Abstract
Biological rhythms are thought to have evolved to enable organisms to organize their activities according to the earth’s predictable cycles, but quantifying the fitness advantages of rhythms is challenging and data revealing their costs and benefits are scarce. More difficult still is explaining why parasites that live exclusively within the bodies of other organisms have biological rhythms. Rhythms exist in the development and traits of parasites, in host immune responses, and in disease susceptibility. This raises the possibility that timing matters for how hosts and parasites interact and, consequently, for the severity and transmission of diseases. Here, we take an evolutionary ecological perspective to examine why parasites exhibit biological rhythms and how their rhythms are regulated. Specifically, we examine the adaptive significance (evolutionary costs and benefits) of rhythms for parasites and explore to what extent interactions between hosts and parasites can drive rhythms in infections. That parasites with altered rhythms can evade the effects of control interventions underscores the urgent need to understand how and why parasites exhibit biological rhythms. Thus, we contend that examining the roles of biological rhythms in disease offers innovative approaches to improve health and opens up a new arena for studying host-parasite (and host-parasite-vector) coevolution.
Keywords: fitness, adaptation, phenotypic plasticity, Plasmodium, transmission, life history, circadian rhythm, chronobiology, host-parasite interactions
Biological rhythms appear to be an elegant evolutionary solution to the challenge of coordinating activities with the consequences of the earth’s daily and seasonal rotation. The genetic and molecular mechanisms that underpin daily (circadian) rhythms have been intensively studied for decades and are well characterized in a number of model systems (Johansson and Staiger, 2015; Takahashi, 2015; Tomioka and Matsumoto, 2010). Yet the evolution and ecology of rhythms and their underlying oscillators (clocks) remain remarkably poorly understood. In particular, evidence for how rhythms shape interactions between organisms is lacking (Martinez-Bakker and Helm, 2015). One of the most ubiquitous interactions between organisms is that between hosts and parasites. Most taxa on the planet act as hosts for a diverse array of parasites, while the remaining organisms are subject to their attack (e.g., Dobson et al., 2008). Rhythms in parasite traits have been known for centuries. For example, the periodicity of fever that accompanies malaria infections was used as a diagnostic tool because different species of malaria (Plasmodium) parasites give rise to fevers with different regularity (every 1, 2, or 3 days; Kwiatkowski and Greenwood, 1989). These fevers are a consequence of the synchronous development of malaria parasites during cycles of asexual replication (Kwiatkowski and Nowak, 1991). More recently, rhythms in host immune responses have been described (Bhardwaj et al., 2011; Scheiermann et al., 2013). But whether rhythms in parasites, rhythms in host immune responses, and their interactions have significant consequences for parasite fitness and for disease transmission is largely unexplored.
Evolutionary and ecological approaches were common in the early days of chronobiology (Daan, 2010), but modern chronobiology tends to focus on the mechanistic basis of oscillators and their rhythms. However, whether the “embodied clocks” observed in parasites are due to parasites controlling their rhythms (i.e., they have evolved oscillators) or are simply the by-product of rhythmic host processes that enforce rhythms (i.e., parasites are intrinsically arrhythmic) is a mystery. We take an evolutionary ecology perspective to ask questions about the fitness costs and benefits of parasite rhythms, because prior knowledge of which party (host and/or parasite) gains advantages from those rhythms will illuminate who controls them, helping to narrow down the search for the genes encoding these embodied clocks to the correct organism. Also, identifying the fitness consequences of rhythms reveals the evolutionary pressures on the mechanisms encoding these embodied clocks, which is important for predicting how rhythms might change in response to efforts to control parasites. We take a parasite-centric view to examine what is known about the fitness consequences (i.e., adaptive significance) of their rhythms during infections, explore putative mechanisms that generate these rhythms, and suggest approaches for solving these mysteries. We then discuss how rhythms could be exploited to better control infectious diseases. We use the term parasite to refer to all infectious disease–causing organisms (i.e., micro- and macro-parasites, from viruses to worms).
Evolutionary Ecology of Rhythms
Circadian rhythms (rhythms of approximately 24 h) may offer two kinds of fitness benefits. First, it may be advantageous to coordinate the internal processes of an organism with each other (intrinsic adaptive value; Sharma, 2003). Second, it may be advantageous to synchronize physiological and/or behavioral processes to cyclic environmental factors (extrinsic adaptive value; Sharma, 2003). One of the major advantages of circadian oscillators is that they allow organisms to anticipate and prepare for an environmental change before it happens—for example, maximizing the opportunity to exploit resources such as food or minimizing exposure to danger such as predators. If parasites have evolved endogenous time-keeping strategies, it is important to understand why rhythmic parasites are fitter than parasites that are arrhythmic or differently phased.
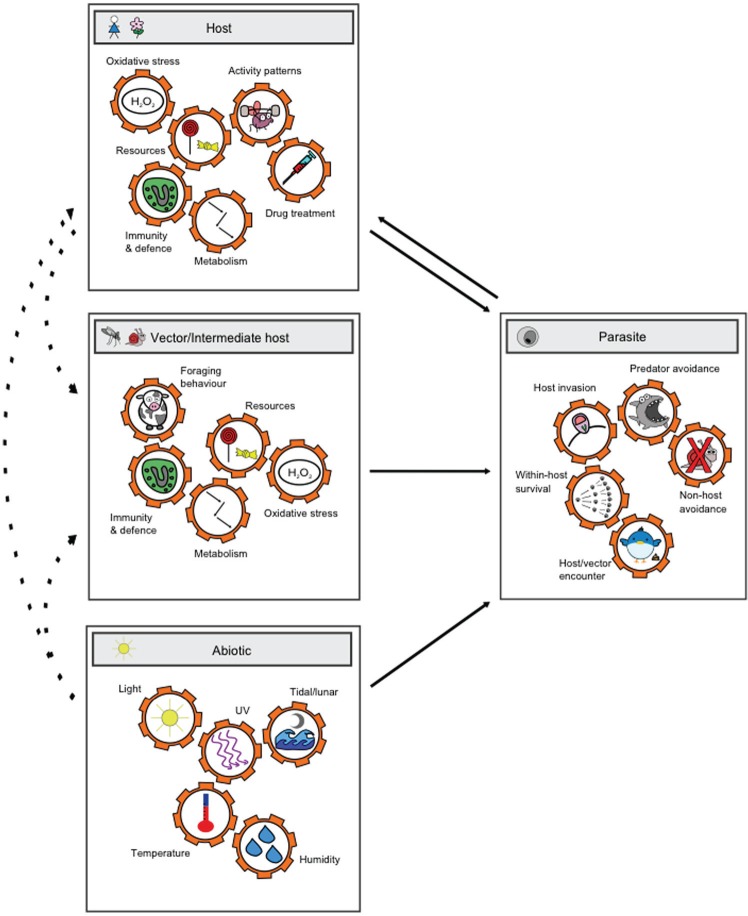
Much like their free-living counterparts, parasites may benefit from rhythms (i.e., be adaptive) because they ensure that host resources are maximally exploited or host immune responses maximally avoided. Alternatively, rhythms in parasite development may benefit hosts, may have no significant fitness consequences for either hosts or parasites (i.e., may be selectively neutral), or may be detrimental to one or both parties (Mideo et al., 2013). The drivers of rhythms in parasites are likely to be complex (Fig. 1) and specific to the host-parasite system. Yet knowing to what extent parasite rhythms are products of host and/or parasite genes is central to understanding the evolution of those phenotypes. If parasite rhythms are under the control of parasite genes, understanding how rhythms vary between genetically distinct individuals and how much the environment can alter those rhythms is also important because this variation is the fuel for evolution (Schlichting and Pigliucci, 1998; Chevin et al., 2010). Even more ambitious is harnessing information about who owns the relevant oscillators, and their effects on fitness, to develop novel antiparasite treatments.
Figure 1.
Selection of the rhythmic factors (cogs) in the various environments that parasites experience during their lifecycles that are hypothesized to shape rhythms in parasite traits. Rhythmic factors within each environment are often correlated and can shape the rhythms of factors in other environments (dotted arrows). The complexity of the possible combinatorial interactions between rhythmic environmental factors and the diversity of parasite rhythms that can be affected poses an interdisciplinary challenge in regard to unravelling what drives parasite rhythms and their consequences for fitness.
Investigating the fitness consequences of rhythms is a surprisingly challenging endeavor. Fitness is variably defined depending on context but generally refers to the contribution of a genotype, relative to other genotypes in the population, to subsequent generations (Acerenza, 2016). Fitness can be decomposed into survival and reproduction, and evolutionary biologists tend to deal in proxies for these components, which are traits (phenotypes, behaviors, physiological processes, etc.) that are expected to underpin survival or reflect lifetime reproductive success. Crucially, proxies are more easily measured and compared than actual genetic contributions to future generations. Fitness may be affected by rhythms in the parasites’ biotic environment (i.e., the host in which they are embodied), rhythms in the vector or the next host species (in effect, “disembodied clocks”), and rhythms in the abiotic environment (Fig. 1, Table 1).
Table 1.
