Abstract
Objective
Mucopolysaccharidosis (MPS) IVA (Morquio syndrome A) is an autosomal-recessive lysosomal storage disorder caused by the deficiency of N-acetylgalactosamine-6-sulfatase (GALNS) resulting in excessive lysosomal storage of keratan sulfate. Treatments for MPS IVA have recently become available with optimal outcomes associated with early diagnosis and treatment which can be achieved by newborn screening.
Design
Newborn screening programme for MPS IVA pilot study.
Setting
MacKay Memorial Hospital (MMH), Taipei and another three branch hospitals in Taiwan.
Participants
A total of 7415 newborns were born in four branch hospitals of MMH and had joined the MPS IVA newborn screening programme. Written informed consents were obtained from parents prior to the screening process (12MMHIS188 approved by MacKay Memorial Hospital Institutional Review Board).
Outcome measures
An alternative newborn screening method for MPS IVA has been performed. Screening involved measuring the quantity of GALNS in dried blood spot (DBS) from newborn infants using the Bio-Plex immunoassay. The amount of fluorescence sorting detected by yttrium aluminium garnet laser was proportional to the quantity of GALNS protein.
Results
Of the 7415 neonates analysed, eight infants whose GALNS levels were below the cut-off value of 8.30 µg/L had been recalled for a second DBS collection. The reference values were 8.30–27.43 µg/L. In patients with confirmed MPS IVA (n=11), the GALNS quantities were far below 5% of the normal population.
Conclusion
The Bio-Plex immunoassay is a validated method used for measuring GALNS protein in DBS and has the potential to be adopted for MPS IVA newborn screening study design.
Keywords: mucopolysaccharidosis IVA, lysosomal storage disorder, N-acetylgalactosamine-6-sulfatase, newborn screening, dried blood spot, Bio-Plex immunoassay.
Strengths and limitations of this study.
The Bio-Plex immunoassay is a well-established, feasible method used for measuring N-acetylgalactosamine-6-sulfatase protein in dried blood spot and has the potential to be adopted for mucopolysaccharidosis IVA (MPS IVA) newborn screening study design.
The early diagnosis of MPS IVA in infantile period is valuable, preventable and treatable.
No positive case has been found is the limitation of this study.
Introduction
Mucopolysaccharidoses (MPSs), a group of rare genetic diseases known as lysosomal storage disorders (LSDs), are caused by the deficiency of enzymes that catalyse the stepwise degradation of glycosaminoglycans (GAGs). Mucopolysaccharidosis IVA (MPS IVA, Morquio syndrome A; OMIM#253000) is a disease characterised by a deficiency in the enzyme N-acetylgalactosamine-6-sulfatase (GALNS; EC 3.1.6.4). This deficiency leads to excessive lysosomal storage of keratan sulfate and chondroitin-6-sulfate resulting in devastating manifestations, such as systemic skeletal dysplasia, short stature, joint abnormalities that limit mobility and endurance, malformation of the thorax that impairs respiratory function, odontoid hypoplasia and ligamentous laxity which causes cervical spinal instability and potentially cord compression. Other symptoms of MPS IVA include hearing loss, corneal clouding and valvular heart disease.1–4 Initial symptoms often present during the first 5 years of life depending on the severity of disease or age of diagnosis.
Few studies have investigated the incidence (or prevalence) of MPS. The overall incidence of MPS was estimated to be 1 in 10 000–25 000 births in Europe.1 5 6 The highest incidence of MPS IVA was reported in Northern Ireland with a rate of 1.31 in 1 00 000 live births,7 which was substantially higher compared with other countries such as Germany, Poland and Tunisia. The birth incidences of MPS IVA in those countries were estimated to be between 0.14 and 0.45 cases in 1 00 000 live births.8–10 In Taiwan, the birth incidence for all MPS cases was 2.04 per 1 00 000 live births investigated from January 1984 to December 2004,11 with MPS IV incidence estimated at 0.33 per 1 00 000 live births, accounting for 16% of all MPS cases in Taiwan.
Recently, several experimental and approved treatments have been reported for some of the MPS subtypes and these include haematopoietic stem cell transplantation,12 13enzyme replacement therapy (ERT),12 14–17 premature stop codon read-through18 19 and vector-mediated gene therapy.20–22 ERTs are available for MPS I, MPS II, MPS IVA and MPS VI. ERT with recombinant human GALNS (elosulfase alfa) was approved in 2014 and is the first systemic treatment available for patients with MPS IVA.
Optimal benefits from ERT, particularly for patients with MPS IVA who suffer from devastating cartilage disease, would require commencement of treatment before the onset of irreversible clinical disease. With the exception of cases where there is a family history of the disease, presymptomatic detection of MPS can only be achieved by newborn screening. Recent progress towards newborn screening for LSDs holds promise for early detection.23–25
MPS is diagnosed by a decrease in or loss of enzyme activity, usually involving a fluorescent-tagged artificial substrate such as 4-methylumbelliferone (4MU) or a natural substrate in which a fragment of biological substrate is labelled with a radio-stable isotope.26–28 Homogenates of cultured fibroblasts and leucocytes have been widely used for definitive diagnoses of LSDs. The use of dried blood spot (DBS) samples offer several advantages in terms of cost, transportation and suitability of sample collection from neonates.
