Abstract
Background
Accurate prevalence figures estimating the number of survivors of poliomyelitis (disease causing acute flaccid paralysis) following poliovirus infection are not available. We aim to undertake a systematic review of all literature concerning the prevalence of survivors of poliomyelitis.
Methods
Electronic databases were searched from 1900 up to May 2016 for peer-reviewed studies using a population-based approach witha defined denominator and some form of diagnostic or clinical verification of polio. Exclusion criteria were any prevalence data that were unable to be extracted or calculated and studies reporting on incidence only. The quality of each included study was assessed using an existing tool modified for use in prevalence studies. Average crude prevalence rates were used to calculate worldwide estimates.
Results
Thirty-one studies met criteria with 90% of studies conducted in low-income to lower middle-income countries. Significant variability in the prevalence of survivors of poliomyelitis was revealed, in low- income to lower middle-income (15 per 100 000 in Nigeria to 1733 in India) and upper-middle to high-income countries (24 (Japan) to 380 per 100 000 (Brazil). The total combined prevalence of survivors of poliomyelitis for those studies at low to moderate risk of bias ranged from 165 (high-income countries) to 425 (low-income to lower middle-income countries) per 100 000 person-years. Historical lameness surveys of children predominated, with wide variation in case definition and assessment criteria, and limited relevance to current prevalence given the lack of incidence of poliovirus infection in the ensuing years.
Conclusions
These results highlight the need for future epidemiological studies of poliomyelitis to examine nationally representative samples, including all ages and greater focus on high-income countries. Such efforts will improve capacity to provide reliable and more robust worldwide prevalence estimates.
Keywords: public health, statistics &, research methods, epidemiology.
Strengths and limitations of this study.
This is the first and largest international systematic review, including 31 studies, of the prevalence of survivors of poliomyelitis.
The study found significant variability in the reported prevalence of survivors across low-income to high-income countries.
There are a lack of studies examining nationally representative samples.
There are no accurate current data on the prevalence of survivors of poliomyelitis worldwide.
Introduction
Poliovirus (polio) is a highly infectious, incurable viral disease caused by a wild or live vaccine-derived virus that remains endemic in Afghanistan, Pakistan and Nigeria.1 Since the creation of the Global Polio Eradication Initiative in 1988, alongside mass vaccination programmes aimed at eradicating polio, the number of new cases has been cut by 99%2 from 350 000 cases to 74 reported cases in 2015.3 Polio, a human enterovirus,4 primarily affects children aged <5 years with infection most commonly spread by the fecal–oral route. Up to 75% of poliovirus infections in children are asymptomatic, while approximately 24% of cases may experience a low-grade fever and sore throat.5 Less than 1% of cases experience viral replication in the central nervous system causing temporary or permanent acute flaccid paralysis (AFP) (known as poliomyelitis).6 While there is wide variability regarding the impact of poliovirus infection, estimates suggest that 12–20 million individuals are living with the consequences of the disease worldwide.7 Up to 40% of all survivors of acute poliomyelitis will experience postpoliomyelitis syndrome (PPS),8 being the delayed appearance of new or worsening disabling neuromuscular symptoms 30–40 years after the original poliomyelitis attack. However, recent accurate prevalence rates estimating the number of survivors of poliomyelitis are not available.
Published international prevalence studies are problematic. These studies have (1)tended to focus on the initial disease and needs in the immediate aftermath, (2) been inconsistent in the definition of ‘polio survivor,’ with it often unclear whether this refers all those infected or only those sustaining some form of residual disability, (3) predominantly focused on health status rather than the everyday effects on people’s lives, their needs and those of their carers, (4) produced inconsistent findings on long-term outcomes, perhaps due to cultural differences, and/or have been (5) limited to lameness surveys of children in mostly low-income to lower middle-income countries. Hence, regional prevalence estimates are often crude and fragmentary. This systematic literature review aims to synthesise current knowledge on the prevalence of survivors of poliomyelitis worldwide using all available population-based polio prevalence studies.
Methods
This review is reported according to the PRISMA Statement.
Search strategy
We searched MEDLINE (1946–May 2016), CINAHL (1937–May 2016), Psychology and Behavioral Sciences Collection (1945–May 2016), ProQuest (1971–May 2016), Scopus (1970–May 2016) and Web of Science (1900–May 2016) databases from inception to May 2016 for relevant studies. A search strategy was developed for Medline using ‘post-polio syndrome’, ‘poliovirus’, ‘polio’, ‘postpolio’, ‘poliomyelitis’, ‘postpoliomyelitis’, ‘PPMA’, ‘PPMD’, ‘LEOP’, or ‘late effects of polio’ and ‘epidemiol’, ‘rate’, ‘proportion’, or ‘prevalence*’, and was then repeated for other database searches. The complete search strategy is available online (see online supplementary table S1). Hand searching of included articles was also undertaken.
bmjopen-2016-015470supp001.pdf (8.8KB, pdf)
Inclusion/exclusion criteria
Inclusion criteria were: peer reviewed; written in English; reporting of prevalence of poliomyelitis survivors; use of a population-based, epidemiology approach with a defined denominator; and some form of diagnostic or clinical verification of polio. Only those studies reporting on cases ascertained from a general population sample (ie, not restricted by gender or ethnicity) were included to enable comparison between populations and with other conditions, and to enhance representativeness of the findings. Studies in which any prevalence data were unable to be extracted or calculated, or studies reporting incidence data only, were excluded. Duplicate publications reporting on the same research data were also removed.