Summary of the state of affairs on the evolutionary ecology of parasite rhythms.
| Parasite | Rhythm | Why? | How? |
|---|---|---|---|
|
Botrytis cinerea
Fungus |
Size of lesions in host leaves | Counteract effects of host immune defenses | Oscillator entrainable by photoperiod and temperature |
|
Dicrocoelium dendriticum
Lancet liver fluke |
Manipulation of host behavior | Match foraging activity of next host | Respond to temperature, not light |
|
Echinostoma caproni
Trematode |
Migration between anterior feeding and posterior egg-laying sites in gut | Maximize food intake/minimize homeostasis, or maximize infective dose | Actively respond to food in gut or digestive processes, or by-product of gut peristalsis |
|
Enterobius vermicularis
Pinworm and other oxyurid nematodes |
Migration out of host anus or posterior gut to lay eggs | Enable larvae to mature in time to encounter host during activity | Respond to food appearing in gut or associated digestive processes |
|
Hymenolepis diminuta
Tapeworm |
Migration between anterior and posterior gut | As for trematodes | Respond to serotonin or gut peristalsis |
|
Isospora spp. Coccidia |
Shedding from host in feces | Minimize exposure to damage by UV light and low humidity, or maximize infective dose | Respond to temperature or diurnal variation in host foraging activity |
| Metastrongyloid nematodes | Larval output in feces | Not known | By-product of rhythms in defecation |
|
Plasmodium spp. Malaria parasite |
Development during asexual replication | Evade immune killing or exploit red blood cell resources. Maximize infectiousness to vector | Respond to melatonin, photoperiod, glucose, temperature, or scheduled by host’s rhythms |
|
Schistosoma japonicum
Fluke |
Emergence from intermediate snail host | Coincide with activity pattern of next host species | Respond to photoperiod (proposed for related species) |
|
Schistosoma haematobium
Fluke |
Egg shedding from definitive human host | Maximize deposition of eggs in habitat of next host species | Respond to host body temperature or locomotor activity |
| Various freshwater trematode flukes | Emergence from intermediate snail host | Cope with rhythms in activity of next host, salinity/tides, UV light, predators, wrong host activity | Respond to photoperiod (proposed for related species) |
|
Trypanosoma brucei
Trypansome |
Expression of metabolic genes | Cope with “metabolic rush” resulting from host foraging | Oscillator entrainable by temperature, not photoperiod |
|
Wuchereria bancrofti, Brugia malayi
Filarial nematodes |
Migration from lungs to peripheral circulation | Maximize availability to mosquito vectors | Respond to oxygen levels in blood |
For each parasite species, the table notes the rhythms that have been documented, hypotheses for how they might affect within-host survival and/or between-host transmission, and how rhythms are thought to be regulated. References are cited in the main text.
Organismal rhythms that are synchronized to environmental rhythms enhance the growth of plants (Dodd et al., 2005) and the replication rate of bacteria (Ouyang et al., 1998). Growth and replication underpin organismal survival and reproductive output and, so, are key components of fitness. For most endoparasites that live inside the bodies of other organisms, the fitness components of survival and reproduction are analogous to “within-host survival” and “between-host transmission.” For macro-parasites (e.g., gastrointestinal worms), within-host survival entails individuals growing and evading elimination, and for micro-parasites (e.g., viruses, malaria parasites), replication inside the host is required to survive. As for plants and bacteria, when the rhythms of malaria parasites are not in synchrony with the rhythms of their environment—the circadian rhythms of their host—the parasites’ capacity to replicate and transmit is reduced by 50% (O’Donnell et al., 2011) (Box 1). While the fitness consequences of parasite rhythms have been most clearly demonstrated for malaria parasites, an outstanding mystery is why it is advantageous for malaria parasites to have a particular phase relative to the rhythm of the host (Mideo et al., 2013). For other parasite species, the theory is ahead of the data: Varied hypotheses for why rhythms are beneficial have been postulated.
Box 1. Rhythms in the Development of Malaria Parasites.
Malaria parasites display rhythms in development during cycles of asexual replication in the host’s red blood cells (RBCs). Each merozoite invades an RBC and develops into a ring stage, which is responsible for remodeling the RBC. Rings stages then begin to feed on hemoglobin inside the RBC and progress through a number of trophozoite stages (early, mid, and late) before replicating their genome and dividing to become schizonts filled with progeny called merozoites (producing ~8 merozoites each in the rodent malaria parasite Plasmodium chabaudi). Schizonts then burst to release their merozoites and initiate the next cycle of asexual replication. For P. chabaudi, each asexual cycle takes ~24 h, parasites develop through the stages synchronously, and schizonts burst over several hours starting around midnight.
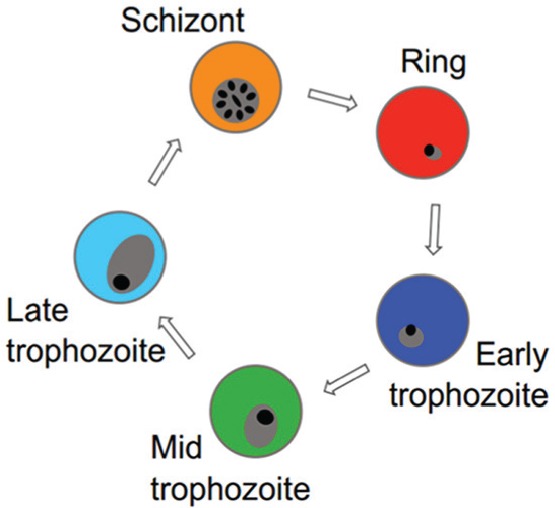
The illustration here above shows rhythms observed in each developmental stage over 3 cycles of asexual replication for P. chabaudi. Murine hosts were entrained to 12-h light:12-h dark and sampled every 3 h. The colors in the graph below match colors in the asexual cycle illustration above. Rhythms exist but appear dampened for late-stage parasites (cyan, orange) because they sequester in host organs; thus, few are collected in blood samples.
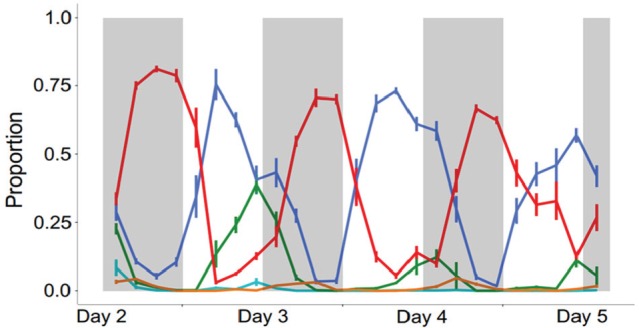
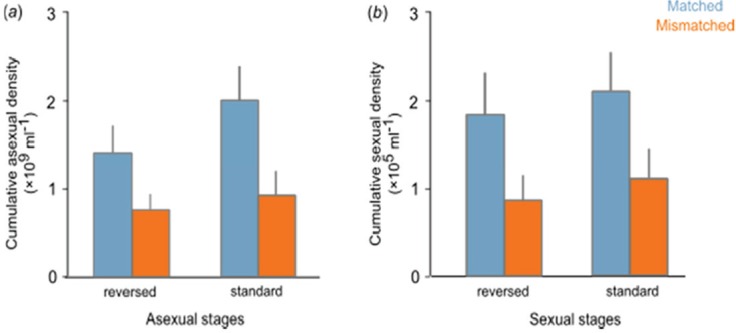
Malaria parasites whose developmental rhythm is not in phase with the circadian rhythms of the host (mismatched) pay fitness costs (O’Donnell et al., 2011). Mismatched infections were created by infecting hosts during their evening with ring stages collected from hosts experiencing their morning. Mismatched infections produce fewer asexual stages (F1,22 = 8.38; p = 0.008) and sexual stages (F1,22 = 6.84; p = 0.016) during the growth phase of infections. Loss of asexual stages puts parasites at risk from the challenges of their lifestyle, including immune responses, antimalarial drugs, and competition with other parasite strains, whereas loss of gametocytes reduces the potential for transmission. The 50% reduction observed in the number of mature gametocytes may not be driven solely by the 50% reduction in asexual numbers because there are complex links between the dynamics of asexual stages and gametocytes.
The graph below shows the cumulative number of asexual and sexual stages during the growth phase of infections. Parasites are either matched (blue bars) or mismatched (orange bars) to the circadian rhythm of the host. Ring stages were used to infect hosts experiencing their mornings in either normal or reverse light conditions (N = 24, mean ± SEM).
Why malaria parasites benefit from synchronizing to the host’s circadian rhythms, and how this is achieved, are unknown. These mysteries should be solvable because malaria parasites are generally a very tractable model system, and rodent hosts offer a wide range of circadian clock knock-out, immune knock-out, and RBC redox reporter phenotypes.
Nonetheless, quantifying rhythms in malaria parasites is difficult. Readouts must be obtained from Giemsa-stained blood smears (shown below), which requires a carefully trained eye and plenty of time.
When considering how rhythms might affect parasite fitness, it is necessary to recognize that endoparasites often exist as populations inside each host. This means that rhythms offer parasites the opportunities to synchronize activities with other individuals in the infection and to express traits at specific times of day (Mideo et al., 2013). Synchrony alone could be favored by natural selection, in which case the time-of-day that parasites express traits does not matter. This is analogous to the emergence behaviors of cicadas and the simultaneous seed production of trees (i.e., masting) where synchrony provides safety in numbers against predators or other dangers (Silvertown, 1980; Williams et al., 1993). Such a phenomenon may apply to the coccidian parasites, Isospora spp., which emerge from their bird host in a coordinated fashion (Dolnik et al., 2011; Martinaud et al., 2009). Alternatively, the time-of-day that parasites express particular traits may be key, and so synchrony is simply a by-product of all parasites doing certain things at a certain time. For example, genes controlling cellular metabolism and drug sensitivity of populations of trypanosomes (which cause sleeping sickness) in the host and in culture are expressed at particular times of day (Rijo-Ferreira et al., 2017). This means that timing and synchrony can be viewed as two traits that can affect parasite fitness independently or additively (Mideo et al., 2013). Whether timing is solely the consequence of the need for synchrony, or timing is the main driver of rhythms, or both timing and synchrony are beneficial, is unclear for any parasite species.
How Might Rhythms Benefit Parasites?
Here, we outline how rhythms help parasites cope with the challenges, and exploit the opportunities, of their lifestyle. We split the lifecycles of parasites into the following components: encountering a suitable host/vector; invading and establishing an infection; evading elimination from the host/vector; and integrating additive effects across the lifecycle. These lifecycle components entail conceptual overlap, so our categorization should be viewed simply as a convenient structure for discussion.
Encountering a Host/Vector
Given that almost all organisms are rhythmic, there is often periodicity in opportunities to transmit to a vector or a new host. Additionally, some parasites must spend time in the abiotic environment before entering the next host. The following sections outline how parasite rhythms facilitate finding a host/vector and surviving in the environment.
