolic derangement occurring as a result of insulin deficiency causes hormone-sensitive lipase activity to increase in adipocytes and eventually the generation of free fatty acids from triglyceride breakdown [1]. The fatty acids are beta oxidized to form acetyl coenzyme A (CoA), which usually enters the tricarboxylic acid (TCA) cycle. However, in this situation of absolute insulin deficiency and fatty acid breakdown, the elevated amount of acetyl CoA entering the TCA cycle overwhelms the enzyme systems, and is then converted into ketone bodies in the liver [2]. These ketones provide an alternative energy substrate, mainly in the form of β-hydroxybutyrate and acetoacetate at an approximate ratio of 10:1 [3]. Figure 1 shows how increased lipolysis results in the liberation of fatty acids and subsequent production of increased acetyl Co-A concentrations. The acetyl CoA acts as the substrate for hepatic ketogenesis, with the predominant ketones being acetoacetate, acetone, and beta-hydroxybutyrate.
Figure 1. A simplified illustration showing the metabolic pathway for ketogenesis.
During insulin deficiency, glucose uptake into cells is limited, and there is a need for an alternative energy substrate. The breakdown of non-esterified fatty acids allows the entry of fatty acid CoA to enter the tricarboxylic acid cycle, thus generating ATP. However, excess fatty acid CoA production leads to the production of acetoacetate (a ketoacid) and beta-hydroxybutyrate (a hydroxyl-acid), causing ketoacidosis in periods of extended insulin deficiency.
Whilst the vast majority of DKA cases occur in those with type 1 diabetes, recent reports have stated that the use of sodium-glucose co-transporter 2 (SGLT-2) increases the risk of developing euglycemic DKA [4]. These drugs have an insulin-independent mode of action, and whilst currently licensed only for use in people with type 2 diabetes, are being trialed in those with type 1 diabetes. As glycemic control improves, there is often a subsequent reduction in insulin dose, which increases the risk of developing DKA [5].
2. Prevalence of DKA
How commonly DKA occurs varies geographically. In the UK, the crude one-year incidence in people with type 1 diabetes has been reported as 3.6%, equating to 4.8 episodes per 100 patient years [6, 7]. In the Western Pacific region, the rate amongst children is 10 per 100 patient years [8], but is much lower in some parts of Northern Europe [9, 10]. In North America, the one-year incidence is between 1% and 5% of people with type 1 diabetes [11, 12], corresponding to about 145,000 cases per year [13]. Most cases occur in those with type 1 diabetes, but in some regions up to 50% of cases may be found in those with type 2 diabetes, depending on ethnicity and family history [14, 15]. However, type 1 diabetes patients tend to have the most extensive metabolic derangements, with a pH lower than that in type 2 diabetes patients [16]. Treatment of DKA remains expensive, with individual admission costs estimated to ~$17,500 in the US [17] and average costs of £886 to £1803 per episode in the UK [18-20].
While in several previous studies DKA was the presenting feature of type 1 diabetes in up to 30% of cases [11, 21-25], recent data from national surveys in the UK have reported that DKA was the first presentation of type 1 diabetes in adults or adolescents in 3-6% of cases only [26, 27]. In addition, the data from the adult survey of over 280 admissions with DKA also showed that mortality was lower than previously reported, with no deaths in the 2016 data, compared with a mortality of 3.9% and 1.8% in UK populations described previously, and 1.7% mortality described recently in a cohort from China [28-30].
3. Prevention of DKA and ongoing management
Patients with insulin-treated diabetes are taught to measure their glucose concentrations by hand-held point-of-care glucometers. Some meters encourage patients to test for ketones if the glucose concentration is higher than 13.9 mmol/l (250 mg/dl). This is the concentration of glucose required to make a diagnosis of DKA according to the ADA guidelines [17]. If the ketone concentration is high, then patients should follow sick-day rules, see their doctor, or seek urgent medical attention, depending on the concentration and their condition.
The use of point-of-care ketone monitoring has been shown to prevent DKA and reduce unnecessary hospital admissions [31, 32]. However, the availability of bedside ketone meters in hospital is limited [33], and even if meters are available, staff is not available over 24 hours or able to use them appropriately [33]. Finally, the same UK national survey showed that quality assurance schemes for ketone meters were not as widely available as those for bedside glucose meters [33]. Despite these limitations, point-of-care testing devices for ketones are becoming more commonly used in the management of inpatient dysglycemia.
4. Confounders and interference
Bedside capillary ketone meters usually measure β-hydroxybutyrate. Previous data have shown that, at high concentrations, these measurements may have a wide coefficient of variation. Therefore, a quality assurance scheme must be in place to enable calibration with laboratory analyzers, using dilution methods if necessary to ensure accuracy [34, 35].
