Abstract
Objective
The aim of this systematic review is to explore the association of South Asian (SA) ethnicity on comorbidities, microvascular and macrovascular complications and mortality compared with other ethnic groups in people with type 1 diabetes mellitus (T1DM).
Design
Systematic review.
Method
A systematic literature search strategy was designed and carried out using Medline and Embase for full-text and abstract studies published in English from 1946 to February 2016. The initial search identified 4722 papers. We assessed 305 full-text articles in detail for potential inclusion. Ten papers met the inclusion criteria for review and an additional one paper was included from our secondary search strategy using the bibliography of included studies. In total, 11 studies were included.
Eligibility criteria for selecting studies
Studies were included if they were published in English, involved SA participants with T1DM and compared them with non-SA participants and assessed one of the outcomes of comorbidities, microvascular complications, macrovascular complications and mortality.
Results
SA with T1DM have higher mortality compared with white Europeans (WE), mainly contributed to by excess cardiovascular disease. SA have significantly higher glycated haemoglobin (HbA1c), lower high-density lipoprotein (HDL) and lower rates of neuropathy compared with WE. There were no differences in rates of retinopathy and nephropathy. Compared with Africans, SA had lower levels of microalbuminuria, HbA1c and systolic blood pressure and higher HDL levels. There were no significant differences in the remaining outcomes: cardiovascular disease, retinopathy, neuropathy and body mass index. Furthermore, SA have higher HbA1c levels than Malay and Chinese and higher waist–hip ratio and lower HDL levels compared with Chinese only.
Conclusion
Our analysis highlights ethnic disparity in macrovascular outcomes that is so evident for type 2 diabetes mellitus may also be present for SA patients with T1DM. We highlight the need for a large, prospective, cohort study exploring the effect of ethnicity in a uniform healthcare setting.
Keywords: type 1 diabetes mellitus, ethnicity, south asian
Strengths and limitations of this study.
The strengths of this analysis are its comprehensive search strategy with clearly defined population and outcomes.
Our search strategy incorporated both full-length papers as well as abstracts and had a secondary search strategy to ensure we did not miss any relevant papers.
We compared the South Asian (SA) group, the largest ethnic group globally with all other indigenous ethnic groups.
The quality of the studies were poor with the majority of studies being retrospective observational or cross-sectional.
Furthermore, the methodology of how outcomes were assessed was not consistently reported, and the numbers of SA in each study were small.
Background
The epidemiology of type 1 diabetes mellitus (T1DM) in South Asians (SA) is poorly understood. Its effects on metabolic control, diabetic complication rate or indeed the underlying pathogenesis has yet to be explored. SA are at higher risk than white Europeans (WE) for the development of obesity and obesity-related diseases including insulin resistance, the metabolic syndrome, type 2 diabetes mellitus (T2DM) and coronary heart disease.1T2DM is two to three times more common in SA than in the WE population in the UK2 and up to three times more common among people of African origin.3 Furthermore, SA with T2DM develop the condition 5–10 years earlier than WE, have increased prevalence of diabetic complications at presentation, worse outcomes and die at a younger age.2 4 These differences have not been explored in people with T1DM.
Willi et al 5 suggested that there were ethnic disparities in the outcomes of children with T1DM with black participants having higher mean HbA1c levels, more diabetic ketoacidosis and severe hypoglycaemic events compared with white or Hispanic participants. A recent systematic review6 identified 16 studies in the current literature that showed racial/ethnic minority youth with T1DM having higher haemoglobin A1c (HbA1c) compared with Caucasian youth. As the majority of these studies are conducted in the USA, their primary focus was on the black and Hispanic ethnic groups and youth with T1DM.
SA comprise 20% of the global population2 and 7% of the UK population.7 Furthermore, the incidence of T1DM appears to be similar in SA as in the background population.7 Therefore, there is a need to understand the effect of ethnicity on the progression of the disease. The aim of this systematic review is to explore the association of SA ethnicity on comorbidities, microvascular and macrovascular complications and mortality compared with other ethnic groups in people with T1DM.
Methods
Terms indicative of T1DM and SA were searched for in MEDLINE (Ovid) and EMBASE using keywords and free text. The search terms included ‘Type 1 Diabetes’, ‘Insulin Dependent Diabetes’ and ‘South Asian’ as well as terms pertaining to ethnicity such as ‘ethnic or racial group’, ‘race’, ‘ethnic or racial aspects’ and ‘ethnic differences’. We also included search terms pertaining to the individual countries from South Asia as listed below. Further information on the search strategy can be found in online supplementary appendix 1. Full-length papers and abstracts published in English were included in the search from 1946 to February 2016. The search was not limited to a particular study design or outcome and the papers did not have to be peer reviewed. A secondary search strategy involved reading bibliographies of the included studies and contacting authors of the included studies and committee members of the South Asian Health Foundation (http://www.sahf.org.uk) enquiring about additional studies or ongoing research.
bmjopen-2016-015005supp001.pdf (304.2KB, pdf)
The inclusion criteria were based on the Population, Intervention, Comparator and Outcome (PICO) framework. The population was SA with T1DM including both children and adults. A clinical diagnosis was accepted for the definition of T1DM. We defined SA ethnicity as persons originating from the following countries: India, Pakistan, Sri Lanka, Bangladesh, Nepal, Bhutan and the Maldives, and compared their comorbidities, complications and mortality with persons of any other ethnicity not classified as SA. We investigated comorbidities (body mass index, systolic and diastolic blood pressure, HbA1c and lipid profile), microvascular complications (retinopathy, neuropathy and nephropathy), macrovascular complications (ischaemic heart disease and cerebrovascular disease) and cause-specific and all-cause mortality.
Identified titles and abstracts were reviewed independently by two researchers (KS and PC). All studies that were deemed suitable for potential inclusion were then further examined in detail by the two researchers independently to create the final list of included studies. Where there were discrepancies between the two researchers (KS and PC) this was resolved by discussion. Quality assessment and data extraction was performed by KS and then checked by PC to identify any missing information (see online supplementary appendix 2). The Newcastle–Ottawa Quality Assessment Scale for observational studies was used for quality assessment.8
bmjopen-2016-015005supp002.pdf (410.7KB, pdf)
We were not able to perform a meta-analysis because the studies were not comparable by outcomes measured, were of poor quality and heterogeneous in the way SA ethnicity was defined. The results have been analysed as a narrative and presented as tabulations with textual description by each comorbidity and complication.
Patient involvement
Patients were not involved.
