Abstract
We aimed to investigate the construct validity of the Timed Up & Go (TUG) test in chronic obstructive pulmonary disease (COPD), to identify characteristics related to an abnormal TUG time and to examine the responsiveness of the TUG to pulmonary rehabilitation (PR). TUG time was assessed before and after comprehensive PR in 500 COPD patients, and compared cross-sectionally in 100 non-COPD subjects. Physical health outcomes, mental health outcomes, symptom-related outcomes and multidimensional indices were assessed in COPD patients only. Good convergent and discriminant validity was demonstrated by fair-to-moderate correlation with physical health outcomes, symptom-related outcomes and multidimensional indices (rs = 0.18–0.70) and by little correlation with mental health outcomes (rs = 0.21–0.26). COPD patients had a worse TUG time than non-COPD subjects, demonstrating known-groups validity. A TUG time of 11.2 seconds had good sensitivity (0.75) and specificity (0.83) for identifying patients with a baseline 6-minute walk distance <350 m. TUG time improved after PR (p < 0.0001) and a change of 0.9–1.4 seconds was identified as clinically important. The TUG is valid and responsive in COPD. An abnormal result is indicative of poor health outcomes. This simple test provides valuable information and can be adopted in clinical and research settings.
Keywords: Chronic obstructive pulmonary disease, outcome assessment (healthcare), rehabilitation
Introduction
The use of simple functional performance tests, such as the five-repetition sit-to-stand and the 4-m gait speed, has gained attention in chronic obstructive pulmonary disease (COPD) patients. Indeed, these tests are reliable, valid and responsive to pulmonary rehabilitation (PR) in COPD patients.1–3
Similarly, the Timed Up & Go (TUG) test is also simple, cheap and reliable.4,5 Subjects are requested to stand up from a chair, walk a distance of 3 m at a comfortable and safe pace, turn and walk back to the chair to sit down again.4 This test has been used for the assessment of functional mobility, walking ability, dynamic balance and risk of falling in subjects with a variety of conditions.4,6,7 Despite its simplicity, the TUG has been shown to predict morbidity and mortality in different populations.8–10 The TUG is even suggested by the American Geriatrics Society and British Geriatrics Society as a gait and balance assessment tool to identify elderly patients who may benefit from a detailed fall risk assessment.11 In addition, the British Geriatrics Society, Age UK and Royal College of General Practitioners suggest it as a measure for the recognition of frailty in older people.12 The European Respiratory Society (statement on nutritional assessment and therapy in COPD) also suggests it as an appropriate measure of physical performance for research and clinical practice in COPD.13
Even though COPD patients may present with lower limb muscle dysfunction,14 limited exercise capacity,15 impaired balance16 and increased risk of falling,17 the TUG has been used scarcely in this population. The TUG is reliable in COPD patients,5 but other measurement properties, such as convergent discriminant and known-groups validity and responsiveness to PR, have not been comprehensively studied. Indeed, a recent systemic review on simple functional tests in COPD found no study investigating TUG’s responsiveness and only one study investigating TUG’s validity.18
Therefore, we aimed: (1) to investigate the construct validity of the TUG in COPD patients referred for PR, (2) to identify characteristics related to an abnormal TUG time and (3) to examine the responsiveness of the TUG to a comprehensive PR programme.
Methods
Design and participants
The present study includes data from the Chance study, a longitudinal observational study aiming to investigate the impact of cardiovascular comorbidities on health status in COPD patients.19 Patients were assessed before and after a comprehensive PR programme between April 2012 and September 2014. Patients had to have stable disease (i.e. no exacerbation in the previous 4 weeks) and complete data on the TUG in order to be included in the current analysis. In addition, a sample of control subjects without COPD or any other debilitating disease was recruited in the primary care setting selected from the Registration Network Family Practices.20 This sample is referred to as non-COPD subjects and had to have a post-bronchodilator forced expiratory volume in the first second/forced vital capacity ratio (FEV1/FVC) ≥ 70% and a healthy condition as determined by the investigator based on medical history and physical examination. The above-mentioned study was registered at the Dutch Trial Register (NTR 3416) and received approval from the Medical Ethical Committee of the Maastricht University Medical Center+ (MUMC+), Maastricht, the Netherlands (METC 11-3-070). All participants gave formal written consent to participate.
Outcome measures and PR programme
The TUG was performed in all participants according to the original protocol: subjects are required to stand up from a chair, walk at a comfortable and safe speed a distance of 3 m, turn and walk back to the chair to sit down again.4 Participants wore their usual footwear and were allowed to use the chair’s arms if they wanted to. If necessary, a demonstration and/or a practice trial were performed and the use of walking aids and/or oxygen was allowed. Participants on oxygen therapy were asked to carry the device for delivering oxygen only when using ambulatory oxygen. The time in seconds to perform the test was recorded by ordinary stopwatches and used as the main outcome of analysis. The timing started when the participant’s back left the back of the chair and ended after the walk, when the participant’s back was positioned against the back of the chair. Three trials were performed by the same assessor and the best trial (i.e. shortest duration) was used for analysis.5 Participants were allowed to rest between the trials, if necessary.
