Abstract
Triptolide (T10), an active component of Tripterygium wilfordii Hook F, is reported to have potent anti-inflammatory and analgesic effects. Additionally, MK-801, a noncompetitive N-methyl-D-aspartate receptor antagonist, can reduce glutamate toxicity and has a significant analgesic effect on chronic pain. In this study, we tested the possible synergistic analgesic ability by intrathecal administration of T10 and MK-801 for the treatment of neuropathic pain. Single T10 (3, 10, or 30 µg/kg), MK-801 (10, 30, or 90 µg/kg), or a combination of them were intrathecally administrated in rats with spinal nerve ligation. We found that single administration of T10 caused a slow-acting but long-term analgesic effect, while single administration of MK-801 caused a fast-acting but short-term effect. Administration of their combination showed obviously synergic analgesia and the 1:3 ratio of T10 to MK-801 reached the peak effect. Furthermore, application of T10 and/or MK-801 significantly inhibited the activation of microglia and astrocyte and phosphorylation of STAT3 and NR2B in the spinal dorsal horn induced by chronic neuropathic pain. Our data suggest that the combination of T10 and MK-801 may be a potentially novel strategy for treatment of neuropathic pain.
Keywords: Triptolide (T10), MK-801, combination medication, neuropathic pain, astrocyte, microglia
Introduction
Neuropathic pain (NP) is a debilitating pain caused by peripheral nerve injury, such as traumatic injury, surgical intervention, diabetes, or infection.1–3 Patients who suffer from NP are essentially intractable to currently available antinociceptive therapies because the underlying cellular and molecular mechanisms are unclear yet. NP can cause both central and peripheral sensitization. Central sensitization is primarily caused by the enhancement of excitatory transmission in nociception.
Glial cells are a major cell type within the central nervous system and consist of diverse cell types, including microglia and astrocyte. Under NP condition, they are highly activated and involved in the nerve injury-induced central sensitization and mechanical allodynia. Microglia is activated within 24 h after peripheral nerve damage, which is commonly associated with the early establishment of NP. Astrocyte is activated as late as three days following microglia activation. 4–6
Tripterygium wilfordii Hook F. (TWHF) is a traditional Chinese herb. T10 is one of the major active components of TWHF and has potent anti-inflammatory and immunosuppressive properties for treatment of various diseases, such as rheumatoid arthritis, nephritic syndrome, lupus, Parkinson's disease, and Alzheimer's disease.7–9 Recent studies have suggested that T10 can inhibit tumor necrosis factor-alpha, interleukin (IL)-1beta, and nitric oxide production in spinal microglia.10,11 Our previous studies have demonstrated that T10 effectively relieves NP by inhibiting the activation of microglia and astrocyte in the spinal dorsal horn (SDH) after spinal cord injury.8 T10 also inhibits the proliferation of reactive astrocyte and blocks the hypertrophy of astrocytic processes after spinal cord injury.8 However, T10 has been investigated to have some side effects along with the increased dosage. Since the effect of T10 is mainly mediated by acting on the glia,8 analgesic drugs that target on neurons may produce a synergistic effect with T10 and reduce its required dose.
MK-801, also named as dizocilpine, is a noncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist. Previous studies have indicated that MK-801 can block neuronal NMDAR-mediated glutamate toxicity during the induction phase of human optic neuritis and highlight a potential reagent for therapeutic neuroprotection.12 However, other studies have reported that a large dose of MK-801 have unacceptable neurotoxic effects.13
Synergistic, additive or antagonistic interactions can be observed when two analgesics are administered at the same time. When acting synergistically, lower doses of each drug can reach an equal or better analgesic effect, but with fewer overall side effects, than the individual compound.14 Because both T10 and MK-801 are associated with an increased risk of side effects, combining the two at a lower dosage to produce a synergistic effect might be a better analgesic strategy. In the current study, by simultaneous intrathecal (i.t.) delivery of T10 and MK-801 at lower dosages in adult rats with spinal nerve ligation (SNL), we did observe a synergistic analgesic effect of the combined components with weak side effects. The analgesic effect may be mediated by the strong inhibition of spinal microcytic and astrocytic activity.
