Abstract
BACKGROUND: Current screening methods for gestational diabetes mellitus (GDM) are insufficient in detecting the risk of GDM in the first trimester of the pregnancy. Recent metabolomic studies have detected altered amino acid and acylcarnitine concentrations in type 2 diabetes (T2D). Because of the similarities between T2D and GDM, the determination of these metabolites may be useful in early screening for GDM. AIM: To evaluate the association between GDM and first-trimester maternal serum concentrations of ten amino acids and 31 acylcarnitines. METHODS: This retrospective case-control study included data from pregnant women screened at Oulu University Hospital between 1.1.2008 and 31.12.2011. A total of 31,146 women participated voluntarily in a first-trimester combined screening (for chromosomal abnormalities). The study population included 69 women who developed GDM during pregnancy and 295 women without diabetes before or after pregnancy. The serum concentrations of ten amino acids and 31 acylcarnitines were analyzed from frozen serum samples taken in the first-trimester screening. Multiple of median (MoM) values were compared between the two groups. RESULTS: In the GDM group, serum levels of arginine were significantly higher (1.13 MoM vs. 0.97 MoM), and those of glycine (0.93 MoM vs. 1.03 MoM) and 3-hydroxy-isovalerylcarnitine (0.86 MoM vs. 1.03 MoM) significantly lower compared to the control group (all p < 0.01). In each case, arginine, glycine, and 3-hydroxy-isovaleryl-carnitine would have detected 46%, 32%, and 39% of GDM cases, with a false-positive rate of 20%. Combining these three metabolites with the first-trimester serum marker pregnancy-associated plasma protein A (PAPP-A) and prior risk (age, BMI, and smoking) achieved a detection rate of 72%. CONCLUSION: There are significant differences in the serum levels of arginine, glycine, and 3-hydroxy-isovalerylcarnitine between controls and women who subsequently develop GDM. These differences were already existent in the first trimester of the pregnancy. The use of metabolites in combination with prior risk and first-trimester PAPP-A represents a reliable method to identify women at risk of GDM.
Keywords: gestational diabetes, acylcarnitine, arginine, glycine, 3-hydroxy-isovalerylcarnitine
Abbreviations: C5OH - 3-hydroxy-isovalerylcarnitine, BCAA - branched-chain amino acid, BCKA - branched-chain keto acid, DR - detection rate, FPR - false-positive rate, fβ-hCG - free β-human chorionic gonadotropin, GDM - gestational diabetes mellitus, HAPO - Hyperglycemia and Adverse Pregnancy Outcomes, HDL - high-density lipoprotein, IFG - impaired fasting glucose, IADPSG - International Association of Diabetes and Pregnancy Study Groups, C5 - isovalerylcarnitine, MoM - multiple of median, NT - nuchal translucency, OGTT - oral glucose tolerance test, PAPP-A - pregnancy associated plasma protein A, PCOS - polycystic ovary syndrome, T2DM - type 2 diabetes mellitus, VLDL - very-low-density lipoprotein
1. Introduction
Gestational diabetes mellitus (GDM) is a condition involving any degree of glucose intolerance with onset or first recognition during pregnancy [1]. The prevalence of GDM is increasing globally; it is currently approximately 8.5% in Finland [2]. An increasing number of fertile women are obese, partly explaining the increasing prevalence of GDM. In Finland, around one third of parturients are overweight (BMI of 25 kg/m2 or higher) and 13% are obese (BMI of 30 kg/m2 or higher) [2]. The incidence of GDM also depends on the definition of GDM, and is as high as 15-20% according to the new screening criteria recommended by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) based on the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [3].
GDM increases maternal and neonatal morbidity. Neonatal complications include hypoglycemia, hyperbilirubinemia, respiratory distress syndrome, polycythemia, hypocalcemia, hypertrophic cardiomyopathy, and fetal macrosomia, which may further cause shoulder dystocia and trauma during delivery [4-6]. Both the GDM patients and their offspring have an increased risk of developing diabetes, obesity, and metabolic syndrome later in life [7]. Up to 70% of GDM patients develop diabetes within 10 years after delivery, mostly type 2 diabetes mellitus (T2DM) [8]. During pregnancy, the risk of morbidity increases in combination with the severity of hyperglycemia.
GDM is diagnosed by an abnormal oral glucose tolerance test (OGTT) during pregnancy. In Finland, a 75g OGTT is recommended for almost all pregnant women during 24-28 weeks of gestation. There is evidence that diet and exercise can decrease the risk of GDM and its complications. The effects of healthy lifestyle changes seem to be more effective the earlier they are initiated during the pregnancy. Preferably, lifestyle changes should be made before the pregnancy [9]. Approximately half of the women developing GDM have no known risk factors, and are therefore not referred for OGTT before mid-gestation. Therefore, new screening methods are necessary to allow earlier identification of women at risk of GDM, their referral to counseling for favorable lifestyle changes, and possible prevention of GDM [10].
Recent metabolomics studies have detected numerous metabolites correlating with insulin resistance and T2DM [11-14]. Metabolic profiles have been shown to be altered as early as 3-12 years before the diagnosis of T2DM [15-17]. Metabolites associated with T2DM include carbohydrates, lipids like lyso-phosphatidylcholines [17, 18], fatty acids [19], acylcarnitines [20], and amino acids, particularly branched-chain amino acids (BCAAs, leucine, isoleucine, and valine) and aromatic amino acids (phenylalanine and tyrosine) [16]. Leucine, isoleucine, and valine are first converted to branched-chain keto acids (BCKAs) 4-methyl-2-oxopentanoate, 3-methyl-2-oxovalerate, and 3-methyl-2-oxobutyrate, respectively, and eventually lead to the production of C3 and C5 acylcarnitines. Both BCKAs and acylcarnitines have been found to be elevated in patients with impaired fasting glucose (IFG) and T2DM [13].