Demographics, anthropometrics, lung function and clinical data, such as comorbidities, smoking status and use of long-term oxygen therapy and/or walking aids, were assessed in all participants. In addition, the number of exacerbations in the previous 12 months (self-reported), physical health outcomes (6-minute walk test (6MWT), cardiopulmonary exercise test and isokinetic quadriceps peak torque), symptom-related outcomes (modified Medical Research Council scale, COPD assessment test and St. George’s Respiratory Questionnaire) and mental health outcomes (Hospital Anxiety and Depression Scale) were assessed in COPD patients only. Furthermore, the Global Initiative for Chronic Obstructive Lung Disease 2007 and 2011 classifications were used, and the multidimensional severity indices age, dyspnoea and obstruction and updated body mass index, airflow obstruction, dyspnoea and exercise capacity (BODE) were calculated. All details about each measurement can be found in the supplemental material.
Patients were enrolled in a 40-session PR programme (inpatient, 8 weeks, 5 days·week−1; or outpatient, 8 weeks, 3 half days·week−1, followed by 8 weeks, 2 half days·week−1). In brief, the programme consisted of moderate-to-high-intensity progressive exercise training (i.e. endurance and strength training); nutritional support, occupational therapy and psychological counselling, if indicated and 20 1-hour educational group sessions. Exercise training prescription was based on a careful characterization of the extra-pulmonary features of patients with COPD performed before PR. The training intensity increased during the rehabilitation period based on dyspnoea and fatigue symptom scores. Moreover, all patients underwent flexibility exercises, general physical exercise for lower and upper extremities and daily supervised 30-minute outdoor walks. Patients who were too dyspnoeic to perform endurance, interval or resistance training received lower limb high-frequency neuromuscular electrical stimulation.21 The programme was implemented by an interdisciplinary team including chest physician, respiratory nurses, dietician, occupational therapist, physiotherapist, psychologist and social worker.
Statistical analysis
Data were expressed as relative frequency, mean ± standard deviation, mean (95% confidence intervals) or median (interquartile range), as appropriate. Construct validity was assessed in terms of convergent, discriminant and known-groups validity. Pearson or Spearman coefficient was used to investigate convergent and discriminant validity. We hypothesized that TUG time would correlate negatively and at least moderately (correlation coefficient > 0.40) with physical health outcomes, and negatively and at least fairly (correlation coefficient > 0.30) with symptom-related outcomes and multidimensional indices. We also hypothesized only positive, negligible-to-little correlation with mental health outcomes (correlation coefficient < 0.30). Mann–Whitney U test was used to investigate known-groups validity by comparing the TUG time between COPD and non-COPD subjects. The hypothesis was that COPD subjects would have a worse (i.e. longer) TUG time than non-COPD subjects.
For responsiveness, paired t-test or Wilcoxon signed-rank test was used to compare pre- and post-PR measurements. In order to test whether changes in TUG time related to relevant changes in 6MWD, changes in TUG time were compared between patients with a clinically important change in 6MWD (≥30 m) and patients with a non-clinically important change (<30 m).22 We hypothesized that a patient with a clinically meaningful change in 6MWD would have larger improvements in TUG time. Effect sizes were estimated with Cohen’s d; logarithmic transformation was applied to substantially skewed data.
For minimal clinically important difference (MCID) estimation, anchor- and distribution-based methods were used. The 6MWT was used as anchor if there was a significant, fair correlation (correlation coefficient ≥ 0.30)23 between change in TUG time and change in anchor. Distribution-based methods included the effect size and the minimal detectable change at 95% confidence (MDC95%). A moderate effect size was calculated as half the standard deviation of the change scores,24 whilst the MDC95% was calculated as 1.96 × √2 × standard error of measurement.25 Kruskal–Wallis test (followed by Dunn post hoc test), Mann–Whitney U test or χ2 test was used for other comparisons, and logistic regression analysis was used to investigate associations with an abnormal TUG time. Receiver operating characteristic curve analysis was used to identify the TUG time with best sensitivity and specificity for identifying patients with a 6MWD < 350 m.26 Statistical significance was considered as p < 0.05, and the analyses were performed using SPSS 17.0 (SPSS, Chicago, Illinois, USA) or GraphPad Prism 5 (GraphPad Software, La Jolla, California, USA). Details on sample size calculations according to the proposed objectives can be found in the supplemental material.