Methods
Experimental animals
Male Sprague-Dawley rats (220–250 g) were used in the present study and housed in cages that were maintained on a standard 12:12 h light/dark cycle. All experiments were conducted in accordance with approved experimental protocols of the Animal Use and Care Committee for Research and Education of the Fourth Military Medical University (Xi'an, China). All efforts were made to minimize animal suffering and reduce the number of animals used.15
The i.t. Implantation
Rats were implanted with a single i.t. catheter for drug delivery. Laminectomy was performed at the level of the thoracic vertebrae, under 7% chloral hydrate (0.4 ml/100 g, i.p.). A polyethylene (PE10) catheter was passed caudally from the T9 to the T12 level, and 2 cm of the free ending was left exposed in the upper thoracic region. After surgery, the animals were injected with 2% lidocaine (10 µl) through the i.t. catheter to ensure that the PE tubing was in the correct location. Only those rats showing complete paralysis of both hind limbs and the tail after the injection of lidocaine were used for the following experiments. Additionally, the position of the PE tubing in the i.t. space at the lumbar enlargement was visually verified by exposing the lumbar spinal cord at the end of experiment. Data from rats with incorrect PE tubing position were discarded from the study.
Spinal nerve ligation
L5 SNL was performed according to Kim and Chung.16 Briefly, rats were anesthetized with 7% chloral hydrate (0.4 ml/100 g, i.p.). Then, the left L6 transverse process was carefully removed with a small rongeur to expose the L4 and L5 spinal nerves. The L5 spinal nerve was then carefully isolated and tightly ligated with 6-0 silk thread. The surgical procedures for the sham group were identical to those of the SNL group, except that the spinal nerves were not ligated. All rats were observed for any visual signs of motor deficits and for the general health and weight maintenance after operation.
The i.t. Administration of T10, MK-801, or their combination and experimental design
T10 was obtained from Fujian Academy of Medical Sciences (Fujian, China) and MK-801 was obtained from Sigma-Aldrich (St. Louis, MO, USA). They were dissolved and diluted with preservative-free normal saline solution for administration. Normal saline (0.9%) was used as the negative control. The doses for i.t. administration of T10 and MK-801 were 3, 10, or 30 µg/kg/day and 10, 30, or 90 µg/kg/day, respectively. In most of the cases, the volume of drug solution was 5 µl. However, the volume would be changed to 2.5 or 7.5 µl according to the indicated experimental requirement. In these cases, the total dosage of drugs will be changed.
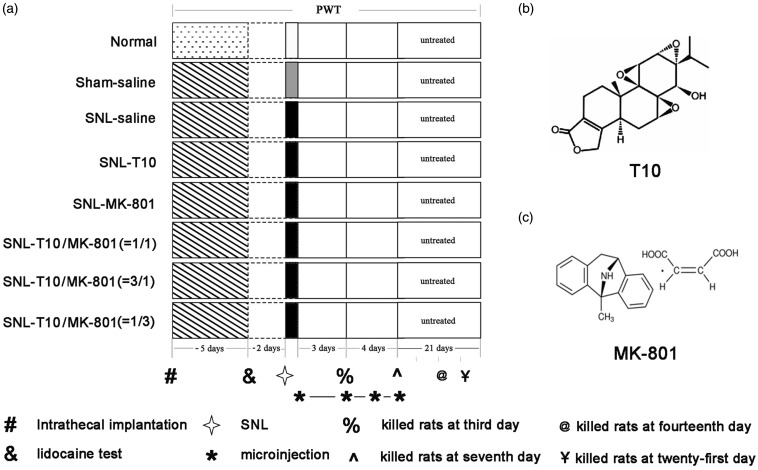
The experimental procedures are shown in Figure 1. A total of 272 animals were used in the study and randomly assigned to one of eight groups: (1) normal group, (2) sham saline group, (3) SNL saline group (a volume of 5–10 µl saline was injected), (4) SNL-T10 group (3, 10 or 30 µg/kg/daily), (5) SNL-MK-801 group (10, 30 or 90 µg/kg/daily), (6) SNL-T10/MK-801 group (1:1, 5 µl T10 + 5 µl MK-801), (7) SNL-T10/MK-801 group (1:3, 2.5 µl T10 + 7.5 µl ΜK-801), and (8) SNL-T10/MK-801 group (3:1, 7.5 µl T10 + 2.5 µl ΜK-801). Each group included 34 rats: 18 for behavioral testing (6 for low concentration, 6 for medium concentration, and 6 for high concentration), 8 for immunohistochemical staining (third day and seventh day), and eight for Western blot analysis (third day and seventh day). The T10 and MK-801 dosages, used singly and combined, for the immunohistochemical staining and Western blot analysis were the effective dose 50 (ED50) of each. Behavioral experiments were conducted by double-blinded technicians and occurred at a fixed time during testing days. The rats were always habituated to the testing room for 30 min before tests. At days 1, 3, 5, 7, 10, 14, 21, and 28, the paw withdrawal thresholds (PWTs) were tested using von Frey filaments. The rats used for immunohistochemical staining and Western blot analysis were sacrificed at day 3 or 7.