Glucose meters may also show inaccurate readings in different circumstances, including abnormal hematocrit, changes in oxygen concentrations or pH, presence of peripheral edema, hyperuricemia, hyperlipidemia, and the use of paracetamol (acetaminophen), vitamin C, or dopamine. Many of these issues are discussed in detail elsewhere [36]. One study has investigated whether ketone meters can also be affected, in particular when hematocrit is low. The authors showed that the Optium Freestyle® meter (Abbott Laboratories Ltd, Berkshire, UK) ketone reading can be artificially elevated [37]. However, the impact of hematocrit has been minimized in the StatStrip® (Nova Biomedical, Cheshire, UK) ketone device because it has an inbuilt correction factor for hematocrit interference [37]. With the use of vitamin C, both types of meters were affected, with the Optium Freestyle® providing falsely high ketone readings, and the StatStrip® showing low readings [37]. What remains unknown is what concentration of vitamin C is needed in the sample to interfere with a reading, or how much needs to be ingested before the accuracy of the meter is degraded. The questions of the kind of substances that interfere with the readings and of their quantitative impacts remain largely unanswered, and need to be investigated in more detail.
Many hand-held ketone meters use β-hydroxybutyrate dehydrogenase coupled with electrochemical detection. This method has a reasonable correlation with the previous standard spectrophotometric method of detection [32, 38]. However, the issue of decreasing precision with large coefficients of variation still exists, especially at high ketone concentrations (>5.0 mmol/l) [39, 40]. However, the measurement of capillary ketone concentrations can provide a rough indication as to whether treatment is working. The measurement of ketone concentrations can be carried out either by using a point-of-care meter or a blood gas machine [41]. The UK DKA guidelines suggest that ketone concentrations should decrease by 0.5 mmol/l per hour when fluid and insulin treatment is working properly [41].
5. Further limitations of urine and blood testing
Limitations of urine and blood testing are shown in Table 1. Urine ketones are no longer recommended for use in the management of DKA for several reasons:
Table 1. Comparison of the advantages and disadvantages of ketone measurement methods.
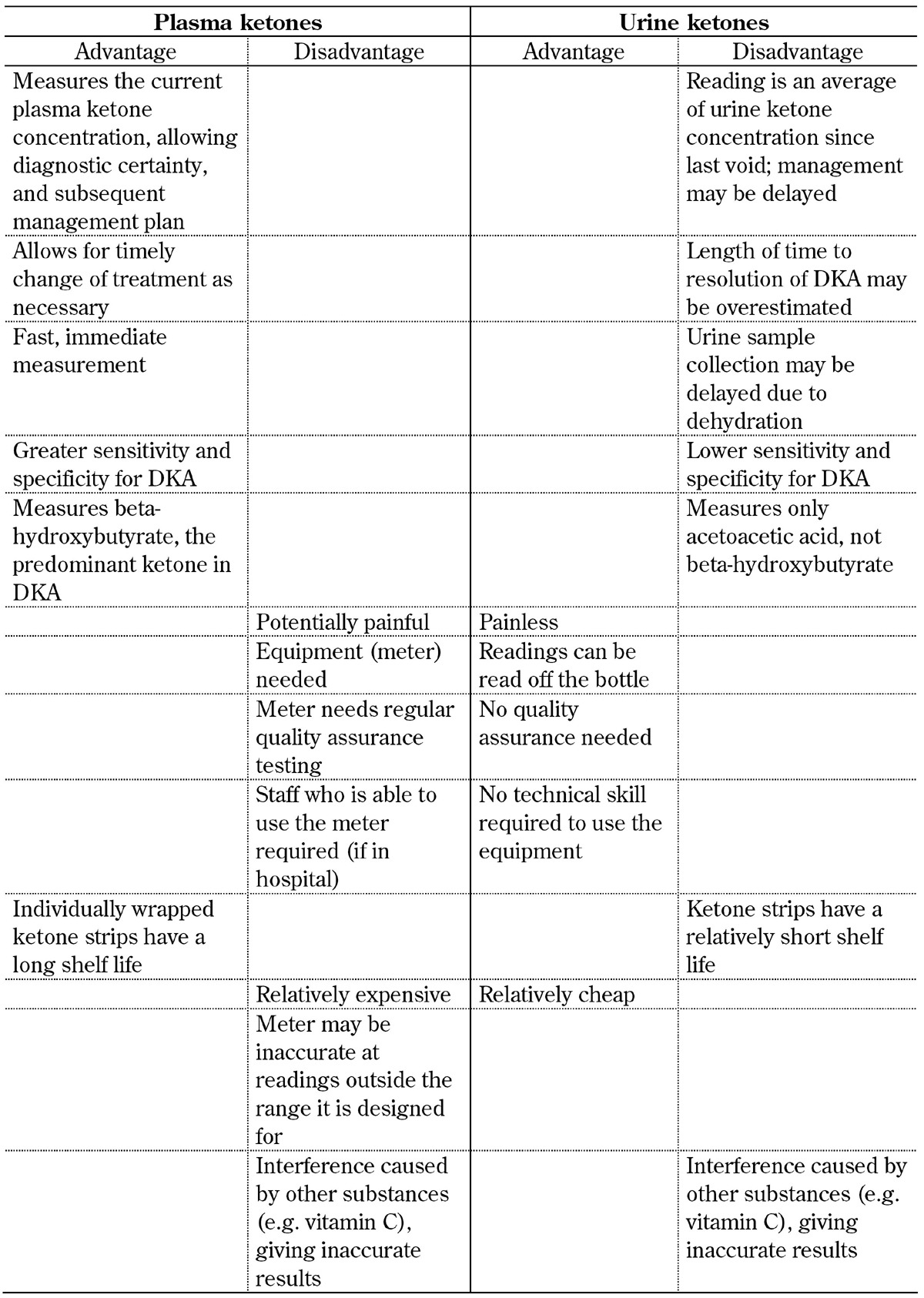
The urine ketone stick test (Ketostix®, Bayer Diabetes, Berkshire, UK) is a relatively cheap method of detection that―unlike plasma ketone measurement―requires no special equipment, and does not require any training to use. The urine test uses a nitroprusside reaction, first described in 1908, and gives a semi-quantitative measure of acetoacetate [42]. However, this test does not detect β-hydroxybutyrate, the predominant metabolite (a hydroxyl-acid, not a ketone) present in DKA. Because of the imbalance between the concentration of acetone and β-hydroxybutyrate, the quantity of ketones measured in the urine does not equate to the plasma ketone concentration.
