Abstract
Objectives
To investigate associations between maternal body mass index (BMI) at delivery (using pregnancy-specific BMI cut-off values 5 kg/m2 higher in each of the WHO groups) and clinical, theatre utilisation and health economic outcomes for women undergoing caesarean section (CS).
Design
A prospective multicentre observational study.
Setting
Seven secondary or tertiary referral obstetric hospitals.
Participants
One thousand and four hundred and fifty-seven women undergoing all categories of CS.
Data collection
Height and weight were recorded at the initial antenatal visit and at delivery. We analysed the associations between delivery BMI (continuous and pregnancy-specific cut-off values) and total theatre time, surgical time, anaesthesia time, maternal and neonatal adverse outcomes, total hospital admission and theatre costs.
Results
Mean participant characteristics were: age 32 years, gestation at delivery 38.4 weeks and delivery BMI 32.2 kg/m2. Fifty-five per cent of participants were overweight, obese or super-obese using delivery pregnancy-specific BMI cut-off values. As BMI increased, total theatre time, surgical time and anaesthesia time increased. Super-obese participants had approximately 27% (17 min, p<0.001) longer total theatre time, 20% (9 min, p<0.001), longer surgical time and 40% (11 min, p<0.001) longer anaesthesia time when compared with normal BMI participants. Increased BMI at delivery was associated with increased risk of maternal intensive care unit admission (relative risk 1.07, p=0.045), but no increased risk of neonatal admission to higher acuity care. Total hospital admission costs were 15% higher in super-obese women compared with normal BMI women and theatre costs were 27% higher in super-obese women.
Conclusions
Increased maternal BMI was associated with increased total theatre time, surgical and anaesthesia time, increased total hospital admission costs and theatre costs. Clinicians and health administrators should consider these clinical risks, time implications and financial costs when managing pregnant women.
Keywords: caesarean section, obesity, Quality in health care, Health economics
Strengths and limitations of this study.
Large multicentre prospective study.
Broad representation of hospitals: two tertiary maternity, two urban general, three regional/rural.
First prospective study examining associations between body mass index and clinical, time and economic outcomes.
All women undergoing caesarean section included.
We were not able to determine the cause of the increased time.
Introduction
Obesity in women of childbearing age, in high-income counties, is a major global health issue. WHO uses the body mass index (BMI) to define categories of size in adults: underweight, normal, overweight, obese (subdivided in to class I, II) and super-obese (class III). BMI is defined a person’s weight in kilograms divided by the square of their height in metres (kg/m2). WHO uses a BMI of ≥25.0–29.9 kg/m2 to define overweight, a BMI value of 30.0–39.9 kg/m2 to define obesity (class I and II) and a BMI value of ≥40.0 kg/m2 to define super-obesity (class III).1 Using these BMI categories, the obesity rate in women of childbearing age has increased in high-income countries from 16% in 1993 to 24% in 2007.2
In pregnancy, an increased BMI is associated with adverse pregnancy outcomes including venous thromboembolism, pre-eclampsia, postpartum haemorrhage and maternal death.2–6 During pregnancy, both prepregnancy BMI and BMI changes that occur as the result of gestational weight gain contribute to the BMI at delivery. When considering BMI at delivery the use of non-pregnant BMI categories leads to over-representation of overweight or obese women in studies undermining analysis of the risks of obesity.7 These limitations in using non-pregnant metrics at delivery has prompted groups to suggest that pregnancy-specific BMI cut-off values be considered with a BMI of 35 kg/m2 or greater as a threshold for obesity at delivery rather than ≥30 kg/m2.8 Following on from defining delivery obesity (class I and II) as a BMI of ≥35 kg/m2, a logical extension is to define delivery super-obesity (class III) as a BMI ≥45 kg/m2.
Regardless of problems in formally defining obesity at delivery, the rates of obesity in pregnancy are increasing, with the rate of prepregnancy obesity increasing, and the rates of women gaining excessive gestational weight during pregnancy increasing.9 Coupled with this are increasing caesarean section rates, especially in women with increased BMI ≥25.0 kg/m2.3 10 When combined with increasing maternal size, the risks associated with caesarean section may be increased leading to adverse maternal and neonatal outcomes, increased total theatre times and increased hospital costs. While there is considerable literature about obesity during pregnancy and postdelivery outcomes,3 there are fewer reports on the relationship between obesity and time it takes to perform a caesarean section and hospital costs in this setting. One small (n=100) single-centre retrospective study from the USA suggested that total theatre times were increased for women undergoing caesarean section with BMI ≥40 kg/m2 compared with women with a lower BMI.11 While clinicians have greater experience in safely caring for obese and super-obese women, anecdotal reports indicate that increased duration of caesarean section for obese women adversely affects operating theatre suite planning and theatre utilisation, and may have resource implications. There are, however, no quantitative data on these effects.
The aim of this study was to investigate the association between maternal size at delivery using pregnancy-specific BMI cut-off values and clinical (maternal and neonatal), theatre utilisation and health economic outcomes for women undergoing caesarean section. We aimed to determine if pregnancy-specific BMI cut-off values of 35 kg/m2 for obesity and 45 kg/m2 for super-obesity are appropriate to assist planning around the time of delivery including resource allocation and theatre scheduling. Our primary hypothesis was that maternal obesity is associated with increased total theatre time. Our secondary hypotheses were that maternal obesity is associated with increased anaesthesia time, increased surgical time, increased length of hospital stay, increased use of intensive care services for women and neonatal services for babies and increased hospital costs.