Transmitting to the vector
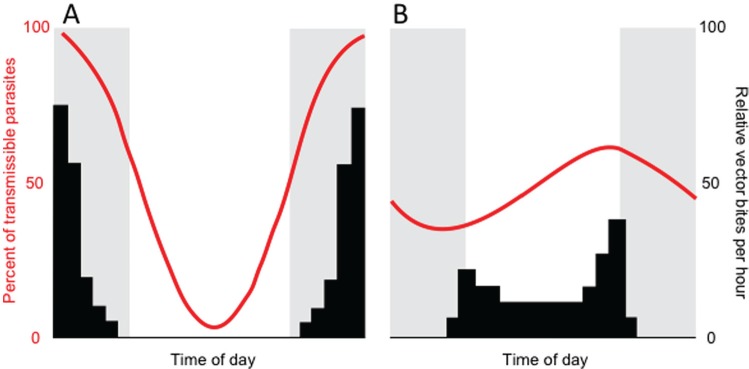
Frank Hawking (1967, 1970) was the first to suggest that parasites match their behaviors to opportunities to transmit to a vector. Hawking studied the filarial nematodes Wuchereria bancrofti and Brugia malayi. These worms, which are causative agents of elephantiasis in humans, produce transmission forms called microfilaria that circulate in the vertebrate host’s blood, ready to be transmitted by an insect vector (Hawking, 1967). Hawking (1967) demonstrated rhythms in the number of microfilariae in the peripheral circulation versus vessels around the lungs across the 24-h day in a manner corresponding to the timing of biting behavior in the local mosquito vector species (Fig. 2). The microfilariae of W. bancrofti and B. malayi appear in peripheral circulation at night and tend to be transmitted by night-biting Anopheles mosquitoes, whereas day-biting Aedes mosquitoes transmit a diurnally circulating form of W. bancrofti (Pichon and Treuil, 2004). Observing the expected rhythms across several Wuchereria species provides compelling support for the idea that activity rhythms in the recipient vector select for parasite rhythms. But why are microfilariae not in the peripheral circulation throughout the day and night? It may be energetically expensive for microfilariae to maintain a location in the peripheral circulation, and/or microfilariae may be better protected from immune killing when located in the lungs, so they only migrate when necessary. More generally, the reason why parasites do not express a trait—essential to survival or transmission—at all times in the circadian cycle may be that the trait is costly (e.g., energetically expensive or risky) and so expressing it only at the most important time-of-day is best (i.e., adaptive).
Figure 2.
The migration of microfilariae from the lungs to the host’s peripheral circulation broadly coincides with the activity rhythms of their mosquito vector species. Red lines illustrate rhythms in the percentage of the maximum number of microfilariae observed in the peripheral blood of hosts, and the bars illustrate vector biting activity. (A) The nocturnally periodic form of Wuchereria bancrofti is transmitted by night-biting Anopheles and Culex, and (B) the diurnally subperiodic form is transmitted by day-biting Aedes. Coinciding migration with vector foraging is thought to maximize parasite transmission, the “Hawking hypothesis.” Adapted from Pichon and Treuil (2004).
The “Hawking hypothesis” has been suggested to apply to malaria parasites as well (Garnham and Powers, 1974; Hawking et al., 1968; Hawking et al., 1966): specifically, that rhythms in the development of malaria parasites (discussed in the next section; see also Box 1) ensure that transmission stages (gametocytes) reach maturity at night, when Anopheline mosquito vectors are foraging for blood. However, once mature, gametocytes circulate for several days, and periodicity in their infectivity to mosquitoes has not been detected (Bray et al., 1976; Githeko et al., 1993; Magesa et al., 2000). These studies would benefit from being revisited because the experiments have confounded parasite and mosquito times of day. This is a problem because if gametocytes are at the optimal age for transmission when vectors are most resistant to infection (Murdock et al., 2013), or gametocytes are immature or senesced when vectors are most susceptible, rhythms in gametocytes and vectors could cancel each other out, making rhythms in infectivity undetectable. Further, if rhythms in vector resistance oppose rhythms in gametocyte transmissibility, a coevolutionary arms race may be occurring (Rund et al., 2016).
Transmitting between hosts
Rhythms in transmission behaviors have been relatively well studied for vector-borne parasites but are likely to be important for all parasites. For parasites that are immediately infective to the recipient host, displaying transmission behaviors during the host’s active phase is advantageous. This is suggested to be the case for the human-infecting fluke Schistosoma haematobium, which lays eggs during the day (McMahon, 1976), and rat metastrongyloid nematodes, whose infective stage larvae accumulate in the feces at night (de Azevedo et al., 2011). However, the latter case may simply be a by-product of rhythms in defecation by the host: The ratio of larvae to fecal mass is not rhythmic, suggesting that the parasites are not intrinsically rhythmic but have a beneficial rhythm imposed on them by the host (de Azevedo et al., 2011).
The transmission forms (cercariae) of Schistosoma spp. emerge from an intermediate aquatic snail host into bodies of water to search for a definitive mammalian host to infect. The times of day that Schistosoma japonicum cercariae exit their snail differ across geographic regions and broadly correspond to whether the most abundant definitive host species is nocturnal or diurnal (Lu et al., 2009; Su et al., 2013). This pattern has been documented for 10 species spanning the 3 main groups of schistosomes (N’Goran et al., 1997). If the activity patterns of the receiving host select for rhythms in exiting the current host, there must be costs or constraints on emerging at other times of day. These remain to be identified, but plausible candidates include that the life expectancy of free-swimming cercariae is short due to a limited resource supply or exposure to rhythmic environmental hazards (e.g., predators) or there is a high risk of inadvertently invading an unsuitable host species. Indeed, recent evidence suggest that such abiotic and extrinsic factors constrain parasite rhythms from perfectly aligning with the timing of activity of their next host. For example, the emergence of trematode cercariae is constrained by the need to avoid environmental stressors (UV light, salinity), unsuitable hosts, and predators (Hannon et al., forthcoming).
Rhythms in the foraging activity of recipient hosts may select for rhythms in egg laying of several nematode worm species that are intestinal parasites. These parasites migrate out of the anus to lay eggs because their eggs require exposure to oxygen to mature. Migration is rhythmic; a well-known example is the (very itchy) human pinworm Enterobius vermicularis, which lays eggs while the host is asleep (Caldwell, 1982; Cook, 1994). Rhythmic migration during the host’s resting phase (i.e., during the night for diurnal hosts and during the day for nocturnal hosts) has been observed in Passalurus ambiguus in rabbits (Rinaldi et al., 2007), Syphacia muris in mice (van der Gulden, 1967), and Thelastoma bulhoesi in the American cockroach (McCallister and Schmidt, 1981). It is thought that laying eggs at the time-of-day hosts are asleep ensures sufficient time for maturation before recipient hosts become active (Lewis and D’Silva, 1980). However, it may also (or instead) be advantageous for adult parasites to undertake dangerous behaviors when the host is asleep and less responsive to itching. The Lancet liver fluke (Dicrocoelium dendriticum) takes over its ant host’s behavior and causes it to climb up and latch onto blades of grass to make it more accessible to grazing by the next host (Hohorst and Graefe, 1960; Schneider and Hohorst, 1971). This manipulation by the fluke is inhibited by high temperatures (Botnevik et al., 2016), raising the possibility that there is daily rhythm in which ants anchor in the evening and return to their nest in the morning (Libersat et al., 2009; van Houte et al., 2013).
Coping with the abiotic environment
Encountering a new host may require spending time in the abiotic environment, which exposes parasites to daily rhythms in UV light, temperature, and humidity. The coccidian parasite of birds, Isospora spp., sporulates (a maturation process that is required before the parasites can infect the next host) in the environment. Exposure to UV light and low daytime humidity can reduce sporulation rates of Isospora oocysts by up to 80% (Martinaud et al., 2009). Hence, Isospora disproportionately exit the host through excretion in feces in the late afternoon rather than in the morning. Interestingly, in the high arctic summer, where environmental Zeitgebers (time cues) are lacking, Isospora-infected snow buntings maintain these rhythms (Dolnik et al., 2011). Despite near-constant daylight, rhythms in UV light are still observed, but fluctuations in humidity are severely reduced. Whether this means that UV rhythms alone determine transmission success or whether humidity is so important that even “dampened” humidity rhythms select for rhythms in Isospora remains unclear. An alternative hypothesis is that environmental rhythms are not important but, instead, synchronized emergence maximizes infective dose to the next host, which facilitates invasion (Dolnik et al., 2011). This explanation requires that parasites synchronize their exit from the host by keeping time.
Invading and Establishing an Infection
Once a parasite has successfully contacted a new host, the parasite may be confronted by rhythmicity in how easy it is to invade. The immune response guards against invading parasites, and there is increasing evidence that immune effectors are rhythmic in healthy hosts. As a general rule, activity patterns of mammals appear to be linked to rhythms in immune effectors since reverse rhythms are generally observed in diurnal and nocturnal creatures (Scheiermann et al., 2013). For example, in the absence of infection, components of innate immunity tend to be upregulated just before or during the active phase (Curtis et al., 2014). This may explain why mice are more vulnerable to bacterial or viral infections when challenged during the resting phase compared with the active phase (Halberg et al., 1960; Feigin et al., 1972; Bellet et al., 2013). To what extent the phase of immune rhythms is due to the host maintaining homeostasis or protecting against invasion is unknown. If these two functions are incompatible, the host faces a trade-off that parasites could exploit by invading hosts when their defenses are down (literally). Hosts may be selected to prioritize the schedule of key defenses in line with infection risk and organize other processes around them. This scenario may apply to Drosophila melanogaster, in which the circadian clock protein “timeless” regulates rhythms in bacterial phagocytosis creating a rhythm in resistance to infection (Stone et al., 2012), and clock mutant flies experience more severe disease (Shirasu-Hiza et al., 2007; Lee and Edery, 2008).
Immune defenses in the plant Arabidopsis are also rhythmic and are proposed to enhance pathogen recognition in the daytime (Bhardwaj et al., 2011). Plants may be more vulnerable to invasion during the daytime because their stomata are open and/or because light can modulate the damage caused by bacterial parasites (Melotto et al., 2006). For parasites attempting to invade hosts at times of day when rhythms in host defenses peak, large infective doses may be required to overwhelm these defenses (as suggested for Isospora). In contrast, the necrotrophic fungus Botrytis cinerea upregulates virulence traits at night, potentially to overcome plant defenses that are enhanced at night (Hevia et al., 2015).