Patients with DKA are dehydrated such that urine output is low. It may take several hours until urine is produced again, which delays treatment instigation unnecessarily.
Any estimation of urine ketones collected in this way will be an average of the concentration within the urine held in the bladder since the last void.
Finally, if treatment is initiated once and acidosis disappears, β-hydroxybutyrate is oxidized to acetoacetate causing urine ketone readings to rise, even if blood β-hydroxybutyrate concentrations are dropping. The paradoxical rise in urinary acetoacetate would give the false impression that the condition is not improving [43].
Despite these limitations, it is clear that urine ketones are still commonly used, particularly in low-resource environments [30]. Some of the differences and limitations associated with measuring ketones in blood and urine are shown in Table 1. Because of the benefits of plasma ketone readings, they should be used as part of the sick-day rules that patients with type 1 diabetes apply when they are not well to ensure that they do not develop ketoacidosis. These sick-day rules are shown in Table 2.
Table 2. Simple sick-day rule.

There has been some debate in the literature as to which concentration of ketones should be considered 'significant'. Because concentrations of up to 0.25 mmol/l may commonly be found after an overnight fast, concentrations above 0.3 mmol/l have been regarded as significantly elevated [44-47]. However, several studies have compared bedside values with laboratory samples [48, 35, 49], and found a consensus that, using either method, a value of 3.0 mmol/l should be used as the cut-off for diagnosing DKA, or at the very least, the concentration at which urgent medical attention should be sought [35, 41, 47, 50].
Previous work has shown that urine ketone testing and capillary testing had sensitivities of 95-100% in diagnosing DKA (using 3.0 mmol/l as cut-off), but that the capillary testing was significantly faster, and had higher specificity (78-94% vs. <50%) [49-55]. Thus, whilst capillary and urine ketone concentrations are both excellent at ruling out DKA, the use of capillary ketones has the advantage of sparing unnecessary time and expensive laboratory investigation because their use is likely to reduce the number of false positive results. This is important because hyperglycemic patients with the hyperosmolar hyperglycemic syndrome need treatment very different from that of DKA [41, 56].
6. Link between ketone concentration and severity
Whilst ketone concentrations may appear to be related to the severity of DKA, it has been shown that this is not the case. Several groups have correlated ketone concentrations (point-of-care and laboratory) with serum bicarbonate, anion gap, and arterial pH. They have reported correlations between -0.33 and -0.76 for pH, between -0.25 and -0.74 for serum bicarbonate, and between 0.16 and 0.59 for anion gap [35, 48, 57-60]. In essence, these correlations―many of which were statistically significant―are not sufficiently robust to allow for the ketone concentrations to be used as a marker for the severity of DKA.
Because of possible wide coefficients of variation at high ketone concentrations, bedside ketone meters should only be used to assess whether treatment is working and to make sure that ketone concentrations are decreasing rather than to judge the severity of DKA. Although it is acknowledged that ketone concentrations should decrease by 0.5 mmol/l/hr [41], at high ketone concentrations, the large coefficient of variation may make more accurate measurements difficult. However, from a clinical and practical perspective, ketone reduction considerations should be taken into account, in combination with the other biochemical and physical findings, when treating a patient with DKA. Certainly, by the time changes need to be carried out in the intravenous insulin infusion rate (e.g. from a fixed rate intravenous insulin infusion to a variable rate) or in fluids (e.g. from 0.9% sodium chloride solution to 10% dextrose), when the glucose concentration falls below 14.0 mmol/l (252 mg/dl) again, the ketone concentration should be <0.6 mmol/l, which is within the range where the meter is accurate. The meter is considered to be accurate up to a ketone concentration of 3.0 mmol/l, which is the threshold for the diagnosis of DKA.
One of the key changes in the UK DKA guidelines has been the move towards the use of venous blood samples rather than arterial blood samples for gas measurement procedures [41]. This is because the differences between the two types of samples are so small that they make no real difference [61, 62]. Also, measurement of arterial blood gases involves painful procedures, and are not free of risk.
7. Cost
Very few of the available point-of-care glucose meters also measure ketones. In the UK, there are currently 38 different glucose meters, of which only 2 can also measure ketones [63]. A full list of glucose meters and their characteristics, which also indicates which meter can measure ketones, can be found elsewhere [64]. The small number of meters that can measure ketones means that they are more expensive than glucose-only meters. Also, the strips themselves cost significantly more than the glucose strips. For example, in the UK, 50 blood glucose testing strips for the FreeStyle Optium Neo® (Abbott Laboratories Ltd, Berkshire UK) cost £15.71, while 50 ketone strips for the same meter cost £105.70. This is in contrast to the £3.06 needed for 50 urine ketone sticks (Ketostix® (Ascensia Diabetes Care, Basel)). However, this high cost has to be weighed against the fact that the use of point-of-care meters in aiding the diagnosis and subsequent management of DKA has major advantages over urine ketone estimation. Further benefits of using blood ketone meters are that readings are available in real time, and that accuracy between venous and capillary samples is comparable [38].