Methods
Study participants
A prospective multicentre observational study was performed in collaboration with the seven obstetric teaching hospitals affiliated with the University of Melbourne: two city tertiary maternity, two outer urban general and three regional and rural. The study protocol was approved through the centralised ethics approval process (see online supplementary appendix 1) with individual hospital site-specific approvals. The study was registered with the Australian Clinical Trial Registry prior to participant recruitment (ACTRN1261300060876; Universal Trial Number: U1111-1143-2500). The study was conducted in accordance with ICH GCP notes for Guidance on Good Clinical Practice (CPMP/ICH/135/95). The study was conducted during a 14-month period from 23 November 2013 to 2 February 2015 during which time consecutive women were recruited at each of the seven hospitals over at least a 3-month period.
bmjopen-2016-015630supp001.pdf (309.9KB, pdf)
Consecutive women undergoing caesarean section, elective and emergency, were eligible if they were aged 18 years or older. Women were not eligible if they were aged <18 years; undergoing planned combined surgery, for example, caesarean and tubal ligation; or the woman requested her data were excluded or either parent requested the baby’s data were excluded. Once eligible participants were identified and included in the study, at a clinically appropriate time (before, during or after delivery), a doctor or trial coordinator sought verbal consent from eligible women using a standardised script approved by the Ethics Committee. A case report form (CRF) was developed to record maternal, neonatal, anaesthesia and surgical details and maternal and neonatal outcomes. Data were recorded in the CRF and entered into the REDCap web-based data system (Vanderbilt University, USA) hosted at the University of Melbourne. Management of anaesthesia, surgery and postdelivery care was at the discretion of the clinical team.
Maternal BMI
Maternal BMI at booking and delivery was calculated. Booking BMI was derived using the recorded weight at the first antenatal appointment, if available, while delivery BMI used the recorded weight and height at the time of the caesarean section. Delivery BMI was grouped into BMI categories of underweight, normal, overweight, obese and super-obese using standard WHO cut-off values (<18.5 kg/m2 underweight, 18.5 to <25 kg/m2 normal, 25 to <30 kg/m2 overweight, 30 to <40 kg/m2 obese (class I and II), ≥40kg/m2 super-obese (class III)) and also pregnancy-specific cut-off values for women at delivery: WHO+5 kg/m2 (<23.5 kg/m2 underweight, 23.5 to <30 kg/m2 normal, 30 to <35 kg/m2 overweight, 35 to <45 kg/m2 obese, ≥45 kg/m2 super-obese).
Classification of urgency of caesarean section
Urgency of caesarean section was defined using Royal College of Obstetricians and Gynaecologists United Kingdom definitions.* 12
Total theatre time, surgical time and anaesthesia time
Total theatre time (min) was defined using the Australian Federal Department of Health and Aging definition of total anaesthesia time: from when the anaesthetist commenced exclusive and continuous care of the patient for anaesthesia until when the anaesthetist was no longer in professional attendance, that is, when the participant was safely placed under the supervision of other personnel, usually recovery nursing staff.13 Start time and finish time were recorded. Surgical time was defined as the time from the start of abdominal prepping until the time the final dressing was applied to the surgical wound. Anaesthesia time was defined as total theatre time-surgical time. This time was when only anaesthesia was being performed and not when anaesthesia and surgery were being undertaken together. The end of the operative day was defined as the next midnight following arrival in the postanaesthesia care unit.
Health economic data and cost analysis
Individual cost data of the study participants from the two largest recruiting centres centres, a specialist centre and an outer urban hospital, were used for the economic analysis and were representative of the type and locality of hospitals in Australia.14 Hospitalisation costs relevant to each participant’s admission for caesarean delivery were extracted from participants’ hospital records retrospectively. Costs, in Australian dollars ($AUD), obtained were based on each participant’s hospital resource use, categorised into relevant-specific subgroups for the entire length of their admission. Total hospital admission cost was the sum of three cost subgroups such that total hospital admission cost=theatre cost+surgical service cost+inpatient cost. The three groups were defined as follows: theatre costs were the total cost of the use of operating room, supplies and staff (both anaesthetist and surgical teams) necessary to perform the caesarean section, surgical service costs were the costs pertaining to the surgical supplies and staff (surgeon’s time) only and inpatient costs were composed of all other costs associated with the hospital admission such as nursing, medical imaging, pathology, allied and pharmacy.
Cost subgroup specifications between the two hospitals were compared, and where necessary, regrouped to ensure comparability. From the two hospitals, to quantify theatre costs and surgical service costs per minute, costs from the theatre and surgical service subgroups were divided by the total theatre times and surgical times, respectively. National costs were estimated to 2020 assuming linear progression based on historical data on number of pregnancies and proportions of caesarean sections and obesity among pregnant women. Costs were discounted at a standard rate of 5% adjusting future costs to reflect present value.15 16
Statistical analysis
Over a 3-month period, we expected that about 1500 women would undergo caesarean section at the seven participating hospitals. We estimated that between a quarter (n=375) to a third (n=500) of those women would be obese at delivery with a pregnancy-specific cut-off BMI ≥35 kg/m2 and about 5% (n=75) to have a BMI ≥45 kg/m2. Therefore, this study would have approximately 80% power to detect a difference of 0.17 hours (~10 min) in the average theatre time between non-obese and obese participants, assuming α=0.05 and approximately 80% power to detect a difference of 0.33 hours (~20 min) between those ≥45 kg/m2 and those <35 kg/m2. These defined BMI classes were part of our secondary end analyses; our primary analysis was to treat BMI as a continuous variable. The nature of the continuous relationship between BMI and time was unclear so we did not perform a sample size calculation on the primary analysis.