Quality appraisal
Each study was assessed for methodological quality and risk of bias using a 10-item assessment tool (external (four items) and internal (six items) validity) specifically designed for population-based prevalence studies.9 Furthermore, a summary assessment evaluates the overall risk of study bias based on the 10 items. A summary assessment deeming a study to be at low risk of bias suggests that ‘further research is very unlikely to change our confidence in the estimate’. A moderate risk of bias rating suggests that ‘further research is likely to have an important impact on our confidence in the estimate and may change the estimate’. The limitations of studies considered to be at high risk of bias suggest that ‘further research is very likely to have an important impact on our confidence in the estimate and is likely to change the estimate’. For this review, a study was considered to have a high risk of bias if the target population was not closely representative of the national population, if there was no use of random selection and if the study had a more than a minimal risk of non-response bias.
Data extraction and synthesis
Two authors (KJ and SB) independently reviewed abstracts for possible inclusion. In cases of non-consensus, an additional independent review was obtained from a third author (VF). Any ongoing discrepancies were resolved via discussion. In cases of incomprehensive study methodology, authors were approached to determine a study's potential inclusion. Where possible, copies of full articles were obtained for studies meeting the inclusion criteria.
Reviewers extracted standard information per study on study characteristics, target population, research design and verification of poliovirus infection. Only those studies considered to be at low to moderate risk of bias were included in the calculation of prevalence estimates. An average prevalence of poliomyelitis is reported for each study as the number of cases per 100 000 people of all ages or a particular age range, depending on the data available. Rates were checked for accuracy where possible, depending on the data provided. Due to a lack of availability of standardised rates, prevalence rates are reported as crude estimate (ie, unadjusted rates). Studies reporting adjusted values only have not been included in the average prevalence calculation. In instances where a range of prevalence rates have been reported in a study and no overall rate reported (eg, across ethnic groups or different geographical regions or years), we have used the average of this range for the purposes of calculating an overall average prevalence. The research protocol was not subject to ethical approval as no such approval was required according to local regulations.
Results
Included studies
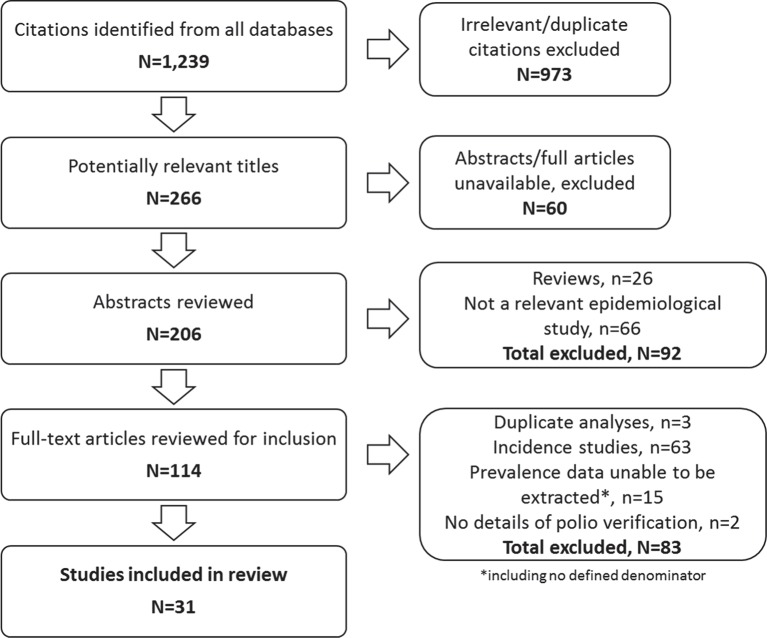
Figure 1 presents an overview of the study selection process. The initial search yielded 1239 citations. Following scanning of the titles for appropriateness for inclusion, those not meeting criteria and duplicate citations were removed (973). Where available, the abstracts of the remaining 266 potentially relevant titles identified across all sources (EBSCO n=25; ProQuest n=17; Scopus n=88; Web of Science n=136) were obtained. Following the availability and review of 206 abstracts, 114 full articles were independently evaluated for inclusion by two reviewers (SB and KJ). This process led to the elimination of 83 studies that did not meet the required inclusion criteria. The remaining 31 articles met inclusion criteria and were included in the review.