Figure 1.
Schematic diagram of the experimental design (a) and the structural formulas of the drugs (b and c). This figure depicted the behavioral tests that were conducted for each group, the time course of i.t. implantation, the time course of the lidocaine test and SNL, the duration time for drugs administration, and the structural formulas of T10 and MK-801. PWT: paw withdrawal threshold, SNL: spinal nerve ligation.
Nociceptive behavioral tests
Rats were habituated to the testing environment for three days before baseline test and were then placed under inverted plastic boxes (30 × 30×50 cm) on an elevated mesh floor for 30 min before the threshold test was conducted to allow for habituation. Briefly, a logarithmic series of von Frey filaments (Stoelting, Kiel, WI, USA) was applied to the hind paw to determine the stimulus intensity threshold stiffness required to elicit a paw withdrawal response. The ipsilateral hind paw was pressed with one of a series of von Frey filaments with increasing stiffness (2, 4, 6, 8, 10, 15, and 26 g) applied to the plantar surface for 5 s for each filament. Rapid pulling back, biting, or shaking of the ipsilateral hind limb was taken as a positive sign of withdrawal responses. The interval between trials was at least 5 min. For each trial, the same hind limb was stimulated 10 times by a single von Frey filament before being stimulated by the next larger filament. The minimal value that resulted in at least six responses to 10 stimulations was recorded as the PWT.
Dose–effect curve and ED50 calculation
The dosages of i.t. T10, MK-801, and their combinations were transformed into logarithm doses, and the non-linear fit was calculated to build the dose-effect curve. Based upon the dose-effect curve, the ED50 value of each agent for analgesia was calculated.
Isobolographic analysis
An isobolographic analysis was further performed to characterize the drug interactions according to the method originally described by Tallarida14 and our previous reports.17 Both drugs in this experiment achieved comparable levels of anti-nociception, such that ED50 values were used to obtain a theoretical dose-response curve for a fixed ratio combination of T10 and MK-801. We calculated a theoretical ED50 (ED50add) based upon the theoretical dose-response curve. Subsequently, an experimental dose-response curve was obtained by treating animals with one of the following combination doses: ED50add*0.3, ED50add*1 and ED50add*3 in ratios of 1:1 for T10 and MK-801. According to this dose-response curve, the ED50 of combinations could be calculated and presented as the ED50comb. An ED50comb that was lower than the ED50add suggested a synergistic effect of these two agents.
Immunofluorescent histochemistry
Rats were deeply anesthetized with 7% chloral hydrate (0.4 ml/100 g, i.p.) and then transcardially perfused with 100 ml of 0.05 M phosphate-buffered saline (PBS, pH 7.2-7.4), followed by 500 ml of 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The L5 spinal cord segment was removed and subsequently immersed in 30% sucrose (w/v) in 0.1 M PB overnight at 4℃. Then, transverse spinal sections (25 µm thickness) were cut on a cryostat (Leica CM1800; Heidelberg, Germany) and processed for immunohistochemistry. For immunofluorescence histochemical staining, the sections were incubated overnight at 4℃ with goat anti-ionized calcium-binding adaptor molecule 1 (Iba1, a marker of microglia, 1:800; ab5067; Abcam, Cambridge, MA, USA) in PBS containing 0.3% (v/v) Triton X-100, 0.25% (w/v) λ-carrageenan, and 5% (v/v) donkey serum (PBS-XCD). After being washed three times in 0.05M PBS (10 min each), all sections were then incubated for 4 h at room temperature with 10 µg/ml of Alexa488-conjugated donkey anti-goat IgG antibody (1:500; Invitrogen, Carlsbad, CA, USA).