Linear regression was used to examine associations between continuous delivery BMI and total theatre time. To determine if maternal obesity was associated with increased total theatre time, we considered categories of BMI (underweight <23.5 kg/m2, normal weight 23.5 to <30 kg/m2, overweight 30 to <35 kg/m2, obese to <45 kg/m2, super-obese ≥45 kg/m2) as a predictor of total theatre time in linear regression models. To assess the assumptions that the residuals are normally distributed with zero mean and constant variance, normality plots and plots of residuals against fitted values will be examined. All models include adjustment for hospital. We used these BMI classifications as underweight, normal weight, overweight, obese and super-obese rather than the usual non-pregnant cut-off points that are 5 kg/m2 lower because our variable of interest was BMI at delivery. Both unadjusted analyses and analyses adjusted for potential confounders (age (years), delivery gestation (weeks), multiple pregnancy (no/yes), pre-eclampsia (no/yes), caesarean section urgency (category 1, category 2, category 3, category 4), previous caesarean section (no/yes), delivery hospital). Bonferroni-adjusted multiple comparisons were conducted to identify where there was evidence of a difference between BMI classifications. We conducted a complete case analysis, omitting participants who were missing data on the outcome or exposure variable, or any of the confounding variables. We conducted secondary analysis of surgery time and anaesthesia time using the same approach as described for the total theatre time. Unadjusted log-binomial regression models were fitted to determine whether there was an association between delivery BMI (BMI at delivery) and the risk of infant admission to a neonatal intensive care unit (NICU) or special care nursery, or the risk of a maternal admission to intensive care unit, readmission to the operating room or red cell transfusion. In these analyses, only three categories of BMI (underweight and normal, overweight, obese and super-obese) were considered due to the small number of cases for some outcomes. For health economic data, all mean costs of hospital resource use were reported with SDs or 95% CIs; t-test was used to test for mean differences for each BMI categories against the normal group and their p values reported. Linear regression was performed to quantify the relationship between BMI and hospitalisation cost. All statistical analysis was conducted using Stata V.13.0. This study is reported using the Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.17
Results
Study participants
At the seven hospitals, during the data collection periods, there were a total of 1978 caesarean section operations; a total of 1505 (76%) women consented to participate. The primary end point of total theatre time was not recorded by the responsible anaesthetist in 48 participants and we did not attempt to retrospectively determine the total theatre time. Therefore, the final sample size was 1457 participants. We were unable to obtain maternal delivery weights for 3% of those who consented to take part. The demographic and obstetric characteristics and clinical outcomes of the participants are shown in table 1. Thirty-eight per cent of the caesarean sections were from the two categories of greatest urgency (categories 1 and 2). General anaesthesia was the initial anaesthesia type in 39 women with similar proportions of women in each BMI category undergoing general anaesthesia (2.4%, 3.8%, 2.0%, 2.5% in normal, overweight, obese and super-obese categories, respectively, p=0.394).
Table 1.
Demographic and obstetric data
| Characteristics | Mean (SD) and range/n (%) |
| Age (years)† | 32.0 (5.2) 18.0–50.0 |
| Gestation at booking visit (weeks) | 17.0 (6.2) 1.0–39.0 |
| Weight at booking visit (kg) | 75.0 (20.2) 35.0–158.0 |
| BMI at booking visit (kg/m²) | 28.0 (7.0) 15.8–62.3 |
| Gestation at delivery (weeks) | 38.0 (2.1) 25.0–42.0 |
| BMI at caesarean section (kg/m²) | 32.0 (6.9) 17.0–66.2 |
| Difference in BMI between delivery and booking visit (kg/m2) | 4.0 (2.7) −3.6–16.9 |
| Comorbidities | |
| Previous caesarean section | 638 (43.8%) |
| Multiple pregnancy | 68 (4.7%) |
| Pre-eclampsia | 62 (4.3%) |
| Classification of urgency of caesarean section* | |
| Category 1 | 116 (8.0%) |
| Category 2 | 433 (29.7%) |
| Category 3 | 261 (17.9%) |
| Category 4 | 647 (44.4%) |
| Maternal and neonatal outcomes | |
| Mother admitted to intensive care unit | 11 (0.7%) |
| Mother received red cell transfusion | 20 (1.4%) |
| Mother returned to the operating room | 9 (0.6%) |
| Neonate admitted to neonatal intensive care unit | 60 (4.1%) |
| Neonate admitted to special care unit | 227 (15.6%) |
*Royal College of Obstetricians and Gynaecologists (RCOG) classification.
†Age at delivery.
‡Sample from 1505 participants excluding those missing data on duration of anaesthesia (n=1; 0.1%), BMI (n=45; 3.0%) and potential confounders: age (n=1; 0.1%), gestation at delivery (no missing), multiple pregnancy (n=1; 0.1%), pre-eclampsia (no missing), caesarean section urgency (n=3; 0.2%) and previous caesarean section (no missing); n=1457.
BMI, body mass index.