We previously reported findings from a pilot study of newborn screening for MPS I in Taiwan using the 4MU fluorescent enzymatic assay and have analysed more than 67 500 samples since 2008.23 The 4MU enzymatic assay for GALNS enzyme activity in DBS has not been proven to be consistently successful since 2010.28–31 Although the recent study showed that GALNS can be reliably and accurately measured by mass spectrometry in DBS,29 the quality and the stability of the synthesised substrate, as well as the commercialisation are all needed to be clarified further. In this study, we reported to conduct a pilot study to determine the utility of the Bio-Plex immunoassay for the quantitation of GALNS protein in DBS as an alternative method used for MPS IVA newborn first-line biochemistry examination in Taiwan. The Bio-Plex immunoassay has many advantages, such as high specificity, high throughput, quantitative assay and easy-to-use 96-well plate-based suspension analysis system. Similar to enzyme-linked immunosorbent assay (figure 1), the Bio-Plex assay uses antibody-coupled magnetic beads (capture beads) to react and bind first with the antigen (GALNS protein) eluted from the DBS sample. The antigen–antibody complex is then treated by biotinylated detection antibodies and a reporter streptavidin–phycoerythrin conjugate. Fluorescence intensity of the reporter conjugate is then measured to determine GALNS quantities. The reading intensity of the fluorescence is proportional to the amount of GALNS protein in the DBS sample.
Figure 1.
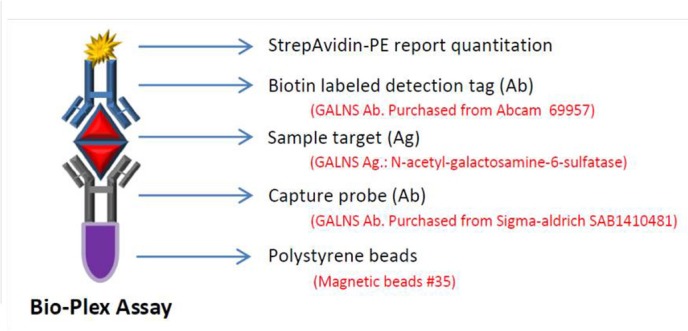
Schematic representation of the Bio-Plex immunoassay. The anti-GALNS antibody-coated magnetic beads (capture beads) reacts and binds with GALNS protein from the DBS sample then reacts with biotinated detection antibodies. DBS, dried blood spot; GALNS, N-acetylgalactosamine-6-sulfatase.
Participants and methods
Preparation of DBS samples
DBS samples were obtained according to the National Committee for Clinical Laboratory Standards protocol (NCCLS standard LA4-A4), ‘Blood Collection on Filter Paper for Neonatal Screening Program’.32 33 Blood samples were collected by heel puncture on the second day of life. Drops of blood were spotted directly on filter paper (ToYo PKU No 545; Toyo Roshi Kaisha, Tokyo, Japan) and dried at room temperature for at least 4 hours. DBS samples were stored at 4°C in zipper lock plastic bags prior to quantitative analysis of GALNS protein by Bio-Plex immunoassay.
Between December 2013 and November 2015, a total of 7415 DBS samples were collected from neonates born in the main medical centre and three other branches of MacKay Memorial Hospital in Taiwan. Written informed consents were obtained from the parents prior to the screening process (12MMHIS188 approved by MacKay Memorial Hospital Institutional Review Board). The normal GALNS levels in DBS of infant examinees were set in 95% of the values lie within two SD. The reference values of GALNS levels in normal infants (n=356) were calculated by using Microsoft Excel 2013, and the Shapiro-Wilk test of normality was also determined. Eleven DBS samples from patients with confirmed MPS IVA without receiving ERT (aged from 4.2 to 29.0 years) were used as positive controls to test the method. All the patients were with significant clinical manifestations, particularly systemic skeletal dysplasia including bone deformities such as gibbus, genu valgum and dysostosis multiplex; and all the patients had no symptom of central nervous system involvement.
Assay validation tests
In the study, 10 DBS samples obtained from the outpatient clinic recruited non-MPS volunteers were run and calculated for within-run and between-run precisions (coefficient of variance % (CV %)). All the 10 samples were run in six consecutive replicates for within-run analysis, and a duplicate analysis of each sample (n=10) was performed on six different days sequentially. In addition, the other EDTA blood samples were collected from non-MPS participants (n=20) and pooled, which were used to make a normal control (C1, n=20) and an inactivated control (C2, n=20), in which the inactivated control DBS samples were placed in a 50°C oven for 96 hours to degrade GALNS. These control DBS samples were used for assay quality control.
In this study, control DBS samples were collected and stored in low gas-permeability plastic bags at −30°C until analysis. The precision of the assay was performed to assess the repeatability. To ensure the stability and the accuracy of Bio-Plex immunoassay in DBS, the within-run and between-run precisions of DBS controls (C1 and C2) were evaluated. All prepared DBS samples were run in six consecutive replicates for the within-run analysis, whereas a duplicate analysis of each sample was performed on six different days sequentially for the between-run precision. Recovery analysis was also performed by spiking aqueous GALNS working standard (25.0 µg/L) into EDTA whole blood obtained from the same participants with appropriate ratio in volumes.