Figure 1.
Flow diagram of included/excluded studies.
The 31 eligible population-based studies reported data from 14 different countries. Data on polio prevalence in low-income to lower middle-income countries were reported in 28 (90%) studies in 11 countries: India (14 studies); Nigeria (2 studies); Ethiopia (4 studies) and one study in each of the following locations: Indonesia, Ghana, Bangladesh, Niger, Cameroon, Sudan, Yemen and Papua New Guinea. Population-based data on polio prevalence in upper-middle to high-income countries were available from three studies in three countries: Japan, Sweden and Brazil.
Study characteristics
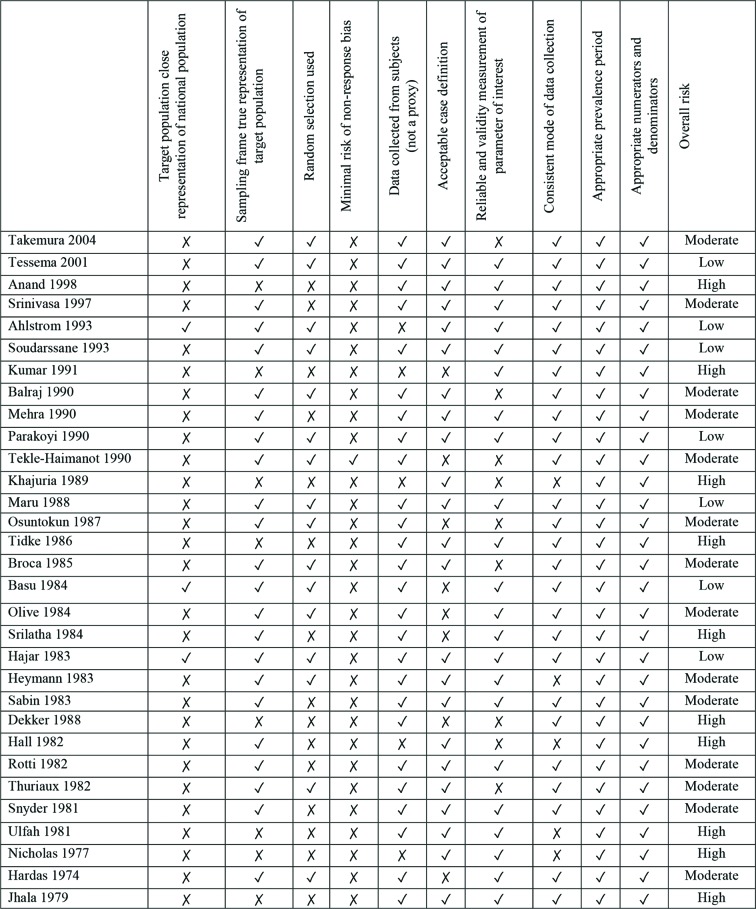
Table 1 provides a summary of the characteristics of those articles included in the review, including details of polio verification, population status, study design and risk of bias. The included studies reported on polio prevalence data collected between 1974 and 2004. In terms of methodologies implemented across the included studies, poliovirus infection was verified in 28 (90%) cases via the use of clinical investigations (ie, examination by a physician or similar). Two of these studies also used laboratory investigations (ie, virological confirmation) to confirm a history of polio. Twenty-nine (94%) studies presented data collected by lameness surveys. These included surveys of schools (5), villages (1), families (1), house-to-house surveys (16), or a mixture thereof (4), and postal questionnaires (1). One lameness survey examined a national population. The remaining two studies (6%) used multiple sources of case ascertainment. Studies most commonly examined urban/rural or semirural populations (12), with eight studies limited to rural populations. Only one study reported a specific focus on an urban population. Of the 31 studies included, 26 (84%) presented data based on children and young persons aged <20 years. In terms of risk of bias, the majority of studies (77%) were at moderate (14 studies, 45%) to high (10 studies, 32%) risk of bias (figure 2). Seven studies (23%) were considered to be at low risk of bias.
Table 1.
