Abstract
Introduction
Low back pain (LBP) and knee osteoarthritis (OA) are highly prevalent and disabling conditions that cause societal and economic impact worldwide. Two randomised controlled trials (RCTs) will evaluate the effectiveness of a multicomponent lifestyle intervention for patients with LBP and knee OA who are overweight or obese. The key targets of this intervention are to improve physical activity, modify diet and correct pain beliefs. These factors may explain how a lifestyle intervention exerts its effects on key patient-relevant outcomes: pain, disability and quality of life. The aim of this protocol is to describe a planned analysis of a mechanism evaluation for a lifestyle intervention for overweight or obese patients with LBP and knee OA.
Methods and analysis
Causal mediation analyses of 2 two-armed RCTs. Both trials are part of a cohort-multiple RCT, embedded in routine health service delivery. In each respective trial, 160 patients with LBP and 120 patients with knee OA waiting for orthopaedic consultation will be randomised to a lifestyle intervention, or to remain part of the original cohort. The intervention consists of education and advice about the benefits of weight loss and physical activity, and the Australian New South Wales Get Healthy Service. All outcome measures including patient characteristics, primary and alternative mediators, outcomes, and potential confounders will be measured at baseline (T0). The primary mediator, weight, will be measured at 6 months post randomisation; alternative mediators including diet, physical activity and pain beliefs will be measured at 6 weeks post randomisation. All outcomes (pain, disability and quality of life) will be measured at 6 months post randomisation. Data will be analysed using causal mediation analysis with sensitivity analyses for sequential ignorability. All mediation models were specified a priori before completing data collection and without prior knowledge about the effectiveness of the intervention.
Ethics and dissemination
The study is approved by the Hunter New England Health Human Research Ethics Committee (13/12/11/5.18) and the University of Newcastle Human Research Ethics Committee (H-2015–0043). The results will be disseminated in peer-reviewed journals and at scientific conferences.
Trial registration number
ACTRN12615000490572 and ACTRN12615000478516; Pre-results.
Keywords: Back pain, Osteoarthritis, Lifestyle, Mechanism evaluation, Mediation Analysis
Strengths and limitations of this study.
Understanding the underlying causal mechanisms of a lifestyle intervention will explain how the intervention works, or why the intervention failed. These findings will have important clinical and policy implications and could guide implementation strategies.
We propose to use contemporary methods for causal mediation analysis with sensitivity analyses to evaluate the robustness of the estimated mediation effects to violation of sequential ignorability—a critical assumption required for causal inference in mechanism evaluations.
The primary mediator (weight) and the outcomes will be captured at the same time point. Thus, it will be challenging to attest the possibility of reverse causation of the mediator–outcome effect.
Putative mediators including diet and physical activity are measured using self-reported questionnaires.
Background
Low back pain (LBP) and knee osteoarthritis (OA) are highly prevalent1 2 and disabling musculoskeletal conditions3 4 that cause societal5–7 and economic8 9 impact worldwide. The lifetime prevalence of LBP is 84%,2 and 40%–47% for knee OA.10 Of all health conditions, LBP is ranked first and OA ranked 11th as contributors to global disability.4 11 Direct costs for the management of LBP are estimated at $A4.7 billion in Australia (2012),7 £2.8 billion in the UK (2013)12 and US$90 billion in the USA (1998)8; and the cost of OA accounts for up to 2.5% of the gross national product in Australia, UK and USA.9
A range of risk factors contribute to the development and persistence of LBP and OA. A large proportion of patients with LBP or OA are physically inactive,13 14 have poor diet14 15 and are overweight or obese.16–19 Targeting factors such as diet and physical activity as part of routine management is a plausible strategy to improve outcomes for these patients.20–22 Two randomised controlled trials (RCTs) will test the effectiveness of a multicomponent lifestyle intervention for patients with LBP23 and knee OA24 who are overweight or obese. However, merely evaluating the effectiveness of these interventions is insufficient25; it is important to understand the underlying causal mechanisms that explain how the intervention works, or why the intervention doesn't work.26 27
Explaining underlying mechanisms
Complex interventions for patients with LBP and knee OA are usually evaluated by their effects on patient-relevant outcomes such as pain, disability and quality of life (QoL).23 24 26 28 29 However, pragmatic interventions such as a lifestyle intervention do not directly target patient-related outcomes; they target intermediate factors (often called mediators), such as diet or physical activity, that are then hypothesised to have a causal effect on patient-relevant outcome(s).26 Therefore, merely evaluating the effect of the intervention leaves a black-box that conceals the underlying mechanism(s) of the intervention. The aim of a mechanism evaluation is to unpack the black box by decomposing the entire intervention effect into indirect and direct effects. The indirect effect is the effect of the intervention on an outcome that is carried through a selected mediator, and the direct effect is the remaining effect of the intervention that is not explained via the selected mediator. For example, the entire effect of the lifestyle intervention on QoL could be decomposed into an effect carried through changes in diet (indirect effect) and remaining unexplained mechanisms (direct effect).