Evading Elimination from the Host/Vector
Once inside a host or vector, parasites live in a rhythmic environment. Given that this environment is usually trying to kill them, rhythms might be part of parasites’ within-host survival strategies. Rhythms may help parasites with the challenges of surviving immune responses, maximizing resource acquisition, or temporally compartmentalizing internal processes. We outline examples where rhythms are implicated in immune evasion and then discuss parasite-specific cases where rhythms are less clearly ascribed to solving a particular challenge inside the host.
Immune evasion
In addition to defending against invading parasites, immune responses also eliminate parasites from established infections. Many parasites, particularly helminths (worms), are able to coerce the host into downregulating the most harmful immune responses. Recent work has revealed that disrupting rhythms in host processes that damage parasites is an immunomodulation tactic that has been adopted by phylogenetically diverse mammalian viruses (Edgar et al., 2016). Host cells with robust circadian rhythms are better able to control replication of influenza A and herpes viruses. These viruses override the host’s time-keeping by inducing expression of the cell’s key clock gene Bmal1 to counter the endogenous decrease in its transcription. This dampens the host’s circadian rhythms, which increases the availability of resources required for viral replication and may also interfere with innate immune defenses (Edgar et al., 2016). Extrapolation of this result suggests that seasonal rhythms in Bmal1 expression may correspond to seasonal transmission of viral infections (Edgar et al., 2016).
Intestinal parasite migration along the gut
The anterior-posterior diurnal migration of the rat tapeworm Hymenolepis diminuta along the host’s gut is proposed to be an adaption to maximize resource intake by following food as it progresses through the gut. The alternative hypothesis, that the parasite is being expelled as a consequence of gut peristalsis, is not supported (Read and Kilejian, 1969; Sukhdeo, 1992). Further, food intake results in substantial changes to environmental conditions experienced by organisms in the intestine, and moving with food may maintain parasites in as constant an environment as possible, minimizing homeostatic challenges (Pappas, 1988). Like tapeworms, the intestinal trematode Echinostoma caproni migrates anteriorly to the intestinal mucosa on which it feeds when hosts are actively foraging, and it migrates posteriorly to where it lays eggs when the host is resting (Platt et al., 2010). This is thought to allow E. caproni to maximize acquisition of the host’s resources, with the consequence that egg laying must be scheduled for a different time-of-day (Platt et al., 2010). Alternatively, migration rhythms may enhance transmission. Because eggs are laid during the host’s resting phase (when defecation rhythms are at their nadir), the eggs accumulate in the feces ready to be released en masse and maximize infective dose to new hosts (Platt et al., 2010).
Host and parasite rhythms in sleeping sickness
Trypanosomes are famous for disrupting host sleep-wake cycles—the disease in humans is called “sleeping sickness.” It is unclear whether this is an adaptive parasite strategy, because disrupting host rhythms requires trypanosomes to have penetrated the blood-brain barrier. This is a “dead end” because parasites cannot be transmitted to the tsetse fly vector from this location (Enanga et al., 2002; Frevert et al., 2012; Rodgers, 2010). It has been suggested that these are sacrificial parasites, carrying out a behavior for the benefit of closely related counterparts in the bloodstream (van Zandbergen et al., 2010). In contrast, rhythms have been discovered recently in trypanosomes themselves. The forms of Trypanosoma brucei that replicate in the host (slenders) and transmit to the tsetse fly vector (stumpies) both exhibit rhythms in more than 200 genes involved in metabolism and redox balance (Rijo-Ferreira et al., 2017). It is not yet clear whether these rhythms are phased to match rhythms in host and vector foraging activity (both are diurnal) and/or to schedule cellular processes in the parasites. Furthermore, rhythms in these genes correspond to rhythms in sensitivity to antiparasite drugs (Rijo-Ferreira et al., 2017).
Developmental rhythms of malaria parasites
Malaria parasites require host RBCs for successive rounds of asexual replication. The asexual cycle (hereafter termed developmental rhythm) of most species of malaria parasite appears coordinated, with parasites progressing through developmental stages in synchrony and transitions between stages occurring at particular times of day (Box 1). Malaria parasites have a great capacity for replication because each asexually replicating parasite produces 6 to 60 progeny (depending on the species) in cycles that last roughly 24, 48, or 72 h (also depending on the species; Gerald et al., 2011). This rapid replication is responsible for the symptoms of malaria and also fuels the production of sexual stage gametocytes for transmission. Mismatching the developmental rhythm to that of the host reduces the number of asexual stages and gametocytes in P. chabaudi (O’Donnell et al., 2011; Box 1). Several non–mutually exclusive hypotheses have been proposed for why malaria parasites benefit from rhythms in asexual development.
First, rhythmic immune responses may differentially affect the survival or developmental rate of parasite stages in a manner that is specific to the time-of-day. For example, if rhythms in fever selectively kill certain stages at certain times of day, parasites would benefit from decoupling the time-of-day that a vulnerable stage develops from the peak of the most damaging immune effector(s) (Kwiatkowski, 1989). This parasite strategy would be successful only if the phase of the relevant immune rhythm is not driven by malaria parasite rhythms themselves. Note that this scenario could explain both how parasite developmental rhythms are established and maintained during infections (rhythmic immune responses schedule parasites) and why parasites benefit from rhythms (they evade immune killing). Similarly, synchrony in developmental rhythms is implicated in helping parasites to survive antimalarial drugs: Early-stage parasites can enter quiescence to become refractory to drugs and restart development up to several weeks later (Teuscher et al., 2010; Witkowski et al., 2010).
Second, RBCs are a key parasite resource that have rhythms in their release from bone marrow (Clark and Korst, 1969) and in redox state (O’Neill and Reddy, 2011). RBC redox state can affect parasite development because within its RBC, each parasite undergoes multiple mitoses toward the end of its development, and DNA replication is sensitive to oxidative damage (Chen and McKnight, 2007; Legorreta-Herrera et al., 2010; Kasozi et al., 2013). Thus, parasite rhythms may be timed to coincide particular developmental stages with time-of-day variation in the quality of RBCs. Furthermore, the redox state of an RBC could affect how permissive it is to invasion by parasites, and so parasites may time the release of progeny in line with when RBCs are most easily invaded. If RBCs permit invasion and/or replication only at certain times of day, this could explain both how parasite rhythms are generated (e.g., RBC rhythms dictate the development of parasites) and why they are beneficial for parasites (e.g. progeny die unless they quickly invade an RBC).
Third, parasites may need to temporally segregate conflicting physiological processes that correspond to the development of different stages (i.e., the intrinsic adaptive value hypothesis; Sharma, 2003). Each developmental stage has a different function: The first stage in the asexual cycle remodels the RBC, the following stages are responsible for feeding, and the later stages undergo mitosis and produce progeny. Time-keeping may allow parasites to carry out these functions in the correct order, and if so, the time-of-day that they occur may not matter. Reconciling this scenario with the costs observed when parasite developmental rhythms are mismatched to the phase of the host rhythm, and the fact that parasites in different RBCs tend to be synchronized, requires that only certain developmental stages tell the time and that time-of-day information is available for only part of the circadian cycle. For example, melatonin has been proposed as a time cue (Hotta et al., 2000; Garcia et al., 2001) and exhibits a brief spike each day in laboratory mice (Vivien-Roels et al., 1998).
Integrating Effects across Lifecycles
For parasites with complex lifecycles involving multiple intermediate hosts or vectors, rhythms may occur in a different part of the lifecycle than when the fitness consequences of the rhythm manifests. For example, rhythms in the development of asexual stage malaria parasites could be explained by the need to match the maturation of gametocytes with vector activity. Likewise, it is possible that the correct timing of transmission behaviors in worms, schistosomes, and Isospora requires rhythmic development during replication within the host. If so, the transmission behaviors of these parasites may be a product of transmission stages doing certain things at specific ages rather than at specific times of day; that is, rhythms in within-host replication are responsible for producing rhythms in transmission stages and sets their phase. In such parasites, teasing apart the contributions of rhythms in multiple lifecycle stages to fitness will be challenging. For malaria parasites (O’Donnell et al., 2011, 2013), viruses (Edgar et al., 2016), bacteria (Stone et al., 2012; Roden and Ingle, 2009), and fungi (Hevia et al., 2015), there is compelling evidence that rhythms in hosts matter for parasite invasion and survival. But whether invasion and survival inside vectors are similarly affected by parasite and/or vector rhythms is unknown (Rund et al., 2016).
How are Parasite Rhythms Generated?
Beyond the questions of whether and why rhythms in infections affect parasite fitness, the question of how parasite rhythms are generated is another mystery. The previous section introduced the notions that rhythmic host processes schedule malaria parasites through stage-specific killing (Kwiatkowski, 1989; Kwiatkowski and Greenwood, 1989, Mideo et al., 2013) and create rhythms in the shedding of infective larvae of metastrongyle nematodes (de Azevedo et al., 2011), and that parasites benefit from being made rhythmic. The underlying assumption of these hypotheses is that parasites are intrinsically arrhythmic and the host’s circadian rhythms are responsible for establishing and maintaining parasite rhythms. This need not be the case: Parasites may have their own time-keeping machinery and may be responsible for organizing their rhythms. If parasites keep time themselves, they could do this using a clock that is entrained by a Zeitgeber or by responding directly to the appearance of time-of-day cues. Thus, there are 3 non–mutually exclusive scenarios by which hosts and parasites could be responsible for parasite rhythms: (1) host rhythms schedule parasites, (2) parasites have evolved an oscillator, or (3) parasites have evolved a plastic response to time-of-day cues. Evidence that parasites have evolved specific mechanisms to generate their rhythms (scenario 2 or 3) adds considerable weight to the notion that rhythms matter for parasite fitness. We discuss each of these scenarios in turn.