A feature of GDM is that the utilization of insulin-dependent cell fuels are altered as a result of increased peripheral insulin resistance [21]. Furthermore, plasma maternal glucose and free fatty acids tend to remain elevated because of the lipolytic effects of placental hormones and insulin resistance, which occur particularly in late pregnancy [22-24]. Whilst a recent review on metabolite profiling in GDM concludes that the existing studies present inconsistent findings regarding metabolite profile characteristics [25], there are pathophysiological similarities between GDM and T2DM, such that the metabolite profile found in T2DM may be used to identify women at risk of GDM.
Therefore, the purpose of this study was to evaluate whether specific first-trimester maternal serum levels of ten amino acids and 31 acylcarnitines, separately or in combination, can effectively identify women at increased risk of GDM.
2. Methods
This was a retrospective case-control study on deliveries that took place between 1.1.2008 and 31.12.2011. In total 31,146 women participated in the first-trimester combined screening (for chromosomal abnormalities) at Oulu University Hospital (Oulu, Finland). In Finland, the National Institute for Health and Welfare records the outcome of all live births and stillbirths with a gestational age of 22 or more or a birth weight of 500 grams or more. Data concerning GDM among the study population was obtained from these records. Participation in the first-trimester combined screening was voluntary. No additional written consent was needed from the patients to use their serum samples taken previously to conduct this retrospective study. The study was approved by the Ethics Committee of Oulu University Hospital (64/2007).
GDM was diagnosed by a 2-h 75g OGTT during gestational weeks 12-16 or 24-28. Diagnostic values were ≥5.3 mmol/l (fasting blood glucose), ≥10.0 mmol/l (1-h) and ≥8.6 mmol/l (2-h). GDM was diagnosed if one or more of the values were abnormal. In general, OGTT was not performed in primipara aged <25 years with no family history of T2DM and pre-pregnancy BMI of 18.5-25, and in multipara aged <40 years with no previous GDM or macrosomia and pre-pregnancy BMI <25 kg/m2. An earlier OGTT during gestational weeks 12-16 was performed in women at increased risk of GDM (i.e. in case of BMI ≥ 35, previous GDM, glucosuria in the first trimester, polycystic ovary syndrome, or having a first-degree relative with T2DM). If early OGTT was negative, it was repeated at gestational weeks 24-28 [26].
The study group consisted of 69 women with GDM. They were selected randomly from the group of all women who were diagnosed with GDM in the second trimester (n = 1,869, 6%), and subsequently subjected to metabolomic analysis. None of the patients had been diagnosed at the time of serum sampling. Serum sample analysis was carried out based on reserved first-trimester serum samples deposited during the screening period. Subsequent GDM diagnosis information was obtained from OGTT testing performed in the second trimester. The control group consisted of 295 women with normal glucose metabolism during pregnancy. They were selected randomly from all women without GDM (n = 29,280) to obtain statistically sufficient data for the generation of reliable multiple of median (MoM) equations for underlying factors (Figure 1).
Figure 1.
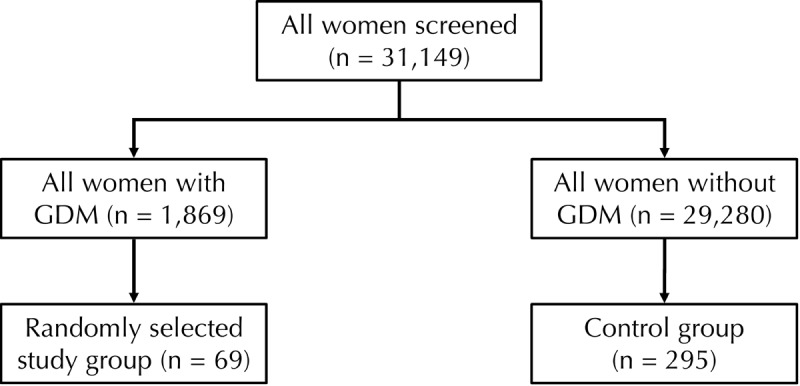
Flow chart of population and selection of study and control group. A stratified sampling was carried out by controlling for the following parameters: sample storage age, maternal BMI class, and gestational age at sampling.
MoM is a value that measures the deviation of an individual test result from the median calculated from values of the general population. The MoM was calculated using the stratified sampling method, where the parameters controlled included serum sample storage time, maternal BMI class, and gestational age at serum sampling (week). Stratified sampling means that the control subjects included attributes that were distributed throughout the scale of each stratum. Serum sample storage time was used to avoid a possible bias due to instability of the analyte. We are not aware of stability issues, but there is a lack supportive data for any analyte. Maternal age was not selected as attribute as it did not affect the levels of markers like maternal weight or BMI.
Some part of the serum samples taken in the first-trimester screening were reserved for measurement of the amino acid and acylcarnitine concentrations. The serum concentrations of the amino acids and acylcarnitines studied were analyzed retrospectively by Quattro micro mass spectrometry (Waters, Milford, MA, USA) using the NeoGram® Amino Acids and Acylcarnitines kit (PerkinElmer, Waltham, MA, USA) applied to 10 µl first-trimester serum. The assays involved a chemical derivatization step, which included the addition of a butyl group to the carboxylate groups of the amino acids, free carnitine, and acylcarnitines to produce butyl esters.
The response of each of the 41 analytes relative to its corresponding stable isotope-labeled internal standard was proportional to its analyte concentration. However, concentrations of surrogate analytes like 3-hydroxy-isovalerylcarnitine (C5OH) were estimated with an internal standard of C5, which has the same chain length and similar performance characteristics. The concentrations (µmol/l) were normalized to maternal weight, gestational age at serum sampling, maternal age, and smoking status, and thereby transferred into MoM values. The study population included Caucasian women only, so ethnicity correction was not required. The characteristics of the participants are shown in Table 1.
Table 1. Maternal characteristics, first-trimester combined screening data, OGTT results, and odds ratios for basic risk factors for GDM.