One way of quantifying causal mechanisms is by conducting causal mediation analysis.25 27 This approach can produce important information about the underlying mechanisms of an intervention. If the intervention is effective, causal mediation analysis informs whether the hypothesised mechanisms actually occurred.27 Conversely, if the intervention is ineffective, causal mediation analysis can identify where the hypothesised mechanism breaks down.27 By using this information, interventions can be refined on the basis of empirical evidence about the underlying mechanism.26 30 Elements of the intervention that aim to target proposed mediators that do not affect the outcome can be eliminated; and elements that influence a mediator that actually affects outcome can be retained and optimised.
Mechanisms of a lifestyle intervention
Causal mechanisms of lifestyle interventions are unknown. However, there is evidence suggesting that weight loss, inactivity and poor diet are important risk factors that should be considered treatment targets for patients with LBP and OA (ie, mediators). For knee OA, being overweight or obese is a modifiable risk factor.18 19 31 32 Further, meta-analyses show that weight loss interventions result in moderate improvements in pain and function for overweight or obese patients with knee OA.33 Similarly for LBP, meta-analyses show significant associations between overweight or obesity and a number of LBP outcomes.16 34 This suggests that weight might be an appropriate treatment target for both of these conditions to improve patient-related outcomes. It is also apparent that physical activity and diet may play a role in this mechanism for both conditions because of their effects on weight.14 35–37 Inaccurate beliefs about pain are also associated with poor LBP and OA outcomes.38 39 Despite evidence for the relationship between weight, physical activity, and pain beliefs and patient-relevant outcomes, these risk factors have not been tested as underlying mechanisms of lifestyle interventions for patients with LBP and knee OA.
To test these underlying mechanisms, we have embedded a priori mechanism evaluations into two RCTs that will test the effectiveness of a lifestyle intervention for patients with LBP23 and knee OA24 who are overweight or obese. Our primary hypothesis is that in patients with either LBP or knee OA who are overweight or obese, a lifestyle intervention will have a causal effect on outcomes (pain, disability and QoL) via a primary mechanism through weight. Our secondary hypothesis is that the causal effect of a lifestyle intervention will also be explained via alternative mechanisms including changes in diet, physical activity and pain beliefs.
Objectives
The objective of this study is to test the underling mechanisms of a lifestyle intervention for patients with LBP or OA who are obese or overweight. The specific objectives of this study vary according to whether the lifestyle intervention is effective (unknown at the time of writing this protocol):
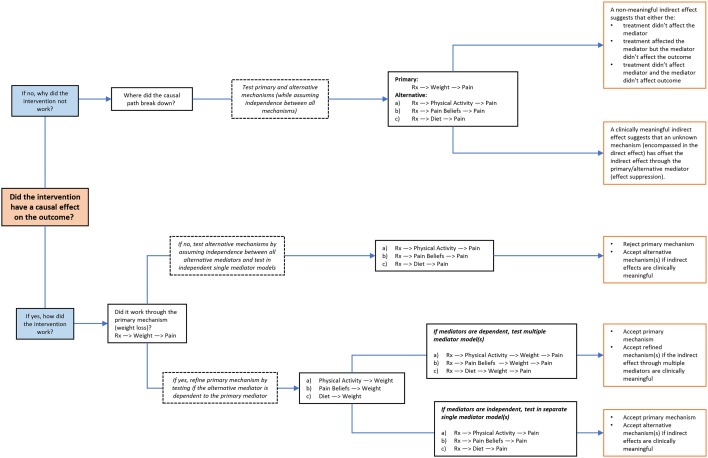
If the intervention is effective, our primary objective is to estimate the extent to which weight mediates this effect. Our secondary objective will be to further refine this mechanism via three serial multiple mediator paths (changes in diet, physical activity and pain beliefs) that then cause changes in weight.
If the intervention is ineffective, our primary objective is to determine where the causal path breaks down. All potential mediators (weight, diet, physical activity and pain beliefs) will be tested independently.
Method
Design
We will conduct a combined causal mediation analysis of 2 two-armed RCTs.23 24 Both trials are part of a cohort multiple RCT,40 embedded in routine health service delivery. In both trials, participants are recruited from an existing cohort of patients waiting for orthopaedic consultation; then randomised to receive a lifestyle intervention (intervention group), or to receive usual care by remaining in the original cohort (control group). The key differences between Williams et al 23 and O’Brien et al 24 are the clinical populations (LBP23 and knee OA),24 and the additional physiotherapy consultations exclusively delivered in the LBP trial.23 Thus, it is plausible that the two different clinical populations may respond differentially to their respective interventions. To accommodate this hypothesis, we will use moderated causal mediation analysis to estimate trial-specific effects, and averaged effects across both trials. If trial assignment (LBP trial vs OA trial) is a significant moderator, we will interpret trial-specific mediation effects in separation; however, if trial assignment is not a significant moderator, we will interpret the averaged mediation effects across both trials.
The trials began recruiting on 11 May 2015 and we expect to close the trial by June 2017. Data collection is still ongoing and all investigators were blind to group allocation at the time of planning and writing this study protocol. Further details of each trial have been outlined by Williams et al 23 (ACTRN12615000478516) and O’Brien et al 24 (ACTRN12615000490572).
Participants and recruitment
One RCT involves 120 patients with OA of the knee,24 and the other, 160 patients with non-specific LBP.23 Patients in both RCTs are those waiting for outpatient orthopaedic consultation at a tertiary referral public hospital in New South Wales (NSW), Australia.