Bio-Plex immunoassay workflow
The MPS IVA-specific Bio-Plex immunoassay was performed using the Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, California, USA) as described.34 35 DBSs (3.175 mm diameter) were punched from the DBS samples for GALNS protein elution. Each DBS was transferred to one well in a 96-well plate and eluted overnight (16 hours) at 4°C in 130 µL phosphate-buffered saline (0.05% Tween, 20.05% bovine serum albumin, 0.05% γ-globulin and 0.05% sodium azide; pH 7.4). A Bio-Plex Promagnetic COOH bead (bead no 35; Luminex, Austin, Texas, USA) was used in this study. The protein coupling was performed according to the instruction manual issued by Bio-Rad Laboratories. The coupling procedure comprises a two-step carbodiimide reaction. The carboxyl groups on the bead surface were first activated to form an active O-acylisourea intermediate by using EDAC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride). Then, the intermediate was further formed a more stable ester by treating with S-NHS (sulfo-N-hydroxysulfosuccinimide), and the stable ester was ready for proceeding the protein coupling reaction. In this study, approximate 6.25×106 carboxylated beads (500 µL) were required and prepared for the coupling reaction. The protein coupling reaction was performed by adding 50 µL (1 mg/mL) capture (primary) GALNS antibody (rabbit polyclonal antibody SAB 1410481, purchased from Sigma-Aldrich, St Louis, Missouri, USA) to 450 µL well-activated bead suspension by gentle agitation on shaker in dark for 2 hours at room temperature.
DBS eluates (50 µL) were then incubated with capture beads (2500 beads/analyte/well) and each with unique spectral address coated with the capture GALNS antibody for 16 hours at 4°C. Subsequently, biotin-labelled detection (secondary) antibody (133 µg/L in concentration; mouse monoclonal antibody ab 69957; Abcam, Cambridge, UK) was added to the wells and incubated for an additional 2 hours. Free capture antibodies were washed out by filtration using a magnetic plate carrier to recover the magnetic beads irrespective of the capture antibody coating. The beads were then incubated in streptavidin–phycoerythrin solution (phycoerythrin (PE); 1.2 mg/L, 50 µL/well) at room temperature for 30 min. After gentle removal of the supernatant from the wells, the beads were resuspended in phosphate-buffered saline (125 µL/well). Specificity of antibodies to GALNS protein was verified by performing dot blot assay (figure 2A) and western blotting (figure 2B),36–39 and the results showed that the specificities of Ag–Ab reactions were good and satisfactory. In figure 2B, the rabbit GALNS polyclonal and the mouse monoclonal antibodies reacted with a full-length human GALNS protein (1 a.a.–522 a.a.), and the results showed distinct bands of ~58 kDa precursor (predicted molecular weight) and 40 kDa mature polypeptides in the cell homogenates from the wild-type by western blotting.
Figure 2.
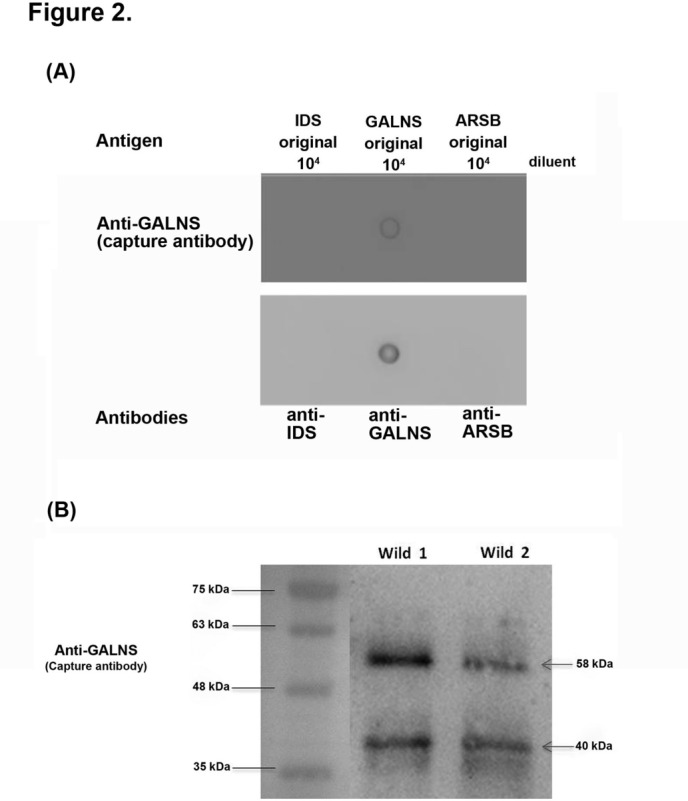
The specificities of capture (primary) and detection (secondary) antibodies to antigen (GALNS) have been assured by employing dot blotting assays (A) and western blotting (B). Both results showed good specificity of this method. Either capture (SAB 1410481; 1:1000) or detection antibody (abcam 69957; 1:1000) showed high specificity to the GALNS antigen. By treating with anti-GALNS antibody, the 58 kDa precursor and 40 kDa mature polypeptides were observed in the wild-type human white blood cell homogenates. The loading control of molecular weight markers are indicated on the left. ARSB, arylsulfatase B; GALNS, N-acetylgalactosamine-6-sulfatase; IDS, iduronate 2-sulfatase.
Fluorescence intensity of the beads was determined using the Bio-Plex protein array system with the Bio-Plex Manager V.3.1 software (Bio-Rad, Hercules, California, USA). The amounts of fluorescence sorting detected by yttrium aluminium garnet laser with wavelengths of 532 (exciting) and 580 nm (emission) was proportional to the quantity of GALNS protein (expressed as μg/L).
Calibration curve
Fresh-made working calibrators were prepared by serial dilutions of GALNS stock solution (1 mg/mL). The human recombinant GALNS protein was purchased from R&D Systems (Minneapolis, Minnesota, USA), and the final concentrations of working standards were 100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78 and 0. 39 µg/L. Biotin was used as a blank. The GALNS calibration curve (fluorescence intensity against the logarithm of concentrations) was made and showed linear between the concentrations of 0.39 and 12.5 µg/L of GALNS working standards, whereas it illustrated non-linear above the concentrations of 25 µg/L according to the logistic fifth party logistic model. The regression analysis (r2) of the calibration curve (fluorescence reading versus concentrations of GALNS calibrations) was excellent (r2=0.991). The correlation of observed versus expected results was 0.999855.