Results
Of the 518 COPD patients included in the Chance study, 18 failed to perform the TUG test: 40% due to musculoskeletal problems, 10% due to injury and 60% due to other medical reasons. Therefore, 500 COPD patients were included, together with 100 non-COPD subjects. Patients had mild to very severe COPD. Patients had worse lung function and higher scores on the Charlson comorbidity index than non-COPD subjects (Table 1). In addition, patients had a slightly lower body mass index and a higher proportion of current smokers.
Table 1.
Characteristics of the samples.a
| Characteristic | COPD | Non-COPD | p Value |
|---|---|---|---|
| N | 500 | 100 | NA |
| Male sex (%) | 55 | 55 | 0.97 |
| Age (years) | 64 (57–71) | 64 (60–69) | 0.64 |
| BMI (kg m−2) | 25.8 (21.7–29.9) | 26.8 (24.4–30.3) | 0.005 |
| FEV1 (% predicted) | 46 (32–63) | 113 (101–123) | <0.0001 |
| GOLD 1/2/3/4 (%) | 7/36/38/19 | NA | NA |
| GOLD A/B/C/D (%)b | 1/22/2/75 | NA | NA |
| Charlson comorbidity index (points) | 1 (1–2) | 0 (0–2) | <0.0001 |
| Smoking status N/F/C (%) | 1/77/22 | 25/63/12 | <0.0001 |
| Long-term oxygen therapy (%) | 24 | 0 | <0.0001 |
| ≥2 Exacerbations in the previous 12 months (%) | 56 | NA | NA |
| Walking aids during the TUG (%) | 9 | 0 | <0.0001 |
BMI: body mass index; C: current smoker; F: former smoker; FEV1: forced expiratory volume in the first second; GOLD: Global Initiative for Chronic Obstructive Lung Disease; N: never smoker; NA: not applicable; COPD: chronic obstructive pulmonary disease.
aData expressed as relative frequency or median (interquartile range).
bIncomplete data for GOLD groups, n = 488.
Construct validity
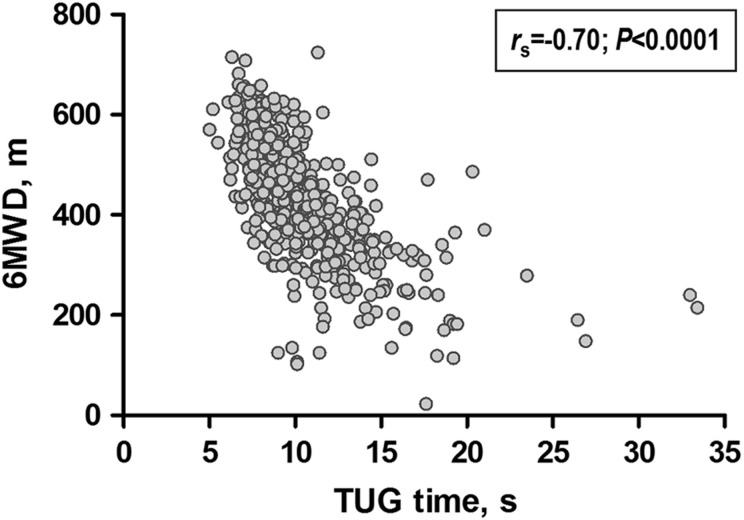
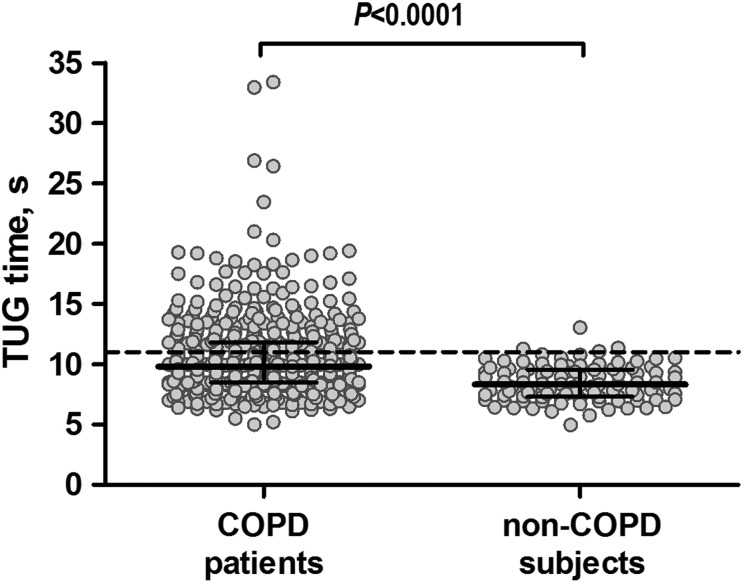
Table 2 presents the correlations between TUG time and other health measures in patients with COPD. In general, fair-to-moderate correlations were found with physical health outcomes, symptom-related outcomes and multidimensional indices, according to convergent validity tests. The strongest correlation was found with functional exercise capacity (Figure 1 and Table 2). Poor correlation was found with symptoms of anxiety and depression, according to discriminant validity tests. As part of known-groups validity analysis, COPD patients showed a worse TUG time (i.e. longer) compared with non-COPD subjects (9.8 (8.5–11.8) vs. 8.3 (7.3–9.6) seconds, respectively; Figure 2), further supporting construct validity.