Not least, it has been shown that the use of ketone meters is associated with reduced emergencies and hospitalizations and a shorter recovery time [65]. Their use can also help in the initial diagnosis of DKA [41]. Whilst the American Diabetes Association guidelines suggest that a diagnosis of DKA should be made only if a blood glucose concentration is >250 mg/dl (13.5 mmol/l), they acknowledge that about 10% of all cases could be associated with euglycemic DKA [17]. In this regard, the use of ketone meters is even more advisable in patients treated with SGLT-2 inhibitors, as this class of antidiabetic agents is associated with an increased risk of developing euglycemic DKA which may increase the prevalence [66]. Thus, the performance of point-of-care ketone measurement is becoming a key element in the diagnosis of DKA independent of the admission glucose values.
It is clear that the provision of a ketone meter alone is not sufficient to reduce DKA. Patients and healthcare professionals need to be educated on how to use the technology. This is important when using ketone measurement as a means of admission avoidance. Previous work has shown that urine ketone strips can be used as a means of admission avoidance in an adult population when combined with advice by telephone [67]. Other authors have looked at the use of capillary ketone meters in a pediatric population and found that overall emergency room visits and admissions were significantly reduced (not just for DKA) [68]. These data suggest that the use of ketone meters in the prevention of DKA and subsequent hospitalization make the technology cost-effective. However, this is a conclusion derived from different findings rather than a hypothesis that has been formally tested in a well-designed clinical trial. Neither has a trial been performed to show that the use of bedside ketone meters in those already admitted with DKA is associated with a shorter length of hospital stay than laboratory ketone measurements.
8. The management of suspected euglycemic DKA with SGLT-2 inhibitor use
Diabetic ketoacidosis should be suspected in any patient who uses this newer class of agent and who presents with classic symptoms. The diagnosis should be made using the three usual criteria:
History of diabetes (regardless of presenting glucose)
A pH of <7.3 with a serum bicarbonate concentration of <15 mmol/l
A plasma ketone concentration of >3.0 mmol/l [41]
The anion gap may also be raised. In this scenario, the drug should be stopped, and the DKA treated as per local guidelines, but using the pH value, bicarbonate concentration, and anion gap as markers, rather than glucose.
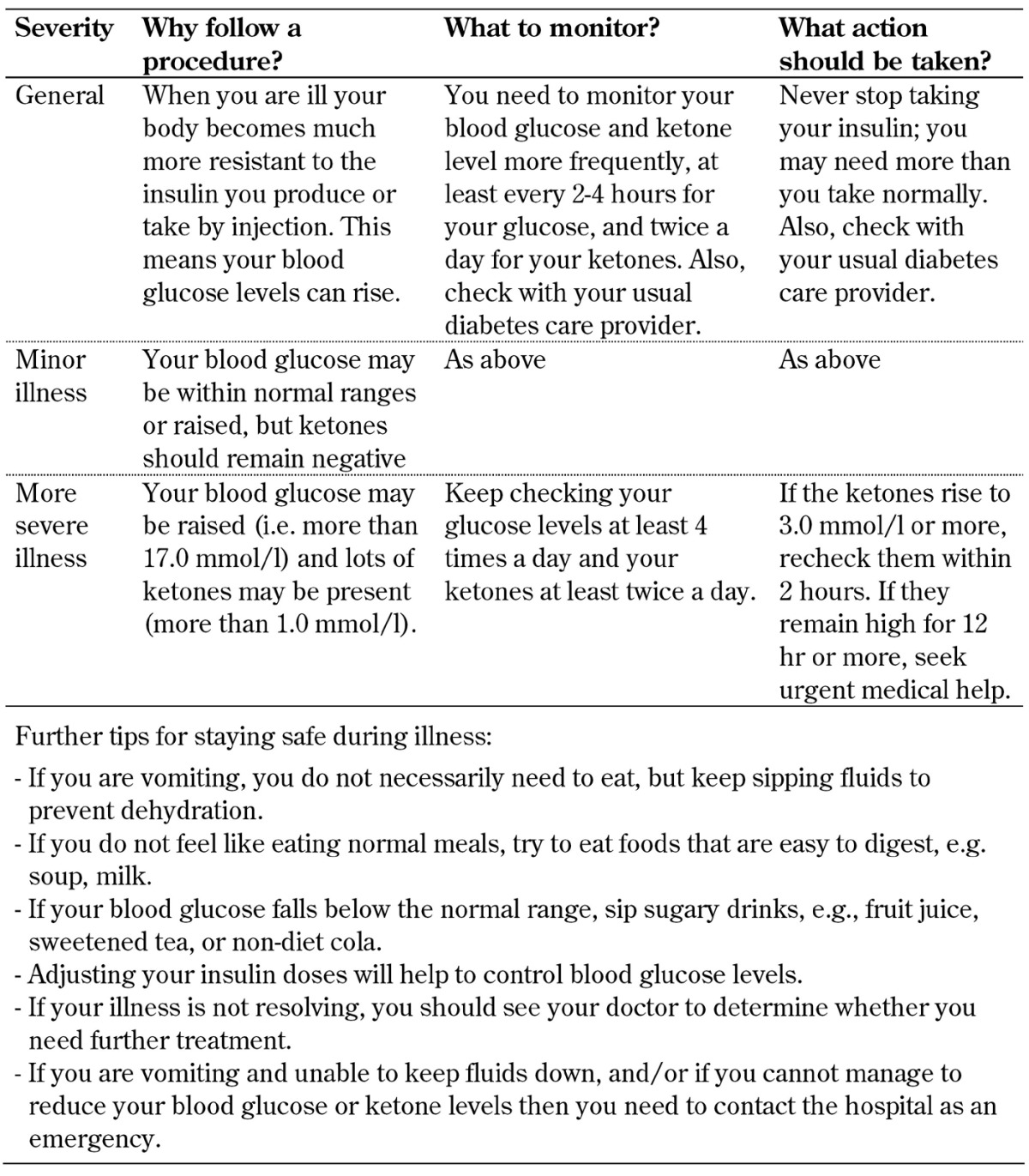
In an attempt to prevent SGLT-2 inhibitor-associated DKA, the drug should be avoided, or stopped, during times of extreme stress, e.g. prior to major surgery, as the patient may be in a catabolic state, or during hospitalization for major illness. Another condition that may predispose to SGLT-2 inhibitor-associated DKA is the very-low-calorie diet. In this regard, it is important that all patients, those with type 1 and type 2 diabetes, are well-informed about the sick-day rules (Tables 2 and 3). Usually, sick-day rules apply to patients on