Maternal BMI
The average BMI at delivery (table 1, figure 1) was 32 kg/m2, ranging from 17 to 66 kg/m2 with 312 (21%) women weighing >100 kg. With the pregnancy-specific cut-off points for women at delivery, normal BMI was defined as being 23.5 to <30 kg/m2; this 5 kg/m2 increase on the usual range is consistent with our finding of a mean BMI increase of 4.0 kg/m2 from booking (mean 17 weeks gestation) to delivery. Using usual WHO BMI criteria, 88% of the participants would have been classified as overweight, obese or super-obese (figure 1). Using the modified BMI criteria, this fell to 55% of pregnant women being overweight, obese or super-obese, consistent with Australian population norms.18 For category 1 caesarean sections, where there is an immediate risk to maternal or fetal life, 54 women (3.7% of total group) were classified as overweight, obese or super-obese according to pregnancy-specific cut-off values for women at delivery (table 2). The incidence of pre-eclampsia ranged from 3% in normal BMI to 14% in the super-obese.
Figure 1.
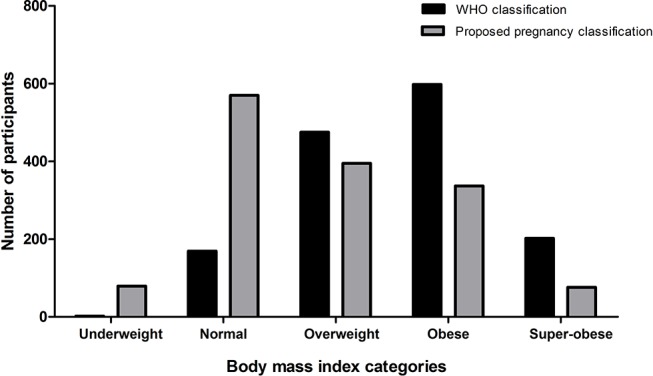
Frequency of body mass index categories according to WHO and proposed pregnancy classifications WHO cut-off points: <18.5 kg/m2 underweight; 18.5 to <25 kg/m2 normal; 25 to <30 kg/m2 overweight; 30 to <40 kg/m2 obese; ≥40 kg/m2 super-obese. Proposed pregnancy cut-off points: <23.5 kg/m2 underweight; 23.5 to <30 kg/m2 normal; 30 to <35 kg/m2 overweight; 35 to <45 kg/m2 obese; ≥45 kg/m2 super-obese.
Table 2.
Descriptive characteristics of the participants by pregnancy proposed BMI category
| Underweight | Normal weight | Overweight | Obese | Super-obese | |
| Mean (SD) and range/n (%) | n=79 | n=570 | n=395 | n=337 | n=76 |
| Total theatre time (min) | 69 (18.7) 34.0–120.0 |
72 (17.4) 36.0–156.0 |
77 (17.9) 35.0–150.0 |
80 (20.1) 40.0–165.0 |
92 (23.5) 49.0–157.0 |
| Surgical time (min) | 44 (13.2) 23.0–75.0 |
45 (13.9) 20.0–126.0 | 48 (14.4) 20.0–115.0 | 50 (14.8) 20.0–115.0 | 54 (15.1) 32.0–111.0 |
| Anaesthesia time (min) | 26 (11.2) 9.0–50.0 | 27 (10.8) 5.0–104.0 | 28 (11.3) 0.0–84.0 | 29 (12.3) 3.0–113.0 | 38 (17.9) 0.0–107.0 |
| BMI at delivery (kg/m2) | 22 (1.5) 17.0–23.4 |
27 (1.8) 23.5–30.0 |
32 (1.4) 30.0–34.9 |
39 (2.9) 35.0–45.0 |
50 (4.4) 45.1–66.2 |
| Age at delivery (years) | 30 (4.7) 20.0–43.3 |
32 (5.1) 18.0–50.0 |
32 (5.0) 19.0–48.0 |
32 (5.5) 19.0–46.0 |
31 (5.5) 20.0–44.0 |
| Gestation at delivery (weeks) | 38 (2.7) 25.0–41.0 |
39 (2.2) 25.0–42.0 |
39 (1.9) 26.0–42.0 |
39 (2.0) 27.0–42.0 |
38 (2.0) 31.0–40.0 |
| Multiple pregnancy | 4 (5.1%) | 33 (5.8%) | 18 (4.6%) | 11 (3.3%) | 2 (2.6%) |
| Pre-eclampsia | 0 (0.0%) | 16 (2.8%) | 18 (4.6%) | 17 (5.0%) | 11 (14.5%) |
| Caesarean section urgency* | |||||
| Category 1 | 4 (5.1%) | 58 (10.2%) | 27 (6.8%) | 26 (7.7%) | 1 (1.3%) |
| Category 2 | 27 (34.2%) | 171 (30.0%) | 116 (29.4%) | 101 (30.0%) | 18 (23.7%) |
| Category 3 | 16 (20.3%) | 91 (16.0%) | 73 (18.5%) | 63 (18.7%) | 18 (23.7%) |
| Category 4 | 32 (40.5%) | 250 (43.9%) | 179 (45.3%) | 147 (43.6%) | 39 (51.3%) |
| Previous caesarean section | 33 (41.8%) | 226 (39.7%) | 168 (42.5%) | 174 (51.6%) | 37 (48.7%) |
| Mother admitted to ICU | 0 (0.0%) | 1 (0.2%) | 4 (1.0%) | 5 (1.5%) | 1 (1.3%) |
| Mother received transfusion | 1 (5.0%) | 4 (20.0%) | 10 (50.0%) | 5 (25.0%) | 0 (0.0%) |
| Mother returned to OR | 1 (11.1%) | 4 (44.4%) | 2 (22.2%) | 2 (22.2%) | 0 (0.0%) |
| NICU | 4 (5.1%) | 28 (4.9%) | 15 (3.8%) | 10 (3.0%) | 3 (3.9%) |
| Special care | 16 (20.3%) | 82 (14.4%) | 50 (12.7%) | 65 (19.3%) | 14 (18.4%) |
*Percentages are calculated from the the number of women in each caesarean section per total number of women in BMI category.