Results
Precision and validation of Bio-Plex immunoassay for GALNS quantitation
A total of 10 samples were run and calculated for within-run and between-run precisions (CV%). The within-run and between-run precisions of DBS Bio-Plex immunoassay were 10.74% and 9.37%, respectively. To ensure the stability and the accuracy of Bio-Plex immunoassay in DBS, the within-run and between-run precisions of control DBSs (C1 and C2) were also evaluated. The within-run and between-run of C1 and C2 were 7.74% and 9.07%, as well as 6.82% and 12.42%, respectively. The averaged quantities of GALNS were 14.09±3.43 µg/L for C1 and 6.58±1.15 µg/L for C2. The recovery of GALNS using Bio-Plex immunoassay was excellent (about 94.3%). The validation of Bio-Plex immunoassay for GALNS quantitation confirmed that the method was capable of meeting the requirements set on it. The sensitivity and specificity of the test according to false positives and false negatives in relation with true positives and true negatives were excellent (about 100% and 99.89%), respectively. The detectable level was as low as 0.39 µg/L; in addition, fine specificity of the immunoassay was also noted which revealed clear identity from the result analysed by western blotting. Either capture or detection antibody showed high specificity to the GALNS antigen. For the cross-reactivity of the test, it was not required to be measured due to the single test (single target) performed in this study. There were no significant effects of the matrix observed in this test because of the use of the same phosphate-buffered saline when diluting samples for the standard curve.
Quantitation of GALNS in DBSs
Of the 7415 samples analysed, 132 (1.78%) samples had GALNS levels below the cut-off value of 13.08 µg/L at the first time of testing. Most of these (n=124) were excluded and results showed normal when the original DBS samples were reanalysed. Only eight had been recalled for a second DBS collection and repeated the analysis (recall rate=0.108%). In those, the GALNS quantities were all >8.30 µg/L (the second time of testing). No positive case has been reported so far in this study.
The reference values of GALNS levels in DBS samples using Bio-Plex immunoassay were 8.30–27.43 µg/L, with a mean of 17.86±4.78 µg/L (figure 3). Sampling to determine the reference values of GALNS in newborn controls showed a 5000 samples simulation (Shapiro-Wilk test, p=0.538) (figure 4). In patients with confirmed MPS IVA who did not receive ERT (n=11), the GALNS quantities were almost near to the lower limit of detection (<0.39 µg/L) that were far below those of 5% of unaffected control. The GALNS quantities in carrier parents identified from two patients with confirmed MPS IVA (n=3) were between 12.34 and 16.53 µg/L (14.17±1.52 µg/L), which were all situated within the normal reference values.
Figure 3.
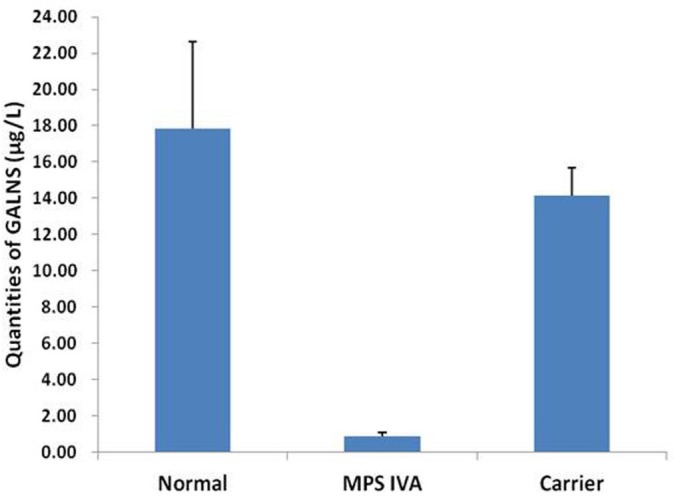
The GALNS concentrations (ranges) in dried blood spots from normal control (17.86±4.78 µg/L, n=356), carriers (14.17±1.52 µg/L, n=3) and patients with confirmed MPS IVA (0.88±0.19 µg/L, n=11). GALNS, N-acetylgalactosamine-6-sulfatase; MPS, mucopolysaccharidosis.
Figure 4.
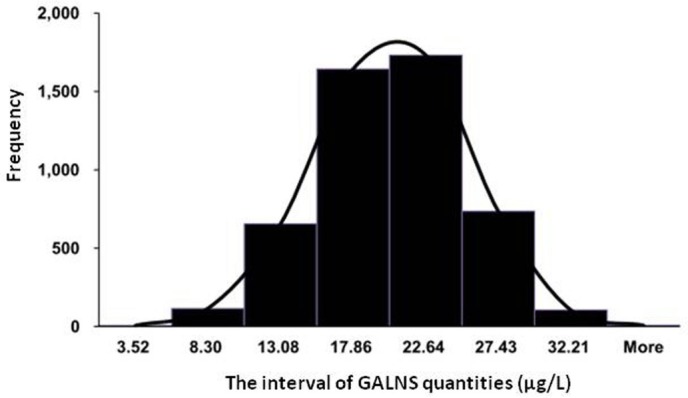
Sampling to determine the reference values of GALNS in newborn controls showed normal distribution (5000 samples, Shapiro-Wilk test, p=0.354). GALNS, N-acetylgalactosamine-6-sulfatase.