For double immunofluorescence histochemical staining, the sections were incubated with a mixture of mouse monoclonal anti-glial fibrillary acidic protein (GFAP, a marker of astrocytes) IgG (1:4000; Chemicon, Temecula, CA, USA), rabbit polyclonal anti-phospho-signal transducer and activator of transcription 3 (pSTAT3) IgG (1:300; Cell Signaling, Danvers, MA, USA) overnight at 4℃. Then, all of the above sections were treated with a mixture of biotin-conjugated donkey anti-rabbit IgG antibody (1:500; Jackson Immuno-Research, PA, USA) for 6 h at room temperature. Finally, the sections were then treated by a mixture of 10 µg/ml Alexa488-conjugated donkey antibody to mouse IgG (1:500; Invitrogen, Carlsbad, CA, USA) and Alexa594-conjugated streptavidin (1:500; Jackson Immuno-Research, West Grove, PA, USA). The sections were observed under a confocal laser scanning microscope (FV1000; Olympus, Tokyo, Japan) with the appropriate laser beams and filter settings for Alexa488 (excitation, 488 nm; emission, 510-530 nm) and Alexa594 (excitation, 543 nm; emission, 590–615 nm).
Western blot analysis
Rats were deeply anesthetized by injection with 7% chloral hydrate (0.4 ml/100 g, i.p.) and then rapidly sacrificed. The L5 spinal cord segments were dissected on ice. Then, the left dorsal portion of the spinal cord was split and homogenized with a hand-held pestle in sodium dodecyl sulfate (SDS) sample buffer (10 ml/mg tissue) that contained a mixture of protease and phosphatase inhibitors (Sigma-Aldrich). The protein concentrations were estimated using the bicinchoninic acid method. The samples were heated in boiling water for 5 min, loaded onto 10% SDS polyacrylamide gels (Bio-Rad Laboratories, CA, USA), and transferred to polyvinylidenedifluoride membranes (Immobilon-P, Millipore, Billerica, MA, USA). Membranes were blocked in a 5% bovine serum albumin solution for 2 h and probed with the following primary antibodies overnight at 4℃: mouse monoclonal anti-GFAP IgG (1:4000; Chemicon, Temecula, CA, USA), rabbit polyclonal anti-pSTAT3 IgG (1:300; Cell Signaling, MA, USA), goat polyclonal anti-Iba-1 (1:800; ab5067; Abcam) and mouse monoclonal anti-β-actin IgG (1:3000, Sigma-Aldrich). The membranes were then incubated with the following secondary antibodies for 2 h: HRP-conjugated anti-rabbit donkey IgG (1:5000; Zhongshan, Beijing, China), HRP-conjugated anti-goat donkey IgG (1:5000; Zhongshan, Beijing, China) and HRP-conjugated anti-mouse donkey IgG (1:5000; Zhongshan, Beijing, China). The membranes were rinsed three times (10 min each) with Tris-buffered saline with Tween-20 between each step. All reactions were detected by the enhanced chemiluminescence detection method (Amersham). Protein blot densities were analyzed using Labworks Software (Ultra-Violet Products, UK). The densities of target proteins and β-actin immunoreactive bands were quantified with background subtraction. The same sized square was drawn around each band to measure the density and the background near that band was subtracted. Target protein levels were normalized against β-actin levels and are expressed as relative fold changes compared with the sham-Veh group. Technicians who were blind to the different treatments quantified the intensity of blots with densitometry.
Statistical analyses
All data were collected and analyzed by researchers who were blinded to the surgery and reagents that were used. One- or two-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was used for multiple comparisons. Data from the Western blot were analyzed using a one-way ANOVA followed by the Newman–Keuls test for post hoc analysis. Pearson correlation analysis was used to identify correlations between the analgesic effect of T10 and the expressions of the related proteins. All data are presented as the mean ± SEM and all statistical analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered significant.
Results
Effects of i.t. alone administration of T10 or MK-801 on SNL-induced mechanical allodynia
Consistent with previous studies,8,17,18 SNL produces rapid and persistent mechanical allodynia as evidenced by significant decreases in ipsilateral PWTs, indicating successfully induced chronic NP by SNL.