Randomisation
In both trials, eligible patients from the cohort are randomised to an intervention or control group (1:1 ratio). The randomisation schedule was a priori generated by an independent statistician using the SURVEYSELECT procedure (SAS V.9.3). Allocation is concealed and all outcome assessors, patients and investigators are blind to group allocation. Patients are blind to group allocation by nature of the cohort multiple design. This design offers the intervention and control as part of a routine clinical service, where patients consent to routine data collection. Patients randomised to the intervention group are not aware of the offer of the control arm. Likewise, patients randomised to the control group are not aware of the offer of the intervention arm. Thus, patients are not able to discriminate whether the intervention or control are being offered as part of a clinical trial. This reduces the risk of performance bias (how well the participants engage with the intervention). Service providers delivering the intervention are blind to treatment status as they are not aware that patients were being referred from a clinical trial. The outcome assessors do not have access to the randomisation schedule, thus blind to group allocation. This reduces the risk of detection bias (differential outcome measurement between groups).
Intervention groups
Participants in both RCTs23 24 will receive advice and education about the benefits of weight loss and physical activity for their conditions by trained interviewers. Participants are then referred to the NSW Get Healthy Information and Coaching Service (GHS; www.gethealthynsw.com.au).41 The GHS is a free, population-wide, telephone-based health coaching service provided by the NSW Government to support adults in NSW to make sustained healthy lifestyle improvements including diet, physical activity and achieving or maintaining a healthy weight. This service consists of 10 individually tailored coaching calls delivered by university-qualified health coaches, including dieticians, exercise physiologists and psychologists, over a 26-week period. All coaches undergo standardised training before delivering the GHS, thus reducing the potential for differential between coach effects. Coaching is provided on a tapered schedule. Six calls are made in the first 12 weeks to guide, monitor and improve uptake; and four calls are dispersed over the remaining 12 weeks to maintain adherence and avoid relapse.42 This tapered schedule will be kept consistent across all participants, reducing the potential for bias.
Participants with LBP23 will receive an additional clinical consultation with the study physiotherapist before beginning the NSW GHS programme. The consultation aims to correct erroneous pain beliefs, highlight the consequences of unhealthy lifestyle factors, and to provide general encouragement and examples of how improving lifestyle factors can influence pain outcomes and QoL. The consultation also involves behaviour change techniques, informed by self-determination theory43 44 that aims to develop autonomous motivation by increasing perceived competence and self-regulation.44
Control groups
Participants allocated to the control group will remain in usual care. The health service does not provide any active management for patients with knee OA or LBP during the orthopaedic consultation waiting period.
Assessment time points
Patient characteristics, outcome measures, primary and alternative mediators, and potential confounders are measured at baseline (T0) prior to randomisation. The primary putative mediator (weight) will be measured 6 months after randomisation. All putative alternative mediators (diet, physical activity and pain beliefs) will be measured 6 weeks after randomisation. Outcomes will be measured 6 months after randomisation. The intervention and assessment time points are outlined in table 1.
Table 1.
Timing of intervention, mediator and outcome assessments
| Week | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 |
| Intervention | Initial consult* Six GHS calls |
Four GHS calls | ||||||||||||
| Primary mediator | ||||||||||||||
| Alternative mediators | ||||||||||||||
| Outcomes | ||||||||||||||
Primary mediator: weight. Alternative mediators: diet, physical activity and pain beliefs. Outcomes: pain, disability and quality of life.
*Patients with low back pain only.
GHS, New South Wales Get Healthy Service.
Primary outcome measures
Average pain intensity over 7 days will be measured using an 11-point pain Numeric Rating Scale (NRS; 0=no pain, 10=pain as bad as it could be).45 We will measure self-perceived disability using the 24-item Roland-Morris Disability Questionnaire in patients with LBP46; and the Western Ontario and McMaster Universities Osteoarthritis Index47 in patients with knee OA. We will measure QoL using the Short Form Health Survey V.2.48
Putative mediators
The primary mediator, weight, will be measured to the nearest 0.1 kg by a trained research assistant using the International Society for the Advancement of Kinanthropometry procedures.49 Physical activity will be measured using the Active Australia Survey,50 which has moderate reliability (Cohen’s kappa=0.52)51 and good face and criterion validity.52 Dietary intake will be measured using a Short Food Frequency Questionnaire,53 which has moderate reliability (weighted kappa range=0.37–0.85)54 55 and criterion validity.55 Pain-related attitudes and beliefs will be measured using the Survey of Pain Attitudes Questionnaire.56 All putative mediators are measured in both control and intervention groups in both trials. These mediators are measured using self-reported questionnaires with known limitations.57
Potential confounders
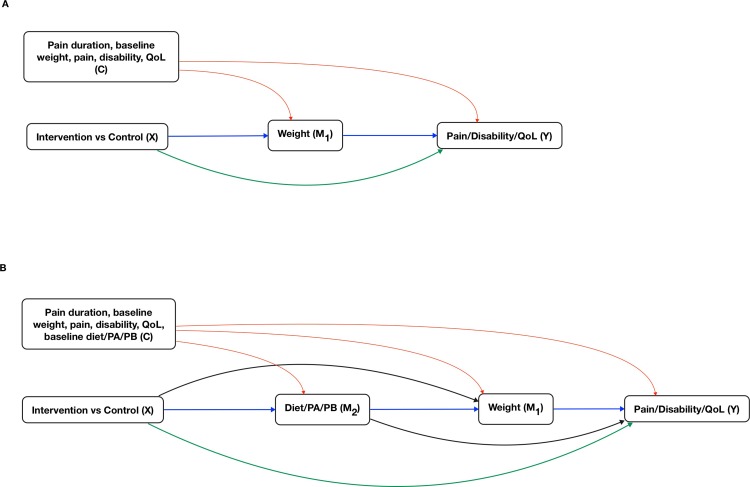
We will control for the following pretreatment confounders: pain duration, baseline pain, disability and QoL. These variables were selected on the basis of their theorised causal relationships with the mediator and outcome variables. We will include baseline measures of the mediators and outcomes in the regression models as covariates.58 Directed acyclic graphs specific to each model are presented in figure 1.