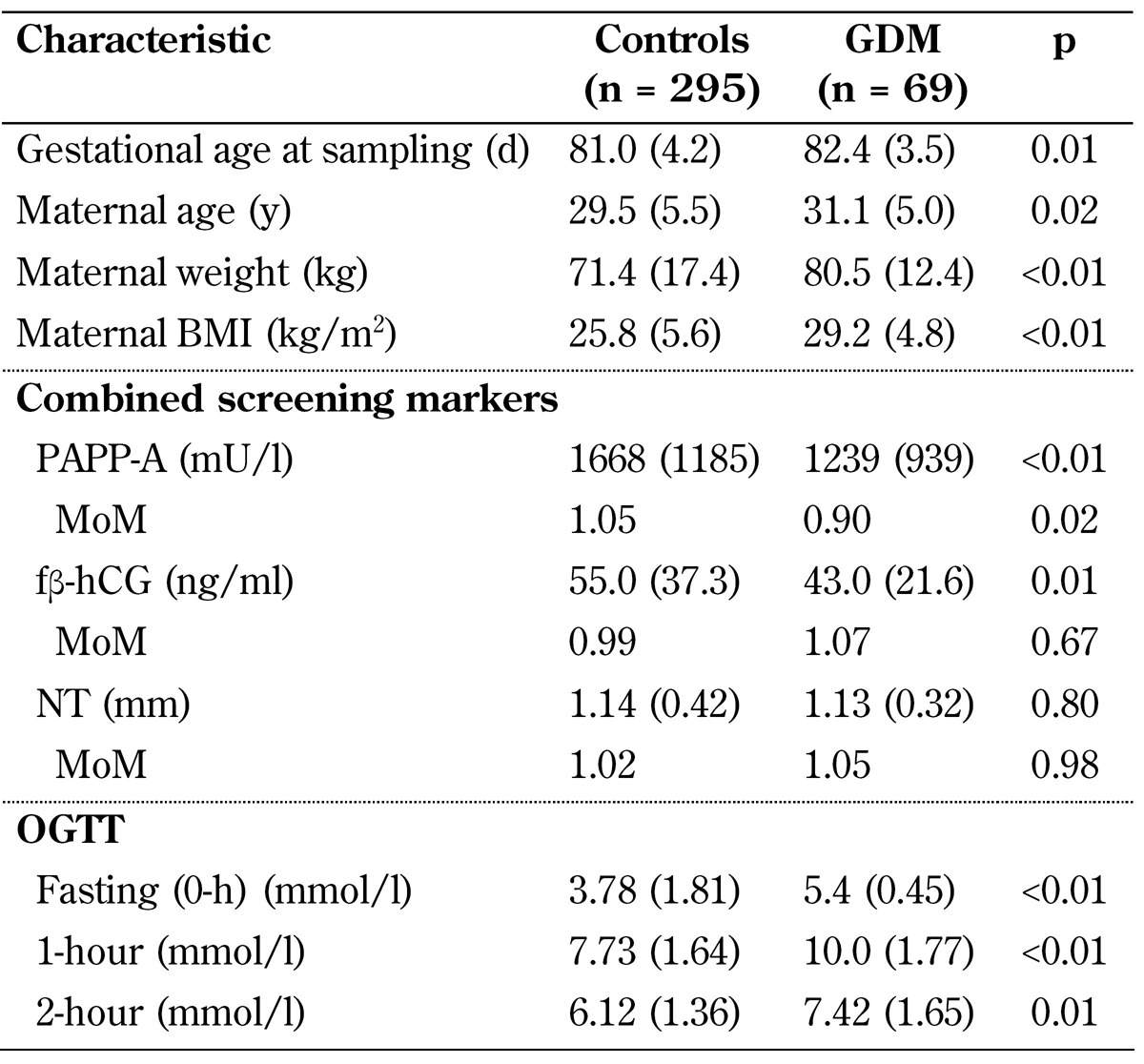
Legend: Data are expressed as mean (SD). Abbreviations: fβ-hCG - free beta human chorionic gonadotropin, MoM - multiple of median, NT - nuchal translucency, OGTT - oral glucose tolerance test, PAPP-A - pregnancy-associated plasma protein A.
MoM values were compared between the GDM and control group (Table 2). The statistical comparison between the groups was performed with the analysis of variance (ANOVA) method using TIBCO®Spotfire® software, version 6.5.1. Combined multivariate logistic regression analysis was performed using Spotfire S+, version 8.1 (TIBCO Spotfire, Boston, US), to estimate the screening performance of the selected markers. Screening performance was evaluated as detection rates (DRs) of different markers and their combinations of GDM and false-positive rates (FPRs) of 10% and 20% (Table 3).
Table 2. Multiple of median (MoM) data for amino acids and carnitines.
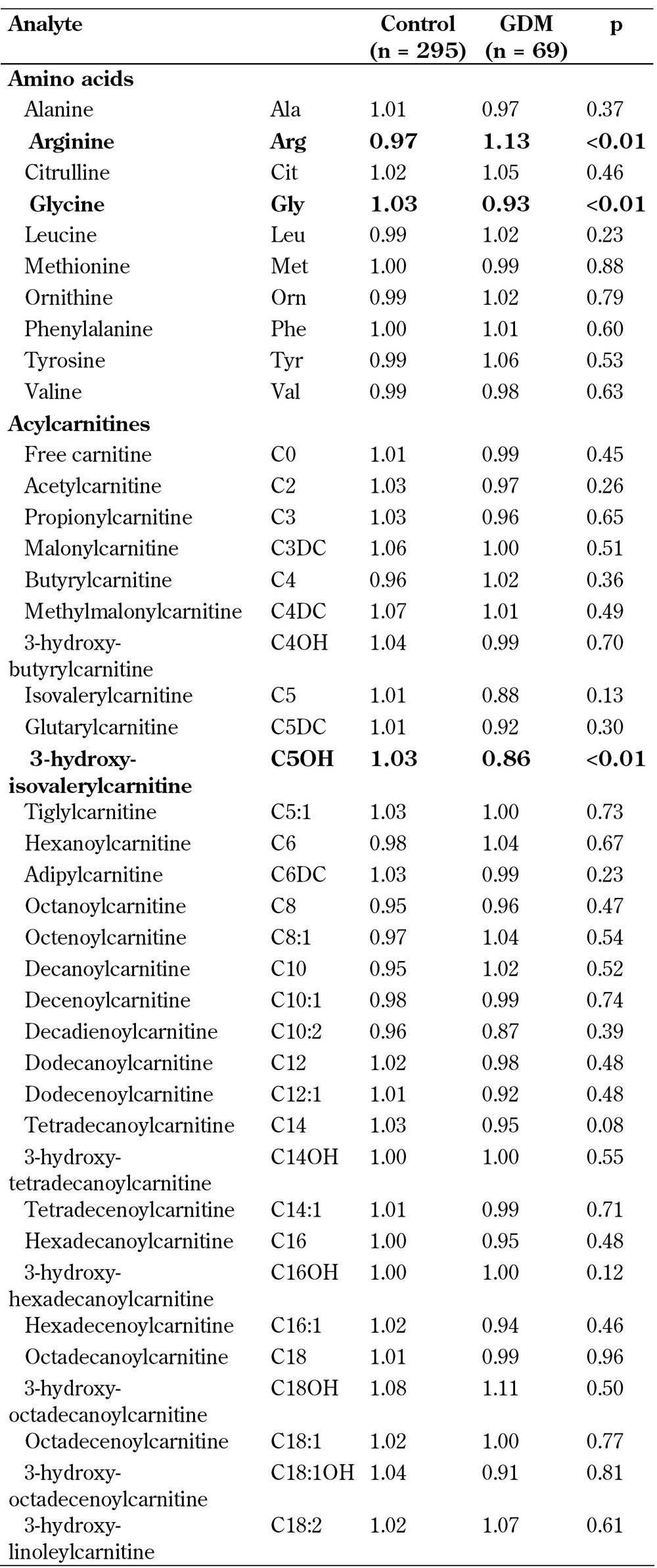
Legend: Data represent multiple of median (MoM) values.