regular insulin therapy and state that the medication must never be stopped irrespective of the patient’s wellbeing. Currently, SGLT-2 inhibitors are not licensed for use in type 1 diabetes, but clinical trials are underway, and an increasing proportion of type 1 diabetes patients is using them already. Many people with type 2 diabetes are also on insulin and SGLT-2 inhibitors. It is thus important to understand the sick-day rules in such a way that the continuance of insulin therapy is ensured, but the use of SGLT-2 inhibitors is stopped when a patient is unwell (Table 2).
Table 3. What to do when you are not well? Practical recommendations for diabetes patients including the monitoring of ketone levels.
9. Summary and future work
In the last few years, national guidelines have embraced the use of new technologies that allow the focus to be on the 'K' and the resultant 'A' in the treatment of DKA, rather than on the 'D'. There are major advantages to measuring capillary ketone concentrations using point-of-care devices, namely equivalent sensitivity and superior specificity to urine ketone testing, when making the initial diagnosis of DKA. Another advantage is the reduced time to diagnosis compared with laboratory methods. The use of bedside point-of-care ketone devices has also changed the ongoing management of DKA. However, there are also concerns about the wide and regular use of ketone meters, namely the cost of testing strips and the degree of accuracy when ketone concentrations are high.
Given the potential difficulties with urine ketone measurements, attention needs to be paid to ensuring that plasma ketone testing is easy to use and affordable even in low-resource environments, and that it does not require specialist equipment other than commonly used hand-held, point-of-care glucose meters. Work needs to be done to ensure the accuracy of ketone meters at a wider concentration to minimize false positive and false negative readings, to avoid potential errors in diagnosis and management of this potentially life-threatening disease. At the same time, technologies need to be developed to minimize the number of substances that interfere with the readings. Finally, further work also needs to be done to determine the best way to use this new technology.
Acknowledgments
Disclosures
The author reported no conflict of interests.
References
- 1.Foster DW, McGarry JD. The metabolic derangements and treatment of diabetic ketoacidosis. N Eng J Med. 1983;309(3):159–169. doi: 10.1056/NEJM198307213090307. [DOI] [PubMed] [Google Scholar]
- 2.McGarry JD, Woeltje KF, Kuwajima M, Foster DW. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- 3.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 4.Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G. et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 5.Peters AL, Henry RR, Thakkar P, Tong C, Alba M. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39(4):532–538. doi: 10.2337/dc15-1995. [DOI] [PubMed] [Google Scholar]
- 6.Health and Social Care Information Centre. National Diabetes Audit 2012-2013. Report 2: Complications and Mortality. [Accessed 6th September 2016]. http://www.hscic.gov.uk/catalogue/PUB16496/nati-diab-audi-12-13-rep2.pdf.