Figure 1.
Directed acyclic graphs. Blue lines represent indirect effects (mechanisms) of interest. Green lines represent direct effects (direct effect of treatment on outcome plus all unspecified indirect effects). Red lines represent possible effects that could induce confounding for indirect and direct effects. (A) A single mediator model where the intervention (X) exerts its effect on the outcome(s) (Y), via an indirect path through the primary mediator (M1), and via a direct path (X to Y). (B) A serial multiple mediator model where the intervention (X) exerts its effect on the outcome (Y), via an indirect path through two mediators—alternative mediator (M2) and primary mediator (M1), and via a direct path (X to Y). This model allows for the potential causal relationship from M2 to M1. PA, physical activity; PB, pain beliefs; QoL, quality of life.
Causal mediation analysis
We plan to construct single and multiple mediator models based on current recommendations for causal mediation analysis.59–61 The details of each model are illustrated in figure 1 and table 2; and the overall analysis plan is outlined in figure 2.
Table 2.
Overview of all mediation models
| Model | Treatment (X) | Alternative mediator (M2) at 6 weeks | Primary mediator (M1) at 6 months | Outcome (Y) at 6 months |
| If the total effect of the intervention on the selected outcome is significant: | ||||
| 1.0 | Rx | Weight | Pain/Disability/QoL | |
| If the indirect effect through weight is significant (from model 1.0): | ||||
| 1.1* | Rx | Diet | Weight | Pain/Disability/QoL |
| 1.2* | Rx | Physical activity | Weight | Pain/Disability/QoL |
| 1.3* | Rx | Pain beliefs | Weight | Pain/Disability/QoL |
| If the indirect effect through weight is not significant (from model 1.0): | ||||
| 1.4 | Rx | Diet | Pain/Disability/QoL | |
| 1.5 | Rx | Physical activity | Pain/Disability/QoL | |
| 1.6 | Rx | Pain beliefs | Pain/Disability/QoL | |
| If the total effect of the intervention on the selected outcome is not significant: | ||||
| 2.0 | Rx | Weight | Pain/Disability/QoL | |
| 2.1 | Rx | Diet | Pain/Disability/QoL | |
| 2.2 | Rx | Physical activity | Pain/Disability/QoL | |
| 2.3 | Rx | Pain beliefs | Pain/Disability/QoL | |
*Multiple mediator models will only be tested if there is a significant relationship between M1 and M2. If the relationship is non-significant, then the alternative mediators will be tested in separate single mediator models with the mediator measured at week 6. Significance levels are set a priori at p<0.05.
QoL, quality of life.
Figure 2.
Overall analysis plan. Note: ‘pain’ is interchangeable with disability and QoL. PA, physical activity; PB, pain beliefs; QoL, quality of life.
Justification for primary and alternative mechanisms
Our hypothesised mechanisms are based on theory and evidence. We selected weight at 6-month follow-up as our primary mediator because the key component of the lifestyle intervention was targeted to reduce weight, and because the target population was overweight or obese. Evidence suggests that weight might have direct causal effects on patient-related outcomes (pain, disability and QoL).15–17 62 The primary mechanism via weight will be tested in a single mediator model (figure 1A).
If we find that the intervention does exert its effect via the primary mechanism (weight), we plan to refine this mechanism to understand how the intervention led to changes in weight (that then affects outcome). Because the intervention includes aspects of lifestyle management (NSW GHS) that aimed to modify diet and increase physical activity, we hypothesise that the intervention will exert its effect on the primary mediator (weight) and outcomes via initial changes in diet and physical activity levels during treatment (captured at week 6). Preliminary evidence supports this hypothesised causal mechanism.63 Finally, we hypothesise that the intervention may also exert its effect through changes in pain beliefs.39 64 This is because initial consultations in the LBP trial23 aimed to reassure patients and reframe erroneous beliefs about pain. Although patients with OA did not receive a clinical consultation that directly targeted pain beliefs, the GHS may have inadvertently changed pain beliefs through the promotion of physical activity. The physical activity component could enable the patients to realise that pain does not need to be a barrier to keeping a physically active lifestyle. This theory is informed by Albert Bandura’s techniques of verbal persuasion, modelling and mastery.65 These refined mechanisms will be tested in serial multiple mediator models (figure 1B).