Table 2.
Correlations between TUG and other measures in COPD patients.a
| Outcome measure | Median (IQR) | rs (95% CI)b |
|---|---|---|
| Physical health outcomes | ||
| 6MWT (m) | 435 (354–512) | −0.70 (−0.75 to −0.65) |
| CPET (W) | 64 (47–87) | −0.44 (−0.52 to −0.37) |
| IQPT (J) | 91.4 (66.0–118.6) | −0.33 (−0.42 to −0.25) |
| Symptom-related outcomes | ||
| mMRC (points) | 2 (2–3) | 0.49 (0.42 to 0.56) |
| CAT (points) | 22 (17–26) | 0.27 (0.19 to 0.36) |
| SGRQ-C | ||
| Symptoms (points) | 63.1 (48.5–76.1) | 0.18 (0.09 to 0.27) |
| Activity (points) | 83.6 (67.8–92.2) | 0.39 (0.31 to 0.46) |
| Impact (points) | 51.2 (34.2–64.1) | 0.40 (0.32 to 0.47) |
| Total (points) | 63.6 (49.9–74.2) | 0.41 (0.33 to 0.48) |
| Multidimensional indices | ||
| ADO index (points) | 4 (3–6) | 0.52 (0.45 to 0.58) |
| Updated BODE index (points) | 3 (2–6) | 0.55 (0.49 to 0.61) |
| Mental health outcomes | ||
| HADS anxiety (points) | 7 (4–11) | 0.21 (0.12 to 0.30) |
| HADS depression (points) | 7 (4–11) | 0.26 (0.17 to 0.34) |
6MWT: 6-minute walk test; BODE: body mass index, airflow obstruction, dyspnoea and exercise capacity index; CAT: COPD assessment test; CPET: cardiopulmonary exercise test; HADS: hospital anxiety and depression scale; IQPT: isokinetic quadriceps peak torque; mMRC: modified medical research council; SGRQ-C: COPD version for the St George’s respiratory questionnaire; COPD: chronic obstructive pulmonary disease; ADO: age, dyspnoea and obstruction; IQR: interquartile range.
aIncomplete data for certain variables: 6MWT, n = 497; CPET, n = 479; IQPT, n = 452; mMRC, n = 495; HADS, n = 485; CAT and SGRQ, n = 488; ADO index, n = 495 and updated BODE index, n = 492.
bAll p-values were < 0.0001.
Figure 1.
Correlation between TUG time and 6MWD in COPD patients. COPD: chronic obstructive pulmonary disease; 6MWD: 6-minute walk distance; TUG: Timed Up & Go.
Figure 2.
TUG time in COPD patients and in non-COPD subjects. The horizontal bars represent median (interquartile range 25–75%). The dotted line corresponds to the 95th percentile in the sample of non-COPD subjects (i.e. 11 seconds), which represents the threshold adopted to identify an abnormal TUG time. TUG: Timed Up & Go; COPD: chronic obstructive pulmonary disease.
Associations with abnormal TUG time
Abnormal TUG time was defined as a TUG time > 11 seconds, as this equals the 95th percentile of the current non-COPD subjects. The proportion of COPD patients with an abnormal TUG time was 33%. Patients with an abnormal TUG time were older, had higher body mass index and Charlson comorbidity index score, worse functional and maximal exercise capacity, worse quadriceps muscle function, more symptoms of dyspnoea, anxiety and depression, more impaired health status, worse disease severity based on multidimensional indices and a higher proportion of former smokers, patients on long-term oxygen therapy and patients using walking aids during the TUG (Table 3). In general, significant associations between a worse TUG time and the above-mentioned outcomes were confirmed in logistic regression models and after stratification for these outcomes (Tables S1 and S2, respectively; supplemental material). Interestingly, a TUG time of 11.2 seconds had the best combination of sensitivity (0.75) and specificity (0.83) for identifying patients with a baseline 6MWD < 350 m (area under the curve = 0.86 (95% CI 0.82 to 0.90), p < 0.0001; Figure S1 in the supplemental material). Similar analyses considering a baseline 6MWD < 200 m revealed that the cut-off with best combination of sensitivity (0.67) and specificity (0.90) was 13.8 seconds (area under the curve = 0.85 (95% CI 0.77 to 0.93), p < 0.0001; Figure S2 in the supplemental material).
Table 3.