Proposed algorithm for MPS IVA newborn screening
On the basis of our findings, we propose a diagnostic algorithm for newborn first-line screening for MPS IVA as described in figure 5. Two levels of testing of the original DBS sample were proposed to rule out the false-positive results, particularly in carriers. The cut-off values of the first time and the second time of testing were determined according to the mean and SD method (mean ± SD). To reduce the number of false-positive cases, the cut-off values of the first time and the second time of testing were set at −1 SD and 2 SD from the mean value, respectively. The cut-off level of GALNS quantity in DBS using Bio-Plex immunoassay was 13.08 µg/L for the first time and 8.30 µg/L for the second time of testing. Infants who had GALNS level below the cut-off point of 8.30 µg/L were likely to be the highly suspicious cases and should be recalled for a second DBS sample collection. The application of the first time of testing was used to rule out the false-positive samples from the highly suspicious cases, including the carriers. For the second time of testing and the recall, the cut-off value was set even narrow in cut-off value in order to separate strictly the affected infants from the healthy individuals. Confirmative diagnoses including leucocyte enzymatic assay and molecular DNA analysis should be performed further regarding the significant reduction of GALNS level being detected by the second time of testing.
Figure 5.
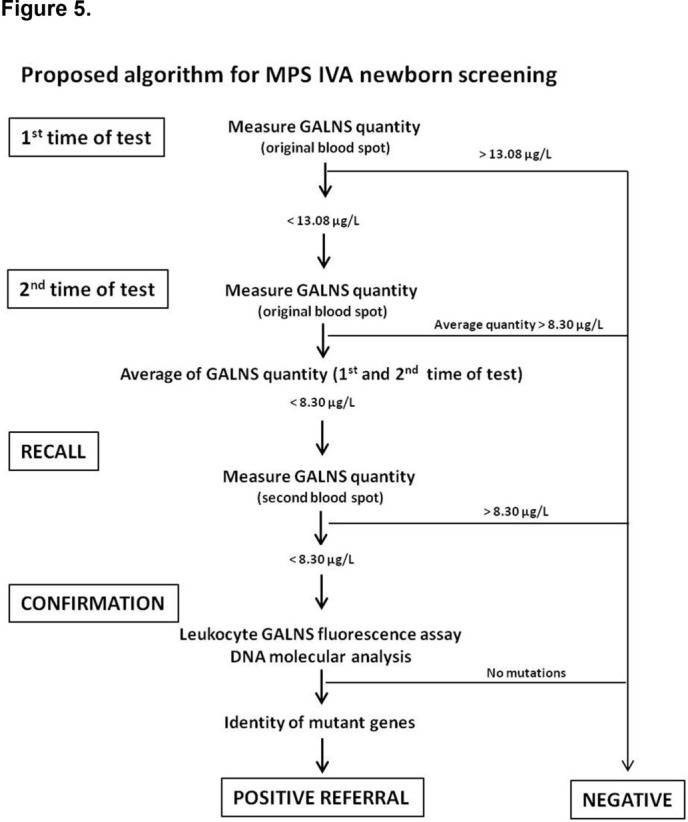
A diagnostic algorithm for MPS IVA newborn screening test. GALNS, N-acetylgalactosamine-6-sulfatase; MPS, mucopolysaccharidosis.
Discussion
Currently, there is no general consensus for the method used for MPS IVA newborn screening. Multiplex assays for quantitative measurement of GALNS activity using tandem mass spectrometry have been developed and reported since 201029–31; however, the 4MU enzymatic assay has not been applied widely due to the stability of the synthesised substrate. Besides, GALNS activity and/or GAG biomarkers, particularly the keratan sulfate in blood, urine or even DBS, can be detected quantitatively using tandem mass spectrometry method, and have shown to be applicable for MPS IVA newborn screening and the prognostic assessment following ERT.40–42 However, the validation of clinical application, particularly for MPS IVA newborn screening purpose, is still uncertain and controversial. Besides, a fluorometric method had been reported by Camelier et al,28 that could be also considered for screening of MPS IVA, but there were no data demonstrated that microplate-based fluorescence analysis for the detection of MPS IVA in dried blood samples was reliable and practicable. In this study, an alternative method, the Bio-Plex immunoassay measuring GALNS protein in DBS, is proposed and it can be another choice to be adopted for MPS IVA first-line screening purpose. The method is with high sensitivity and high specificity which is compulsory for the development and implementation of newborn screening. Several similar immunoassays such as immune-quantification assay, immune-capture method and time-resolved fluorometry with the dissociation-enhanced lanthanide fluorescence immunoassay (DELFIA) method had been developed to screen populations for LSDs, including MPS.43–46 However, the experimental applications and diagnostic purposes of LSD are different. The principle of immune-quantification assay is similar with the method we presented in this study, which can accurately quantify lysosomal proteins in DBS.44
Using immunoassay for LSD screening and diagnostic purpose raised many questions, such as, the stability of protein level in DBS? protein level changing through the life? and protein level correlated with clinical severity?. According to literatures reported,28 43 the LSD enzyme activities in DBS decreased notably when DBS samples stored under different temperatures like 4°C or room temperature, but protein quantity will not be affected significantly. To verify any difference in the amount of GALNS protein by age, we had collected both plasma and DBS samples from 34 unaffected control in variety of ages, in which three age groups were divided including group 1 (<2 years old; n=9), group 2 (2–17 years old; n=12) and group 3 (over 18 years old; n=13). All the samples were performed measuring GALNS protein based on the same method described above. From the results obtained in both plasma and DBS samples, there were no significant differences between group 1 and group 2, as well as between group 2 and group 3, whereas the results showed small biostatistic differences (p<0.05) between group 1 and group 3 (data not shown). The conclusion is that the amount of GALNS protein is changing through the life, but not very noticeable, and the changing of protein level in different age groups will not influence the judgement of attenuated phenotype by using Bio-Plex immunoassay for measuring GALNS protein under this circumstance. Whether or not the protein level correlates with clinical severity? The prediction of clinical severity with a single marker is somewhat limited. Combined markers including immune-detectable protein and enzyme activity are partially predictive of clinical severity.44 45 Many research evidences revealed that the effect of GALNS gene mutations on the GALNS tertiary structure was existent that indicated a good correlation among protein level, residual activity and clinical phenotype.36–39