Host Rhythms Schedule Parasites
Observations of rhythmic immune effectors in a broad range of taxa suggest that rhythms are important for host-parasite interactions (Martinez-Bakker and Helm, 2015) and could have significant effects on parasite fitness. These observations include that arrhythmic host cells are less able to restrict viral replication, which may be due to disruption of innate immune responses (Edgar et al., 2016). In Drosophila, bacterial phagocytosis by hemocytes peaks at night during the rest phase, and flies die sooner when challenged with Streptococcus pneumoniae during the day (Stone et al., 2012). Plant pathogen recognition (Bhardwaj et al., 2011) and phytohormones that play key roles in activating biotic stress response pathways (jasmonates and salicylates) are under circadian control (Goodspeed et al., 2012) and are also shaped by the phase of their parasite’s rhythm. Laboratory mice develop higher bacterial loads and produce a greater inflammatory response when challenged in the day compared with the night (Bellet et al., 2013). Rhythms also exist in the resources that parasites need, ranging from food (e.g., in the gut for intestinal worms, blood glucose for trypanosomes) to homes (e.g., red blood cells for malaria parasites). Rhythms in host factors that harm parasites and rhythms in the resources parasites need could impose rhythms on parasite activities in the host and vector. The hypothesis that parasite rhythms are a product of the “host’s footprint” has been most thoroughly considered in the case of immune rhythms and malaria parasites.
Immune rhythms and malaria parasites
The dynamics of malaria infections show that processes operating within the first few days of infection are responsible for the costs of phase mismatch to the host’s circadian rhythm (O’Donnell et al., 2013). The immune effectors upregulated early in infection are the components of the innate response, which includes inflammation (Iwasaki and Medzhitov, 2010; Stevenson and Riley, 2004). The effects of the inflammatory cytokines tumor necrosis factor (TNF) and interferon γ (IFN-γ) are particularly harmful to malaria parasites (Artavanis-Tsakonas and Riley, 2002; Long et al., 2006) and are rhythmic (Scheiermann et al., 2013; Curtis et al., 2014). Late-stage parasites that are undergoing mitosis (Box 1) are thought to be most sensitive to damage by the immune system (Kwiatkowski, 1989; Kwiatkowski and Greenwood, 1989). Further, the parasite stage that elicits the host response may not be the stage most vulnerable to that response (Rouzine and McKenzie, 2003). Thus, it appears there is a limited window each day when parasites at these vulnerable stages are permitted to survive and replicate (analogous to a “gate”). How many asexual cycles are required for the effects of circadian immunity to generate rhythms in parasite development will depend on the efficacy of immune effectors, their duration of action, and the sensitivity of parasite developmental stages (Kwiatkowski and Nowak, 1991; Rouzine and McKenzie, 2003).
In theory, immune rhythms could impose schedules on parasite development, but several major challenges arise for this hypothesis. First, while some species are synchronous (e.g., P. chabaudi and P. vinckei), other closely related species are asynchronous and have non-24 h cycle durations (e.g., P. berghei and P. yoelii) despite infecting the same host strain (Killick-Kendrick and Peters, 1978). Could some species escape the scheduling effect of the host’s immune response? This would require that all developmental stages of asynchronous parasites are equally sensitive to circadian immune effectors or that each species is vulnerable to a different suite of host responses. Second, when parasites are mismatched to the host’s circadian rhythm by 12 h, they suffer a small initial loss in number which is propagated over successive cycles of replication to generate a large reduction compared with parasites in infections that are in phase with the host’s rhythm (O’Donnell et al., 2013). Even if the small and initial reduction in number is due to stage-specific killing, the decrease is not sufficient to reschedule parasites within 7 cycles of replication (Gautret et al., 1995). Third, for immune rhythms to dictate the phase of parasite rhythms, the host circadian oscillator must control immune rhythms. This is not the case during infection: The bursting of mature parasites elicits a strong inflammatory response, typified by fever in humans and paroxysm in mice (Netea et al., 2000; Kwiatkowski and Greenwood, 1989). Thus, immune responses elicited as a result of parasite rhythms could increase synchrony of parasites but not change their phase. Ruling in or out a role for host immune rhythms should be straightforward using arrhythmic and immune knock-out mice or by perturbing immune effectors to change their rhythms and testing for knock-on effects to parasite rhythms.
Immune rhythms and other taxa
We contend that immune rhythms are also unlikely to enforce rhythms in other parasite species. For example, explaining rhythms in microfilariae requires that they are released into the capillaries and lungs at all times of day and that immune effectors in the lungs clear them at certain times in the circadian cycle, whereas effectors in the capillaries clear them at other times. On top of this, it must be coincidental that the time-of-day that microfilariae survive in the capillaries matches vector foraging rhythms. It also seems remote that the rhythms of snail hosts are responsible for releasing schistosome cercariae at different times because parasites appear to use the same subspecies of snail host (Oncomelania hupensis hupensis [O. h. hupensis]) regardless of whether their next host is nocturnal or diurnal (Lu et al., 2009; Su et al., 2013). Finally, as we discuss in the following section, increasing evidence that parasites have their own oscillators suggests that they are actively in control of their rhythms, rather than allowing the host to generate them.
Parasites Have Evolved an Endogenous Oscillator
If host rhythms do not create parasite rhythms, parasites must have a mechanism to organize their own rhythms. A common solution to the problem of time-keeping is to evolve an endogenous circadian oscillator (circadian clock). A circadian clock allows its owner to anticipate and prepare for rhythmic changes in the environments (in the host, vector, and abiotic environment) and confers robustness to temperature changes. Given that the need to cope with rhythms in the rotation of the earth is a more ancient evolutionary challenge than the appearance of a parasitic lifestyle, it may be assumed that parasites simply retain an oscillator from their free-living ancestors. This may be the case, but identifying the workings of oscillators is a major challenge. There is little homology in the genes underpinning oscillators driven by transcription-translation feedback loops (TTFLs) across divergent free-living taxa (Johansson and Staiger, 2015; Takahashi, 2015; Tomioka and Matsumoto, 2010). Furthermore, TTFLs may be more modern than parasitism. Thus, the absence of canonical clock genes in parasites may be a case of “absence of evidence” rather than “evidence of absence”. Nonetheless, evidence of endogenous clocks exists.
Evidence of oscillators
A functional clock has been identified in the fungus B. cinerea, which involves components (white collar complex) central to the clock of the model organism Neurospora crassa. As for N. crassa, this clock is entrained by light via a blue light photoreceptor (Hevia et al., 2015). TTFL homologues have not been reported in other parasite taxa, although nontranscriptional oscillators (NTOs) may exist. An NTO drives rhythms in the redox state of peroxiredoxins, which are a class of highly conserved antioxidants (Edgar et al., 2012). Rhythms in peroxiredoxins could provide an accessible marker for the presence of an NTO in nonmodel organisms. For example, peroxiredoxins are essential for the development of malaria parasites (Koncarevic et al., 2009), and rhythms in peroxiredoxins have been reported in RBCs (O’Neill and Reddy, 2011).
In addition to genetic and molecular signatures of oscillators, their presence can be inferred indirectly because clock-controlled rhythms have several key features (Pittendrigh, 1960). If a rhythm can be entrained, temperature can be compensated, and the rhythm persists (free-runs) with a circadian period of approximately 24 h in constant conditions, then the rhythm is driven by a clock. These criteria are fulfilled for rhythms in the expression of more than 200 metabolic and redox genes in T. brucei (Rijo-Ferreira et al., 2017). The authors revealed that rhythms in these genes are entrained by temperature cycles of 32 to 37 °C (as likely to be experienced inside the host) but not by light, the rhythms are temperature compensated, and they free-run with a period of slightly less than 24 h when the temperature is maintained at 37 °C. The workings of the clock driving these rhythms are unknown but are suggested to involve a novel posttranscriptional mechanism (Rijo-Ferreira et al., 2017).
Some clock criteria are also fulfilled for B. cinerea. Larger lesions in the leaves of its Arabidopsis host occur when infections are initiated at dusk, compared with dawn, and these time-of-day differences still occur when arrhythmic Arabidopsis kept in constant darkness are infected (Hevia et al., 2015). Temperature compensation of B. cinerea’s clock has not yet been reported. Temperature compensation in the developmental rhythms of malaria parasites has not been investigated in a clock context, but some observations are relevant. In cell culture, transcription rate during the asexual cycle appears temperature compensated (Q10 < 1.5; Fang and McCutchan, 2002), but this may not translate into compensation for developmental rhythms because the duration of the replication cycle in vivo has been observed to vary by 25% to 50% when body temperature varies by only 3 °C (from 34 °C to 37 °C; Hawking et al., 1968). Observations also suggest that temperature is not a Zeitgeber for malaria parasites, although it is difficult to distinguish between free running and the possibility that the minimum or maximum biologically possible durations for the asexual cycle are, by coincidence, around 24 h or multiples thereof.
Parasites Respond (Plastically) to Time-of-day Cues
An alternative to using an oscillator to organize rhythms is to respond directly when reliable time-of-day cues appear in the environment. In evolutionary ecology, this is called “phenotypic plasticity” and is defined very broadly as a change in phenotype (which includes traits, behaviors, etc.) in response to environmental change (Pigluicci, 2001). To illustrate how a clock-controlled behavior differs from a plastic cue-response system, consider the following (playful) example. A clock allows a nocturnally foraging rodent to evade the dangerous predators that are active during the day by ensuring it is safe in its burrow before dawn. In contrast, a rodent without a clock waits until it starts to get light before retreating to its burrow. If the rodents are given sunglasses, the rodent with the clock will still return to its burrow before dawn, but the other will be late. Similarly, the level of cloud cover may influence the time-of-day when the plastic rodent perceives dawn approaching. Thus, because it is sensitive to environmental fluctuations, a plastic strategy is more likely to result in timing errors than a true clock. Conversely, if the rodents go on holiday to a different time zone, the rodent with a clock will experience jet lag but the other could seamlessly adjust by having a short night (or day, depending in the direction of travel).