Outcome measures in COPD patients with and without an abnormal baseline TUG time.a
| Characteristic | Normal (n = 333) | Abnormal (n = 167) | p Value |
|---|---|---|---|
| Male sex (%) | 56 | 53 | 0.63 |
| Age (years) | 61 (56–69) | 69 (62–75) | <0.0001 |
| BMI (kg·m−2) | 25.0 (21.1–28.9) | 26.4 (23.1–31.1) | 0.001 |
| FEV1 (% predicted) | 46 (34–63) | 46 (30–60) | 0.24 |
| GOLD 1/2/3/4 (%) | 8/35/40/17 | 6/37/34/23 | 0.34 |
| GOLD A/B/C/D (%) | 2/23/3/72 | 0/19/0/81 | 0.06 |
| Charlson comorbidity index (points) | 1 (1–2) | 2 (1–3) | <0.0001 |
| Smoking status N/F/C (%) | 1/74/25 | 2/82/16 | 0.02 |
| Long-term oxygen therapy (%) | 16 | 39 | <0.0001 |
| ≥2 Exacerbations in the previous 12 months (%) | 51 | 67 | 0.001 |
| Walking aids during the TUG (%) | 2 | 23 | <0.0001 |
| 6MWT (m) | 480 (415–545) | 340 (273–396) | <0.0001 |
| CPET (W) | 69 (54–97) | 51 (41–66) | <0.0001 |
| IQPT (J) | 97.3 (71.3–124.2) | 77.0 (58.7–98.0) | <0.001 |
| mMRC (points) | 2 (1–3) | 3 (2–4) | <0.0001 |
| HADS anxiety (points) | 6 (4–11) | 8 (5–12) | 0.005 |
| HADS depression (points) | 6 (3–10) | 9 (6–12) | <0.0001 |
| CAT (points) | 21 (17–25) | 23 (19–28) | <0.0001 |
| SGRQ-C | |||
| Symptoms (points) | 61.1 (47.7–74.0) | 67.5 (53.6–79.5) | 0.002 |
| Activity (points) | 77.0 (60.4–91.8) | 91.8 (83.6–100.0) | <0.0001 |
| Impact (points) | 46.3 (29.0–60.3) | 59.9 (45.3–74.4) | <0.0001 |
| Total (points) | 58.6 (43.7–70.2) | 68.8 (59.5–79.9) | <0.0001 |
| ADO index (points) | 4 (3–5) | 6 (5–7) | <0.0001 |
| Updated BODE index (points) | 3 (2–4) | 7 (4–10) | <0.0001 |
| TUG time (seconds) | 8.8 (7.9–9.8) | 13.1 (11.8–14.9) | <0.0001 |
Responsiveness to PR and MCID estimation
A total of 500 patients started PR, of which 378 patients (76%) had complete data after PR, 92 patients dropped-out (18%) and 30 patients (6%) did not perform the TUG after PR. Patients who did not complete PR had worse baseline TUG time, a higher proportion of current smokers and tended to have worse forced expiratory volume in the first second at baseline (Table S3; supplemental material).
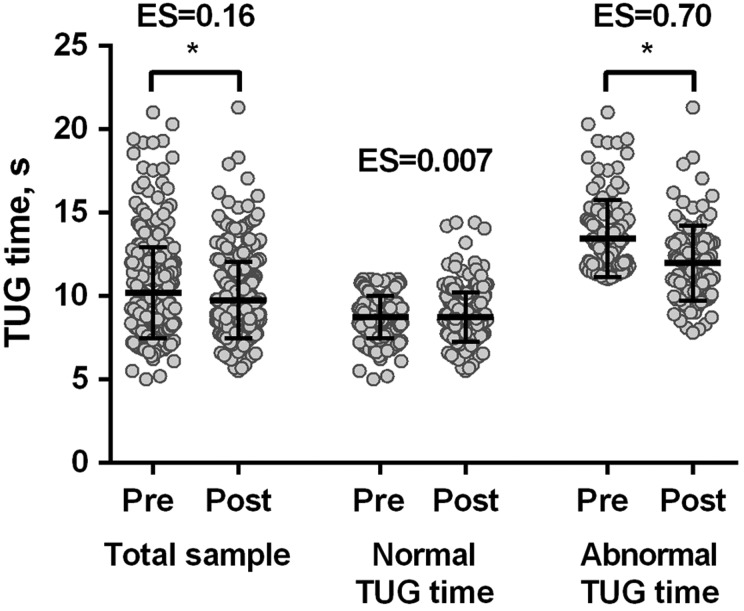
Mean TUG time improved significantly from 10.2 ± 2.7 to 9.7 ± 2.3 seconds after PR (mean change −0.5 (95% CI −0.6 to −0.3) seconds, p < 0.0001), with an effect size of 0.16 (Figure 3). Significant improvements in 6MWD (445 ± 112 to 469 ± 114 m, p < 0.0001; effect size of 0.21) and St. George’s Respiratory Questionnaire (COPD version) total score (61.3 (49.0–72.3) to 50.7 (40.1–62.0) points, p < 0.0001; effect size of 0.45) were also observed following PR. A significant correlation was observed between changes in TUG time and in 6MWD (r = −0.32; p < 0.0001). Patients with a clinically important change in 6MWD of 30 m or more22 had a larger change in TUG time than patients with a non-clinically important change: −1.0 (95% CI −1.3 to −0.7) vs. −0.1 (95% CI −0.3 to 0.2) seconds, respectively (p < 0.0001). The moderate effect size for the TUG time was 0.9 seconds, whilst the absolute MDC95% was 1.4 seconds, which corresponds to a relative MDC95% of 14%. After stratifying for normal or abnormal baseline TUG time, only the latter group showed significant improvements after PR: −1.5 (95% CI −1.9 to −1.0) vs. 0.01 (95% CI −0.2 to 0.2) seconds (p < 0.0001; Figure 3).