Sample size
Both trials are sufficiently powered (90%) to detect clinically meaningful between-group changes in pain (1.5-point reduction on NRS) and weight (6% reduction).23 24 To gain a general appreciation for the required sample size to detect an indirect effect through the primary mediator (weight), we used the sample size estimator for joint indirect effects developed by Vittinghoff and Neilands.66 With a two-sided alpha of 0.05, exposure-mediator error term correlation coefficient of 0, and mediator-outcome error term correlation coefficient of 0.2, a sample of 71 per group provides 80% power to detect a proportion mediated of 50%, with clinically meaningful treatment-mediator (r=0.5) and mediator-outcome (r=0.3) effects. The sample sizes for both trials were primarily estimated to detect the main effect of the intervention on pain and weight. Therefore, this post hoc power calculation provides indication that both trials would be powered to detect an indirect effect that consists of moderate treatment-mediator and mediator-outcome effects. Moderate effects would be considered clinically meaningful effects based on previous work.67 68 Sample size estimators for multiple mediator models are currently unavailable.69 O’Rourke and Mackinnon provide evidence that multiple mediator models have more power than single mediator models.70 Thus we expect this study to have sufficient power for multiple mediator models.
Methodological considerations
No-confounding assumption (sequential ignorability)
Estimating indirect effects that have causal meaning relies on satisfying the ‘no-confounding’ assumption, often termed ‘sequential ignorability.’60 It is critical that the treatment-mediator effect and the mediator-outcome effect are not confounded.25 In mediation analyses of standard RCTs, this assumption only holds for the treatment-mediator and treatment-outcome effects (due to randomisation). However, since the mediators cannot be randomised, this assumption does not hold for the mediator-outcome relationship.60 There may be unknown or unmeasured confounders that might induce a spurious relationship between the mediator and outcome. Recent advances in causal mediation analysis have developed sensitivity analysis techniques that can estimate the impact of violating this assumption, which we will employ in this study.71 These methods are an extension of the traditional methods (Baron and Kenny)72 and reflect contemporary advances in causal mediation analysis.61
Alternative mediator as a post-randomisation confounder in multiple mediator models
In mediation analyses, post-randomisation confounders are variables that are affected by the treatment that then simultaneously influence the mediator and outcome. The presence of a post-randomisation confounder effectively induces bias for indirect and direct effects.73 By construction of the multiple mediator model, an alternative mediator (M2) is a post-randomisation confounder for the primary mediator-outcome relationship (ie, the alternative mediator that is affected by the treatment might causally affect both the primary mediator and the outcome and induce a spurious relationship). For example, changes in diet caused by the treatment can subsequently have a causal effect on weight and QoL, thereby inducing a spurious relationship between weight and QoL. To overcome this problem, we will assess the dependence between the alternative mediators (diet, physical activity, pain beliefs) and the primary mediator (weight). If an alternative mediator and a primary mediator are significantly correlated, we will build serial multiple mediator models, as recommended by Imai et al.59 If the alternative and primary mediators are not related, then we will not treat the alternative mediator as a post-randomisation confounder, and test the alternative mediators in independent single mediator models.
Data analysis
Analyses will be performed in R (The R Foundation for Statistical Computing) using the mediation package.74
Single mediator models
A model-based inference approach will be used to estimate the average causal mediation effect (ACME), average direct effect (ADE) and the average total effect.74 First, we will fit two regression models: the mediator model and the outcome model. The mediator model is constructed with the treatment status as the independent variable and the mediator as the dependent variable. The outcome model is constructed with the treatment status and the mediator as independent variables, the outcome as the dependent variable, and the set of observed pretreatment confounders as covariates. Continuous mediators and outcomes that are normally distributed will be modelled using linear models (lm); but if skewed, they will be modelled using generalised linear models (glm) with appropriate family and link functions.75 The ordinal mediator (diet) will be modelled using the proportional odds logistic model (polr).74
Because it is plausible that the indirect and direct effect sizes might depend on treatment allocation (treated and non-treated), we will include a treatment-mediator interaction term in the outcome model. We will calculate two separate ACMEs that are conditional on treatment status (x=1 and x=0) and their marginal effects. We will interpret both conditional effects to generalise to their respective treatment group (treated and non-treated) and the marginal effect to generalise to the overall population. Not accounting for small non-significant interaction effects can dramatically influence the indirect and direct effect estimates.69
The mediates function will use the mediator and outcome models to estimate the potential values of the mediator and outcome. The simulated potential values of the mediator and the outcome will be used to compute the ACME, ADE and average total effects. We will use 1000 bootstrap stimulations to generate 95% CIs. We will interpret the unstandardised point estimate of ACME and its 95% CIs.
Trial assignment (OA trial vs LBP trial) could moderate indirect and direct effects. Therefore, we will test the moderating effect of trial assignment by using the test.modmed function. This function directly tests the difference in the ACME and ADE between two levels of the hypothesised moderator (OA trial vs LBP trial). If the ACME and ADE are statistically different, we will analyse the two trials separately to estimate the ACME and ADE that are specific to each trial. However, if they are not different, we will estimate an averaged ACME and ADE across both trials.