Results
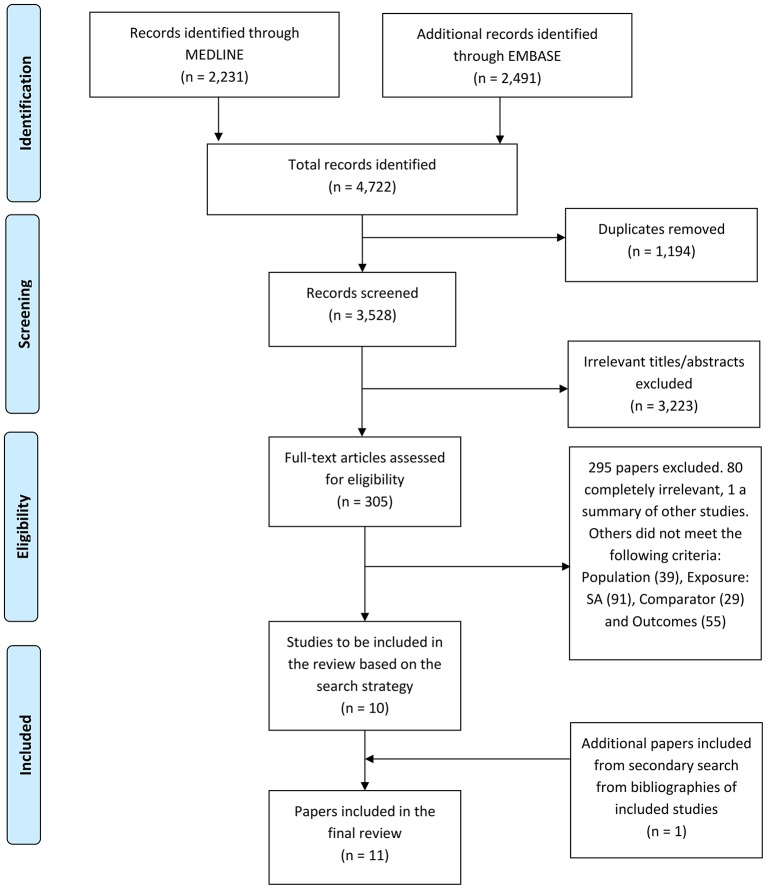
The initial search identified 4722 papers. After removing duplicates (1194), the remaining 3528 titles and abstracts were screened. After excluding 3223 papers in this initial screening process, 305 full-text articles were assessed in detail for potential inclusion into the analysis. Ten papers met the inclusion criteria for review. A secondary search using the bibliographies of included studies yielded an additional one paper (figure 1). A total of 11 studies were therefore included: 6 studies were from the UK, 4 from South Africa and 1 from Malaysia. Nine of the papers were full-length papers and two were abstracts. Of the included articles, one was a prospective cohort study, two were retrospective analysis of observational data and eight studies were cross-sectional analyses. The results are summarised in tables 1 and 2.
Figure 1.
Flowchart demonstrating study selection.
Table 1.
Data extraction of studies included in systematic review
| Study & year published | Country | Design | Method and Description | Number of articipants & Ethnic Group | Age description | Duration of Study | Key Outcomes | ||||
| Papers assessing T1DM comorbidities | |||||||||||
| Brabarupan et al 12 (2013) | UK | Cross-sectional study |
Ethnicity
Grouped into WE, African and SA T1DM Diagnosis of T1DM and diagnosed <35 years of age Method Data from patients from WE, African or SA ancestry were obtained from an electronic database in a large multiethnic London diabetes clinic |
642 individuals in total WE: 564 SA: 39 African: 39 |
Median age at diagnosis (years) WE: 16.7 African: 19.4 SA: 19.1 |
N/A | Parameters median (IQR) BMI (kg/m2) Systolic BP (mm Hg) Diastolic BP (mm Hg) HbA1c (%) Microalbuminuria (mg/mmol) Total cholesterol (mmol/L) HDL (mmol/L) Triglyceride (mmol/L) |
WE 25.0 (22.3–27.7) 130 (119–141) 75 (69–81) 8.0 (7.1–8.9) 1.2 (−0.5–3.0) 4.50 (3.90–5.10) 1.49 (1.21–1.77) 0.93 (0.59–1.28) |
African 25.7 (22.5–28.9) 135 (121–149) 80 (72–88) 9.1 (7.6–10.7) 3.7 (−44.5–51.9) 4.40 (3.90–4.90) 1.25 (0.95–1.56) 0.99 (0.58) |
SA 25.3 (22.2–28.5) 122 (1120133) 73 (67–79) 8.3 (7.5–9.2) 1.2 (−1.4–3.8) 4.00 (3.2–4.8) 1.30 (1.47–1.14) 1.07 (0.76–1.39) |
Significant p<0.05 p<0.05 p<0.05 p<0.05 p<0.05 p<0.05 |
| Sarwar et al 9 (2015) | UK | Cross-sectional study |
Ethnicity
SA and WE T1DM Coding of T1DM from the clinical database of two centres—no diagnostic criteria included Method Data analysed from two centres in the West Midlands (QEH and NCH) |
WE: 278 SA: 139 |
Median age (years) NCH WE: 34 NCH SA: 33.5 QEH WE: 36 QEH SA: 36 |
N/A |
Characteristic (no of patients)
HbA1c (mmol/mol) Systolic BP (mm Hg) Diastolic BP (mm Hg) BMI (kg/m2) Total cholesterol (mmol/L) HDL (mmol/L) Cholesterol/HDL Creatinine level (µmol/L) eGFR (mL/min/1.73 m2) Albumin/creatinine ratio (mg/mmol) |
NCH South Asian (80)
75 (61.5–88.5) 121 (113–132) – 25.6 (22.55–28.4) 4.7 (3.9–5.45) 1.3 (1.0–1.6)* 3.6 (2.9–4.5)* 75 (66–87) 97.3 (82.2–109.9) 2.4 (0.7–3.6) |
NCH Caucasian (160)
76 (63–91) 125 (115–132) – 25.7 (22.5–30.4) 4.6 (4–5.3) 1.4 (1.2–1.65)* 3.2 (2.7–4.0)* 78 (69–87) 91.2 (79.3–103.9) 2.5 (0.75–3.5) |
QEH South Asian (59)
66.1 (55.25–81.75) 130 (120.5–141.5) 86 (80.5–90)* 30.9 (22.8–37) 4.45 (3.8–5.45) – – – – – |
QEH Caucasian (118)
70.5 (61–83.6) 131.5 (120.3–144) 82 (77.25–88.75)* 25 (22.6–28) 4.1 (3.7–4.95) – - - – – |
| Shenoy et al 10 (2004) | UK | Retrospective observational study |
Ethnicity
SA and WE T1DM Children coded as T1DM in a centre in Leicestershire—no diagnostic criteria included Method Rates of obesity/overweight in WE and SA groups and to correlate these with age, duration of diagnosis, daily insulin requirement and HbA1c Included children between the ages of 2 and 18 years and who had been diagnosed more than a year ago |
WE: 112 SA: 38 |
Age group (n)
2–4 years (3) 5–9 years (33) 10–15 years (90) 16–18 years (24) |
N/A |
Demographic data
No statistically significant difference in the two subgroups in relation to age, duration of diagnosis, daily insulin requirement and metabolic control (median HbA1c 8.4% vs 8.8%, respectively, for white Caucasian/SA) Obesity in children No statistically significant differences noted in the rates of overweight or obesity between white Caucasian and SA children at any age grouping. |
||||
| Asmal et al 16 (1981) | South Africa | Cross-sectional analysis |
Ethnicity
Two groups: Indians and Black African T1DM Clinic patients who fulfilled the following criteria: age of diagnosis of diabetes <35 years, development of symptoms with/without ketosis in the absence of insulin therapy and duration of diabetes of at least 12 months Method Case records examined, clinical assessments and biochemical tests carried out |