BMI, body mass index; ICU, intensive care unit; NICU, neonatal intensive care unit.
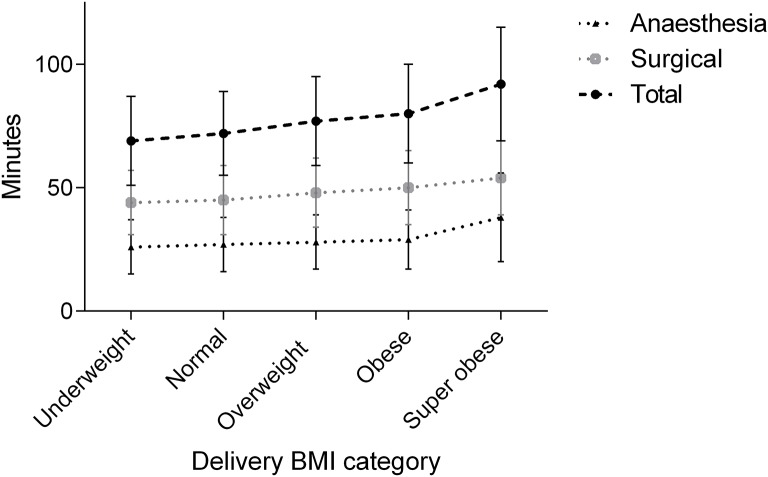
Total theatre time
The average total theatre time for caesarean section was 76 min (SD 19.3, range 34–165 min). We found a positive association between BMI at delivery and total theatre time: for every 1 kg/m2 increase in BMI, total theatre time increased, on average, by 0.6 min (95% CI 0.51 to 0.77). Using pregnancy-specific BMI categories for women at delivery, the mean total theatre time increased with increasing BMI category (table 2 and figure 2). Women classed as obese at delivery had a mean increase in total theatre time of 7.7 min (10%) compared with those classed as normal BMI, while women classed as super-obese at delivery had a total theatre time 19.8 min (26%) longer than those who were of normal BMI (table 3 and figure 2). Both surgical and anaesthesia time increased in a linear fashion with BMI: for every 1 kg/m2 increase in BMI, surgical time increased on average by 0.3 min (95% CI 0.23 to 0.44) and anaesthesia time by 0.3 min (95% CI 0.22 to 0.39). However, considering the pregnancy BMI thresholds, there was a marked increase in the mean anaesthesia time between the obese and super-obese groups (mean increase of 8.4 min, 95% CI 4.38 to 12.38), which was not the case for the mean surgery time (mean increase of 3.3 min, 95% CI −1.66 to 8.26).
Figure 2.
Anaesthesia alone, surgical and total operating room times (mean and SD) by delivery BMI category. BMI, body mass index.
Table 3.
Mean time differences by body mass index category compared with normal body mass index
| Paired comparison | Difference mins (95% CI)* | p Value |
| Total theatre time | ||
| Normal—underweight | 2.7 (−3.6 to 9.0) | 1.000 |
| Overweight—normal | 4.7 (1.3 to 8.2) | 0.001 |
| Obese—normal | 7.7 (4.1 to 11.3) | <0.001 |
| Super-obese—normal | 19.8 (13.4 to 26.2) | <0.001 |
| Surgical time | ||
| Normal—underweight | 1.6 (−3.2 to 6.4) | 1.000 |
| Overweight—normal | 2.9 (0.3 to 5.6) | 0.017 |
| Obese—normal | 4.9 (2.2 to 7.7) | <0.001 |
| Super-obese—normal | 8.7 (3.8 to 13.7) | <0.001 |
| Anaesthesia time | ||
| Normal—underweight | 1.1 (−2.9 to 5.1) | 1.000 |
| Overweight—normal | 1.8 (−0.38 to 3.95) | 0.207 |
| Obese—normal | 2.8 (0.5 to 5.1) | 0.006 |
| Super-obese—normal | 11.1 (7.0 to 15.1) | <0.001 |
*Bonferroni adjusted.
Maternal and neonatal outcomes
No mother or neonate died within 5 days of delivery. While numbers were small, there was some evidence that greater BMI was associated with increased maternal admission to ICU (relative risk (RR) 1.07, 95% CI 1.00 to 1.14; p=0.045). Of 11 women (0.7%) admitted to ICU after delivery (table 2), 6 were obese or super-obese (54.5%) compared with 1 in the normal weight or underweight group (9.1%) (overweight/obese vs normal/underweight RR 1.55, 95% CI: −0.04 to 3.15; p=0.057). There was no evidence of an difference between receiving a red cell transfusion or return to the operating room between those who were classified as obese/super-obese and those who were normal or underweight (red cell transfusion: overweight/obese vs normal/underweight RR 1.57, 95% CI 0.46 to 5.39; p=0.47; return to operating room: overweight/obese vs normal/underweight RR 0.63, 95% CI 0.12 to 3.22; p=0.58). Furthermore, we did not find evidence of an association between delivery BMI and increased admission to NICU. Overall 60 neonates (4.1%) were admitted to NICU. Of these, 13 were the babies of obese or super-obese women (21.7%) compared with 32 in the normal weight or underweight group (53.3%) (overweight/obese vs normal/underweight RR 0.64, 95% CI 0.34 to 1.20; p=0.16). Overall, 227 neonates (15.6%) were admitted to special care. Of these, 79 were the babies of obese or super-obese women (34.8%) compared with 82 in the normal or underweight BMI group (43.2%) (overweight/obese vs normal weight/underweight RR 1.26, 95% CI 0.96 to 1.65; p=0.09).