- 7.Karges B, Rosenbauer J, Holterhus PM, Beyer P, Seithe H, Vogel C, Bockmann A, Peters D, Muther S, Neu A. et al. Hospital admission for diabetic ketoacidosis or severe hypoglycemia in 31,330 young patients with type 1 diabetes. Eur J Endocrinol. 2015;173(3):341–350. doi: 10.1530/EJE-15-0129. [DOI] [PubMed] [Google Scholar]
- 8.Craig ME, Jones TW, Silink M, Ping YJ. Diabetes care, glycemic control, and complications in children with type 1 diabetes from Asia and the Western Pacific Region. J Diabetes Complications. 2016;21(5):280–287. doi: 10.1016/j.jdiacomp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Henriksen OM, Roder ME, Prahl JB, Svendsen OL. Diabetic ketoacidosis in Denmark. Diabetes Res Clin Pract. 2007;76(1):51–56. doi: 10.1016/j.diabres.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Rosilio M, Cotton JB, Wieliczko MC, Gendrault B, Carel J, Couvaras O, Ser N, Gillet P, Soskin S, Garandeau P. et al. Factors associated with glycemic control: A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. Diabetes Care. 1998;21(7):1146–1153. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 11.Faich GA, Fishbein HA, Ellis SE. The epidemiology of diabetic acidosis: a population-based study. Am J Epidemiol. 1983;117(5):551–558. doi: 10.1093/oxfordjournals.aje.a113577. [DOI] [PubMed] [Google Scholar]
- 12.Ginde AA, Pelletier AJ, Camargo CA. National study of U.S. emergency department visits with diabetic ketoacidosis, 1993-2003. Diabetes Care. 2006;29(9):2117–2119. doi: 10.2337/dc06-0627. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Number (in thousands) of hospital discharges with diabetic ketoacidosis (DKA) as first-listed diagnosis, United States, 1988-2009. [Accessed 6th September 2016]. http://www.cdc.gov/diabetes/statistics/dkafirst/fig1.htm.
- 14.Wang ZH, Kihl-Selstam E, Eriksson JW. Ketoacidosis occurs in both Type 1 and Type 2 diabetes - a population-based study from Northern Sweden. Diabetic Med. 2008;25(7):867–870. doi: 10.1111/j.1464-5491.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 15.Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: Ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350–357. doi: 10.7326/0003-4819-144-5-200603070-00011. [DOI] [PubMed] [Google Scholar]
- 16.Newton CA, Raskin P. Diabetic ketoacidosis in type 1 and type 2 diabetes mellitus: Clinical and biochemical differences. Arch Intern Med. 2004;164(17):1925–1931. doi: 10.1001/archinte.164.17.1925. [DOI] [PubMed] [Google Scholar]
- 17.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allan J, Howarth S. [Accessed 6th September 2016];Diabetes and DKA in England's primary care trusts. http://dwed.org.uk/s/DwedReportDKAPCT.pdf. [Google Scholar]
- 19.Byrne ML, Mills LS, Saunders S, Garrett CJ. The economic burden and mortality of recurrent diabetic ketoacidosis: a 3 year cost analysis and mortality follow-up at a district general hospital. Poster 496, 2016. [Accessed 9th February 2017]. http://onlinelibrary.wiley.com/doi/101111/dme51_13048/epdf.
- 20.Mohindru B, Dhatariya KK, Fordham R. Diabetic ketoacidosis in the UK: a cost of illness study based on nationally collected data and use of Joint British Diabetes Societies Inpatient Care Group guidelines. Submitted for publication. [Google Scholar]
- 21.Ellemann K, Soerensen JN, Pedersen L, Edsberg B, Andersen OO. Epidemiology and treatment of diabetic ketoacidosis in a community population. Diabetes Care. 1984;7(6):528–532. doi: 10.2337/diacare.7.6.528. [DOI] [PubMed] [Google Scholar]
- 22.Bui TP, Werther GA, Cameron FJ. Trends in diabetic ketoacidosis in childhood and adolescence: a 15-yr experience. Pediatr Diabetes. 2002;3(2):82–88. doi: 10.1034/j.1399-5448.2002.30204.x. [DOI] [PubMed] [Google Scholar]
- 23.Dunger DB, Sperling MA, Acerini CL, Bohn DJ, Daneman D, Danne TP, Glaser NS, Hanas R, Hintz RL, Levitsky LL. et al. European Society for Paediatric Endocrinology/Lawson Wilkins Pediatric Endocrine Society Consensus Statement on diabetic ketoacidosis in children and adolescents. Pediatrics. 2004;113(2):e133–e140. doi: 10.1542/peds.113.2.e133. [DOI] [PubMed] [Google Scholar]
- 24.Rewers A, Dong F, Slover RH, Klingensmith G, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA. 2015;313(15):1570–1572. doi: 10.1001/jama.2015.1414. [DOI] [PubMed] [Google Scholar]
- 25.Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–2894. doi: 10.1007/s00125-012-2690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhatariya KK, Nunney I, Higgins K, Sampson MJ, Iceton G. A national survey of the management of diabetic ketoacidosis in the UK in 2014. Diabetic Med. 2016;33(2):252–260. doi: 10.1111/dme.12875. [DOI] [PubMed] [Google Scholar]
- 27.Edge JA, Nunney I, Dhatariya KK. Diabetic ketoacidosis in an adolescent and young adult population in the UK in 2014: a national survey comparison of management in paediatric and adult settings. Diabetic Med. 2016;33(10):1352–1359. doi: 10.1111/dme.13065. [DOI] [PubMed] [Google Scholar]