Figure 3.
TUG time before and after pulmonary rehabilitation in COPD patients. The horizontal bars represent mean (standard deviation). *p < 0.05. TUG: Timed Up & Go; COPD: chronic obstructive pulmonary disease; ES: effect size.
Discussion
This is the first study to investigate different measurement properties of the TUG in COPD patients. The TUG was shown to be valid for the assessment of functional performance and responsive to PR. COPD patients with a baseline TUG time > 11 seconds showed poorer health outcome measures but were more responsive to PR in terms of the performance on the TUG. Moreover, a TUG time of 11.2 seconds had the best combination of sensitivity and specificity for identifying patients with a baseline 6MWD < 350 m.
In the study of TUG’s development,4 the authors already identified this test as a valid outcome measure. Other studies have also supported the validity of TUG in different populations,27–29 but no study so far has comprehensively investigated this property in patients with COPD. This gap was highlighted in a recent systemic review on simple functional tests in COPD which found only one study investigating the validity of the TUG in this population.18 Nevertheless, only the correlation with lower limb maximal strength was investigated, and in fact this correlation did not reach statistical significance.18 Fair-to-moderate significant correlation was found between TUG time and other measures of functional performance (correlation coefficients 0.33–0.70), suggesting that the test is indeed valid for the assessment of functional performance in COPD. Although the strongest correlation was found with the 6MWT (rs = 0.70), we do not believe that the TUG would be able to replace the 6MWT. These tests have different designs and the correlation coefficient between the tests was not excellent (i.e. > 0.90). Nonetheless, a TUG time of 11.2 seconds had good sensitivity and specificity for identifying patients with a baseline 6MWD < 350 m, the discriminatory threshold for mortality.26 So, the TUG could be very useful to have a first insight into the patient’s exercise capacity, especially in situations with limited time and space for a 6MWT. If a patient had a TUG time > 11 seconds, then an additional 6MWT would be recommended. Of note, the 6MWT should be preferred if one wants to estimate specific parameters, such as exercise-induced desaturation and 6-minute walk work.30 Further supporting construct validity and confirming our hypotheses, only weak correlations were found with mental health outcomes, and COPD subjects performed worse on the TUG than non-COPD subjects.
COPD is related to multi-systemic consequences, such as comorbidities and lower limb muscle dysfunction,14,31 which can compromise the performance on functional tests. Indeed, in our study COPD patients were found to have worse functional mobility (i.e. longer TUG time) when compared with healthy subjects. Although the median TUG time by COPD patients could be considered relatively low (i.e. good functional mobility) if compared with previous findings,4 we believe this reflects the fact that not all COPD patients seem to have an impaired TUG performance since only around one-third of the patients were found to have an abnormal TUG time, that is, a TUG time > 11 seconds. This threshold was derived from the sample of healthy subjects and is supported by the receiver operating characteristic curve analysis, which revealed a similar value (i.e. 11.2 seconds) for identifying patients with a baseline 6MWD < 350 m. Previous studies also suggest that 11 seconds is a reasonable cut-off point.4,32 Nevertheless, if one aims to identify patients with a very poor baseline 6MWD (<200 m), a higher cut-off point should be used instead (i.e. 13.8 seconds). COPD patients with an abnormal TUG time showed worse health outcomes, but the opposite was also observed (i.e. patients with worse health outcomes showed a worse TUG time; Table S2). These associations support the ability of the TUG to reflect the multi-systemic consequences of COPD.