Economic outcomes
We performed the economic analysis on 768 participants from one of the specialist obstetric hospitals (325) and one of the outer urban hospitals (443); 53% of the total study sample. With the exception of women who were underweight at delivery, women with above normal BMI incurred higher total hospital admission cost (table 4). The mean total hospital admission cost for a woman of normal BMI was $AUD7359 (SD, $AUD3039) while women in the super-obese category had total costs of $AUD8488 (SD, $AUD3564) (table 4), which translates to a 15% increase in total hospital admission costs between a normal BMI and super-obese women of $AUD1129 (95% CI $AUD95 to $AUD2163). Approximately three-quarters of the total hospital admission cost was attributable to inpatient costs including nursing, medications and all other resources used during the patient’s hospital stay while theatre costs accounted for a quarter of the total cost (table 4). The approximate average theatre cost per minute for women undergoing caesarean section in general, regardless of BMI, was $AUD35/min.
Table 4.
Mean costs and hospital length of stay, across BMI categories.
| N | BMI categories | ||||
| Underweight | Normal | Overweight | Obese | Super-obese | |
| 52 | 320 | 192 | 165 | 39 | |
| Total hospital admission costs, | |||||
| Mean ($AUD) | 7605 | 7359 | 7442 | 7530 | 8487 |
| SD | 3589 | 3039 | 2543 | 2680 | 3564 |
| Cost subgroups | |||||
| Theatre, mean ($AUD) | 2531 | 2306 | 2466 | 2556 | 2814 |
| SD | 1788 | 724 | 836 | 795 | 1103 |
| Length of hospital stay | |||||
| Mean (days) | 3.8 | 4.0 | 4.0 | 3.9 | 4.4 |
| Min-max (days) | 1–11 | 1–15 | 1–20 | 1–14 | 3–9 |
BMI, body mass index.
Mean theatre cost increased progressively as BMI increased; there was evidence of a difference in cost between each of the higher BMI categories compared with women with normal BMI. Compared with normal BMI women, theatre costs were increased by 7% in the overweight, 11% in the obese and 22% in super-obese women. Women who were classified as super-obese incurred the greatest cost in all the other subgroups, except for imaging, when compared with women in other BMI categories with costs related to pathology services being 55% greater than normal BMI women. The mean length of hospital stay was the longest for a super-obese patient: 4.4 days (95% CI 3.82 to 4.90); however, the differences between each of the BMI categories were small (p=0.18 for normal vs super-obese; 95% CI of the mean difference: −0.96 to 0.18) (table 4).
Discussion
We conducted a prospective multicentre study of the relationship between maternal BMI and outcomes for caesarean section. The major findings were that increased BMI was associated with increased total theatre time, increased surgical time, increased anaesthesia time, increased risks of maternal admission to ICU, increased total hospital admission costs and increased theatre costs. Using our predetermined pregnancy-specific cut-off values for BMI (WHO classes+5 kg/m2) for women at the time of delivery, we found that approximately 1 in 20 women were super-obese at delivery, and had more than 25% longer total theatre time, 20% longer surgical time and 40% longer anaesthesia time, compared with normal weight women. Super-obese women also had a 15% increase in total hospital admission costs and a nearly 30% theatre costs compared with normal BMI women. These findings have important implications for understanding clinical care, operating theatre use and health service costs, for both clinicians and health services managing pregnant women. These clinical and cost findings support arguments for increased allocated theatre time and increased funding for care of super-obese pregnant women.
While the recording of prepregnancy BMI and gestational weight gain are important, our study supports routinely recording height and weight measurements throughout pregnancy so that BMI can be used as part of care planning around the time of delivery with pregnancy-specific BMI ranges 5.0 kg/m2 greater than the current WHO ranges. While we found that the average BMI increase during pregnancy was 4.0 kg/m2, it was most likely >4.0 kg/m2 due to the late average booking gestation of 17 weeks, leading to the pragmatic use of 5.0 kg/m2 incremental changes in BMI classes.
We found that total hospital admission costs increased by 15% (about $AUD1129 per woman), including theatre costs by 22% (about $AUD500) in super-obese women compared with normal BMI women. These findings support the argument for increased funding of super-obese pregnant women. Based on our data, and using conservative estimates, additional hospital resources to manage super-obesity for Australian women undergoing caesarean section currently exceeds $AUD3.8 million annually and will continue to rise to over $AUD5 million per year by 2020 with cumulative costs of over $AUD50 million over the next 10 years.