Details of included population-based studies in reverse chronological order
| First author | Year | Country (region) | Population status | Population base | Study design/date | Full article | Verification of diagnosis | Risk rating |
| Takemura14 | 2004 | Japan (Kitakyushu) | Registry of 13 000 physically disabled persons | 342 | Lameness survey (postal questionnaire) |
Yes | Clinical investigation | Moderate |
| Tessema15 | 2001 | Ethiopia* (Gondar Zuria district) | General population (urban/rural) | 12 000 children aged 1–15 years | Lameness survey (house-to-house) July–August 1993 |
Yes | Clinical investigation | Low |
| Anand16 | 1998 | India* (Ballabgarh, Haryana) | General population (rural) | 28 464 children aged <15 years | Lameness survey (house-to-house) December 1996 |
Yes | Clinical and laboratory investigation | High |
| Srinivasa17 | 1997 | India* (Pondicherry) |
General population (urban/rural) |
Approx. 11 000 children aged <60 months | Lameness survey (house-to-house) January–February of each year between 1989 and 1991 |
Yes | Clinical investigation | Moderate |
| Ahlström18 | 1993 | Sweden (Orebro) |
General population (urban/rural) | 269 341 | Multiple sources (hospital, outpatient, institutional, insurance, medical records) 1 January 1988 |
Yes | Search of clinical records | Low |
| Soudarssanane19 | 1993 | India* (Pondicherry) |
General population | 47 960 children aged 0–6 years | Lameness survey (house-to-house) April–July 1989 |
Yes | Clinical investigation | Low |
| Kumar20 | 1991 | India* (Ambala, Haryana State) |
General population | 15 761 children aged <15 years | Lameness survey (house-to-house) January 1989 |
Yes | Clinical investigation | High |
| Balraj21 | 1990 | India* (Northern Arcot District, Tamil Nadu State) |
General population (rural) |
42 045 children aged <5 years | Lameness survey (house-to-house) February–October 1988 |
Yes | Clinical investigation | Moderate |
| Mehra22 | 1990 | India* (New Delhi) |
General population (urban/rural) |
7318 children aged 5–15 years | Lameness survey (house-to-house) June–August 1986 |
Yes | Clinical investigation | Moderate |
| Parakoyi23 | 1990 | Nigeria* (Ilorin, Kwara State) | General population (urban/rural) | 4576 children aged 5–9 years | Lameness survey (house-to-house) March 1988 |
Yes | Clinical investigation | Low |
| Tekle-Haimanot24 | 1990 | Ethiopia* (Meskan and Moreko subdistricts) |
General population (rural) |
60 820 adults | Lameness survey (house-to-house) 1986–1988 |
Yes | Clinical investigation | Moderate |
| Khajuria25 | 1989 | India* (Haryana) | General population (rural) | 37 851 children aged 1–11 years | Lameness survey (village) 1985 |
Yes | Clinical investigation | High |
| Dekker26 | 1988 | Ethiopia* (Addis Ababa) |
General population | 256 092 children aged<20 years | Lameness survey (school) Unknown date |
Yes | Clinical investigation | High |
| Maru27 | 1988 | Ethiopia* (Gondar) |
General population (urban/rural) | 17 941 children aged 5–9 years | Lameness survey (house-to-house) February–July 1983 |
Yes | Clinical investigation | Low |
| Osuntokun28 | 1987 | Nigeria* (Igbo-Ora) |
General population | 18 954 | Lameness survey (house-to-house) Unknown date |
Yes | Clinical investigation | Moderate |
| Tidke29 | 1986 | India* (Bombay) |
General population (slums) | 15 165 children aged <6 years | Lameness survey (house-to-house) Unknown date |
Yes | Clinical investigation | High |
| Broca30 | 1985 | India* (Ajmer City, Rajasthan) |
General population | 6000 children aged 5–15 years | Lameness survey (house-to-house) August 1981–March 1982 |
Yes | Clinical investigation | Moderate |
| Basu31 | 1984 | India (National)* |
General population (urban/rural) | 715 039 children aged 5–9 years | Lameness survey (national) 1981–1982 |
Yes | Clinical investigation | Low |
| Heymann32 | 1983 | Rep. of Cameroon* (Yaounde, Bamenda, Eseka) |
General population (urban/rural) |
37 130 children aged 5–11 years | Multiple sources (hospital and clinical registers, house-to-house and school lameness survey) | Yes | Clinical investigation | Moderate |
| Olive33 | 1984 | Sudan* (Port Sudan and Juba, Khartoum) | General population (urban/semirural) | 45 499 children aged 5–13 years | Lameness survey (house-to-house/school) July–September 1982 |
Yes | Clinical investigation | Moderate |
| Srilatha34 | 1984 | South India* (North Arcot District, Tamil Nadu) | General population (rural) | 14 643 children aged 5–17 years | Lameness survey (school) June-December 1979 |
Yes | Unknown | High |
| Hajar35 | 1983 | Yemen Arab Republic* | General population (urban/rural) | 12 443 children aged 5–13 years | Lameness survey (school and community) November 1980–January 1981 |
Yes | Clinical investigation | Low |