Table 3. Screening performance of different marker combinations for gestational diabetes mellitus in the first trimester of pregnancy.
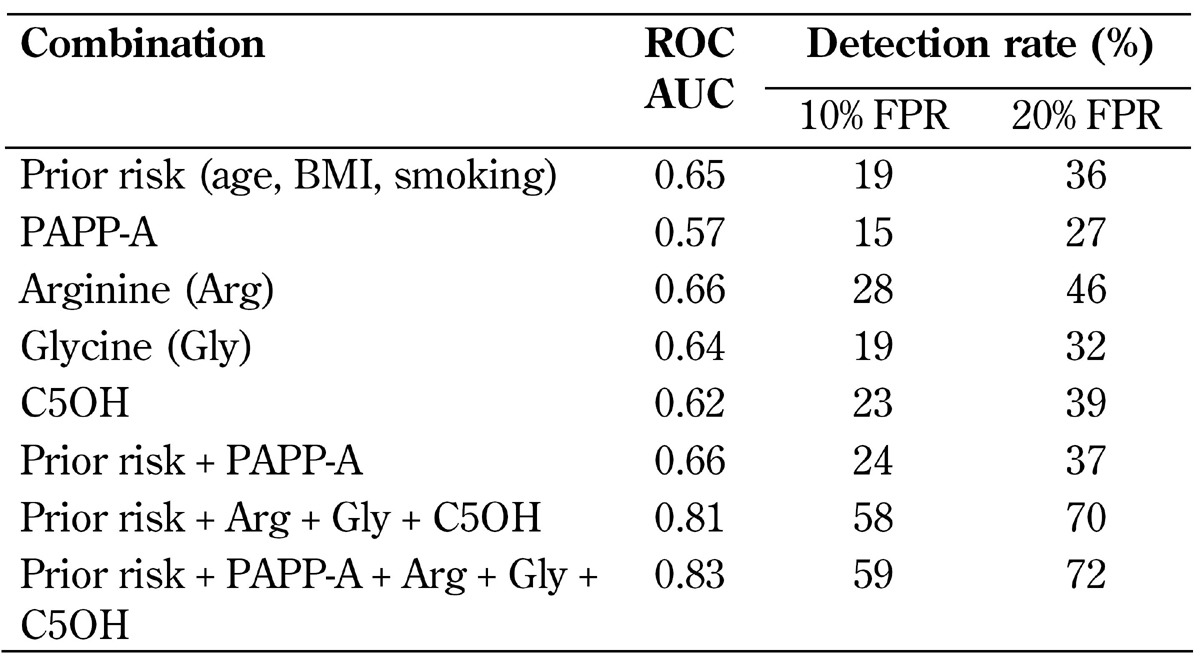
Legend: AUC - area under the curve, BMI - body mass index, C5OH - 3-hydroxy-isovalerylcarnitine, FPR - false-positive rate, PAPP-A - pregnancy-associated plasma protein A, ROC - receiver operating characteristics.
Higher FPRs can be justified as the prevalence is high (up to 15-20%), especially if the new IADPSG guidelines are applied for diagnosis. In Finland, diagnostic cutoff values are slightly higher than IADPSGG guideline values (≥5.3 mmol/l vs. ≥5.1 mmol/l, ≥10.0 mmol/l vs. ≥10.0 mmol/l and ≥8.6 mmol/l vs. 8.5 mmol/l, respectively) [27]. However, using the current diagnostic criteria, the prevalence of GDM (6%) in our study was in line with corresponding figures from other countries, for example with those in the USA (5-6%) [25]. In our study, we collected data from 69 women who subsequently developed GDM and 295 control women. The prevalence of GDM was 6% in the entire population studied.
3. Results
Table 1 shows the maternal characteristics in the GDM and control group. There were 18 (26.1%) women in the GDM group and 40 (13.6%) women in the control group over 35 years of age (OR 2.3). Twenty-nine (42.0%) women in the GDM group and 75 (25.4%) women in the control group had a BMI of ≥30.0 (OR 2.1). There were 37 (12.5%) smokers in the control group and 14 (20.3%) in the GDM group (p-value 0.09). There were no women with polycystic ovary syndrome (PCOS) among cases or controls.
Table 2 shows the MoM values and p values of each analyzed amino acid and acylcarnitine in GDM and control women. We found statistically significant differences in arginine (p = 23 x 10-6), glycine (p = 0.0013), and C5OH levels (p = 0.0029) between GDM and control. Arginine levels were significantly higher, while glycine and C5OH levels were significantly lower, in the GDM group compared to controls. The analyses were conducted with an in vitro diagnostic medical device system; individual analytes have been verified to fulfill clinically relevant performance criteria. Box plot distribution of arginine, glycine, and C5OH MoM values in the GDM group and controls are shown in Figure 2.