To date, no study has comprehensively investigated the responsiveness of the TUG to PR in COPD.18 TUG time improved significantly after PR, but a small effect size was observed (i.e. 0.16). Previous studies identified larger effects sizes (0.48 to 1.0) following PR.33,34 However, these studies used the modified version of the test in which subjects are instructed to walk as fast as possible. This modified version seems to be more responsive to PR. When using the same version used in the current study, Beauchamp et al. also found a small/modest effect size (i.e. 0.30).35 Nevertheless, the mean change found in their study (i.e. 1.5 seconds) seems to be clinically relevant as it exceeds the MCID values identified in our study (0.9–1.4 seconds). The mean change in the total sample in our study did not exceed the identified MCID values, but this can also be found in other MCID studies.1,3 Moreover, patients with an abnormal baseline TUG time did exceed the identified MCID values. The difference in the magnitude of change between our study and the study by Beauchamp et al. is probably due to the different baseline values, which are worse in the latter study.35
To the best of our knowledge, this is the first study to identify MCID values for the TUG in COPD. Importantly, these MCID values were derived according to anchor and distribution-based methods. After performing a sub-analysis in patients with normal or abnormal TUG time at baseline, we observed the greatest improvements in those with an abnormal TUG time (Figure 3). Although this could suggest a possible ceiling effect by this test, it does not diminish its clinical utility. In fact, this finding is more indicative that PR is especially indicated for patients with an abnormal baseline TUG time.
TUG is a simple, cheap and quick-to-perform test. Basically, only a chair, an ordinary stopwatch and a 3-m course are necessary, which makes this test possible to be adopted in different settings (e.g., patient’s home, hospitals). Moreover, considering that COPD patients take a median time of 9.8 seconds only to perform the test, that we have previously shown that only 2 trials are necessary in COPD patients5 and that most patients do not need any rest between the trials, the test itself can be done in less than 1 minute in the vast majority of patients. Besides being reliable,5 valid, responsive to PR and able to identify patients with worse health outcomes in COPD, previous findings have shown that the TUG is also able to discriminate between fallers and non-fallers.17 Other simple and quick functional performance tests, such as the five-repetition sit-to-stand and the 4-m gait speed, have also been explored and are increasingly being used in COPD.1,2 Theoretically, the advantage of the TUG over the five-repetition sit-to-stand and the 4-m gait speed is that TUG includes both walking and sitting/standing manoeuvres, besides turning, which can challenge the balance of the patients. Nevertheless, to the best of our knowledge, no study so far has performed a proper head-to-head comparison between these tests in COPD. Noteworthy, based on the current findings and on previous studies, all these tests have been shown to be reliable, valid and responsive to PR in COPD.1–3,5
The current study has some methodological considerations. Probably the most important limitation is that other relevant outcomes, such as physical activity, falls and other measures of balance, were not available. Nevertheless, findings from previous studies do suggest that the TUG time is related to some of these outcomes in COPD.17,36 Another limitation is that we were not able to include a measure of patient experience as an anchor for the calculation of the MCID. Nevertheless, the MCID of the 6MWT, which was used as anchor in our study, is in agreement with studies which took into account patient experience.
In conclusion, this study demonstrated that the TUG is valid for the assessment of functional performance in COPD. Patients with a TUG time > 11 seconds have poorer health outcomes than patients with a TUG time below this threshold. A very similar threshold had good sensitivity and specificity for identifying patients with a baseline 6MWD < 350 m, which has been associated with worse prognosis in COPD. Moreover, the TUG is responsive to PR, especially in patients with a baseline TUG time > 11 seconds. The TUG is a simple functional performance test which provides valuable information and can be adopted in both clinical and research settings.
Supplementary Material
Acknowledgement
We would like to thank the Registration Net Family Medicine (RNH).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RM is supported by CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil (246704/2012-8). This study was supported, in part, by The Lung Foundation Netherlands, The Netherlands (grant 3.4.10.015); and GlaxoSmithKline (grant SCO115406).
Supplemental material: The online data supplements are available at http://crd.sagepub.com/supplemental.