The overall recall rate of the method was <0.108%. The results suggest that the Bio-Plex immunoassay is practicable for MPS IVA newborn first-line screening purpose due to the rational percentage of false-positive cases. The only concerned query we raised for Bio-Plex immunoassay is the false-negative results that may be happened due to the production of enzymatic non-functional protein. According to the literature reported,36 most GALNS mutations identified were point mutations, particularly the missense mutation, and small deletions. Transient expression analysis of some mutations showed deficiency or low residual GALNS activity due to nonsense codon in the transcribed mRNA and thus the protein to be incorrectly translated. Results also revealed that the configuration of GALNS protein might be distinctly changed because of the destruction of hydrophobic core, salt bridge removal and modification of active site.36 47 48 Rivera-Colóna et al reported that most of the mutations (76.4%; 120/157 mutations) have been identified in the GALNS gene in patients with MPS IVA were missense mutations leading to a change of a single side chain residue in the protein. On the basis of the data reported, GALNS mutations in MPS IVA being mapped to their location on the protein showed that most of the mutations (65%; 78/120 mutations) lead to changes in buried residues of the hydrophobic core, and it means that MPS IVA is most often a protein-folding disease. Only six mutations (5%) affect active site residues and 32 mutations (27%) that map to surface residues. The GALNS protein fails to fold into its normal configuration and can lose its normal function.49 On the basis of the above evidences, false-negative results would be happened, but should be very rare. In addition, a crucial point of false-negative result that needs to be concerned by performing this method is, when patients have large deletions on an area where there is no epitope, neither the primary nor the reporter GALNS antibodies would not bind on the GALNS antigen, and the GALNS quantity will be very low or non-detectable. In this study, we used the rabbit GALNS polyclonal antibody (the primary antibody) and the mouse GALNS monoclonal antibody (the reporter antibody) that all showed satisfactory specificity to the protein by performing western blotting. The rabbit GALNS polyclonal and the mouse monoclonal antibody reacted with a full-length human GALNS protein (1 a.a.–522 a.a.), any variations of the gene may change the correct amino acid sequence and thus alter the configuration either at the active site or the surface of the protein structure. It may not be detectable by using immunoassay if the change of protein molecule is distinct.
Conclusion
To date, very few methods for MPS IVA newborn screening have been reported and none have been clinically adopted. The Bio-Plex immunoassay proposed in this pilot study is a reliable, specific, validated, simple and cost-effective method for measuring GALNS protein in DBS and has the potential to be adopted for MPS IVA newborn screening purpose. The cost of this technique (per sample) is about US$3.27 which is lower than that of developing a tandem mass spectrometry method for MPS type IVA (about 6.39 US$) provided by one of the newborn screening centres in Taiwan. More collaborating with other newborn screening centres in Taiwan are required to confirm the validation of the method that provides appropriate methodology to screen out more suspicious patients to enable early diagnosis and treatment before the onset of irreversible symptoms.
Supplementary Material
Acknowledgments
All authors have disclosed potential conflicts of interest and all authors have read the journal’s policy on conflicts of interest and the journal’s authorship agreement. The authors express their sincere thanks to the staff of the Department of Clinical Laboratory and the Department of Pediatrics at MacKay Memorial Hospital for the collection of samples. This study was supported by the research grants from the Ministry of Science and Technology, Executive Yuan, Taiwan (MOST-105–2628-B-195–001-MY3, MOST-105–2314-B-195–013, MOST-104–2314-B-195–019 and MOST-102–2314-B-195–006) and Mackay Memorial Hospital (MMH-103–13 and MMH-103–115). The writing assistance was provided by Stefanie Chuah, from Mudskipper Shanghai, funded by BioMarin.
Footnotes
Contributors: C-KC and H-YL are equal contributors and participated in this pilot study design, immunoassay development by using dried blood spot filter paper, statistical analysis, data interpretation and drafting of the manuscript. S-PL performed acquisition and interpretation of the data, as well as helped to revise the manuscript. T-JW and S-FH coordinated and supervised data collection at MacKay Memorial Hospital, Taipei and another three branch hospitals in Taiwan, and performed Bio-Plex immunoassay and data collection.
Funding: This study was supported by research grants from the Ministry of Science and Technology, Executive Yuan, Taiwan (MOST-102-2314-B-195-006, MOST-104-2314-B-195-019, MOST-105-2628-B-195-001-MY3, and MOST-105-2314-B-195-013) and Mackay Memorial Hospital (MMH-103-13 and MMH-103-115).