Why might an organism use a plastic response instead of an oscillator, especially if an oscillator was inherited from a free-living ancestor? It may be energetically costly to maintain the machinery required for a clock. Furthermore, if an inherited oscillator is entrained by light, this might not prove useful for parasites infecting large organisms (e.g., vertebrates) because light cannot penetrate very far. In this case, the oscillator either is lost (via genetic drift) or is altered by natural selection to respond to a more appropriate Zeitgeber. If the best new Zeitgeber is the host’s body temperature, it may be difficult to adjust a clock to respond to temperature because the inherited system has evolved to be robust to temperature variation.
Just as an oscillator must be set by a Zeitgeber that reliably indicates time-of-day, a plastic strategy will evolve to respond to a reliable cue. For parasites, this could be information provided by the host’s circadian rhythms or the abiotic environment. The cue used by parasites to stimulate a response may be the rhythmic factor that is responsible for the fitness consequences of the parasite’s rhythm, or it may be a proxy that temporally correlates with this factor. For example, if matching an immune effector rhythm is important for malaria parasite fitness, parasites may detect the appearance of this effector and adjust their developmental rate accordingly, or they may detect a proxy (e.g., a glucocorticoid) whose rhythm is in the same phase as the effector. Alternatively, by using a proxy whose rhythm has a slightly earlier phase relative to the effector rhythm that matters, parasites could also anticipate rhythmic changes in their environment. Distinguishing between parasites using time-of-day information as a Zeitgeber to set an oscillator or as a cue for a plastic response could be achieved with a phase response curve (PRC). Once a factor is found to influence a parasite rhythm, it can be supplied at different points across the circadian cycle during constant conditions. The shape of the PRC will shed light on the extent to which parasites experience “jet lag” (have a clock) or seamlessly adjust (have a plastic response).
Evidence of plastic strategies
No time-of-day cues have been unequivocally identified for parasites with plastic responses, but several are hypothesized (Table 1). Malaria parasites are proposed to respond to the increase in melatonin at night (in humans and mice) by completing the asexual cycle (Hotta et al., 2000; Hotta et al., 2003) and to respond to light (Engelbrecht and Coetzer, 2015). However, parasites in the latter study may have been subject to additional differences in temperature. Neither of these studies can explain why malaria parasite rhythms become disrupted during the peak of infections because host melatonin rhythms and photoperiod remain unchanged. Other hormones such as glucocorticoids and vasodilators are also rhythmic and available in the blood for parasites to monitor (Cuesta et al., 2015; Kalsbeek and Strubbe, 1998; Luna-Moreno et al., 2009; Stephenson et al., 1984) but again, rhythms in these factors are unlikely to be affected by illness. Alternatively, rhythmic factors associated with host activity and foraging, such as insulin, glucose, other metabolites, or body temperature, are likely to be disrupted by illness and so could be the information that parasites use to keep time. Glucose is an essential resource for malaria parasites, and temperature change is used as a cue to make developmental transitions in the mosquito (Blanford et al., 2013; Chao and Ball, 1962), so glucose uptake and/or temperature-sensing mechanisms may also be used to organize rhythms in the host.
Daily rhythms in levels of oxygen in the blood and tissues have recently been shown to reset circadian clocks (Adamovich et al., 2016), and microfilariae are proposed to migrate between the lung vessels and peripheral capillaries based on the venous-arterial difference in oxygen tension caused by host activity patterns (Hawking, 1967). The migration of nematodes and other gut parasites may be based on cues from the upregulation of digestive processes (Platt et al., 2010). Specifically, the rat tapeworm H. diminuta is thought to migrate in response to mechanical pressure and serotonin (5-HT) resulting from gut peristalsis (Mettrick and Cho, 1981; Sukhdeo, 1992) in response to food. Despite being inside a snail host, trematode cercariae may use light to time their exit: O. h. hupensis snails are small enough for light to penetrate, and cercariae are shed when light pulses are applied to snails kept in the dark (Nojima and Sato, 1982; Raymond and Probert, 1987). This highlights a potential challenge for parasites with complex lifecycles: If there is a need for timekeeping in multiple host/vector species, can the same system be used because it is sensitive to a general cue or Zeitgeber?
Parasite Offense and Host Defense
How host rhythms affect a parasite’s within-host survival and between-host transmission, and how parasite rhythms affect hosts, are mysterious. Given that parasites must perform their rhythmic behaviors at some point in the day, does the time-of-day they do it matter for the host? For example, do schistosome cercariae damage snails when they are shed, and are snails better able to repair themselves at a certain time-of-day? Since immune responses are involved in wound healing and are known to be circadian (Curtis et al., 2015; Davies et al., 2013; Keller et al., 2009; Petrovsky et al., 2003; Stevenson et al., 1992), it is likely that time-of-day matters to the host. Such effects on the host can create feedback to select for parasites to time their behaviors to modulate the damage they cause. For example, the synchronized bursting of malaria parasites induces a strong inflammatory response, which causes immunopathology to the host (Kwiatkowski and Greenwood, 1989; Netea et al., 2000). Because a rhythm in bursting benefits parasites but is costly to the host, the stage is set for antagonistic coevolution between host and parasite rhythms—hosts may be under counter selection to disrupt parasite rhythms or dampen rhythms in their inflammatory response. Similarly, simian immunodeficiency virus affects host body temperature and activity rhythms, which impairs cognition (Huitron-Resendiz et al., 2007). Better data on the fitness consequences of the “embodied” parasite rhythm for hosts are needed, and it is important to note that health and fitness are not always synonymous.
Interactions between host and parasite rhythms are determinants of the severity and spread of diseases. Harnessing the host’s circadian systems to enhance immune responses, or precisely timing drug administration, could reduce symptoms and parasite transmission (Fortier et al., 2011; Long et al., 2016). In the case of malaria parasites, antimalarial drugs are most effective against a subset of developmental stages. Moreover, some antimalarial drugs induce a subpopulation of young developmental stages to become dormant and restart development 3 to 20 days later when drugs are absent (Teuscher et al., 2010; Witkowski et al., 2010). Dormancy is assumed to be a within-host survival strategy, but its benefits are unclear because the immediate reduction in replication is a loss of “safety in numbers” and puts maintenance of the infection at risk. If dormancy does enhance parasite survival, drug treatment will be more effective if interventions disrupt parasite rhythms or target the developmental stages best able to recover from dormancy. Interventions that alter parasite rhythms could operate by disrupting the mechanisms that generate rhythms (e.g., remove time-of-day information) or by reducing the benefits garnered by rhythmic parasites. Similarly, because innate immune defenses and the mammalian clock are linked (Silver et al., 2012), preventing herpes virus from interacting with the host’s circadian clock (Kalamvoki and Roizman, 2011; Benegiamo et al., 2013) and preventing harmful alteration to lung rhythms during influenza (Sundar et al., 2015) could help control viral replication and dissemination.
Interactions between parasite, host, and vector rhythms also matter (Marques, 2013). For example, the use of bed nets is changing the biting time of mosquito populations (Moiroux et al., 2012), yet the consequences for malaria parasite transmission are unknown. Changing the time-of-day that hosts, vectors, and parasites interact could alter the likelihood of mosquitoes becoming infected as well as the likelihood of mosquitoes transmitting their parasites to new hosts. Circadian rhythms in the host’s transmission-blocking immune responses, the infectiousness of parasite transmission stages, and the mosquito’s ability to metabolize blood meals could interact to directly affect infection prevalence and intensity in mosquitoes (Rund et al., 2016). These rhythms could also have indirect effects on disease transmission through altering mosquito lifespan and biting rate (Rund et al., 2016). The worst-case scenario is an additive, positive effects of these interactions—for example, if during the daytime, parasites are most infective, mosquitoes are most susceptible, and blood is most nutritious. Understanding rhythms in tri-trophic interactions is therefore required to make vector control strategies sustainable.
Conclusion
Remarkably, despite initial documentation centuries ago and a flurry of activity several decades ago, the study of rhythms in parasites is still in its infancy. The conceptual frameworks for how circadian oscillators operate, how to interrogate them and their behavioral outputs, and how to analyze rhythmic data are now well developed. We suspect that rhythms in parasites are actually widespread, but in many cases, it is more challenging to study embodied clocks than the clocks of hosts. The lack of canonical clock genes in parasite genomes does not preclude oscillators, or parasites may possess simple mechanisms to plastically respond to diverse signals in their in-host environment, or they may use ancient, nontranscriptional oscillators. Unlocking the mysteries of how parasite rhythms are organized would greatly facilitate investigation of the roles of rhythms in interactions between host, parasites, and vectors and the fitness consequences of these interactions. We have discussed data and concepts from evolutionary biology, ecology, parasitology, immunology, and chronobiology; moving forward requires greater cultivation of this interdisciplinary effort.
Acknowledgments
We thank M. Greischar, A. O’Donnell, S. Rund, P. Schneider, the 2016 cohort of the “Hosts, Pathogens, and Global Health” students, and two anonymous reviewers for insightful comments on the manuscript. We thank the following organizations for funding: The Royal Society (S.E.R.), the Natural Environment Research Council (S.E.R.), the Wellcome Trust (S.E.R.), the Human Frontier Science Program (S.E.R., K.F.P., N.M.), and the Natural Sciences and Engineering Research Council of Canada (N.M.).