Black African: 52 Indians: 38 |
Mean age at onset (years) Blacks: 21.8 Indians: 18.0 |
4 weeks |
Basic biochemical data
Glucose (mmol/L) Growth hormone (ng/mL) Cortisol (µg/dL) Cholesterol (mmol/L) Triglyceride (mmol/L) Creatinine (µg/dL) Complications Chronic complications associated with microangiopathy were detected in 12 Indians (33%) and 2 blacks (4%). The most common complication was neuropathy found in 19% of Indians with diabetesand in 4% of blacks with diabetes. Two Indians had evidence of diabetic triopathy. |
Indians
15.80±1.50 3.00±0.76 16.20±1.47 5.17±0.32 2.81±0.97 68.90±4.10 |
Black African
14.20±1.50 1.76±0.41 15.80±1.40 4.78±0.26 2.27±0.83 79.40±6.70 |
||
| Ismail et al 14 (2001) | Malaysia | Cross-sectional study |
Ethnicity
Three groups: Indians, Malay and Chinese Each ethnic group identified by appearance, language and religion T1DM T1DM defined as acute symptoms associated with heavy ketonuria (>3+) or ketoacidosis at diagnosis, or continuous treatment with insulin within 1 year of diagnosis Method Patients recruited from seven centres throughout Peninsular Malaysia Blood taken for lipid levels, clinical history and physical examination performed |
Indians: 154 Malay: 297 Chinese: 128 |
Mean age (years) All: 28.8 Indians: 29.1 Chinese: 29.8 Malay: 27.7 |
June 1997– June 1998 |
Demographic features
BMI (kg/m2) Waist–hip ratio HbA1c (%) Lipid profiles (mmol/L, mean ± SEM) Total cholesterol: Indians (5.74±1.25), Chinese (5.64±1.42), Malay (5.58±1.38) LDL cholesterol: Indians (3.89±1.20), Chinese (3.52±1.22), Malay (3.48±1.12) HDL cholesterol (mean (95% CI)): Indians (1.28 (1.19 to 1.38)), Chinese (1.57 (1.48 to 1.67)), Malay (1.37 (1.28 to 1.46)) Triglycerides (mean (95% C)): Indians (1.02 (0.9 to 1.16)), Chinese (0.82 (0.74 to 0.91)), Malay (1.11 (0.99 to 1.23)) |
Malay (n=297)
26.8±4.9 All: 0.88±0.06 Male: 0.91±0.06 Female: 0.86±0.06 8.8 (8.6–9.1) |
Chinese (n=128)
25.4±4.5 All: 0.88±0.07 Male: 0.90±0.06 Female: 0.85±0.07 8.0 (7.7–8.3) |
Indians (n=154)
25.5±4.3 All: 0.89±0.06 Male: 0.93±0.06 Female: 0.85±0.06 8.5 (8.2–8.8) |
|
| Omar et al 13 (1984) | South Africa | Cross-sectional analysis |
Ethnicity
Indians and Africans T1DM Classification of diabetes based on criteria by National Diabetes Data Group and WHO Expert Committee Patients with T1DM had always depended on insulin for control of symptoms and prevention of basal ketosis. All patients diagnosed <35 years of age |
African T1DM: 86 Indian T1DM: 40 |
Mean age of onset (range) African: 23.5 (1–35) years Indians: 17 (1-35) |
2-year period |
Clinical characteristics of patients with T1DM
Characteristic Male: female Mean % ideal body weight Mean duration of disease (years) Mean age of onset |
African (n=86)
21: 25 106 (68–153) 3.8 (1–27) 23.5 (1–35) |
Indians (n=40)
17: 24 91 (71–136) 5.4 (1–22) 17 (1–35) |
||
| Papers assessing T1DM complications | |||||||||||
| Swerdlow et al 19 (2004) | UK | Prospective cohort study |
Ethnicity
Grouped into SA and non-SA SA identified by computer algorithm (SANGRA) followed by a clerical check by an individual with expertise in this area T1DM Patients with IDDM diagnosed <30 years Method SMRs calculated, comparing mortality in the cohort to the corresponding mortality rates in the general population |
Non-SA: 23, 326 SA: 424 |
N/A | 1972– 1999 |
Mortality
The SMRs for SA patients diagnosed <30 years were 3.9 (95% CI 2.0 to 6.9) in men and 10.1 (95% CI 5.6 to 16.6) in women and in the corresponding non-SA were 2.7 (95% CI 2.6 to 2.9) in men and 4.0 (95% CI 3.6 to 4.3) in women. |
||||
| Mehta et al 11 (2011) | UK | Cross-sectional study |
Ethnicity
Ethnicity was categorised as SA or WE based on patient record documentation or by analysis of their name using a validated name recognition software ‘Nam Pechan’ supplemented by a visual inspection of surnames and forenames T1DM Patient coded as having T1DM in the clinical database of a specialist outpatient diabetes clinic in Leicestershire, UK—no diagnostic criteria included Method Patient characteristics and other data were extracted from the clinical workstation, a clinical database of patients attending a specialist outpatient diabetes clinic in Leicestershire |
WE: 1169 SA: 163 |
Mean age (years)
WE: 45.3 SA: 41.9 |
2003– 2005 |
No of comorbidities (n (%))
0 1 ≥2 Macrovascular (n (%)) CVD Ischaemic heart disease Peripheral vascular disease Cerebrovascular disease TIA Microvascular (n (%)) Retinopathy Neuropathy Nephropathy Glycaemic control (n (%)) HbA1C <7% HbA1C ≥7% |
SA (N=163)
114 (69.9) 36 (22.1) 13 (8.0) 25 (15.3) 20 (12.3) 3 (1.8) 6 (3.7) 0 63 (38.7) 24 (14.7) 22 (13.5) (N=163) 19 (12.0) 144 (88.0) |