| Sabin36 | 1983 | Brazil (Federal District of Brazil) |
General population | 20 807 children aged 6–7 and 10–11 years | Lameness survey (school) 1980 |
Yes | Clinical investigation | Moderate |
| Hall37 | 1982 | Papua New Guinea* (Gulf Province) |
General population | 3368 children (ages unspecified) | Lameness survey (school) 1979 |
Yes | Unknown | High |
| Rotti38 | 1982 | South India* (Pondicherry) | General population (urban) | 6683 children aged 6–15 years | Lameness survey (house-to-house and school) July 1980–September 1981 |
Yes | Clinical investigation | Moderate |
| Thuriaux39 | 1982 | Niger* (Niamey, Kollo, Tillaberry, Gotheye) |
General population (rural/urban) | 46 772 children aged 5–14 years | Lower limb motor disorders survey (school) February–May 1981 |
Yes | Clinical investigation | Moderate |
| Snyder40 | 1981 | Bangladesh* (Matlab) |
General population (rural) | 25 000 children aged 5–14 years | Lameness survey (house-to-house) May–June 1979 |
Yes | Clinical investigation | Moderate |
| Ulfah41 | 1981 | Indonesia* (Yogyakarta) |
Philanthropic private agency | 94 376 children and adolescents aged <20 years | Lameness survey (families) | Yes | Clinical and laboratory investigation | High |
| Jhala42 | 1979 | India* (Patan Taluka, Gujarat) | General population (rural) |
57 435 adults | Lameness survey (house-to-house) | Yes | Clinical investigation | High |
| Nicholas43 | 1977 | Ghana* (Danfa) |
General population (rural) | 13 232 children aged 0–15 years | Lameness survey (school, village) | Yes | Clinical investigation | High |
| Hardas44 | 1974 | India* (Nagpur) |
General population (urban/rural) | 36 826 children aged <12 years | Lameness survey (house-to-house) | Yes | Clinical investigation | Moderate |
| Low-income to lower middle-income countries:average prevalence† | 425 | |||||||
| Upper-middle to high-income countries: average prevalence‡ | 165 | |||||||
| International: total average prevalence | 295 | |||||||
*Low-income to lower-middle income countries.
†Based on 18 studies with low to moderate risk of bias.
‡Based on three studies with low to moderate risk of bias.
Figure 2.
Risk of bias summary of included population-based studies in reverse chronological order.
Poliomyelitis prevalence
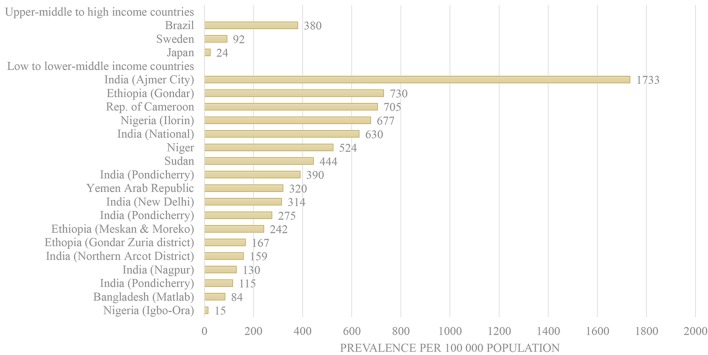
In the general population, crude average prevalence across all included studies ranged from 15 per 100 000 in Nigeria to 1733 in Ajmer City, India (figure 3). Among all low-income to lower middle-income countries, crude rates of poliomyelitis prevalence ranged from 15 per 100 000 (Igbo-Ora, Nigeria) to 1733 per 100 000 (Ajmer City, Rajasthan, India). Among all high-income counties, crude rates of poliomyelitis prevalence ranged from 24 (PPS) per 100 000 (Japan) to 380 per 100 000 (Brazil). For those studies considered to be at low to moderate risk of bias, prevalence estimates ranged from 92 in Sweden to 730 in Ethiopia. The total combined prevalence of poliomyelitis for those studies at low to moderate risk of bias ranged from 165 (high-income countries) to 425 (low-income to lower middle-income countries) per 100 000 person-years. The estimated average crude worldwide prevalence is 295 per 100 000 person-years.
Figure 3.
Poliomyelitis prevalence by country income level.
Discussion
This study reviews all the available data from population-based poliomyelitis prevalence studies. Findings reveal significant discrepancies in average crude unadjusted prevalence rates, both between and within countries. Across all included studies and within low-income to lower middle-income countries, prevalence rates ranged from 15 per 100 000 person-years in Igbo-Ora, Nigeria to 1733 in Ajmer City, India. Within high-income countries, rates ranged from 24 in Japan to 380 in Brazil.
Worldwide variations in prevalence ratings could be attributed to the diversity (and at times a lack of clarity) of applied case definitions and assessment processes to determine a history of poliomyelitis. For example, while the majority of studies used lameness surveys, some studies were limited to the examination of lower extremity disabilities. Other studies included the examination of upper extremity disabilities in their efforts to identify those affected by poliovirus leading to poliomyelitis. Three studies were limited to the examination of PPS only. Such inconsistencies are of concern given incomplete case ascertainment or disease misclassification can significantly skew the reported prevalence. Even assuming that all cases were ascertained in a given study, data would still omit those survivors of poliomyelitis who are now free from any observable, physical ailments. However, perhaps more problematic are the risks for over-reporting due to the inclusion of cases of non-polio AFP.