Footnotes
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Acerenza L. (2016) Constraints, trade-offs and the currency of fitness. J Mol Evol 82:117-127. [DOI] [PubMed] [Google Scholar]
- Adamovich Y, Ladeuix B, Golik M, Koeners MP, Asher G. (2016) Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab 25:1-9. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas K, Riley EM. (2002) Innate immune response to malaria: rapid induction of IFN-γ from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169:2956-2963. [DOI] [PubMed] [Google Scholar]
- Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, et al. (2013) Circadian clock regulates the host response to Salmonella. Proc Natl Acad Sci U S A 110:9897-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benegiamo G, Mazzoccoli G, Cappello F, Rappa F, Scibetta N, Oben J, Greco A, Williams R, Andriulli A, Vinciguerra M, et al. (2013) Mutual antagonism between circadian protein Period 2 and hepatitis C virus replication in hepatocytes. PLoS One 8:e60527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj V, Meier S, Petersen LN, Ingle RA, Roden LC. (2011) Defence responses of Arabidopsis thaliana to infection by Pseudomonas syringae are regulated by the circadian clock. PLoS One 6:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford JI, Blanford S, Crane RG, Mann ME, Paaijmans KP, Schreiber KV, Thomas MB. (2013) Implications of temperature variation for malaria parasite development across Africa. Sci Rep 3:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botnevik CF, Malagocka J, Jensen AB, Fredensborg BL. (2016) Relative effects of temperature, light, and humidity on clinging behaviour of metacercariae-infected ants. J Parasitol 102:495-500. [DOI] [PubMed] [Google Scholar]
- Bray RS, McCrae AWR, Smalley ME. (1976) Lack of a circadian rhythm in the ability of the gametocytes of Plasmodium falciparum to infect Anopheles gambiae. Int J Parasitol 6:399-401. [DOI] [PubMed] [Google Scholar]
- Caldwell JP. (1982) Pinworms (Enterobius vermicularis). Can Fam Physician 28:306-309. [PMC free article] [PubMed] [Google Scholar]
- Chao J, Ball G. (1962) The effect of low temperature on Plasmodium relictum in Culex tarsalis. J Parasitol 48:252-254. [PubMed] [Google Scholar]
- Chen Z, McKnight SL. (2007) A conserved DNA damage response pathway responsible for coupling the cell division cycle to the circadian and metabolic cycles. Cell Cycle 6:2906-2912. [DOI] [PubMed] [Google Scholar]
- Chevin L-M, Lande R, Mace GM. (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PloS Biol 8(4):e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Korst DR. (1969) Circadian periodicity of bone marrow mitotic activity and reticulocyte counts in rats and mice. Science 166:236-237. [DOI] [PubMed] [Google Scholar]
- Cook GC. (1994) Tropical infection of the gastrointestinal tract and liver series. Gut 35:1159-1162.7959218 [Google Scholar]
- Cuesta M, Cermakian N, Boivin DB. (2015) Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J 29:1360-1370. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LAJ. (2014) Circadian clock proteins and immunity. Immunity 40:178-186. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, et al. (2015) Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc Natl Acad Sci U S A 112:7231-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S. (2010) A history of chronobiological concepts. In: The Circadian Clock. Protein Reviews, Albrecht U. ed, vol. 12, pp 1-35, New York: Springer. [Google Scholar]
- Davies LC, Jenkins SJ, Allen JE, Taylor PR. (2013) Tissue-resident macrophages. Nat Immunol 14:986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo GV, Rodriguez R, Porto SM, Graeff-Teixeira C, Fornari F. (2011) Elimination of Angiostrongylus costaricensis larvae in feces from experimentally infected Swiss mice: circadian rhythm and correlation with survival. Parasitol Res 108:537-540. [DOI] [PubMed] [Google Scholar]
- Dobson A, Lafferty KD, Kuris AM, Hechinger RF, Jetz W. (2008) Homage to Linnaeus: how many parasites? How many hosts? Proc Natl Acad Sci U S A 105:11482-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630-633. [DOI] [PubMed] [Google Scholar]
- Dolnik OV, Metzger BJ, Loonen MJJE. (2011) Keeping the clock set under the midnight sun: diurnal periodicity and synchrony of avian Isospora parasites cycle in the high arctic. Parasitology 138:1077-1081. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, et al. (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A 113:10085-10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enanga B, Burchmore RJ, Stewart ML, Barrett MP. (2002) Sleeping sickness and the brain. Cell Mol Life Sci 59:845-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht D, Coetzer TL. (2015) Sunlight inhibits growth and induces markers of programmed cell death in Plasmodium falciparum in vitro. Malar J 14:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, McCutchan TF. (2002) Malaria: thermoregulation in a parasite’s life cycle. Nature 418(6899):742. [DOI] [PubMed] [Google Scholar]
- Feigin RD, Middelkamp JM, Reed C. (1972) Circadian rhythmicity in susceptibility of mice to sublethal Coxsackie B3 infection. Nature 240:57-58. [DOI] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H, Hardy M-P, Labrecque N, Cermakian N. (2011) Circadian variation of the response of T cells to antigen. J Immunol 187:6291-6300. [DOI] [PubMed] [Google Scholar]
- Frevert U, Movila A, Nikolskaia OV, Raper J, Mackey ZB, Abdulla M, McKerrow J, Grab DJ. (2012) Early invasion of brain parenchyma by African trypanosomes. PLoS One 7:e43913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CRS, Markus RP, Madeira L. (2001) Tertian and quartan fevers: temporal regulation in malarial infection. J Biol Rhythms 16:436-443. [DOI] [PubMed] [Google Scholar]
- Garnham PCC, Powers KG. (1974) Periodicity of infectivity of plasmodial gametocytes: the Hawking phenomenon. Int J Parasitol 4:103-106. [DOI] [PubMed] [Google Scholar]
- Gautret P, Deharo E, Tahar R, Chabaud AG, Landau I. (1995) The adjustment of the schizogonic cycle of Plasmodium chabaudi chabaudi in the blood to the circadian rhythm of the host. Parasite 2:69-74. [DOI] [PubMed] [Google Scholar]
- Gerald N, Mahajan B, Kumar S. (2011) Mitosis in the human malaria parasite Plasmodium falciparum Eukaryot Cell 10:474-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Brandling-Bennet AD, Beir M, Mbogo CM, Atieli FK, Owaga ML, Juma F, Collins FH. (1993) Confirmation that Plasmodium falciparum has aperiodic infectivity to Anopheles gambiae. Med Vet Entomol 7:373-376. [DOI] [PubMed] [Google Scholar]
- Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. (2012) Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci U S A 109:4674-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. (1960) Susceptibility rhythm to E. coli endotoxin and bioassay. Proc Soc Exp Biol Med 103:142-144. [DOI] [PubMed] [Google Scholar]
- Hannon E, Calhoun D, Chadalawada S, Johnson P. (forthcoming) Circadian rhythms of trematode parasites: applying mixed models to test underlying patterns. Parasitology. [DOI] [PubMed] [Google Scholar]
- Hawking F. (1967) The 24-hour periodicity of microfilaria. Proc R Soc Lond (Biol) 169:59-76. [Google Scholar]
- Hawking F. (1970) The clock of the malaria parasite. Sci Am 222:123-131. [DOI] [PubMed] [Google Scholar]
- Hawking F, Worms ME, Gammage K. (1968) 24 and 48 hour cycles of malaria parasites in the blood: their purpose and control. Trans R Soc Trop Med Hyg 62:731-760. [DOI] [PubMed] [Google Scholar]
- Hawking F, Worms MJ, Gammage K, Goddard PA. (1966) The biological purpose of the blood-cycle of the malaria parasite Plasmodium cynomolgi. Lancet 288:422-424. [Google Scholar]
- Hevia MA, Canessa P, Müller-Esparza H, Larrondo LF. (2015) A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc Natl Acad Sci U S A 112:201508432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohorst W, Graefe G. (1960) Ameisen—obligatorische Zwischenwirte des Lanzettegels (Dicrocoelium dendriticum). Naturwissenschaften 7:229-230. [Google Scholar]
- Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, Markus RP, Pozzan T, Garcia CRS. (2000) Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nat Cell Biol 2:466-468. [DOI] [PubMed] [Google Scholar]
- Hotta CT, Markus RP, Garcia CRS. (2003) Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Braz J Med Biol Res 36:1583-1587. [DOI] [PubMed] [Google Scholar]
- Huitron-Resendiz S, Marcondes MCG, Flynn CT, Lanigan CMS, Fox HS. (2007) Effects of simian immunodeficiency virus on the circadian rhythms of body temperature and gross locomotor activity. Proc Natl Acad Sci U S A 104:15138-15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327:291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Staiger D. (2015) Time to flower: interplay between photoperiod and the circadian clock. J Exp Bot 66:719-730. [DOI] [PubMed] [Google Scholar]
- Kalamvoki M, Roizman B. (2010) Circadian CLOCK histone acetyl transferase localizes at ND10 nuclear bodies and enables herpes simplex virus gene expression. Proc Natl Acad Sci U S A 107:17721-17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Strubbe JH. (1998) Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav 63:553-558. [DOI] [PubMed] [Google Scholar]
- Kasozi D, Mohring F, Rahlfs S, Meyer AJ, Becker K. (2013) Real-time imaging of the intracellular glutathione redox potential in the malaria parasite Plasmodium falciparum. PLoS Pathog 9:e1003782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. (2009) A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 106:21407-21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killick-Kendrick R, Peters W. (1978) Rodent Malaria. New York: Academic Press. [Google Scholar]
- Koncarevic S, Rohrbach P, Deponte M, Krohne G, Helena Prieto J, Yates J, Rahlfs S, Becker K. (2009) The malarial parasite Plasmodium falciparum imports the human protein peroxiredoxin 2 for peroxide detoxification. Proc Natl Acad Sci U S A 106:13323-13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D. (1989) Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med 169:357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D, Greenwood BM. (1989) Why is malaria fever periodic? A hypothesis. Parasitol Today 5:264-266. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D, Nowak M. (1991) Periodic and chaotic host-parasite interactions in human malaria. Proc Natl Acad Sci U S A 88:5111-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Edery I. (2008) Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol 18:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legorreta-Herrera M, Retana-Ugalde R, Ventura-Gallegos JL, Narváez V. (2010) Pyrimethamine induces oxidative stress in Plasmodium yoelii 17XL-infected mice: a novel immunomodulatory mechanism of action for an old antimalarial drug? Exp Parasitol 126:381-388. [DOI] [PubMed] [Google Scholar]