Figure 2.
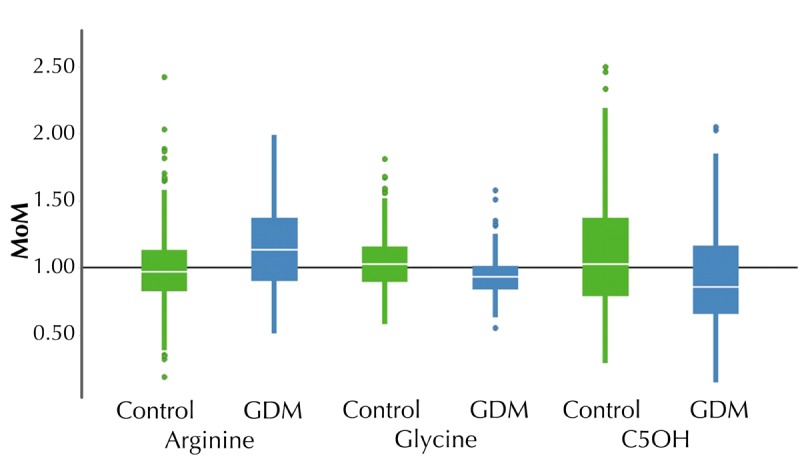
Box plot of metabolites with statistically significant changes between GDM and control.
First-trimester combined screening markers, maternal serum PAPP-A, free β-human chorionic gonadotropin (fβ-hCG), and nuchal translucency (NT) measurements were also compared between the two groups. There was a significant reduction in PAPP-A in the GDM group compared to controls (0.90 MoM vs. 1.05 MoM, p < 0.01), but no significant difference in fβ-hCG and NT MoMs between the groups.
We calculated detection rates (DRs) for GDM using combinations of maternal prior risk (maternal age, BMI, smoking status), arginine, glycine, C5OH, and PAPP-A. Fixed false-positive rates (FPRs) were 10% and 20%. As a single marker, arginine achieved the best prediction performance with DRs of 28% and 46% for FPRs of 10% and 20%, respectively. The most efficient combination included all markers, although adding PAPP-A increased the DR only marginally from 58% to 59% with an FPR of 10% and from 70% to 72% with an FPR of 20% (Table 3). Figure 3 shows a ROC-plot of the best marker combination. Ethnicity was not included in the prior risk model since all women were Caucasian.
Figure 3.
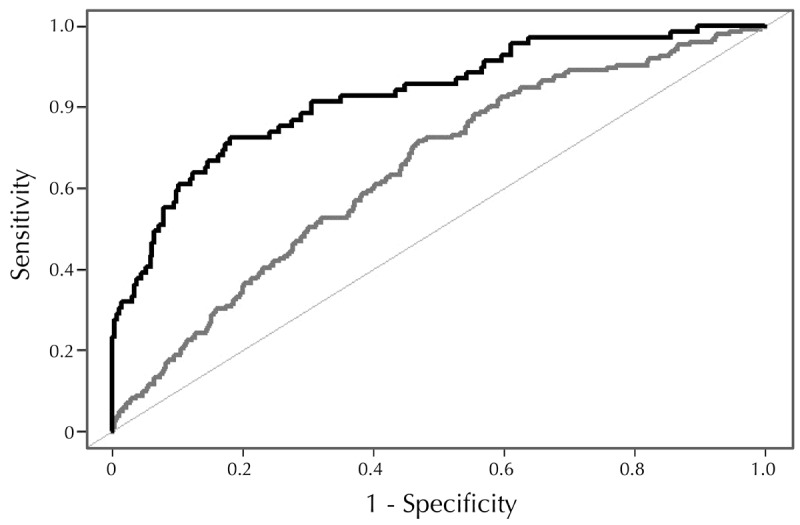
ROC-plot for the screening panel consisting of basic maternal characteristics (i.e. prior risk), PAPP-A from aneuploidy screening, and three selected metabolites (arginine, glycine, and C5OH) from the MSMS panel (black curve). The grey curve includes prior risk only.
4. Discussion
GDM is a risk for both maternal and neonatal health. While the risk of GDM can be lowered significantly by physical activity before pregnancy and during early pregnancy [28], there are conflicting results on the effects of lifestyle changes in GDM women after gestational weeks 26-28. It appears that lifestyle changes are more effective the sooner they are carried out [9, 29]. Therefore, the focus of screening for GDM should be on the early identification of risk pregnancies. Most women can be managed with diet and exercise, but if a normal glucose balance is not achieved, anti-diabetic drugs, mainly insulin and/or metformin are needed. As effective treatment for GDM is available, and benefits are greater the sooner it is started, screening for GDM should be performed as early as the first trimester [30].
Metabolomic profiling studies have found changes in serum metabolites of diabetic patients. Altered metabolite levels are believed to reflect the underlying pathomechanisms of insulin resistance and impaired glucose tolerance. Most consistent findings relate to amino acids and lipids, and one of the mechanisms leading to insulin resistance is thought to be lipotoxicity [31, 32]. Changes in metabolic pathways lead to accumulation of intermediary metabolites like acylcarnitines which may interfere with insulin sensitivity. Amino acid-derived acylcarnitines, C3- and C5-carnitine, fatty acid-derived C6- and C8-carnitine, and long-chain acylcarnitine levels have been found to be elevated in obese and T2DM individuals compared with lean controls [33-35]. However, it is unclear whether high acylcarnitine levels cause insulin resistance or just reflect a result of it [36]. In contrast to previous data in obese and T2DM individuals, our study analyzed the concentrations of 31 acylcarnitines, and found that C5OH-acylcarnitine levels were significantly lower in GDM than in controls. C5 presented similar decreased levels as C5OH, but the difference was not statistically significant. C5 and C5OH are catabolites of leucine.