Alongside methodological variations and shortcomings discussed above, rather than informing estimates of the prevalence of survivors of poliomyelitis worldwide, limitations in the literature render this review largely of the historical prevalence of residual AFP that may be due to poliomyelitis. AFP is a clinical syndrome with a broad array of possible etiologies (ie, spinal cord compression, trauma, exposure to chemicals and recent illness) that serves as a proxy for poliomyelitis.10 Figures from AFP surveillance surveys, an essential strategy of the Global Polio Eradication Initiative, suggest that non-polio AFP affects one to three cases per 100 000 children aged <15 years per year.11 Subsequently lameness surveys, most common in this review, risk overstating the prevalence of survivors of polio. Such risks are especially high in areas such as Afghanistan, India and Nigeria who have the highest annualised non-polio AFP rate compared with the number of poliovirus cases.12 Furthermore, few studies examined the prevalence of survivors of poliomyelitis across all age groups, nor included samples that were representative of the respective national population being studied to inform appropriate gender, age and ethnicity estimates. Findings from lameness surveys in the current review also had considerable variation in the range of age groups surveyed (ie, 5–15 years, 0–6 years).
Our findings suggest the average crude worldwide prevalence of 295/100 000 person-years. However, many of the studies included in this review were undertaken in geographical areas where rates of non-polio AFP are high. Alongside the dated nature of studies (many being published more than 30 years ago), since ageing population, and a 99% reduction in the more recent incidence of poliovirus infection, it must be noted that the actual worldwide prevalence is likely much lower.
This review has provided an overview of studies to date that have endeavoured to examine the prevalence of survivors of poliomyelitis. While all efforts were made to identify and access all articles relevant to the review, it is important to acknowledge the likelihood that some studies (ie, unpublished or inaccessible studies) were not identified by the search strategy and therefore excluded. Well-designed epidemiological studies are clearly required to accurately determine the current prevalence of poliomyelitis survivors, living either with or without AFP.
Future epidemiological polio studies can reduce bias noted in this review by including the use of random or cluster sampling, the examination of populations that are representative of the national population where possible and the application of clear case definitions and diagnosis. In addition to recommending that future studies adhere to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement,13 we offer specific recommendations for the pursuit of future studies examining prevalence of survivors of poliomyelitis in box 1.
Box 1. Suggested recommendations for future epidemiological studies of poliomyelitis.
External validity
Examine nationally representative populations to enhance the generalisability of findings.
Further prevalence studies are required in high-income countries.
Examine all age groups (ie, adults and children).
Estimate prevalence by age, sex, residency (urban/rural) and include ethnic-specific rates.
Undertake a census OR use some form of random selection (ie, cluster sampling).
Extend findings of lameness surveys by also capturing lame-free cases of poliomyelitis (ie, using multiple sources of case ascertainment including review of medical records).
Use an established risk of bias tool specifically designed for use in population-based studies.
Internal validity
Standard case definition and clinical evaluation.
Describe any efforts to address potential sources of bias.
Appropriate numerator(s) and denominator(s).
Conclusions
In conclusion, this review reported prevalence of poliomyelitis survivors worldwide from all identified studies. The majority of research to date has been limited to the examination of children and adolescents in low-income to lower middle-income countries (predominantly India) who reside in geographical regions that are not representative of the national population (eg, in terms of age, sex, ethnic distributions) and face high rates of non-polio AFP. Further research of the prevalence of survivors of poliomyelitisis is required using a population-based approach, examining nationally representative samples of all ages, particularly in high-income countries including those declared to be polio free. Such efforts will reduce risks for sampling and measurement bias and improve capacity to provide reliable and more robust worldwide prevalence estimates.
Supplementary Material
Footnotes
Contributors: GJ conceived the study. SB conducted searches and extracted the data. KJ and SB compiled and analysed data. VLF, AT and GJ advised throughout the study. KJ prepared the initial draft of the manuscript. All authors provided conceptual input and critical review of drafts.
Funding: This work was funded by The Sir Thomas and Lady Duncan Trust Fund
Competing interests: None declared.