- 28.Basu A, Close CF, Jenkins D, Krentz AJ, Natrass M, Wright AD. Persisting mortality in diabetic ketoacidosis. Diabetic Med. 1993;10(3):282–284. doi: 10.1111/j.1464-5491.1993.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 29.Wright J, Ruck K, Rabbitts R, Charlton M, De P, Barrett T, Baskar V, Kotonya C, Saraf S, Narendran P. Diabetic ketoacidosis (DKA) in Birmingham, UK, 2000-2009: an evaluation of risk factors for recurrence and mortality. Br J Diabetes Vasc Dis. 2009;9(6):278–282. [Google Scholar]
- 30.Xu Y, Bai J, Wang G, Zhong S, Su X, Huang Z, Chen G, Zhang J, Hou X, Yu X. et al. Clinical profile of diabetic ketoacidosis in tertiary hospitals in China: a multicentre, clinic-based study. Diabetic Med. 2016;33(2):261–268. doi: 10.1111/dme.12820. [DOI] [PubMed] [Google Scholar]
- 31.Weber C, Kocher S, Neeser K, Joshi SR. Prevention of diabetic ketoacidosis and self-monitoring of ketone bodies: an overview. Curr Med Res Opin. 2009;25(5):1197–1207. doi: 10.1185/03007990902863105. [DOI] [PubMed] [Google Scholar]
- 32.Voulgari C, Tentolouris N. The performance of a glucose-ketone meter in the diagnosis of diabetic ketoacidosis in patients with type 2 diabetes in the emergency room. Diabetes Technol Ther. 2010;12(7):529–535. doi: 10.1089/dia.2010.0011. [DOI] [PubMed] [Google Scholar]
- 33.Dhatariya K, Nunney I, Iceton G. Institutional factors in the management of adults with diabetic ketoacidosis in the UK: results of a national survey. Diabetic Med. 2016;33(2):269–270. doi: 10.1111/dme.12877. [DOI] [PubMed] [Google Scholar]
- 34.Noyes KJ, Crofton P, Bath LE, Holmes A, Stark L, Oxley CD, Kelnar CJ. Hydroxybutyrate near-patient testing to evaluate a new end-point for intravenous insulin therapy in the treatment of diabetic ketoacidosis in children. Pediatr Diabetes. 2007;8(3):150–156. doi: 10.1111/j.1399-5448.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh-Ali M, Karon BS, Basu A, Kudva YC, Muller LA, Xu J, Schwenk WF, Miles JM. Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31(4):643–647. doi: 10.2337/dc07-1683. [DOI] [PubMed] [Google Scholar]
- 36.Rebel A, Rice MA, Fahy BG. The accuracy of point-of-care glucose measurements. J Diabetes Sci Technol. 2012;6(2):396–411. doi: 10.1177/193229681200600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceriotti F, Kaczmarek E, Guerra E, Mastrantonio F, Lucarelli F, Valgimigli F, Mosca A. Comparative performance assessment of point-of-care testing devices for measuring glucose and ketones at the patient bedside. J Diabetes Sci Technol. 2015;9(2):268–277. doi: 10.1177/1932296814563351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Byrne HA, Tieszen KL, Hollis S, Dornan TL, New JP. Evaluation of an electrochemical sensor for measuring blood ketones. Diabetes Care. 2000;23(4):500–503. doi: 10.2337/diacare.23.4.500. [DOI] [PubMed] [Google Scholar]
- 39.Yu HY, Agus M, Kellogg MD. Clinical utility of Abbott Precision Xceed Pro® ketone meter in diabetic patients. Pediatr Diabetes. 2011;12(7):649–655. doi: 10.1111/j.1399-5448.2011.00768.x. [DOI] [PubMed] [Google Scholar]
- 40.Khan AS, Talbot JA, Tieszen KL, Gardener EA, Gibson JM, New JP. Evaluation of a bedside blood ketone sensor: the effects of acidosis, hyperglycaemia and acetoacetate on sensor performance. Diabetic Med. 2004;21(7):782–785. doi: 10.1111/j.1464-5491.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- 41.Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, Hilton L, Dyer PH, Hamersley MS for the Joint British Diabetes Societies. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabetic Med. 2011;28(5):508–515. doi: 10.1111/j.1464-5491.2011.03246.x. [DOI] [PubMed] [Google Scholar]
- 42.Rothera AC. Note on the sodium nitro-prusside reaction for acetone. J Physiol. 1908;37(5-6):491–494. doi: 10.1113/jphysiol.1908.sp001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace TM, Matthews DR. Recent advances in the monitoring and management of diabetic ketoacidosis. QJM. 2004;97(12):773–780. doi: 10.1093/qjmed/hch132. [DOI] [PubMed] [Google Scholar]
- 44.Samuelsson U, Ludvigsson J. When should determination of ketonemia be recommended? Diabetes Technol Ther. 2002;4(5):645–650. doi: 10.1089/152091502320798286. [DOI] [PubMed] [Google Scholar]
- 45.Laffel L. Sick-day management in type 1 diabetes. Endocrinol Metab Clin North Am. 2000;29(4):707–723. doi: 10.1016/s0889-8529(05)70160-2. [DOI] [PubMed] [Google Scholar]
- 46.Guerci B, Tubiana-Rufi N, Bauduceau B, Bresson R, Cuperlier A, Delcroix C, Durain D, Fermon C, Le Floch JP, Le Devehat C. et al. Advantages to using capillary blood beta-hydroxybutyrate determination for the detection and treatment of diabetic ketosis. Diabetes Metab. 2005;31(4 Pt 1):401–406. doi: 10.1016/s1262-3636(07)70211-2. [DOI] [PubMed] [Google Scholar]