According to the literature, BCAAs, leucine, isoleucine, and valine, are the amino acids that are most consistently associated with insulin resistance and impaired glucose tolerance [13, 14, 16]. The Finnish population-based Metabolomic Syndrome in Men (METSIM) study, which included 9,369 male individuals (non-diabetic and newly diagnosed type 2 diabetic), investigated the possible correlation of 8 amino acids with hyperglycemia. BCAAs, aromatic amino acids, and alanine levels were found to increase while levels of glutamine and histidine decrease with glycemia. Even mild hyperglycemia induced these results. The effects were more pronounced in obese than in lean men [37].
As GDM has pathophysiological similarities to T2DM, the metabolite profiles in GDM were considered to be similar to those in T2DM. Some studies have focused on metabolomics in GDM. For example, in a nested case-control study of 96 women with GDM and 96 matched controls with normal glucose tolerance, Bentley-Lewis et al. (2015) measured the levels of 91 metabolites from maternal serum samples obtained at the first prenatal visit. Alanine, allantoin, anthranilic acid, glutamate, and serine levels were significantly higher (p < 0.05), and creatinine levels were significantly lower (p < 0.05) in women who later developed GDM than in controls, even after adjustment for gestational age. No difference was found in BCAA levels. This showed that the metabolites studied were not altered in GDM to a similar degree as in T2DM [38].
A review of 17 articles concluded that the analyzed studies on metabolic profiling in GDM found metabolic biomarkers and derangements in lipid, amino acid, and carbohydrate metabolism similar to T2DM, but also that the results are inconsistent. The metabolites most consistently associated with GDM were asymmetric dimethylarginine (ADMA) and NEFAs (major components of triacylglycerols) [25]. The latter have been found to be elevated in the third trimester of pregnancy [24, 39-41]. The inconsistency between the studies arose because they varied in several aspects, including the timing of serum sampling (early, mid, or late gestation), number of participants, fasting status, selection of metabolites, and differing glycemic control or treatment. One of the studies included in the review detected a significant difference in vitamin B12 serum concentrations between GDM women (n = 15, 160.4 ± 32.1 pmol/l) and control women (n = 78, 234.5 ± 295.9 pmol/l, p = 0.003). However, no significant difference was found in high-density lipoprotein (HDL) and total cholesterol [42]. In another study, no difference in vitamin B levels between GDM women and controls was found. Similarly, the changes for total cholesterol converged in both groups, with non-significant changes between the groups, although very-low-density lipoprotein (VLDL) was upregulated in the GDM group [43].
In our study, we found significant changes in maternal serum first-trimester levels of arginine, glycine, and C5OH-acylcarnitine in women who eventually developed GDM. This result is consistent with the literature on GDM. In contrast, we found no differences in BCAA, leucine, and valine levels between GDM patients and controls, which is not consistent with some previous reports on T2DM [13, 14, 16]. However, this finding is in agreement with Bentley-Lewis et al. (2015) who found that there are differences in metabolic levels, despite several similarities between T2DM and GDM [38]. Ferrannini et al. (2012) observed that patients who developed T2DM later had increased BCAA levels, including those of leucine and valine, and of three major glucogenic amino acids (including arginine), whereas glycine was significantly decreased [44].
Scholtens et al. (2014) found higher arginine levels in women with high fasting plasma glucose (FPG) (n = 67, 96.7 ± 20.6 µmol/l) than in women with low FPG (n = 49, 90.2 ± 15.7 µmol/l, p < 0.01). The serum samples in this study were taken when OGTT was carried out, at gestational weeks 24-32 [43]. In the study of Pappa et al. (2007), arginine levels in GDM women (n = 25, 75.47 ± 15.25 µmol/l) were slightly lower than in controls (n = 46, 81.51 ± 28.99 µmol/l), but the difference was not statistically significant. In contrast, glycine levels were significantly lower in pregnant women with diet-treated GDM (n = 25, 144.29 ± 19.93 µmol/l) than in controls (n = 46, 204.92 ± 75.84 µmol/l, p < 0.001), which is consistent with our results. However, Pappa et al. (2007) used serum samples collected later, at gestational weeks 30-33, which makes a comparison difficult. These authors also compared fasting free fatty acid concentrations, and they found a significant difference (0.40 ± 0.33 vs. 0.52 ± 0.34, respectively, p = 0.02) [41]. Similarly, Chen et al. (2010) found maternal serum total fatty acids to be elevated in women with GDM when compared to controls [45].
In a study that used the data from the HAPO study, Scholtens et al. (2014) showed that amino acid degradation pathways were altered in women with high FPG (>90th percentile) compared to women with low FPG (<10th percentile) at gestational weeks 24-32. There were 67 women with high FPG and 49 women with low FPG. The glycine levels did not differ between the two groups (191.5 ± 46.6 µmol/l in the high-FPG group vs. 189.4 ± 31.1 in the low-FPG group, p = 0.60) [46].
Overall, our findings on glycine and arginine levels in first-trimester serum samples of patients with subsequent GDM correspond with previous findings obtained from studies in GDM and T2DM. However, our results on C5OH, C5, valine, and leucine point to a difference in BCAA metabolism between T2DM and GDM. We hypothesize that GDM and T2DM are similar in pathophysiology, with GDM resembling pre-T2DM. These findings are not completely consistent with previous data, although the identified markers have been associated with T2DM. The differences may be due to the fact that in the T2DM studies the serum samples were taken from patients already living with the disease, and we have analyzed serum samples from women before the onset of GDM.
Generally, it is difficult to compare the results from the metabolic profiling studies as the variability in the concentration levels may be reflected by differences in the methods and between the study populations, including ethnic ancestry, maternal BMI, weight gain during pregnancy, lipid status, individual risk for developing GDM, and diagnostic criteria for GDM and gestational ages at sampling. Some metabolites correlate with age and BMI [46, 47]. For example, glutamate and serine have been found to correlate with BMI and parity, while glutamate also correlates with gravidity [38].