WE (N=1169)
878 (75.1) 235 (20.1) 56 (4.8) 132 (11.3) 97 (8.3) 31 (2.7) 21 (1.8) 2 (0.2) 561 (48.0) 325 (27.8) 118 (10.1) (N=1169) 193 (17.0) 976 (83.0) |
p Value
0.166 0.133 0.093 0.790 0.130 1.000 0.025 <0.001 0.184 0.113 |
|
| Sivaprasad et al 18 (2012) | UK | Cross-sectional study |
Ethnicity
Self-reported ethnicity based on UK census standard (Census 2001): categorised as ‘White European’, ‘African', ‘South Asian’, ‘Mixed’, ‘other ethnic group’ and ‘not known’ T1DM Patients coded as T1DM in the database of the local DR screening service—no diagnostic criteria included Method To assess ethnic variations of the prevalence of DR and visual impairment in two multiracial cohorts in the UK (Yorkshire and South East London) |
WE: 2628 African: 344 SA: 120 |
Mean age of T1DM population: 39.4 years |
2008– 2009 |
Ethnic group
Any diabetic retinopathy WE African South Asian Any maculopathy (M1) WE African SA CSMO (M1P1) WE African SA STDR (R2 or R3 or M1P1) WE African SA |
Prevalence: n (%)
1446 (55.0) 154 (44.8) 64 (53.3) 371 (14.1) 47 (13.7) 171 (6.5) 17 (14.2) 35 (10.20 12 (10.0) 318 (12.1) 53 (15.4) 19 (15.8) |
Age-standardised prevalence: % (95% CI)
55.0 (53.2 to 56.9) 42.8 (37.3 to 48.3) 54.0 (44.8 to 63.2) 13.1 (9.4 to 16.8) 16.6 (10.0 to 23.2) 6.5 (5.6 to 7.4) 10.0 (6.7 to 13.3) 11.2 (5.4 to 16.9) 12.1 (10.9 to 13.3) 15.9 (11.8 to 20.0) 17.5 (10.6 to 24.3) 14.1 (12.8 to 15.4) |
||
| Thomas et al 15 (2012) | South Africa | Retrospective observational study |
Ethnicity
Caucasian, indigenous African, Asian and mixed race T1DM Classified as having T1DM on clinical assessment according to the American Diabetes Association classification of diabetes Method Retinal photography was conducted using a non-mydriatic digital camera without mydriasis and graded by one of three senior graders. |
Caucasian: 1247 Indigenous African: 117 Asian: 118 Mixed race: 49 |
Mean age (years) Caucasian: 35.7 Indigenous African: 36.3 Asian: 32.2 Mixed race: 32.6 |
2001– 2010 |
DR
Caucasian (1247) Indigenous African (117) Asian (118) Mixed race (49) |
Any DR (n=541)
Crude OR (95% CI) 1.00 0.71 (0.46 to 1.09) 1.10 (0.74 to 1.63) 1.01 (0.56 to 1.84) |
Adjusted OR (95% CI)
1.00 1.72 (1.00 to 2.97) 2.02 (1.23 to 3.29) 1.29 (0.62 to 2.69) |
RDR (n=142)
Crude OR (95% CI) 1.00 0.95 (0.49 to 1.84) 1.05 (0.54 to 2.04) 1.10 (0.42 to 2.88) |
Adjusted OR (95% CI)
1.00 3.40 (1.40 to 8.26) 2.07 (0.90 to 4.75) 1.06 (0.36 to 3.18) |
| Omar et al 17 1984 | South Africa | Crosssectional analysis |
Ethnicity
2 groups: Indians and Black African. T1DM Patients with onset of IDDM <35 years at King Edward Hospital in Durban. Diagnosis of IDDM based on the criteria recommended by WHO. Method Both case records obtained and a physical examination performed to assess complications. |
Black African: 92Indians: 41 | Mean age at onset (yrs) Blacks: 17 Indians: 23.5 | Not mentioned |
Complications
Keto-acidosis Neuropathy peripheral autonomic Retinopathy Nephropathy Triopathy Ischaemic heart disease Hypertension Cataracts Tuberculosis |
Black African
53 (58%) 20 (22%) 4 (4%) 13 (14%) 3 (3%) 1 (1%) - 4 (4%) 5 (5%) 6 (7%) |
Indians
22 (54%) 13 (32%) 2 (5%) 9 (22%) 3 (7%) 2 (5%) - 2 (5%) 2 (5%) 1 (2%) |
Total
75 (56%) 33 (25%) 6 (5%) 22 (17%) 6 (5%) 3 (2%) - 6 (5%) 7 (5%) 7 (5%) |
|
bp, blood pressure; BMI, body mass index; CSMO, clinically significant macular oedema;CVD, cardiovascular disease; DR, diabetic retinopathy; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein;eGFR, estimated glomerular filtration rate; IDDM, insulin-dependent diabetes mellitus; LDL, low-density lipoprotein;M1, maculopathy; NCH, New Cross Hospital;P1, macular laser;QEH, Queen Elizabeth Hospital; R1, mild to moderate non-proliferative diabetic retinopathy; R2, preproliferative diabetic retinopathy; R3, proliferative diabetic retinopathy; SA, South Asian; SANGRA, South Asian Names and Group Recognition Algorithm;SMR, standardised mortality ratio;STDR, sight-threatening diabetic retinopathy; T1DM, type 1 diabetes mellitus; TIA, transient ischaemic attack; WE, white European.
*p Value <0.05.
Table 2.
Summary of findings
|
Findings in the SASA population when compared with the specified ethnicity
(eg, SA have the same BMI as WE but higher HbA1c) |
|||
| WE | African | Chinese | |
| BMI | → | → | |
| HbA1c | ↑ | ↓ | ↑ |
| SBP | ↓ | ↓ | |
| DBP | → | → | |
| HDL | ↓ | ↑ | ↓ |
| Total cholesterol | → | → | → |
| Retinopathy | → | → | → |
| Nephropathy | → | ↓ | |
| Neuropathy | ↓ | → | |
| CVD | → | → | |
| Mortality | ↑ | ||
BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; HbA1c, haemoglobin A1c; HDL, high-density lipoprotein; SA, South Asian; SBP, systolic blood pressure; WE, white European.