Patient consent: This submission is a systematic literature review of published data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Toole MJ. So close: remaining challenges to eradicating Polio. BMC Med 2016;14:43 10.1186/s12916-016-0594-6 10.1186/s12916-016-0594-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Polio Eradication Initiative. Global Polio eradication initiative annual report 2007: impact of the intensified eradication effort. Switzerland: World Health Organisation, 2008. [Google Scholar]
- 3. World Health Organisation. Poliomyelitis Fact Sheet Updated April 2016, 2016. [Google Scholar]
- 4. Dowdle WR, Birmingham ME. The biologic principles of poliovirus eradication. J Infect Dis 1997;175 Suppl 1(Suppl 1):S286–S292. 10.1093/infdis/175.Supplement_1.S286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Poliomyelitis : H J Kroger J, Wolfe C, Epidemiology and Prevention of Vaccine-Preventable Diseases. (The Pink Book). 13 ed Washington DC: Public Health Foundation, 2015. [Google Scholar]
- 6. Shibuya K, Murray CJL, C.L. M Poliomyelitis. In:, Lopez AD, Mathers C, et al. ; The global epidemiology of infectious diseases. Geneva: World Health Organisation, 2004:111-–49. [Google Scholar]
- 7. Gonzalez H, Olsson T Borg K. Management of postpolio syndrome. Lancet Neurol 2010;9:6–634 10.7861/clinmedicine.6-6-536 [DOI] [PubMed] [Google Scholar]
- 8. Lin KH, Lim YW. Post-poliomyelitis syndrome: case report and review of the literature. Ann Acad Med Singapore 2005;34:447. [PubMed] [Google Scholar]
- 9. Hoy D, Brooks P, Woolf A, et al. . Assessing risk of Bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 10. Growdon JH, Fink JS. Paralysis and movement disorder : Isselbacher KJ, Braunwald E, Wilson JD, Harrison's principles of internal medicine. New York: : McGraw-Hill Book Company, 1994:115–25. [Google Scholar]
- 11. Snider CJ, Diop OM, Burns CC, et al. . Surveillance Systems to Track Progress toward Polio Eradication--Worldwide, 2014-2015. MMWR Morb Mortal Wkly Rep 2016;65:346–51. 10.15585/mmwr.mm6513a3 [DOI] [PubMed] [Google Scholar]
- 12. Mateen FJ, Black RE. Expansion of acute flaccid paralysis surveillance: beyond Poliomyelitis. Trop Med Int Health 2013;18:1421–2. 10.1111/tmi.12181 [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, et al. . The strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007;147:573–7. 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 14. Takemura J, Saeki S, Hachisuka K, et al. . Prevalence of post-polio syndrome based on a cross-sectional survey in Kitakyushu, Japan. J Rehabil Med 2004;36:1–3. 10.1080/16501970310017423 [DOI] [PubMed] [Google Scholar]
- 15. Tessema T, Hailu A. Epidemiology of Poliomyelitis in Northwestern Ethiopia. East Afr Med J 2001;78:430–2. 10.4314/eamj.v78i8.8996 [DOI] [PubMed] [Google Scholar]
- 16. Anand K, Kant S, Kumar G, et al. . Thirty year trend (1967-1996) in prevalence of Poliomyelitis and vaccine coverage in Ballabgarh, Haryana, India. J Epidemiol Community Health 1998;52:823–5. 10.1136/jech.52.12.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srinivasa DK, Sahai A, Rotti SB, et al. . Poliomyelitis trends in Pondicherry, South India, 1989-91. J Epidemiol Community Health 1997;51:443–8. 10.1136/jech.51.4.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahlström G, Gunnarsson LG, Leissner P, et al. . Epidemiology of neuromuscular diseases, including the postpolio sequelae, in a swedish county. Neuroepidemiology 1993;12:262–9. 10.1159/000110327 [DOI] [PubMed] [Google Scholar]
- 19. Soudarssanane MB, Rotti SB, Srinivasa DK, et al. . Paralytic Poliomyelitis in children under 6 years in Pondicherry: a community survey. J Epidemiol Community Health 1993;47:210–4. 10.1136/jech.47.3.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar R, Kumar V. Poliomyelitis control by annual immunization campaigns with oral polio-virus vaccine in a rural area of India. Trop Geogr Med 1991;43:215–9. [PubMed] [Google Scholar]
- 21. Balraj V, John TJ, Thomas M, et al. . Efficacy of oral poliovirus vaccine in rural communities of North Arcot District, India. Int J Epidemiol 1990;19:711–4. 10.1093/ije/19.3.711 [DOI] [PubMed] [Google Scholar]
- 22. Mehra M, Bansal Y. Prevalence of Paralytic Poliomyelitis in a Rural and Urban community of Delhi. Indian Pediatr 1990;27:915–7. [PubMed] [Google Scholar]
- 23. Parakoyi B, Babaniyi OA. Prevalence of Paralytic Poliomyelitis in children of Kwara State, Nigeria: report of a house-to-house survey. East Afr Med J 1990;67:545–9. [PubMed] [Google Scholar]