A limitation is that we were not able to determine the underlying causes of the increased total theatre time, surgical time and anaesthesia time. The current association between anaesthesia difficulty and maternal obesity is unclear. Two recent studies could not clearly associate maternal obesity with anaesthetic difficulty.8 19 In 2009, Bamgbade et al conducted a single-centre study of 1477 women having caesarean section in the UK.19 They found no evidence of an association between obesity and increased difficulty in spinal anaesthesia, increased block failure or increased use of general anaesthesia. This study may have been limited by using a delivery obesity definition of ≥30 kg/m2, which was potentially overinclusive. These authors speculated that a BMI of 35 kg/m2 (that we used) may be better to define obesity at delivery. In another 2009 single-centre study of 427 women, Ellinas et al found evidence to demonstrate that obesity was associated with difficulty with neuraxial blockade for labour.8 They did, however, find that obesity was associated with the two factors associated with difficult neuraxial block: inability to palpate landmarks and limited patient flexion. In a recent multicentre Australian study, McDonnell et al did not find that general anaesthesia for caesarean section was more likely for patients weighing >100 kg; they did not, however, consider BMI.20 Similarly, in a single-centre study Kinsella did not find evidence of an association between increased maternal weight and anaesthetic difficulty during caesarean section.21
It is also important to note that some anaesthesia times were recorded as zero minutes. This occurred when surgical prepping and anaesthesia commenced at the same time. Additionally according to our definition of anaesthesia time, in some cases this may not reflect the total time to establish anaesthesia if there is a delay between surgical prepping and incision time due to establishment of anaesthesia.
An older single-centre retrospective study of predominantly African-American women from the USA found that maternal obesity, defined as BMI >30 kg/m2, was one of the several factors associated with increased operative time for caesarean delivery.22 They did not examine anaesthetic factors nor did they examine how total time varied with increasing body size. Because anaesthetists, and the rest of the delivery team, are caring for more women who are obese, there is growing expertise, and possibly efficiency, in managing obese pregnant women. Added to this growing experience and expertise are new technologies such as use of ultrasound to guide neuraxial blockade23 24 and video-laryngoscopes to aid difficult intubation.25 The combined effect of greater experience and new technologies may to some extent counteract challenges of maternal obesity.
While we were primarily looking at overweight and obesity, we noted that women who were underweight had higher average costs and theatre times than those classified as normal weight. Mungo et al, in a study investigating outcomes of pulmonary resection for lung cancer, also found that underweight adults had a greater risk-adjusted length of stage compared with normal weight patients.26 Our findings may be explained by the presence of maternal comorbidities. Therefore, further research is required to confirm this unexpected finding.
Conclusions
Pregnancy-specific BMI cut-off values for women at delivery are justified and enable correct classification of maternal size at delivery. Obesity is common among Australian women of childbearing age and was found to be associated with increased total theatre time, surgical and anaesthesia time, increased maternal risk of ICU admission, increased total hospital admission costs and theatre costs. There was no evidence that mothers who were obese had increased risk of blood transfusion, re-admission to the operating room, neonatal admission to higher acuity care or neonatal admission to special care nursery compared with those of normal weight. Clinicians and health administrators need to consider these clinical risks, the time implications and financial costs when managing pregnant women. To do so, we need to record maternal BMI during the antenatal period and at delivery, increase communication between clinical teams and increase funding for women with increased BMI.
Supplementary Material
Acknowledgments
We would like to thank the MUM SIZE investigators at the seven participating hospitals: Ballarat Base Hospital: Michael Shaw, Balvindar Kaur, Lucy Johnson, Gabriel Jones, Alyce Burgess; The Mercy Hospital for Women: Scott Simmons, Katrina Pirie, Anne Galati-Laguda, Leanne Pilkington; The Northern Hospital: Joseph Lew, Yasmin Lennie; Goulburn Valley Health: Arnold Beeton, Gwendolyn Liow; Sunshine Hospital: Elizabeth Hessian, Samantha Bates, Mari Kawamata, Miriam Towns, Anna Tippett, Jenny Vo; North Eastern Hospital Wangaratta: Andrew Haughton, Ben Kabbabe; The Royal Women’s Hospital: Alicia Dennis, Sarah Grant, Jacqueline de Gabriel, Ingrid Walkley, Hannah Barker. This work was presented in part at the Australian and New Zealand College of Anaesthetists Annual Scientific Meeting in Auckland New Zealand, May 2016 as a poster in the Obstetric Anaesthesia 2016 congress 19–20 May 2016, Manchester Central Convention Complex UK) http://www.epostersonline.com/oaa2016/node/46 and the Society of Obstetric Anesthesiologists and Perinatologists Meeting in Boston in May 2016.
Footnotes
Category 1 = maternal or fetal compromise H immediate threat to life of woman or fetus; Category 2 = maternal or fetal compromise H no immediate threat to life of woman or fetus; Category 3 = no maternal or fetal compromise – requires early delivery; Category 4 = no maternal or fetal compromise – delivery at a time to suit woman and maternity services
Contributors: ATD, KT: design; acquisition, analysis and interpretation of data; drafting and revising manuscript. KEL: design; analysis and interpretation of data; drafting and revising manuscript. DS: conception and design; acquisition, analysis and interpretation of data; drafting and revising manuscript. KD: design; analysis and interpretation of data; revising manuscript. PC: conception and design; interpretation of data; drafting and revising manuscript. JL: conception and design; acquisition and interpretation of data; revising manuscript. AP: design; acquisition and analysis of data; drafting and revising manuscript. EH: acquisition, analysis and interpretation of data; drafting and revising manuscript. GT: conception and design; interpretation of data; revising manuscript. SS: design; acquisition of data; revising manuscript. DC: design; interpretation of data; drafting and revising manuscript.
Funding: ANZCA Pilot Grant to develop case report form.
Competing interests: None declared.
Patient consent: Verbal consent for this prospective study was approved by Human Research Ethics Committee.
Ethics approval: Monash Health HREC/13/SHB/2B.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data from the study.