Results
Comorbidities
Body mass index
Six studies explored body mass index (BMI) and general weight measurements as an outcome: three comparing SA with WE only, one comparing with WE and Africans, one comparing with Africans only and one comparing to Malay and Chinese. The three papers comparing SA to only WE demonstrated no statistically significant difference in BMI.9–11 Mehta et al 11 in the UK showed a mean BMI of 27.5 kg/m2 in SA (n=163) compared with 27.4 in WE (n=1169) (p=0.835). Similarities in BMI (kg/m2) between SA and WE have previously been reported in two different centres (median BMI 25.6 kg/m2 vs 25.7 kg/m2, respectively, and 30.9 kg/m2 vs 25 kg/m2, respectively).9 The results were not significant due to the small number of participants. Shenoy et al 10 also in the UK showed no statistically significant differences in the rates of overweight or obesity between WE (n=112) and SA (n=38) children with T1DM at any age grouping.
Brabarupan et al 12 in the UK showed no statistically significant difference in BMI in SA (n=39) compared with WE (n=565) and Africans (n=38) (median 25.3 kg/m2 vs 25.0 kg/m2 vs 25.7 kg/m2, respectively). Omar et al 13 in South Africa also showed no difference between SA (n=40) and Africans (n=86) in mean % ideal body weight (91 kg/m2 vs 106 kg/m2, respectively).
Lastly, a study by Ismail et al 14 in Malaysia showed that there was no difference in BMI when comparing SA to Malay and Chinese (mean 22.0 kg/m2 vs 22.3 kg/m2 vs 22.0 kg/m2, respectively). However, there were significant differences in waist–hip ratio between the ethnic group males with SA having significantly higher waist–hip ratio compared with Chinese (mean 0.88 vs 0.84, respectively, p=0.007).
In summary, there are no demonstrable differences in BMI between SA, WE and African ethnic groups with T1DM. However, SA males compared with Chinese males with T1DM had a higher waist–hip ratio.
Glycaemic control
Seven studies explored glycaemic control as an outcome: three comparing SA with WE only, two comparing with WE and Africans, one comparing to Africans only and one comparing with Malay and Chinese. Mehta et al 11 in the UK, demonstrated higher HbA1c levels in SA (n=163) (mean 9.1%) compared with WE (n=1169) (mean 8.5%) (p<0.001). This is similar to the results from Brabarupan et al 12 in the UK who demonstrate SA (n=39) having higher HbA1c levels (median 8.3%) compared with WE (n=565) (median 8.0) but lower than African (n=38) (median 9.1) (p<0.05). Another UK study analysed SA and WE at two different hospitals9 and demonstrated similar HbA1c (median 9.0% vs 9.1%, respectively, and 8.2% vs 8.6%, respectively, at the two different hospitals). Shenoy et al in10 the UK found no significant difference in metabolic control between WE (n=112) and SA (n=38) children (median HbA1c 8.4% vs 8.8%, respectively). Thomas et al 15 in South Africa also found no statistically significant differences between SA (n=118), WE (n=1247) and Africans (n=117) in HbA1c levels (8.7% vs 8.2% vs 9.5%, respectively). A study by Asmal et al 16 in South Africa showed that SA (n=38) had similar mean glucose concentrations to Africans (n=52) (15.80 mmol/L vs 14.20 mmol/L, respectively).
Ismail et al 14 in Malaysia showed that SA (n=76) have significantly higher HbA1c levels compared with Chinese (n=91) and Malay (n=102) (mean 9.3% vs 7.8% vs 9.0%, respectively, p<0.001).
In summary, studies suggest SA have higher HbA1c levels compared with WE, Malay and Chinese but lower than Africans.
Blood pressure
Four studies determined blood pressure/hypertension as an outcome: two comparing SA with WE only, one comparing with WE and Africans and one comparing to Africans only. The three papers with a WE group all showed that SA have lower blood pressure than the comparator groups. Mehta et al 11 in the UK, showed a significantly lower systolic blood pressure in SA (n=163) compared with WE (n=1169) (mean value 136.4 mm Hg vs 141.6 mm Hg, respectively, p=0.004). However, there was no difference in diastolic blood pressure between SA (mean 75.4 mm Hg vs 75.4 mm Hg, respectively, p=0.41). Brabarupan et al 12 in the UK also showed that SA (n=39) compared with WE (n=565) and Africans (n=38) had a lower systolic blood pressure (median 120 mm Hg vs 130 mm Hg vs 135 mm Hg, respectively, p<0.05) and a lower diastolic blood pressure (median 73 mm Hg vs 75 mm Hg vs 80 mm Hg, respectively, p<0.05). We have previously noted that there was no significant difference in systolic blood pressure between SA and WE (median 121 mm Hg vs 125 mm Hg, respectively, and 130 mm Hg vs 131.5 mm Hg, respectively, in two different centres) in a UK population.9 However, we reported that SA (n=59) had a higher diastolic blood pressure than WE (n=118) (median 86 mm Hg vs 82 mm Hg, respectively, p<0.05).9 Lastly, Omar et al 17 in South Africa showed absence of difference between SA (n=41) and Africans (n=92) in the prevalence of hypertension (5% vs 4%, respectively). The analyses in these studies were not adjusted.
In summary, studies suggest SA have lower systolic blood pressure compared with WE and Africans, but there is no difference in the diastolic blood pressure across these three ethnic groups.
Lipid profile
Five studies examined differences in lipid profiles: two comparing SA to WE only, one comparing to WE and Africans, one comparing to Africans only and one comparing to Malay and Chinese. A UK study has previously shown that SA (n=80) have lower levels of HDL (median 1.3 mmol/L vs 1.4 mmol/L, respectively, p<0.05) and higher cholesterol/HDL ratio (median 3.6 vs 3.2, respectively, p<0.05) than WE (n=160).9 There were no statistically significant differences in the levels of total cholesterol in SA compared with WE (median 4.7 mmol/L vs 4.6 mmol/L, respectively, and 4.45 mmol/L vs 4.1 mmol/L, respectively). Another UK study12 also showed that SA (n=39) had lower levels of HDL compared with WE (n=565) but higher levels than Africans (n=38) (median 1.30 mmol/L vs 1.49 mmol/L vs 1.25 mmol/L, respectively, p<0.05). They also demonstrate absence of difference in total cholesterol levels between SA, WE and Africans (median 4.00 mmol/L vs 4.50 mmol/L vs 4.40 mmol/L, respectively) and triglyceride levels (median 1.07 mmol/L vs 0.93 mmol/L vs 0.99 mmol/L, respectively). Mehta et al 11 in the UK also show similar levels of total cholesterol in SA (n=163) (mean value 4.6 mmol/L) compared with WE (n=1169) (mean value 4.8 mmol/L) (p=0.132).