- 24. Tekle-Haimanot R, Abebe M, Gebre-Mariam A, et al. . Community-based study of neurological disorders in rural central Ethiopia. Neuroepidemiology 1990;9:263–77. 10.1159/000110783 [DOI] [PubMed] [Google Scholar]
- 25. Khajuria R, Datta N, Kumar R, et al. . Impact of annual immunisation programme with oral Polio vaccine on the prevalence of paralytic Poliomyelitis. Indian J Pediatr 1989;56:343–7. 10.1007/BF02722297 [DOI] [PubMed] [Google Scholar]
- 26. Dekker PA, Green-Abate C. Prevalence of residual paralysis due to Poliomyelitis in schoolchildren in Addis Ababa. Ethiop Med J 1988;26:133–8. [PubMed] [Google Scholar]
- 27. Maru M, Getahun A, Hoshna S. Prevalence of Paralytic Poliomyelitis in Rural and Urban populations in Ethiopia: report of a house-to-house survey. Am J Trop Med Hyg 1988;38:633–5. 10.4269/ajtmh.1988.38.633 [DOI] [PubMed] [Google Scholar]
- 28. Osuntokun BO, Adeuja AO, Schoenberg BS, et al. . Neurological disorders in nigerian africans: a community-based study. Acta Neurol Scand 1987;75:13–21. 10.1111/j.1600-0404.1987.tb07883.x [DOI] [PubMed] [Google Scholar]
- 29. Tidke RW, Joshi U, Patel RB. Paralytic Poliomyelitis in slums of Bombay. Indian J Pediatr 1986;53:109–13. 10.1007/BF02787081 [DOI] [PubMed] [Google Scholar]
- 30. Broca JS, Chaturvedi SK, Mathur GM. Prevalence of Residual Polio paralysis in children of 5-15 years age group in Ajmer City. Indian J Public Health 1985;29:193–200. [PubMed] [Google Scholar]
- 31. Basu RN, Sokhey J. Prevalence of Poliomyelitis in India. Indian J Pediatr 1984;51:515–9. 10.1007/BF02776613 [DOI] [PubMed] [Google Scholar]
- 32. Heymann DL, Floyd VD, Lichnevski M, et al. . Estimation of incidence of Poliomyelitis by three survey methods in different regions of the United Republic of Cameroon. Bull World Health Organ 1983;61:501–7. [PMC free article] [PubMed] [Google Scholar]
- 33. Olive JM, Gadir A, Abbas M. Prevalence of residual paralysis from paralytic Poliomyelitis in some urban and semi-rural areas of the Sudan, November 1982. J Trop Pediatr 1984;30:329–33. 10.1093/tropej/30.6.329 [DOI] [PubMed] [Google Scholar]
- 34. Srilatha V, Mukarji D, John TJ. The prevalence of Poliomyelitis in rural school children in South India. J Trop Pediatr 1984;30:68–9. 10.1093/tropej/30.2.68 [DOI] [PubMed] [Google Scholar]
- 35. Hajar MM, Zeid AS, Saif MA, et al. . Prevalence, incidence, and epidemiological features of Poliomyelitis in the Yemen Arab Republic. Bull World Health Organ 1983;61:353. [PMC free article] [PubMed] [Google Scholar]
- 36. Sabin AB, Silva E. Residual Paralytic Poliomyelitis in a Tropical region of Brazil, 1969-1977: prevalence surveys in Different age groups as indicators of changing incidence. Am J Epidemiol 1983;117:193–200. 10.1093/oxfordjournals.aje.a113530 [DOI] [PubMed] [Google Scholar]
- 37. Hall AJ. A survey of lameness in school children in Gulf Province. P N G Med J 1982;25:26–8. [PubMed] [Google Scholar]
- 38. Rotti SB, Satpathy SK, Mehta SP. Prevalence of Paralytic Poliomyelitis in Pondicherry, South India. J Epidemiol Community Health 1982;36:279–81. 10.1136/jech.36.4.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thuriaux MC. A prevalence survey of lower limb motor disorders in school-age children in Niger and an estimation of Poliomyelitis incidence. Trop Geogr Med 1982;34:163–8. [PubMed] [Google Scholar]
- 40. Snyder JD, Black RE, Baqui AH, et al. . Prevalence of residual paralysis from paralytic Poliomyelitis in a rural population of Bangladesh. Am J Trop Med Hyg 1981;30:426–30. 10.4269/ajtmh.1981.30.426 [DOI] [PubMed] [Google Scholar]
- 41. Ulfah NM, Parastho S, Sadjimin T, et al. . Polio and lameness in Yogyakarta, Indonesia. Int J Epidemiol 1981;10:171–5. 10.1093/ije/10.2.171 [DOI] [PubMed] [Google Scholar]
- 42. Jhala CI, Goel RK, Dave SK. Epidemiology of Poliomyelitis in rural area of Gujarat -- a report of house to house survey in Patan Taluka. Indian J Med Sci 1979;33:143–9. [PubMed] [Google Scholar]
- 43. Nicholas DD, Kratzer JH, Ofosu-Amaah S, et al. . Outside Europe. is Poliomyelitis a serious problem in developing countries?--the danfa experience. Br Med J 1977;1:1009–12. 10.1136/bmj.1.6067.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hardas U, Waikar A. Pilot survey of disabled children in and around Nagpur. Indian J Pediatr 1974;41:267–71. 10.1007/BF02829306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015470supp001.pdf (8.8KB, pdf)