- Lewis JW, D’Silva J. (1980) Rhythmic egg deposition by the oxyurid nematode Syphacia muris in the rat. J Zool 191:429-433. [Google Scholar]
- Libersat F, Delago A, Gal R. (2009) Manipulation of host behavior by parasitic insects and insect parasites. Annu Rev Entomol 54:189-207. [DOI] [PubMed] [Google Scholar]
- Long GH, Chan BH, Allen JE, Read AF, Graham AL. (2006) Parasite genetic diversity does not influence TNF-mediated effects on the virulence of primary rodent malaria infections. Parasitology 133:673-684. [DOI] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DB, Wang TP, Rudge JW, Donnelly CA, Fang GR, Webster JP. (2009) Evolution in a multi-host parasite: chronobiological circadian rhythm and population genetics of Schistosoma japonicum cercariae indicates contrasting definitive host reservoirs by habitat. Int J Parasitol 39:1581-1588. [DOI] [PubMed] [Google Scholar]
- Luna-Moreno D, Aguilar-Roblero R, Díaz-Muñoz M. (2009) Restricted feeding entrains rhythms of inflammation-related factors without promoting an acute-phase response. Chronobiol Int 26:1409-1429. [DOI] [PubMed] [Google Scholar]
- Magesa SM, Mdira YK, Akida JA, Bygbjerg IC, Jakobsen PH. (2000) Observations on the periodicity of Plasmodium falciparum gametocytes in natural human populations. Acta Trop 76:239-246. [DOI] [PubMed] [Google Scholar]
- Marques MD. (2013) Biological rhythms and vector insects. Mem Inst Oswaldo Cruz 108:59-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinaud G, Billaudelle M, Moreau J. (2009) Circadian variation in shedding of the oocysts of Isospora turdi (Apicomplexa) in blackbirds (Turdus merula): an adaptive trait against desiccation and ultraviolet radiation. Int J Parasitol 39:735-739. [DOI] [PubMed] [Google Scholar]
- Martinez-Bakker M, Helm B. (2015) The influence of biological rhythms on host-parasite interactions. Trends Ecol Evol 30:314-326. [DOI] [PubMed] [Google Scholar]
- McCallister GL, Schmidt GD. (1981) Diurnal migration of the female of Thelastoma bulhoesi (Oxyurata: Thelastomida) in the American cockroach, Periplaneta americana. Proc Helminthol Soc Wash 48:127-129. [Google Scholar]
- McMahon JE. (1976) Circadian rhythm in Schistosoma haematobium egg excretion. Int J Parasitol 6:373-377. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969-980. [DOI] [PubMed] [Google Scholar]
- Mettrick D, Cho C. (1981) Migration of Hymenolepis diminuta (Cestoda) and changes in 5-HT (serotonin) levels in the rat host following parenteral and oral 5-HT administration. Can J Physiol Pharmacol 59:281-286. [DOI] [PubMed] [Google Scholar]
- Mideo N, Reece SE, Smith AL, Metcalf CJ. (2013) The Cinderella syndrome: why do malaria-infected cells burst at midnight? Trends Parasitol 29:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux N, Gomez MB, Pennetier C, Elanga E, Djènontin A, Chandre F, Djègbé I, Guis H, Corbel V. (2012) Changes in Anopheles funestus biting behaviour following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis 206:1622-1629. [DOI] [PubMed] [Google Scholar]
- Murdock CC, Moller-Jacobs LL, Thomas MB. (2013) Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proc Biol Sci 280:20132030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Van der Meer JW. (2000) Circulating cytokines as mediators of fever. Clin Infect Dis 31:S178-S184. [DOI] [PubMed] [Google Scholar]
- N’Goran E, Brémond P, Sellin E, Sellin B, Théron A. (1997) Intraspecific diversity of Schistosoma haematobium in West Africa: chronobiology of cercarial emergence. Acta Trop 66:35-44. [DOI] [PubMed] [Google Scholar]
- Nojima H, Sato A. (1982) Schistosoma mansoni and Schistosoma haematobium: emergence of schistosome cercariae from snails with darkness and illumination. Exp Parasitol 53:189-198. [DOI] [PubMed] [Google Scholar]
- O’Donnell AJ, Mideo N, Reece SE. (2013) Disrupting rhythms in Plasmodium chabaudi: costs accrue quickly and independently of how infections are initiated. Malar J 12:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell AJ, Schneider P, McWatters HG, Reece SE. (2011) Fitness costs of disrupting circadian rhythms in malaria parasites. Proc Biol Sci 278:2429-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Reddy A. (2011) Circadian clocks in human red blood cells. Nature 469:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. (1998) Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A 95:8660-8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas PW. (1988) The relative roles of the intestines and external surfaces in the nutrition of monogeneans, digeneans and nematodes. Parasitology 96:S105-S121. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Socha L, Silva D, Grossman AB, Metz C, Bucala R. (2003) Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol 81:137-143. [DOI] [PubMed] [Google Scholar]
- Pichon G, Treuil JP. (2004) Genetic determinism of parasitic circadian periodicity and subperiodicity in human lymphatic filariasis. C R Biol 327:1087-1094. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. (2001) Phenotypic Plasticity: Beyond Nature and Nurture (Syntheses in Ecology and Evolution). Baltimore (MD): The John Hopkins University Press. [Google Scholar]
- Pittendrigh CS. (1960) Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol 25:159-184. [DOI] [PubMed] [Google Scholar]
- Platt TR, Graf E, Kammrath A, Zelmer DA. (2010) Diurnal migration of Echinostoma caproni (Digenea: Echinostomatidae) in ICR mice. J Parasitol 96:1072-1075. [DOI] [PubMed] [Google Scholar]
- Raymond K, Probert A. (1987) The effect of light and darkness on the production of cercariae of Schistosoma haematobium from Bulinus globosus. J Helminthol 61:291-296. [DOI] [PubMed] [Google Scholar]
- Read CP, Kilejian AZ. (1969) Circadian migratory behavior of a cestode symbiote in the rat host. J Parasitol 55:574-578. [PubMed] [Google Scholar]
- Rijo-Ferreira F, Pinto-Neves D, Barbosa-Morais NL, Takahashi JS, Figueiredo LM. (2017) Trypanosoma brucei metabolism is under circadian control. Nat Microbiol 2:17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi L, Russo T, Schioppi M, Pennacchio S, Cringoli G. (2007) Passalurus ambiguus: new insights into copromicroscopic diagnosis and circadian rhythm of egg excretion. Parasitol Res 101:557-561. [DOI] [PubMed] [Google Scholar]
- Roden LC, Ingle RA. (2009) Lights, rhythms, infection: the role of light and the circadian clock in determining the outcome of plant-pathogen interactions. Plant Cell 21:2546-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. (2010) Trypanosomiasis and the brain. Parasitology 137:1995-2006. [DOI] [PubMed] [Google Scholar]
- Rouzine IM, McKenzie FE. (2003) Link between immune response and parasite synchronization in malaria. Proc Natl Acad Sci U S A 100:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund SSC, O’Donnell AJ, Gentile JE, Reece SE. (2016) Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS. (2013) Circadian control of the immune system. Nat Rev Immunol 13:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting CD, Pigliucci M. (1998) Phenotypic Evolution: A Reaction Norm Perspective. Sunderland (MA): Sinauer. [Google Scholar]
- Schneider G, Hohorst W. (1971) Wanderung der Metacercarien des Lanzett-Egels in Ameisen. Naturwissenschaften 6:327-328. [DOI] [PubMed] [Google Scholar]
- Sharma VK. (2003) Adaptive significance of circadian clocks. Chronobiol Int 20:901-919. [DOI] [PubMed] [Google Scholar]
- Shirasu-Hiza MM, Dionne MS, Pham LN, Ayres JS, Schneider DS. (2007) Interactions between circadian rhythm and immunity in Drosophila melanogaster. Curr Biol 17:353-355. [DOI] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. (2012) The circadian clock controls Toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36:251-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvertown JW. (1980) The evolutionary ecology of mast seeding in trees. Biol J Linnean Soc 14:235-250. [Google Scholar]
- Stephenson L, Wenger C, O’Donovan B, Nadel E. (1984) Circadian rhythm in sweating and cutaneous blood flow. Am J Physiol 246:R321-R324. [DOI] [PubMed] [Google Scholar]
- Stevenson MM, Huang DY, Podoba JE, Nowotarski ME. (1992) Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infect Immun 60:1193-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MM, Riley EM. (2004) Innate immunity to malaria. Nat Rev Immunol 4:169-180. [DOI] [PubMed] [Google Scholar]
- Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. (2012) The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS Pathog 8:e1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Zhou F, Lu DB. (2013) A circular analysis of chronobiology of Schistosoma japonicum cercarial emergence from hilly areas of Anhui, China. Exp Parasitol 135:421-425. [DOI] [PubMed] [Google Scholar]
- Sukhdeo MVK. (1992) Intestinal contractions and migration behaviour in Hymenolepis diminuta. Int J Parasitol 22:813-817. [DOI] [PubMed] [Google Scholar]
- Sundar IK, Ahmad T, Yao H, Hwang JW, Gerloff J, Lawrence BP, Sellix MT, Rahman I. (2015) Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci Rep 29:9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS. (2015) Molecular components of the circadian clock in mammals. Diabetes Obes Metab 17:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. (2010) Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis 202:1362-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka K, Matsumoto A. (2010) A comparative view of insect circadian clock systems. Cell Mol Life Sci 67:1397-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gulden WJI. (1967) Diurnal rhythm in egg production by Syphacia muris. Exp Parasitol 21:344-347. [DOI] [PubMed] [Google Scholar]
- van Houte S, Ros VID, van Oers MM. (2013) Walking with insects: molecular mechanisms behind parasitic manipulation of host behaviour. Mol Ecol 22:3458-3475. [DOI] [PubMed] [Google Scholar]
- van Zandbergen G, Luder CG, Heussler V, Duszenko M. (2010) Programmed cell death in unicellular parasites: a prerequisite for sustained infection? Trends Parasitol 26:477-483. [DOI] [PubMed] [Google Scholar]
- Vivien-Roels B, Malan A, Rettori MC, Delagrange P, Jeanniot JP, Pévet P. (1998) Daily variations in pineal melatonin concentrations in inbred and outbred mice. J Biol Rhythms 13:403-409. [DOI] [PubMed] [Google Scholar]
- Williams KS, Smith KG, Stephen MS. (1993) Emergence of 13-yr periodical cicadas (Cicadidae: Magicicada): phenology, mortality and predator satiation. Ecology 74:1143-1152. [Google Scholar]
- Witkowski B, Lelièvre J, Barragán MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. (2010) Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother 54:1872-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]