A sensitivity analysis will be conducted to determine the robustness of the ACME to the influence of violating the no-confounding assumption (sequential ignorability). The level of confounding due to unknown confounders is represented by the correlation between the residuals (error terms) from the mediator and outcome models, denoted ρ (rho). If ρ=0 (ie, no correlation between residuals), then this can be hypothetically interpreted as no unmeasured confounding. We will use the medsens function to explore how varying levels of ρ (between the extremes of −1 and +1) influence the ACME. The output will provide the values of ρ at which the CIs for the ACME include 0 (a non-significant ACME). That is, how strong the effect of unmeasured confounding would need to be to invalidate the estimated ACME.
Multiple mediator models
For multiple mediator models, we will use an expanded mathematical framework.59 Multiple mediator models will only be constructed if the alternative mediator (diet, physical activity and pain beliefs) and primary mediator (weight) are related.59 We will use the multimed function from the mediation package to estimate the ACME and ADE, and the sensitivity parameters. We will use 1000 bootstrap stimulations to generate 95% CIs.
Conclusion
We present an analysis plan for a mechanism evaluation of a lifestyle intervention for patients with knee OA and LBP who are overweight or obese. In the event that the intervention is effective, this investigation will provide evidence for hypothesised causal mechanisms through changes in weight, diet, physical activity and pain beliefs. If the intervention is ineffective it will provide explanations for why the intervention did not work. These results will help refine the intervention and guide implementation strategies.
Supplementary Material
Footnotes
Contributors: HL, JW, SK, AW, KMO, RKH, LW, SLY, EC, RH, EKR, JHM and CMW were responsible for the design of the study. CMW and JW procured funding. All authors contributed to developing the intervention and data collection protocols and materials, and reviewing, editing, and approving the final version of the paper. HL drafted the manuscript, and all authors subsequently contributed to the manuscript. All authors have read and approved the final manuscript.
Funding: This work is funded by Hunter New England Local Health District, and the Hunter Medical Research Institute. The project also received infrastructure support from the University of Newcastle.
Competing interests: None declared.
Ethics approval: Hunter New England Health Human Research Ethics Committee (13/12/11/5.18) and the University of Newcastle Human Research Ethics Committee (H-2015-0043)
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Croft P, Blyth F, van der Windt D. Chronic pain Epidemiology: from aetiology to Public Health. Oxford: Oxford University Press, 2010. [Google Scholar]
- 2. Balagué F, Mannion AF, Pellisé F, et al. . Non-specific low back pain. Lancet 2012;379:482–91. 10.1016/S0140-6736(11)60610-7 [DOI] [PubMed] [Google Scholar]
- 3. Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 4. Vos T, Barber RRM, Bell B, et al. . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. 10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borghouts JA, Koes BW, Vondeling H, et al. . Cost-of-illness of neck pain in The Netherlands in 1996. Pain 1999;80:629–36. 10.1016/S0304-3959(98)00268-1 [DOI] [PubMed] [Google Scholar]
- 6. Buchbinder R, Blyth FM, March LM, et al. . Placing the global burden of low back pain in context. Best Pract Res Clin Rheumatol 2013;27:575–89. 10.1016/j.berh.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 7. A problem worth solving - The rising cost of musculoskeletal conditions in Australia. Victoria: Arthritis and Osteoporosis, 2013. [Google Scholar]
- 8. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008;8:8–20. 10.1016/j.spinee.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 9. March LM, Bachmeier CJ. Economics of osteoarthritis: a global perspective. Baillieres Clin Rheumatol 1997;11:817–34. 10.1016/S0950-3579(97)80011-8 [DOI] [PubMed] [Google Scholar]
- 10. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. 10.1016/j.berh.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Cross M, Smith E, Hoy D, et al. . The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease Study 2010. Ann Rheum Dis 2014;73:1323–30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 12. Hong J, Reed C, Novick D, et al. . Costs associated with treatment of chronic low back pain: an analysis of the UK General Practice Research Database. Spine 2013;38:75–82. 10.1097/BRS.0b013e318276450f [DOI] [PubMed] [Google Scholar]
- 13. Verbunt JA, Westerterp KR, van der Heijden GJ, et al. . Physical activity in daily life in patients with chronic low back pain. Arch Phys Med Rehabil 2001;82:726–30. 10.1053/apmr.2001.23182 [DOI] [PubMed] [Google Scholar]
- 14. Messier SP, Loeser RF, Miller GD, et al. . Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and activity Promotion Trial. Arthritis Rheum 2004;50:1501–10. 10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 15. Messier SP, Mihalko SL, Legault C, et al. . Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263–73. 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leboeuf-Yde C. Body weight and low back pain. A systematic literature review of 56 journal articles reporting on 65 epidemiologic studies. Spine 2000;25:226–37. [DOI] [PubMed] [Google Scholar]