In our study, serum samples were taken for routine first-trimester combined screening where fasting was not required, so that samples were not based on fasting. While the acylcarnitine profile is usually not affected by fasting status, fasting is recommended for the amino acid profile. However, the purpose of our study was to evaluate the use of first-trimester maternal combined screening serum samples for further screening, and therefore unfavorable fasting status could not be avoided for amino acid measurement. We found that C5OH was the only one out of 31 acylcarnitines that was significantly different between the study groups. Therefore, in future studies, multiple testing for C5OH should be considered.
In the present study, serum samples were taken during routine first-trimester combined screening, a procedure that is completed by most women in Finland. At this occasion, one serum sample is obtained from all pregnant women at the same gestational age; no other routine serum sampling is carried out during normal pregnancy. Therefore, precisely one serum sample from each woman was available for the first-trimester serum metabolite measurement conducted in this retrospective analysis. Adjustments for multiple comparisons were not performed (see for example http://beheco.oxfordjournals.org/content/15/6/1044.full), but we used a p value of 0.01 as a cutoff for statistically significant difference instead of 0.05. This reduced chance findings from 1:20 to 1:100.
In our study, we normalized the serum concentrations for underlying factors such as maternal age and weight (Table 2). The use of MoM values enabled us to evaluate the true change in the measured metabolites. Normalization of results according to patient’s weight is important given that the women in our study group were significantly heavier than the controls. The use of MoM values ensured that the differences in the metabolites were independent from the underlying factors such as weight. The analysis of arginine and C5OH values in different maternal BMI groups (normal, overweight, and obese) showed similar differences in each group. The glycine values showed that the difference between GDM women and controls increased with BMI.
In our study, the study group consisted of 69 women with comprehensive pregnancy outcome data, including OGTT and the diagnosis of GDM. The prevalence of GDM in our study (6%) was slightly below the national level (8.5%), which may be because first-trimester combined screening was not made available to all pregnant women. We randomly chose 69 GDM women for complete metabolic analysis. This number was estimated to be large enough to detect differences between GDM women and controls. Controls were selected by stratified sampling to ensure that these women represented the background population. A limitation of the study is that we could not use "prior GDM" and "T2DM in first-degree relatives" as risk factors in the risk model as we did not have this information from all control subjects. Using these data would have overestimated the prediction performance of the model. On the other hand, the use of history information will further improve the prediction model for multiparous women.
During the study period, national legislation on prenatal screening was established in 2007, but there was a three-year transition period for implementation at local government level. However, there is still no reliable screening method for GDM to date, and the diagnostic OGTT is carried out in mid-gestation only, although we know that lifestyle changes are more effective in preventing adverse pregnancy outcomes the earlier they are initiated [9]. Maternal serum metabolites could offer a new way to detect women at risk of developing GDM at a point in time when lifestyle changes would be more effective, namely in the first trimester. In our study, we analyzed 41 metabolites and found that arginine was significantly elevated, while glycine and C5OH levels were significantly reduced in women who subsequently developed GDM, which is a confirmation of earlier studies. In addition, the first-trimester combined screening marker PAPP-A was significantly reduced in the GDM group.
We used these markers alone and in combination with each other and maternal prior risk to calculate the detection rates and false-positive rates (FPRs) of 10% and 20% for GDM (Table 3, Figure 3). We found that an FPR of 20% is applicable to high-prevalence GDM, especially if the new IADPSG guidelines are applied, while 5% or 10% FPR is more applicable to less prevalent adverse outcomes of pregnancy such as aneuploidies or pre-eclampsia. The best prediction performance was achieved by using all markers. For an FPR of 20%, this marker combination could detect 72% of the women who developed GDM subsequently. Since the first-line treatment for GDM is a healthy diet and exercise, women false-positively identified would not be harmed by the treatment. Instead, women with true-positive screening results could potentially benefit from the earlier treatment.
In conclusion, maternal metabolites may be useful in screening for GDM in combination with maternal characteristics and other data available (e.g. PAPP-A or history of previous pregnancies). More studies evaluating the first-trimester levels of these markers in prospective settings and larger populations are needed to establish their value as an early screening method for GDM.
Acknowledgments
Disclosures
HA and MS are employees at PerkinElmer which is the company that provided the NeoGram® amino acids and acylcarnitines kits used in this study. The other authors have no conflict of interests to declare.
References
- 1.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Report No. 99.2. 1999:19. Geneva, Switzerland: [Google Scholar]
- 2.National Institute for Health and Welfare. Perinatal statistics: parturients, deliveries and newborns 2012, Statistical Report 24/2013. Helsinki: [Google Scholar]
- 3.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 4.Yessoufou A, Moutairou K. Maternal diabetes in pregnancy: early and long-term outcomes on the offspring and the concept of “metabolic memory”. Exp Diabetes Res. 2011;2011:218598. doi: 10.1155/2011/218598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36(6 Pt 2):617–627. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Suhonen L, Hiilesmaa V, Kaaja R, Teramo K. Detection of pregnancies with high risk of fetal macrosomia among women with gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2008;87(9):940–945. doi: 10.1080/00016340802334377. [DOI] [PubMed] [Google Scholar]