Ismail et al 14 in Malaysia demonstrate that SA (n=76) compared with Malay (n=102) and Chinese (91) had no statistically significant differences in total cholesterol levels (mean 5.74 mmol/L vs 5.58 mmol/L vs 5.64 mmol/L, respectively) and LDL cholesterol levels (mean 3.89 mmol/L vs 3.48 mmol/L vs 3.52 mmol/L, respectively). SA had significantly lower HDL cholesterol compared with Chinese (mean 1.28 mmol/L vs 1.57 mmol/L, respectively, p<0.01) and significantly higher triglyceride levels (mean 1.02 mmol/L vs 0.82 mmol/L, respectively, p<0.03). Lastly, Asmal et al 16 in South Africa found that SA (n=38) compared with Africans (n=52) had no statistically significant differences in cholesterol levels (mean 5.17 mmol/L vs 4.78 mmol/L, respectively) and triglyceride levels (2.81 mmol/L vs 2.27 mmol/L, respectively).
In summary, SA have lower HDL levels compared with WE and Chinese but higher than Africans. SA have higher triglyceride levels compared with Chinese. There are no differences in total cholesterol between SA and WE, African, Malay or Chinese ethnic groups.
Microvascular disease
Retinopathy
Four studies examined retinopathy; one comparing SA with WE only, two comparing to WE and Africans and one comparing to Africans only. The most relevant study by Sivaprasad et al 18 investigated retinopathy in T1DM in the UK cohort consisting of 2626 WE, 344 Africans and 120 SA. The mean age in this study was 39.4±16.3 years. The study found no statistically significant differences between SA, WE and Africans with T1DM in the age-standardised prevalence of maculopathy (95% CI) (16.6% (10% to 23.2%) vs 14.1% (12.8% to 15.4%) vs 13.1% (9.4% to 16.8%), respectively), clinically significant macular oedema (11.2% (5.4% to 16.9%) vs 6.5% (5.6% to 7.4%) vs 10.0% (6.7% to 13.3%), respectively), sight threatening diabetic retinopathy (17.5% (10.6% to 24.3%) vs 12.1% (10.9% to 13.3%) vs 15.9% (11.8% to 20.0%), respectively) and any diabetic retinopathy (54.0% (44.8% to 63.2%) vs 55.0% (53.2% to 56.9%) vs 42.8% (37.3% to 48.3%), respectively).
Thomas et al,15 in South Africa, reported that SA (n=118) were at increased risk of any diabetic retinopathy (OR 2.02, 95% CI 1.23 to 3.29) when compared with WE (n=1247), after adjustment for age at diagnosis, sex, duration of diabetes, HbA1c, hypertension and smoking status. Mehta et al 11 in the UK showed that SA (n=163) compared with WE (n=1169) had decreased prevalence of retinopathy (38.7% vs 48.0%, respectively, p=0.025). Lastly, Omar et al,17 a South African study, compared SA (n=41) to Africans (n=92) and were unable to demonstrate a statistically significant difference in the prevalence of retinopathy (22% vs 14%, respectively).
In summary, there is no difference in the prevalence of retinopathy between SA, WE and African ethnic groups.
Nephropathy
Five studies explored nephropathy and renal function as an outcome in SA with T1DM: two papers comparing to WE only, one comparing to WE and Africans and two papers comparing to Africans only. The largest study, by Mehta et al 11 in the UK did not show any differences between SA (n=163) and WE (n=1169) in the prevalence of nephropathy (13.5% vs 10.1%, respectively, p=0.184).
In another UK study, no statistically significant differences were found between SA (n=80) and WE (n=160) in creatinine levels (median 76 µmol/L vs 78 µmol/L, respectively), albumin/creatinine ratio (median 2.4 mg/mmol vs 2.5 mg/mmol, respectively) and eGFR (median 97.3 mL/min/1.732 vs 91.2 mL/min/1.732, respectively).9 Brabarupan et al 12 in the UK showed no difference in the prevalence of microalbuminuria between SA (n=39) and WE (n=565) (median 1.2 mg/mmol vs 1.2 mg/mmol, respectively); however, Africans (n=38) had significantly higher levels (median 3.7 mg/mmol) (p<0.05). There were two studies in South Africa comparing SA to Africans. The first by Omar et al 17 showed in their cohort of SA (n=41) and Africans (n=92), there was absence of difference in the prevalence of nephropathy (7% vs 3%, respectively). Asmal et al 16 also showed no statistically relevant difference between SA (n=38) and Africans (n=52) in creatinine levels (mean 68.90 µmol/L vs 79.40 µmol/L, respectively).
In summary, there is no difference in the prevalence of nephropathy or difference in renal function between SA and WE. However, in one study, SA had lower levels of microalbuminuria compared with Africans.
Neuropathy
Three studies included neuropathy as an outcome in SA: one comparing to WE only and two comparing to Africans only. The most relevant study, Mehta et al 11 in the UK, showed that SA (n=163) compared with WE (n=1169) have a lower prevalence of neuropathy (14.7% vs 27.8%, respectively, p<0.001). Omar et al 17 compared SA (n=41) to Africans (n=92) in South Africa demonstrating no statistically significant differences in the prevalence of peripheral neuropathy (32% vs 22%, respectively) and autonomic neuropathy (5% vs 4%, respectively). Asmal et al 16 in South Africa showed increased prevalence of neuropathy in SA (n=38) compared with Africans (n=52) (19% vs 4%, respectively); however, no statistical tests were performed.
In summary, SA have lower prevalence of neuropathy that WE. There is no difference noted in the prevalence of neuropathy between SA and Africans.
Macrovascular disease
Two studies reported cardiovascular outcomes: one comparing to WE only and the other comparing to Africans only. The largest of these studies, by Mehta et al 11 in the UK, did not show evidence of difference between SA (n=163) and WE (n=1169) with T1DM in prevalence of cardiovascular disease (15.3% vs 11.3%, respectively, p=0.133). Subanalysis also did not reveal a difference between SA and WE in ischaemic heart disease (12.3% vs 8.3%, respectively, p=0.093), peripheral vascular disease (1.8% vs 2.7%, respectively, p=0.79) and cerebrovascular disease (3.7% vs 1.8%, respectively, p=0.13). It is important to note that the mean age in the T1DM group was lower (mean age of SA 41.9 years and WE 45.3 years) compared with T2DM (mean age 59.2 years SA and 66.2 years WE) which may have led to an under-representation of cardiovascular outcomes in the T1DM group.