- 17. Shiri R, Karppinen J, Leino-Arjas P, et al. . The association between obesity and low back pain: a meta-analysis. Am J Epidemiol 2010;171:135–54. 10.1093/aje/kwp356 [DOI] [PubMed] [Google Scholar]
- 18. Anandacoomarasamy A, Caterson I, Sambrook P, et al. . The impact of obesity on the musculoskeletal system. Int J Obes 2008;32:211–22. 10.1038/sj.ijo.0803715 [DOI] [PubMed] [Google Scholar]
- 19. Blagojevic M, Jinks C, Jeffery A, et al. . Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 2010;18:24–33. 10.1016/j.joca.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 20. Wai EK, Rodriguez S, Dagenais S, et al. . Evidence-informed management of chronic low back pain with physical activity, smoking cessation, and weight loss. Spine J 2008;8:195–202. 10.1016/j.spinee.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 21. Roffey DM, Budiansky A, Coyle MJ, et al. . Obesity and low back pain: is there a Weight of evidence to support a positive relationship? Curr Obes Rep 2013;2:241–50. 10.1007/s13679-013-0058-7 [DOI] [Google Scholar]
- 22. Graves N, Barnett AG, Halton KA, a HK, et al. . Cost-effectiveness of a telephone-delivered intervention for physical activity and diet. PLoS One 2009;4:e7135 10.1371/journal.pone.0007135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams A, Wiggers J, O'Brien KM, et al. . A randomised controlled trial of a lifestyle behavioural intervention for patients with low back pain, who are overweight or obese: study protocol. BMC Musculoskelet Disord 2016;17:70 10.1186/s12891-016-0922-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Brien KM, Wiggers J, Williams A, et al. . Randomised controlled trial of referral to a telephone-based weight management and healthy lifestyle programme for patients with knee osteoarthritis who are overweight or obese: a study protocol. BMJ Open 2016;6:e010203 10.1136/bmjopen-2015-010203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2010;19:237–70. 10.1177/0962280209105014 [DOI] [PubMed] [Google Scholar]
- 26. Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: medical research council guidance. BMJ 2015;350:h1258 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imai K, Keele L, Tingley D, et al. . Unpacking the Black Box of Causality: learning about Causal mechanisms from Experimental and Observational studies. Am Political Sci Rev 2011;105:765–89. 10.1017/S0003055411000414 [DOI] [Google Scholar]
- 28. Eriksson MK, Hagberg L, Lindholm L, et al. . Quality of life and cost-effectiveness of a 3-year trial of lifestyle intervention in primary health care. Arch Intern Med 2010;170:1470–9. 10.1001/archinternmed.2010.301 [DOI] [PubMed] [Google Scholar]
- 29. Norris SL, Zhang X, Avenell A, et al. . Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med 2004;117:762–74. 10.1016/j.amjmed.2004.05.024 [DOI] [PubMed] [Google Scholar]
- 30. Lange T, Starkopf L. Commentary: mediation analyses in the real world. Epidemiology 2016;27:1 10.1097/EDE.0000000000000518 [DOI] [PubMed] [Google Scholar]
- 31. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41:1343–55. [DOI] [PubMed] [Google Scholar]
- 32. Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol 2010;22:533–7. 10.1097/BOR.0b013e32833b4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christensen R, Bartels EM, Astrup A, et al. . Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 2007;66:433–9. 10.1136/ard.2006.065904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiri R, Karppinen J, Leino-Arjas P, et al. . The association between smoking and low back pain: a meta-analysis. Am J Med 2010;123:87.e7–87.e35. 10.1016/j.amjmed.2009.05.028 [DOI] [PubMed] [Google Scholar]
- 35. Curioni CC, Lourenço PM. Long-term weight loss after diet and exercise: a systematic review. Int J Obes 2005;29:1168–74. 10.1038/sj.ijo.0803015 [DOI] [PubMed] [Google Scholar]
- 36. Goodpaster BH, Delany JP, Otto AD, et al. . Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. Jama 2010;304:1795–802. 10.1001/jama.2010.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu T, Gao X, Chen M, et al. . Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009;10:313–23. 10.1111/j.1467-789X.2008.00547.x [DOI] [PubMed] [Google Scholar]
- 38. Turner AP, Barlow JH, Buszewicz M, et al. . Beliefs about the causes of osteoarthritis among primary care patients. Arthritis Rheum 2007;57:267–71. 10.1002/art.22537 [DOI] [PubMed] [Google Scholar]
- 39. Buchbinder R, Jolley D, Wyatt M. Population based intervention to change back pain beliefs and disability: three part evaluation. BMJ 2001;322:1516–20. 10.1136/bmj.322.7301.1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Relton C, Torgerson D, O'Cathain A, et al. . Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design. BMJ 2010;340:c1066–967. 10.1136/bmj.c1066 [DOI] [PubMed] [Google Scholar]
- 41. O'Hara BJ, Phongsavan P, Venugopal K, et al. . Effectiveness of Australia's Get Healthy Information and Coaching Service®: translational research with population wide impact. Prev Med 2012;55:292–8. 10.1016/j.ypmed.2012.07.022 [DOI] [PubMed] [Google Scholar]
- 42. Larimer ME, Palmer RS, Marlatt GA. Relapse prevention. an overview of Marlatt's cognitive-behavioral model. Alcohol Res Health 1999;23:151–60. [PMC free article] [PubMed] [Google Scholar]
- 43. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol 2000;55:68–78. 10.1037/0003-066X.55.1.68 [DOI] [PubMed] [Google Scholar]