References
- 1. World Health Organization classification of Body Mass Index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed 23 Aug 2015).
- 2. Cantwell R, Clutton-Brock T, Cooper G, et al. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118(Suppl 1):1–203. 10.1111/j.1471-0528.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 3. Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Aust N Z J Obstet Gynaecol 2000;40:139–55. 10.1111/j.1479-828X.2000.tb01137.x [DOI] [PubMed] [Google Scholar]
- 4. Lewis G. The Confidential Enquiry into Maternal and Child Health(CEMACH). Saving mothers' Lives:reviewing maternal deaths to make motherhood safer - 2003-2005. London: CEMACH, 2007. [Google Scholar]
- 5. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ Care - Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12. Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2014. [Google Scholar]
- 6. Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet 2006;93:269–74. 10.1016/j.ijgo.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 7. Mace HS, Paech MJ, McDonnell NJ. Obesity and obstetric anaesthesia. Anaesth Intensive Care 2011;39:559–70. [DOI] [PubMed] [Google Scholar]
- 8. Ellinas EH, Eastwood DC, Patel SN, et al. The effect of obesity on neuraxial technique difficulty in pregnant patients: a prospective, observational study. Anesth Analg 2009;109:1225–31. 10.1213/ANE.0b013e3181b5a1d2 [DOI] [PubMed] [Google Scholar]
- 9. Helms E, Coulson CC, Galvin SL. Trends in weight gain during pregnancy: a population study across 16 years in North Carolina. Am J Obstet Gynecol 2006;194:e32–4. 10.1016/j.ajog.2006.01.025 [DOI] [PubMed] [Google Scholar]
- 10. Callaway LK, Prins JB, Chang AM, et al. The prevalence and impact of overweight and obesity in an australian obstetric population. Med J Aust 2006;184:56–9. [DOI] [PubMed] [Google Scholar]
- 11. Butwick A, Carvalho B, Danial C, et al. Retrospective analysis of anesthetic interventions for obese patients undergoing elective cesarean delivery. J Clin Anesth 2010;22:519–26. 10.1016/j.jclinane.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 12. Tuffnell DJ SA, Waugh JJS, Walker JJ. The management of severe pre-eclampsia/eclampsia. In: Guideline No 10(A) Royal College of Obstetricians and Gynaecologists 2006;11. [Google Scholar]
- 13. Relative Value Guide. 18th Edition North Sydney NSW: The Australian Society of Anaesthetists Limited, 2016. 2059. [Google Scholar]
- 14. AIHW 2015. Australia's mothers and babies 2013—in brief. Perinatal statistics series no. 31. Cat. no. PER 72. Canberra: AIHW; http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129554140 Viewed 15/3/3017. [Google Scholar]
- 15. McIntyre HD, Gibbons KS, Flenady VJ, et al. Overweight and obesity in Australian mothers: epidemic or endemic? Med J Aust 2012. 2012;196:184–8. 10.5694/mja11.11120 [DOI] [PubMed] [Google Scholar]
- 16. Australian Institute of Health and Welfare. Australia’s Mothers and Babies. 2012. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=60129550054 (accessed 23 Sep 2015).
- 17. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. AIHW. Cardiovascular disease, diabetes and chronic kidney disease—Australian facts: risk factors. Cardiovascular, diabetes and chronic kidney disease series no. 4 Cat. no. CDK 004. Canberra: AIHW, 2015. [Google Scholar]
- 19. Bamgbade OA, Khalaf WM, Ajai O, et al. Obstetric anaesthesia outcome in obese and non-obese parturients undergoing caesarean delivery: an observational study. Int J Obstet Anesth 2009;18:221–5. 10.1016/j.ijoa.2008.07.013 [DOI] [PubMed] [Google Scholar]
- 20. McDonnell NJ, Muchatuta NA, Paech MJ. Management of a super-morbidly obese parturient requiring caesarean delivery (again!). Anaesth Intensive Care 2008;36:751. [PubMed] [Google Scholar]
- 21. Kinsella SM. A prospective audit of regional anaesthesia failure in 5080 caesarean sections. Anaesthesia 2008;63:822–32. 10.1111/j.1365-2044.2008.05499.x [DOI] [PubMed] [Google Scholar]
- 22. Doherty DA, Magann EF, Chauhan SP, et al. Factors affecting caesarean operative time and the effect of operative time on pregnancy outcomes. Aust N Z J Obstet Gynaecol 2008;48:286–91. 10.1111/j.1479-828X.2008.00862.x [DOI] [PubMed] [Google Scholar]
- 23. Carvalho JC. Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol Clin 2008;26:145 vii–158 viii. 10.1016/j.anclin.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 24. Balki M, Lee Y, Halpern S, et al. Ultrasound imaging of the lumbar spine in the transverse plane: the correlation between estimated and actual depth to the epidural space in obese parturients. Anesth Analg 2009;108:1876–81. 10.1213/ane.0b013e3181a323f6 [DOI] [PubMed] [Google Scholar]
- 25. Cook TM, MacDougall-Davis SR. Complications and failure of airway management. Br J Anaesth 2012;109(Suppl 1):i68–i85. 10.1093/bja/aes393 [DOI] [PubMed] [Google Scholar]
- 26. Mungo B, Zogg CK, Hooker CM, et al. Does obesity affect the outcomes of pulmonary resections for lung Cancer? a national surgical quality improvement program analysis. Surgery 2015;157:792–800. 10.1016/j.surg.2014.10.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015630supp001.pdf (309.9KB, pdf)