- 47.Wallace TM, Meston NM, Gardner SG, Matthews DR. The hospital and home use of a 30-second hand-held blood ketone meter: guidelines for clinical practice. Diabetic Med. 2001;18(8):640–645. doi: 10.1046/j.1464-5491.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- 48.Turan S, Omar A, Bereket A. Comparison of capillary blood ketone measurement by electrochemical method and urinary ketone in treatment of diabetic ketosis and ketoacidosis in children. Acta Diabetol. 2008;45(2):83–85. doi: 10.1007/s00592-008-0026-y. [DOI] [PubMed] [Google Scholar]
- 49.Naunheim R, Jang TJ, Banet G, Richmond A, McGill J. Point-of-care test identifies diabetic ketoacidosis at triage. Acad Emerg Med. 2006;13(6):683–685. doi: 10.1197/j.aem.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Harris S, Ng R, Syed H, Hillson R. Near patient blood ketone measurements and their utility in predicting diabetic ketoacidosis. Diabetic Med. 2005;22(2):221–224. doi: 10.1111/j.1464-5491.2004.01374.x. [DOI] [PubMed] [Google Scholar]
- 51.Schwab TM, Hendey GW, Soliz TC. Screening for ketonemia in patients with diabetes. Ann Emerg Med. 1999;34(3):342–346. doi: 10.1016/s0196-0644(99)70128-9. [DOI] [PubMed] [Google Scholar]
- 52.Taboulet P, Haas L, Porcher R, Manamani J, Fontaine JP, Gautier JF. Urinary acetoacetate or capillary ß-hydroxybutyrate for the diagnosis of ketoacidosis in the Emergency Department setting. Eur J Emerg Med. 2004;11(5):251–258. doi: 10.1097/00063110-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Charles RA, Bee YM, Eng PH, Goh SY. Point-of-care blood ketone testing: screening for diabetic ketoacidosis at the emergency department. Singapore Med J. 2007;48(11):986–989. [PubMed] [Google Scholar]
- 54.Arora S, Menchine M. The role of point-of-care beta-hydroxybutyrate testing in the diagnosis of diabetic ketoacidosis: A review. Hosp Pract. 2012;40(2):73–78. doi: 10.3810/hp.2012.04.972. [DOI] [PubMed] [Google Scholar]
- 55.Arora S, Henderson SO, Long T, Menchine M. Diagnostic accuracy of point-of-care testing for diabetic ketoacidosis at emergency-department triage. Diabetes Care. 2011;34(4):852–854. doi: 10.2337/dc10-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott A on Behalf of the Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperosmolar hyperglycaemic state in adults with diabetes. Diabetic Med. 2015;32(6):714–724. doi: 10.1111/dme.12757. [DOI] [PubMed] [Google Scholar]
- 57.Wijaya IP, Soewondo P, Widodo D, Sudoyo AW. Beta-hydroxybutyrate levels as a determinant for the success of diabetic ketoacidosis management. Acta Med Indones. 2004;36(2):70–77. [PubMed] [Google Scholar]
- 58.Rewers A, McFann K, Chase HP. Bedside monitoring of blood b-hydroxybutyrate levels in the management of diabetic ketoacidosis in children. Diabetes Technol Ther. 2006;8(6):671–676. doi: 10.1089/dia.2006.8.671. [DOI] [PubMed] [Google Scholar]
- 59.Ham MR, Okada P, White PC. Bedside ketone determination in diabetic children with hyperglycemia and ketosis in the acute care setting. Pediatr Diabetes. 2004;5(1):39–43. doi: 10.1111/j.1399-543X.2004.00032.x. [DOI] [PubMed] [Google Scholar]
- 60.Arora S, Probst MA, Agy C, Menchine M. Point-of-care beta-hydroxybutyrate testing for assessing diabetic ketoacidosis severity prior to treatment in the emergency department. Diabetes Res Clin Pract. 2011;94(3):e86–e88. doi: 10.1016/j.diabres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10(8):836–841. doi: 10.1111/j.1553-2712.2003.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 62.Herrington WG, Nye HJ, Hammersley MS, Watkinson PJ. Are arterial and venous samples clinically equivalent for the estimation of pH, serum bicarbonate and potassium concentration in critically ill patients? Diabet Med. 2012;29(1):32–35. doi: 10.1111/j.1464-5491.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 63.British Medical Association; Royal Pharmaceutical Society of Great Britain. British National Formulary. 2016. [Google Scholar]
- 64.Diabetes UK. Meds and kits. [Accessed 6th September 2016]. https://shop.diabetes.org.uk/usr/downloads/Meds%20and%20Kit%20low%20res.pdf.
- 65.Klocker AA, Phelan H, Twigg SM, Craig ME. Blood beta-hydroxybutyrate vs. urine acetoacetate testing for the prevention and management of ketoacidosis in type 1 diabetes: a systematic review. Diabet Med. 2013;30(7):818–824. doi: 10.1111/dme.12136. [DOI] [PubMed] [Google Scholar]
- 66.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: A potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans NR, Richardson L, Dhatariya KK, Sampson MJ. Diabetes specialist nurse telemedicine: admissions avoidance, costs and casemix. Europ Diabetes Nurs. 2012;29(1):17–21. [Google Scholar]
- 68.Laffel LM, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278–284. doi: 10.1111/j.1464-5491.2005.01771.x. [DOI] [PubMed] [Google Scholar]