A second study compared peripheral arterial disease between SA and Africans in South Africa. Omar et al 17 showed that none of their participants in either the SA (n=41) or African group (n=92) had peripheral vascular disease or ischaemic heart disease. This may also be due to their younger cohort of patients and small sample size.
In summary, the prevalence of cardiovascular disease between the SA, WE and African populations do not differ.
Mortality
Only one study examined the association of SA ethnicity on mortality in people with T1DM. Swerdlow et al 19 in a UK study investigated mortality of SA patients compared with the non-SA population, approximately 97% of which were Caucasian. The patients were followed for up to 28 years. In their cohort of 424 SA patients there were 27 deaths (6.4%) and in 23 326 non-SA there were 1293 deaths (5.5%). Mortality in SA and non-SA with T1DM was calculated independently by comparing with the general population mortality using standardised mortality ratios (SMRs). Compared with the reference population, the SMR for SA patients were 3.9 (95% CI 2.0 to 6.9) in men and 10.1 (6.6 to 16.6) in women. The SMR for the corresponding non-SA were 2.7 (2.6 to 2.9) in men and 4.0 (3.6 to 4.3) in women. No details are provided as to the age of death in these patients. The most common causes of death in SA patients were cardiovascular disease (29.6%) and renal disease (14.8%). The ‘other’ causes of death accounted for eight deaths (29.6%) and included septicaemia, systemic lupus erythematosus, bronchopneumonia, unspecified urinary tract infection and congenital malformation. The most common causes of death in non-SA were cardiovascular disease (n=474, 36.7%) and diabetes and hypoglycaemia (n=239, 18.5%). There was 1 death due to neoplasm in SA (3.7%) and 89 in non-SA (6.9%).
In summary, mortality is higher in SA with T1DM than non-SA when compared with the reference population in the UK. SA females were in particular affected, with an SMR that was over twice that of the non-SA female T1DM population. The the most common cause of death was cardiovascular disease.
Discussion
This is the first systematic review to examine the differences in comorbidities, microvascular complications, macrovascular complications and mortality between SA and other ethnic groups with T1DM. In summary (see table 2), mortality is higher in SA with T1DM when compared with a largely WE reference population. Female SA were in particular affected, with a SMR that was over twice that of the non-SA female T1DM population. The the most common cause of death is cardiovascular disease.
Overall, the studies suggest that cardiovascular disease itself is no more common in SA T1DM compared with WE. The study by Mehta et al 11 that examined cardiovascular disease most clearly, studied a population with a mean age in their early 40s, and is likely to be too young for cardiovascular disease to manifest clinically. While they observed a 50% higher risk of ischaemic heart disease (12.3% vs 8.3%) and twice the risk of cerebrovascular disease (1.8% vs 3.7%) in SA compared with WE, the study had less than 30% power to detect a statistically significant difference. Some risk factors for cardiovascular disease appear greater in SA, with lower HDL than WE and the Chinese and higher HbA1c. However, the most powerful risk factor for cardiovascular disease of systolic BP is lower than in WE.
Most studies also suggest SA have higher HbA1c levels than WE,20 Malay and Chinese but lower than African ethnic groups. Despite this, rates of retinal and nephropathic microvascular disease were the same as the WE population and some (neuropathy) even lower. There is an issue around competing risk however, as SA with T1DM may die at a younger age before developing retinopathy.
Compared with Africans, SA had lower levels of microalbuminuria, lower HbA1c, lower systolic blood pressure and higher HDL levels. There were no statistically significant differences between these two ethnic groups in the remaining complications: cardiovascular disease, retinopathy and neuropathy. There was also no difference in BMI.
Weaknesses
There are several weaknesses with the analysis. The quality of the studies was poor with most studies being retrospective observational or cross-sectional. It was not possible to undertake a meta-analysis of the combined studies because the results were heterogeneous in nature.
The studies included in the analysis are derived from a large range of years (1981 until 2015), a time period during which diabetes treatment and prevention of complications has changed dramatically. Ideally, the analysis should specifically consider studies which compared the different ethnic groups during the same period of observation with similar standards of therapy to eliminate bias.
Furthermore, we accepted a clinical diagnosis for T1DM in the included studies. Some studies simply relied on coding of T1DM in their clinical systems as inclusion criteria with other studies accepting a younger age of diagnosis (<30/35 years of age) and insulin dependency as their inclusion criteria. As we did not have a standardised criterion for the diagnosis of T1DM for the included studies, it may well be that some patients with juvenile-onset T2DM requiring insulin treatment may have been wrongly coded as having T1DM.
Moreover, the papers in our review did not include data on medication use which makes it unclear whether differences in blood pressure, hbA1c and lipid profiles were primarily due to ethnicity or because of differences in medication use.
Lastly, data from patients with SA ethnicity living in the UK and abroad were pooled. Prevalence of T2DM is higher in migrant SA compared with native SA thought to be secondary to urbanisation and lifestyle.21 It is likely that prevalence and complication rates of T1DM would also be different in migrant and native SA and therefore grouping them together may cause inaccuracy of reporting of the results.
Strengths
The strengths of this analysis are its comprehensive search strategy with clearly defined population and outcomes. Our search strategy incorporated both full-length papers as well as abstracts, included all languages and had a secondary search strategy to ensure we did not miss any relevant papers. We compared the SA group, the largest ethnic group globally with all other indigenous ethnic groups.
Implications
Our analysis highlights two areas. First, the ethnic disparity in mortality that has previously been described in T2DM22 is also present for SA patients with T1DM. This disparity is most likely due to cardiovascular disease but this association remains to be proven. Given the close association between glycaemic control with cardiovascular disease and excess mortality in T1D,23 and the higher HbA1c in the SA population, the findings of this systematic review call for more aggressive glycaemic control in the SA T1D population. Previous literature has demonstrated how SA have increased adiposity in comparison with WE and have advocated lower cut-offs for BMI in SA; BMI >23 kg/m2 as overweight and BMI >25 kg/m2 as obese.1 24 These culturally tailored programmes that have been attempted for T2DM may also be required for T1DM.25
In addition, we may require more stringent control of other comorbidities such as hyperlipidaemia and hypertension,26 though this needs to be formally addressed. Second, we highlight a need for a large, ideally prospective, multinational study exploring the effect of ethnicity in a uniform healthcare setting. This will enable consistent methodology, and standardised reporting of comorbidities and complications such as those mentioned previously, but also complications such as peripheral vascular disease, depression and bone fractures that have not previously been addressed.