References
- 1. Jones SE, Kon SS, Canavan JL, et al. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013; 68: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 2. Kon SS, Patel MS, Canavan JL, et al. Reliability and validity of 4-metre gait speed in COPD. Eur Respir J 2013; 42: 333–340. [DOI] [PubMed] [Google Scholar]
- 3. Kon SS, Canavan JL, Nolan CM, et al. The 4-metre gait speed in COPD: responsiveness and minimal clinically important difference. Eur Respir J 2014; 43: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 4. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 5. Mesquita R, Janssen DJ, Wouters EF, et al. Within-day test-retest reliability of the timed up & go test in patients with advanced chronic organ failure. Arch Phys Med Rehabil 2013; 94: 2131–2138. [DOI] [PubMed] [Google Scholar]
- 6. Flansbjer UB, Holmback AM, Downham D, et al. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med 2005; 37: 75–82. [DOI] [PubMed] [Google Scholar]
- 7. Resnik L, Borgia M. Reliability of outcome measures for people with lower-limb amputations: distinguishing true change from statistical error. Phys Ther 2011; 91: 555–565. [DOI] [PubMed] [Google Scholar]
- 8. Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg 2011; 213: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol 2012; 30: 1829–1834. [DOI] [PubMed] [Google Scholar]
- 10. Laflamme GY, Rouleau DM, Leduc S, et al. The Timed Up & Go test is an early predictor of functional outcome after hemiarthroplasty for femoral neck fracture. J Bone Joint Surg Am 2012; 94: 1175–1179. [DOI] [PubMed] [Google Scholar]
- 11. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society. Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc 2011; 59: 148–157. [DOI] [PubMed] [Google Scholar]
- 12. Turner G, Clegg A, British Geriatrics S; Age UK and Royal College of General P. Best practice guidelines for the management of frailty: a British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014; 43: 744–747. [DOI] [PubMed] [Google Scholar]
- 13. Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014; 44: 1504–1520. [DOI] [PubMed] [Google Scholar]
- 14. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189: e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spruit MA, Franssen FM, Rutten EP, et al. Age-graded reductions in quadriceps muscle strength and peak aerobic capacity in COPD. Rev Bras Fisioter 2012; 16: 148–156. [DOI] [PubMed] [Google Scholar]
- 16. Beauchamp MK, Sibley KM, Lakhani B, et al. Impairments in systems underlying control of balance in COPD. Chest 2012; 141: 1496–1503. [DOI] [PubMed] [Google Scholar]
- 17. Beauchamp MK, Hill K, Goldstein RS, et al. Impairments in balance discriminate fallers from non-fallers in COPD. Respir Med 2009; 103: 1885–1891. [DOI] [PubMed] [Google Scholar]
- 18. Bisca GW, Morita AA, Hernandes NA, et al. Simple lower limb functional tests in patients with Chronic Obstructive Pulmonary Disease: a systematic review. Arch Phys Med Rehabil 2015; 96: 2221–2230. [DOI] [PubMed] [Google Scholar]
- 19. Smid DE, Wilke S, Jones PW, et al. Impact of cardiovascular comorbidities on COPD Assessment Test (CAT) and its responsiveness to pulmonary rehabilitation in patients with moderate to very severe COPD: protocol of the chance study. BMJ open 2015; 5: e007536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metsemakers JF, Hoppener P, Knottnerus JA, et al. Computerized health information in The Netherlands: a registration network of family practices. Br J Gen Pract 1992; 42: 102–106. [PMC free article] [PubMed] [Google Scholar]
- 21. Spruit MA, Augustin IM, Vanfleteren L, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J 2015; 46: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 22. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society Technical Standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- 23. Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008; 61: 102–109. [DOI] [PubMed] [Google Scholar]
- 24. Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Dis 1987; 40: 171–178. [DOI] [PubMed] [Google Scholar]
- 25. Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005; 19: 231–240. [DOI] [PubMed] [Google Scholar]
- 26. Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest 2007; 132: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 27. Schoppen T, Boonstra A, Groothoff JW, et al. The Timed “up and go” test: reliability and validity in persons with unilateral lower limb amputation. Arch Phys Med Rehabil 1999; 80: 825–828. [DOI] [PubMed] [Google Scholar]
- 28. Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil 2005; 86: 1641–1647. [DOI] [PubMed] [Google Scholar]
- 29. Lin MR, Hwang HF, Hu MH, et al. Psychometric comparisons of the timed up & go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc 2004; 52: 1343–1348. [DOI] [PubMed] [Google Scholar]
- 30. Andrianopoulos V, Wouters EF, Pinto-Plata VM, et al. Prognostic value of variables derived from the six-minute walk test in patients with COPD: Results from the ECLIPSE study. Respir Med 2015; 109: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 31. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. [DOI] [PubMed] [Google Scholar]
- 32. Kamide N, Takahashi K, Shiba Y. Reference values for the Timed Up & Go test in healthy Japanese elderly people: determination using the methodology of meta-analysis. Geriatr Gerontol Int 2011; 11: 445–451. [DOI] [PubMed] [Google Scholar]
- 33. Marques A, Jacome C, Cruz J, et al. Family-based psychosocial support and education as part of pulmonary rehabilitation in COPD: a randomized controlled trial. Chest 2015; 147: 662–672. [DOI] [PubMed] [Google Scholar]
- 34. Marques A, Gabriel R, Jacome C, et al. Development of a family-based pulmonary rehabilitation programme: an exploratory study. Disabil Rehabil 2015; 37: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 35. Beauchamp MK, O’Hoski S, Goldstein RS, et al. Effect of pulmonary rehabilitation on balance in persons with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91: 1460–1465. [DOI] [PubMed] [Google Scholar]
- 36. Marques A, Jacome C, Cruz J, et al. Effects of a pulmonary rehabilitation program with balance training on patients with COPD. J Cardiopulm Rehabil Prev 2015; 35: 154–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.