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Ethics approval: 12MMHIS188 approved by MacKay Memorial Hospital Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Neufeld E, Muenzer J, et al. . The mucopolysaccharidoses. : Scriver C, Beaudet A, Sly W, The metabolic and molecular bases of inherited disease. New York, USA: McGraw-Hill, 2001:3421–52. [Google Scholar]
- 2. Montaño AM, Tomatsu S, Gottesman GS, et al. . International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis 2007;30:165–74. 10.1007/s10545-007-0529-7 [DOI] [PubMed] [Google Scholar]
- 3. Wood TC, Harvey K, Beck M, et al. . Diagnosing mucopolysaccharidosis IVA. J Inherit Metab Dis 2013;36:293–307. 10.1007/s10545-013-9587-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin HY, Chuang CK, Chen MR, et al. . Natural history and clinical assessment of Taiwanese patients with mucopolysaccharidosis IVA. Orphanet J Rare Dis 2014;9:21 10.1186/1750-1172-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Besley GTN, Wraith JE. Lysosomal disorders. Current Paediatrics 1997;7:128–34. 10.1016/S0957-5839(97)80195-9 [DOI] [Google Scholar]
- 6. Wraith JE. Mucopolysaccharidoses. Curr Paediatrics 1996;6:74–9. 10.1016/S0957-5839(96)80065-0 [DOI] [Google Scholar]
- 7. Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet 1997;101:355–8. 10.1007/s004390050641 [DOI] [PubMed] [Google Scholar]
- 8. Baehner F, Schmiedeskamp C, Krummenauer F, et al. . Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis 2005;28:1011–7. 10.1007/s10545-005-0112-z [DOI] [PubMed] [Google Scholar]
- 9. Jurecka A, Ługowska A, Golda A, et al. . Prevalence rates of mucopolysaccharidoses in Poland. J Appl Genet 2015;56:205–10. 10.1007/s13353-014-0262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben Turkia H, Tebib N, Azzouz H, et al. . Incidence of mucopolysaccharidoses in Tunisia. Tunis Med 2009;87:782–5. [PubMed] [Google Scholar]
- 11. Lin HY, Lin SP, Chuang CK, et al. . Incidence of the mucopolysaccharidoses in Taiwan, 1984-2004. Am J Med Genet A 2009;149A:960–4. 10.1002/ajmg.a.32781 [DOI] [PubMed] [Google Scholar]
- 12. Patel P, Suzuki Y, Tanaka A, et al. . Impact of enzyme replacement therapy and hematopoietic stem cell therapy on growth in patients with hunter syndrome. Mol Genet Metab Rep 2014;1:184–96. 10.1016/j.ymgmr.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanjuakio J, Suzuki Y, Patel P, et al. . Activities of daily living in patients with Hunter syndrome: impact of enzyme replacement therapy and hematopoietic stem cell transplantation. Mol Genet Metab 2015;114:161–9. 10.1016/j.ymgme.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wraith JE, Clarke LA, Beck M, et al. . Enzyme replacement therapy for mucopolysaccharidosis I: a randomized, double-blinded, placebo-controlled, multinational study of recombinant human alpha-L-iduronidase (laronidase). J Pediatr 2004;144:581–8. 10.1016/j.jpeds.2004.01.046 [DOI] [PubMed] [Google Scholar]
- 15. Tomatsu S, Alméciga-Díaz CJ, Barbosa H, et al. . Therapies of mucopolysaccharidosis IVA (Morquio A syndrome). Expert Opin Orphan Drugs 2013;1:805–18. 10.1517/21678707.2013.846853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Decker C, Yu ZF, Giugliani R, et al. . Enzyme replacement therapy for mucopolysaccharidosis VI: growth and pubertal development in patients treated with recombinant human N-acetylgalactosamine 4-sulfatase. J Pediatr Rehabil Med 2010;3:89–100. 10.3233/PRM-2010-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomatsu S, Montaño AM, Oikawa H, et al. . Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions? Mol Genet Metab 2015;114:195–202. 10.1016/j.ymgme.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brooks DA, Muller VJ, Hopwood JJ. Stop-codon read-through for patients affected by a lysosomal storage disorder. Trends Mol Med 2006;12:367–73. 10.1016/j.molmed.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 19. Hein LK, Bawden M, Muller VJ, et al. . alpha-L-iduronidase premature stop codons and potential read-through in mucopolysaccharidosis type I patients. J Mol Biol 2004;338:453–62. 10.1016/j.jmb.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 20. Sands MS, Haskins ME. CNS-directed gene therapy for lysosomal storage diseases. Acta Paediatr 2008;97:22–7. 10.1111/j.1651-2227.2008.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Domenico C, Villani GR, Di Napoli D, et al. . Intracranial gene delivery of LV-NAGLU vector corrects neuropathology in murine MPS IIIB. Am J Med Genet A 2009;149A:1209–18. 10.1002/ajmg.a.32861 [DOI] [PubMed] [Google Scholar]
- 22. Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci 2007;27:9928–40. 10.1523/JNEUROSCI.2185-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin SP, Lin HY, Wang TJ, et al. . A pilot newborn screening program for mucopolysaccharidosis type I in Taiwan. Orphanet J Rare Dis 2013;8:147 10.1186/1750-1172-8-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scott CR, Elliott S, Buroker N, et al. . Identification of infants at risk for developing fabry, pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr 2013;163:498–503. 10.1016/j.jpeds.2013.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruijter GJ, Goudriaan DA, Boer AM, et al. . Newborn screening for hunter disease: a small-scale feasibility study. JIMD Rep 2014;14:23–7. 10.1007/8904_2013_279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Diggelen OP, Zhao H, Kleijer WJ, et al. . A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A). Clin Chim Acta 1990;187:131–9. 10.1016/0009-8981(90)90339-T [DOI] [PubMed] [Google Scholar]