- 7.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 8.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 9.Rönö K, Stach-Lempinen B, Klemetti MM, Kaaja RJ, Pöyhönen-Alho M, Eriksson JG, Koivusalo SB RADIEL group. Prevention of gestational diabetes through lifestyle intervention: study design and methods of a Finnish randomized controlled multicenter trial (RADIEL) BMC Pregnancy Childbirth. 2014;14:70. doi: 10.1186/1471-2393-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pöyhönen-Alho MK, Teramo KA, Kaaja RJ, Hiilesmaa VK. 50gram oral glucose challenge test combined with risk factor-based screening for gestational diabetes. Eur J Obstet Gynecol Reprod Biol. 2005;121:34–37. doi: 10.1016/j.ejogrb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Stancakova A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, Paananen J, Pihlajamäki J, Bonnycastle LL, Morken MA. et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. 2012;61(7):1895–1902. doi: 10.2337/db11-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, Fritsche A, Häring HU, Hrabe de Angelis M, Peters A. et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ. et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276. doi: 10.2337/db13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Hu FB. Comprehensive metabolomic profiling of type 2 diabetes. Clin Chem. 2015;61:453–455. doi: 10.1373/clinchem.2014.235986. [DOI] [PubMed] [Google Scholar]
- 15.Tabak AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373(9682):2215–2221. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C. et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B. et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drogan D, Dunn WB, Lin W, Buijsse B, Schulze MB, Langenberg C, Brown M, Floegel A, Dietrich S, Rolandsson O. et al. Untargeted metabolic profiling identifies altered serum metabolites of type 2 diabetes mellitus in a prospective, nested case control study. Clin Chem. 2015;61(3):487–497. doi: 10.1373/clinchem.2014.228965. [DOI] [PubMed] [Google Scholar]
- 19.Kotronen A, Velagapudi VR, Yetukuri L, Westerbacka J, Bergholm R, Ekroos K, Makkonen J, Taskinen MR, Oresic M, Yki-Järvinen H. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia. 2009;52(4):684–690. doi: 10.1007/s00125-009-1282-2. [DOI] [PubMed] [Google Scholar]
- 20.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivan E, Boden G. Free fatty acids, insulin resistance, and pregnancy. Curr Diab Rep. 2003;3:319–322. doi: 10.1007/s11892-003-0024-y. [DOI] [PubMed] [Google Scholar]
- 23.Reece EA, Homko C, Wiznitzer A. Metabolic changes in diabetic and nondiabetic subjects during pregnancy. Obstet Gynecol Surv. 1994;49:64–71. doi: 10.1097/00006254-199401000-00027. [DOI] [PubMed] [Google Scholar]
- 24.Metzger BE, Phelps RL, Freinkel N, Navickas IA. Effects of gestational diabetes on diurnal profiles of plasma glucose, lipids, and individual amino acids. Diabetes Care. 1980;3:402–409. doi: 10.2337/diacare.3.3.402. [DOI] [PubMed] [Google Scholar]
- 25.Huynh J, Xiong G, Bentley-Lewis R. A systematic review of metabolite profiling in gestational diabetes mellitus. Diabetologia. 2014;57:2453–2464. doi: 10.1007/s00125-014-3371-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaaja R, Alenius H, Kinnunen T, Komulainen J, Peränen N, Rönnemaa T, Saramies J, Soukka H, Teramo K, Vuorela P. et al. Update on current care guideline: gestational diabetes. Duodecim. 2013;129:1798–1799. [PubMed] [Google Scholar]
- 27.International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Springer; New York: 2014. [Google Scholar]
- 28.Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care. 2011;34:223–229. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, Mansikkamäki K, Lamberg S, Vasankari T, Komulainen T. et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. Plos Med. 2011;8(5):e1001036. doi: 10.1371/journal.pmed.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moses RG, Cheung NW. Point: universal screening for gestational diabetes mellitus. Diabetes Care. 2009;32:1349–1351. doi: 10.2337/dc09-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65:S39–S46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 33.Bene J, Marton M, Mohas M, Bagosi Z, Bujtor Z, Oroszlán T, Gasztonyi B, Wittmann I, Melegh B. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann Nutr Metab. 2013;62(1):80–85. doi: 10.1159/000345759. [DOI] [PubMed] [Google Scholar]
- 34.Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 2010;18(9):1695–1700. doi: 10.1038/oby.2009.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62:1–8. doi: 10.2337/db12-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stancakova A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, Paananen J, Pihlajamäki J, Bonnycastle LL, Morken MA. et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes. 2012;61(7):1895–1902. doi: 10.2337/db11-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bentley-Lewis R, Huynh J, Xiong G, Lee H, Wenger J, Clish C, Nathan D, Thadhani R, Gerszten R. Metabolomic profiling in the prediction of gestational diabetes mellitus. Diabetologia. 2015;58(6):1329–1332. doi: 10.1007/s00125-015-3553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care. 2010;33:2049–2054. doi: 10.2337/dc10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idzior-Walus B, Cyganek K, Sztefko K, Seghieri G, Breschi MC, Walus-Miarka M, Kawalec E, Seretny M, Sieradzki J. Total plasma homocysteine correlates in women with gestational diabetes. Arch Gynecol Obstet. 2008;278(4):309–313. doi: 10.1007/s00404-008-0571-1. [DOI] [PubMed] [Google Scholar]
- 41.Pappa KI, Vlachos G, Theodora M, Roubelaki M, Angelidou K, Antsaklis A. Intermediate metabolism in association with the amino acid profile during the third trimester of normal pregnancy and diet-controlled gestational diabetes. Am J Obstet Gynecol. 2007;196:65.e61–e65. doi: 10.1016/j.ajog.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 42.Seghieri G, Breschi MC, Anichini R, De Bellis A, Alviggi L, Maida I, Franconi F. Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metabolism. 2003;52(6):720–723. doi: 10.1016/s0026-0495(03)00032-5. [DOI] [PubMed] [Google Scholar]
- 43.Tarim E, Bagis T, Kilicdag E, Erkanli S, Aslan E, Sezgin N, Kuscu E. Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2004;83(6):543–547. doi: 10.1111/j.0001-6349.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 44.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T. et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62(5):1730–1737. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care. 2010;33(9):2049–2054. doi: 10.2337/dc10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholtens DM, Muehlbauer MJ, Daya NR, Stevens RD, Dyer AR, Lowe LP, Metzger BE, Newgard CB, Bain JR, Lowe WL Jr HAPO Study Cooperative Research Group. Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care. 2014;37(1):158–166. doi: 10.2337/dc13-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soda E, Miura I, Hoshino H, Kanno-Nozaki K, Ota T, Oguchi H, Watanabe K, Yang Q, Mashiko H, Niwa S. Impacts of age on plasma monoamine metabolite concentrations in a large cohort of healthy individuals. Psychiatry Res. 2014;15;220(1-2):639–645. doi: 10.1016/j.psychres.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 48.Gogna N, Krishna M, Oommen AM, Dorai K. Investigating correlations in the altered metabolic profiles of obese and diabetic subjects in a South Indian Asian population using an NMR-based metabolomic approach. Mol Biosyst. 2015;11:595–606. doi: 10.1039/c4mb00507d. [DOI] [PubMed] [Google Scholar]