Supplementary Material
Acknowledgments
KK acknowledges support from the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East Midlands and the NIHR Leicester-Loughborough Biomedical Research Unit.
Footnotes
Contributors: KNS: involvement in the design of the work, data collection, data analysis, writing the paper, drafting and revision of the paper. PC:
involvement in the design of the work, data collection, data analysis, helped with drafting and revision of the paper. PS:
involvement in the design of the work, reviewed all drafts of the paper, helped with revision of the paper. KK:
involvement in the design of the work, reviewed all drafts of the paper, helped with revision of the paper. KN:
responsible for the conception and design of the work, reviewed all drafts of the paper, helped with revision of the paper. PN:
responsible for the conception and design of the work, reviewed all drafts of the paper, helped with revision of the paper.
Competing interests: KK (co-chair), PS and PN are the members of the South Asian Health Foundation Working group on diabetes.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1. Misra A, Khurana L. Obesity-related non-communicable diseases: south Asians vs White Caucasians. Int J Obes 2011;35:167–87. 10.1038/ijo.2010.135 [DOI] [PubMed] [Google Scholar]
- 2. Gholap N, Davies M, Patel K, et al. Type 2 diabetes and cardiovascular disease in South Asians. Prim Care Diabetes 2011;5:45–56. 10.1016/j.pcd.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 3. Department of Health. National Service Framework for Diabetes: standards, 2001. [Google Scholar]
- 4. Tillin T, Hughes AD, Mayet J, et al. The Relationship Between Metabolic Risk Factors and Incident Cardiovascular Disease in Europeans, South Asians, and African Caribbeans. Journal of the American College of Cardiology 2013;61:1777–86. 10.1016/j.jacc.2012.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willi SM, Miller KM, DiMeglio LA, et al. Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics 2015;135:424–34. 10.1542/peds.2014-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borschuk AP, Everhart RS. Health disparities among youth with type 1 diabetes: A systematic review of the current literature. Fam Syst Health 2015;33:297–313. 10.1037/fsh0000134 [DOI] [PubMed] [Google Scholar]
- 7. Office for National Statistics. Ethnicity and national identity in England and Wales. 2011, 2012. [Google Scholar]
- 8. Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Ottawa Hospital Research Institute.
- 9. Sarwar K, Gillani S, Singh B, et al. A comparison of diabetes type 1 in south asian and caucasian patients in the UK: the type 1 in Minority ethnic populations (TIME) study. European Association for the study of Diabetes. Abstract 2015. [Google Scholar]
- 10. Shenoy S, Waldron S, Cody D, et al. Ethnic group differences in overweight and obese children with type 1 diabetes mellitus. Arch Dis Child 2004;89:1076–7. [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta RL, Davies MJ, Ali S, et al. Association of cardiac and non-cardiac chronic disease comorbidity on glycaemic control in a multi-ethnic population with type 1 and type 2 diabetes. Postgrad Med J 2011;87:763–8. 10.1136/postgradmedj-2011-130298 [DOI] [PubMed] [Google Scholar]
- 12. Brabarupan T, Misra S, Oliver N. A comparison of type 1 diabetes phenotype in a young multi-ethnic urban population. European Association for the study of Diabetes. Abstract 2013. [Google Scholar]
- 13. Omar MA, Asmal AC. Patterns of diabetes mellitus in young africans and Indians in Natal. Trop Geogr Med 1984;36:133–8. [PubMed] [Google Scholar]
- 14. Ismail IS, Nazaimoon W, Mohamad W, et al. Ethnicity and glycaemic control are Major determinants of diabetic dyslipidaemia in Malaysia. Diabet Med 2001;18:501–8. [DOI] [PubMed] [Google Scholar]
- 15. Thomas RL, Distiller L, Luzio SD, et al. Ethnic differences in the prevalence of diabetic retinopathy in persons with diabetes when first presenting at a diabetes clinic in South Africa. Diabetes Care 2013;36:336–41. 10.2337/dc12-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asmal AC, Jialal I, Leary WP, et al. Insulin-dependent diabetes mellitus with early onset in blacks and Indians. S Afr Med J 1981;60:91–3. [PubMed] [Google Scholar]
- 17. Omar MA, Asmal AC. Complications of early-onset insulin-dependent diabetes mellitus in Blacks and Indians. S Afr Med J 1984;65:75–8. [PubMed] [Google Scholar]
- 18. Sivaprasad S, Gupta B, Gulliford MC, et al. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdom (DRIVE UK). PLoS One 2012;7:e32182 10.1371/journal.pone.0032182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swerdlow AJ, Laing SP, Dos Santos Silva I, Silva dosS I, et al. Mortality of south asian patients with insulin-treated diabetes mellitus in the United Kingdom: a cohort study. Diabet Med 2004;21:845–51. 10.1111/j.1464-5491.2004.01253.x [DOI] [PubMed] [Google Scholar]
- 20. Mostafa SA, Davies MJ, Webb DR, et al. Independent effect of ethnicity on glycemia in South Asians and white Europeans. Diabetes Care 2012;35:1746–8. 10.2337/dc11-2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–82. 10.1056/NEJMoa1408214 [DOI] [PubMed] [Google Scholar]
- 22. Bellary S, O'Hare JP, Raymond NT, et al. Premature cardiovascular events and mortality in south Asians with type 2 diabetes in the United Kingdom Asian Diabetes Study - effect of ethnicity on risk. Curr Med Res Opin 2010;26:1873–9. 10.1185/03007995.2010.490468 [DOI] [PubMed] [Google Scholar]
- 23. Bellary S, O'Hare JP, Raymond NT, et al. Enhanced diabetes care to patients of South Asian Ethnic origin (the United Kingdom Asian Diabetes Study : a cluster randomised Controlled trial. Lancet 2008;371:1769–76. 10.1016/S0140-6736(08)60764-3 [DOI] [PubMed] [Google Scholar]
- 24. Bodicoat DH, Gray LJ, Henson J, et al. Body mass index and waist circumference cut-points in multi-ethnic populations from the UK and India: the ADDITION-Leicester, Jaipur heart watch and New Delhi cross-sectional studies. PLoS One 2014;9::9 10.1371/journal.pone.0090813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah A, Kanaya AM. Diabetes and associated complications in the south asian population. Curr Cardiol Rep 2014;16:476 10.1007/s11886-014-0476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2â diabetes: ukpds 38. BMJ 1998;317 703-713. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015005supp001.pdf (304.2KB, pdf)
bmjopen-2016-015005supp002.pdf (410.7KB, pdf)