- 44. Silva MN, Markland D, Minderico CS, et al. . A randomized controlled trial to evaluate self-determination theory for exercise adherence and weight control: rationale and intervention description. BMC Public Health 2008;8:234 10.1186/1471-2458-8-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Von Korff M, Ormel J, Keefe FJ, et al. . Grading the severity of chronic pain. Pain 1992;50:133–49. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 46. Roland M, Morris R. A study of the natural history of back pain. part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 1983;8:141–4. [DOI] [PubMed] [Google Scholar]
- 47. Bellamy N. WOMAC® user guide IX, 2009. [Google Scholar]
- 48. Maruish ME. User’s manual for the SF-12v2 Health Survey. Lincoln, RI: Quality Metric incorporated, 2012. [Google Scholar]
- 49. International Standards for Anthropometric Assessment. Underdale, SA: 2001. [Google Scholar]
- 50. The Active Australia survey: a Guide and Manual for Implementation, analysis and Reporting, 2003. [Google Scholar]
- 51. Brown WJ, Trost SG, Bauman A, et al. . Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport 2004;7:205–15. 10.1016/S1440-2440(04)80010-0 [DOI] [PubMed] [Google Scholar]
- 52. Booth M, Bauman AE, Timperio A, et al. . Measurement of adult physical activity: reliability, comparison and validity of Self-Report surveys for population surveillance Summary and Recommendations, 2002. [Google Scholar]
- 53. Centre for epidemiology and research NSW population health survey. Sydney, 2014. [Google Scholar]
- 54. Population Health monitoring and Surveillance: question development field testing, 2004. [Google Scholar]
- 55. Flood VM, Wen LM, Hardy LL, et al. . Reliability and validity of a short FFQ for assessing the dietary habits of 2-5-year-old children, Sydney, Australia. Public Health Nutr 2014;17:498–509. 10.1017/S1368980013000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jensen MP, Karoly P, Huger R. The development and preliminary validation of an instrument to assess patients' attitudes toward pain. J Psychosom Res 1987;31:393–400. 10.1016/0022-3999(87)90060-2 [DOI] [PubMed] [Google Scholar]
- 57. Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 2003;37:197–206. 10.1136/bjsm.37.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Landau S, Emsley R, Dunn G. Beyond total treatment effects in RCTs: why we need to measure outcomes at baseline when investigating mediation. Trials 2015;16(Suppl 2):O42 10.1186/1745-6215-16-S2-O42 [DOI] [Google Scholar]
- 59. Imai K, Yamamoto T. Identification and sensitivity analysis for multiple causal mechanisms: revisiting evidence from framing experiments. Political Analysis 2013;21:141–71. 10.1093/pan/mps040 [DOI] [Google Scholar]
- 60. Imai K, Keele L, Yamamoto T, Identification YT. Identification, inference and sensitivity analysis for Causal Mediation Effects. Statistical Science 2010;25:51–71. 10.1214/10-STS321 [DOI] [Google Scholar]
- 61. Dunn G, Emsley R, Liu H, et al. . Evaluation and validation of social and psychological markers in randomised trials of complex interventions in mental health: a methodological research programme. Health Technol Assess 2015;19:1–116. 10.3310/hta19930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cicuttini FM, Wluka AE, loading Njust. Not just loading and age: the dynamics of osteoarthritis, obesity and inflammation. Med J Aust 2016;204:47–47.e1. 10.5694/mja15.01069 [DOI] [PubMed] [Google Scholar]
- 63. O'Hara BJ, Bauman AE, Eakin EG, et al. . Evaluation framework for translational research: case study of Australia's get healthy information and coaching service(R). Health Promot Pract 2013;14:380–9. 10.1177/1524839912456024 [DOI] [PubMed] [Google Scholar]
- 64. Meeus M, Nijs J, Van Oosterwijck J, et al. . Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a double-blind randomized controlled trial. Arch Phys Med Rehabil 2010;91:1153–9. 10.1016/j.apmr.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 65. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- 66. Vittinghoff E, Neilands TB. Sample size for Joint Testing of indirect effects. Prev Sci 2015;16:1128–35. 10.1007/s11121-014-0528-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Warkentin LM, Majumdar SR, Johnson JA, et al. . Weight loss required by the severely obese to achieve clinically important differences in health-related quality of life: two-year prospective cohort study. BMC Med 2014;12:175 10.1186/s12916-014-0175-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salaffi F, Stancati A, Silvestri CA, et al. . Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283–91. 10.1016/j.ejpain.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 69. VanderWeele T. Explanation in causal inference: methods for mediation and Interaction: Oxford University Press, 2015. [Google Scholar]
- 70. O’Rourke HP, Mackinnon DP. When the test of mediation is more powerful than the test of the total effect. Behav Res 2014:424–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods 2010;15:309–34. 10.1037/a0020761 [DOI] [PubMed] [Google Scholar]
- 72. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–82. 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 73. Manson JE, Shufelt CL, Robins JM. The potential for Postrandomization Confounding in Randomized clinical trials. JAMA 2016;315:2273–4. 10.1001/jama.2016.3676 [DOI] [PubMed] [Google Scholar]
- 74. Tingley D, Yamamoto T, Hirose K, et al. . Mediation: R package for Causal Mediation analysis. J Stat Softw 2014;59:1–38. 10.18637/jss.v059.i05 26917999 [DOI] [Google Scholar]
- 75. Vky N, Cribbie RA. Using the Gamma Generalized Linear Model for modeling continuous, skewed and heteroscedastic outcomes in psychology. Curr Psychol 2016:1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.