- 27. Tylki-Szymańska A, Czartoryska B, Bunge S, et al. . Clinical, biochemical and molecular findings in a two-generation Morquio A family. Clin Genet 1998;53:369–74. 10.1111/j.1399-0004.1998.tb02747.x [DOI] [PubMed] [Google Scholar]
- 28. Camelier MV, Burin MG, De Mari J, et al. . Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio syndrome type A) in dried blood samples. Clin Chim Acta 2011;412(19-20):1805–8. 10.1016/j.cca.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 29. Kumar AB, Masi S, Ghomashchi F, et al. . Tandem mass spectrometry has a larger analytical range than fluorescence assays of lysosomal enzymes: application to newborn screening and diagnosis of mucopolysaccharidoses types II, IVA, and VI. Clin Chem 2015;61:1363–71. 10.1373/clinchem.2015.242560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duffey TA, Khaliq T, Scott CR, et al. . Design and synthesis of substrates for newborn screening of Maroteaux-Lamy and Morquio A syndromes. Bioorg Med Chem Lett 2010;20:5994–6. 10.1016/j.bmcl.2010.08.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khaliq T, Sadilek M, Scott CR, et al. . Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem 2011;57:128–31. 10.1373/clinchem.2010.149880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. National Committee for Clinical Laboratory Standards (NCCLS). Blood collection on filter paper for newborn screening programs; approved standard-fourth edition, NCCLS document LA4-A4. 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA: National Committee for Laboratory Standards, 2003. [Google Scholar]
- 33. De Jesus VR, Zhang XK, Keutzer J, et al. . Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin Chem 2009;55:158–64. 10.1373/clinchem.2008.111864 [DOI] [PubMed] [Google Scholar]
- 34. Meikle PJ, Grasby DJ, Dean CJ, et al. . Newborn screening for lysosomal storage disorders. Mol Genet Metab 2006;88:307–14. 10.1016/j.ymgme.2006.02.013 [DOI] [PubMed] [Google Scholar]
- 35. Parkinson-Lawrence EJ, Muller VJ, Hopwood JJ, et al. . N-acetylgalactosamine-6-sulfatase protein detection in MPS IVA patient and unaffected control samples. Clin Chim Acta 2007;377:88–91. 10.1016/j.cca.2006.08.030 [DOI] [PubMed] [Google Scholar]
- 36. Sukegawa K, Nakamura H, Kato Z, et al. . Biochemical and structural analysis of missense mutations in N-acetylgalactosamine-6-sulfate sulfatase causing mucopolysaccharidosis IVA phenotypes. Hum Mol Genet 2000;9:1283–90. 10.1093/hmg/9.9.1283 [DOI] [PubMed] [Google Scholar]
- 37. Montaño AM, Sukegawa K, Kato Z, et al. . Effect of 'attenuated' mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J Inherit Metab Dis 2007;30:758–67. 10.1007/s10545-007-0702-z [DOI] [PubMed] [Google Scholar]
- 38. Tomatsu S, Montaño AM, Nishioka T, et al. . Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A). Hum Mutat 2005;26:500–12. 10.1002/humu.20257 [DOI] [PubMed] [Google Scholar]
- 39. Tomatsu S, Gutierrez M, Nishioka T, et al. . Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum Mol Genet 2005;14:3321–35. 10.1093/hmg/ddi364 [DOI] [PubMed] [Google Scholar]
- 40. Tomatsu S, Fujii T, Fukushi M, et al. . Newborn screening and diagnosis of mucopolysaccharidoses. Mol Genet Metab 2013;110:42–53. 10.1016/j.ymgme.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tomatsu S, Kubaski F, Sawamoto K, et al. . Newborn screening and diagnosis of mucopolysaccharidoses: application of tandem mass spectrometry. Nihon Masu Sukuriningu Gakkai Shi 2014;24:19–37. [PMC free article] [PubMed] [Google Scholar]
- 42. Tomatsu S, Shimada T, Mason RW, et al. . Assay for glycosaminoglycans by tandem mass spectrometry and its applications. J Anal Bioanal Tech 2014;2014(Suppl 2):006 10.4172/2155-9872.S2-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tan MA, Dean CJ, Hopwood JJ, et al. . Diagnosis of metachromatic leukodystrophy by immune quantification of arylsulphatase A protein and activity in dried blood spots. Clin Chem 2008;54:1925–7. 10.1373/clinchem.2008.108456 [DOI] [PubMed] [Google Scholar]
- 44. Fuller M, Tucker JN, Lang DL, et al. . Screening patients referred to a metabolic clinic for lysosomal storage disorders. J Med Genet 2011;48:422–5. 10.1136/jmg.2010.088096 [DOI] [PubMed] [Google Scholar]
- 45. Parkinson-Lawrence E, Fuller M, Hopwood JJ, et al. . Immunochemistry of lysosomal storage disorders. Clin Chem 2006;52:1660–8. 10.1373/clinchem.2005.064915 [DOI] [PubMed] [Google Scholar]
- 46. Fuller M, Brooks DA, Evangelista M, et al. . Prediction of neuropathology in mucopolysaccharidosis I patients. Mol Genet Metab 2005;84:18–24. 10.1016/j.ymgme.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 47. Bond CS, Clements PR, Ashby SJ, et al. . Structure of a human lysosomal sulfatase. Structure 1997;5:277–89. 10.1016/S0969-2126(97)00185-8 [DOI] [PubMed] [Google Scholar]
- 48. Lukatela G, Krauss N, Theis K, et al. . Crystal structure of human arylsulfatase A: the aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis. Biochemistry 1998;37:3654–64. 10.1021/bi9714924 [DOI] [PubMed] [Google Scholar]
- 49. Rivera-Colón Y, Schutsky EK, Kita AZ, et al. . The structure of human GALNS reveals the molecular basis for mucopolysaccharidosis IV A. J Mol Biol 2012;423:736–51. 10.1016/j.jmb.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