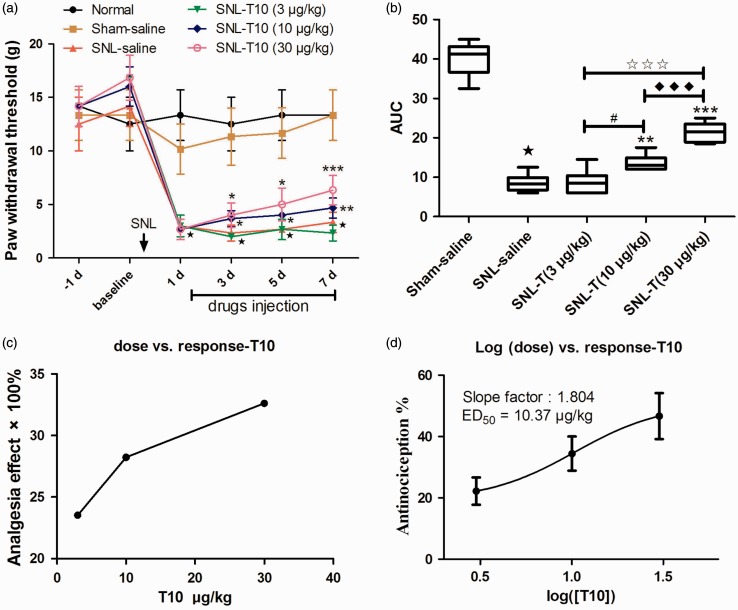
In the present study, the PWTs of the ipsilateral hind limb decreased significantly at post-operational day (POD) 1 and remained decreased until POD 7 (Figure 2(a)). When compared with the SNL saline group, i.t. administration of 5 µl of 10 and 30 µg/kg doses of T10 significantly elevated the threshold value of mechanical allodynia (P < 0.05), although a low dose of T10 (3 µg/kg) could not reverse the mechanical allodynia (P > 0.05) (Figure 2(a)). Based on the summarized values of area under curves (AUCs) and PWTs, the analgesia of i.t. T10 presented a significant group difference among the three dose regimes with a dose-dependent manner (Figure 2(b) and (c); P < 0.05). The effects of different doses of i.t. T10 on the PWTs were then calculated as log (dose) versus response curve (Figure 2(d)), from which the ED50 of i.t. T10 analgesia was calculated as 10.37 µg/kg.
Figure 2.
T10 dose dependently inhibited SNL-induced mechanical allodynia of the ipsilateral hind paw. (a) The analgesia effects of different doses of i.t. T10. (b) The AUCs for the different groups were calculated for statistical analysis. (c–d) The dose-effect and log (dose)-effect curves for the analgesic effects in attenuating SNL-induced mechanical allodynia after i.t. saline and T10, respectively. ⋆P < 0.001, SNL saline group versus sham saline group; *P < 0.05, **P < 0.01, and ***P < 0.001, SNL-T10 groups versus SNL saline group; #P < 0.05, SNL-T10 (10 µg/kg) versus SNL-T10 (3 µg/kg) group; ♦♦♦P < 0.001, SNL-T10 (30 µg/kg) versus SNL-T10 (10 µg/kg) group; ★★★P < 0.001, SNL-T10 (30 µg/kg) versus SNL-T10 (3 µg/kg) group. AUC: area under curve; SNL: spinal nerve ligation; ED50: effective dose 50.
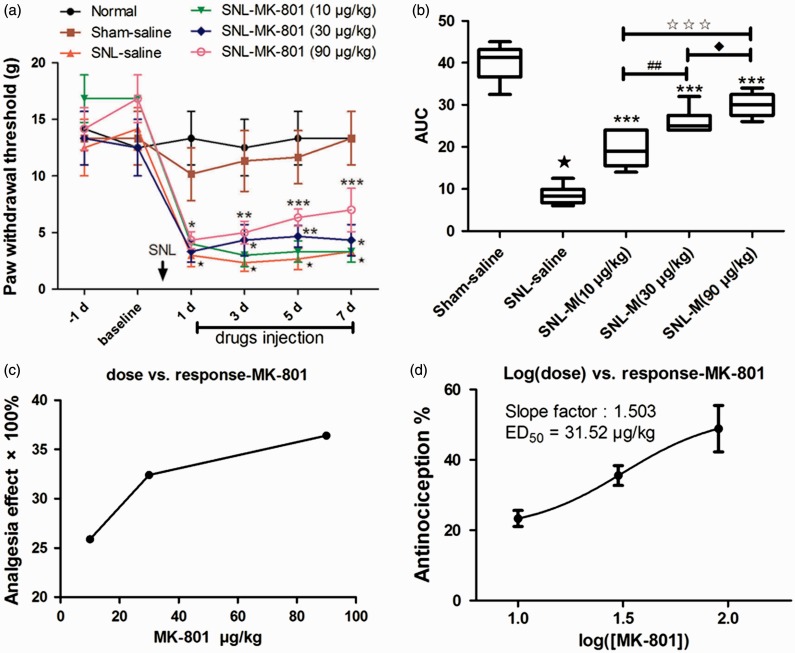
When compared with the SNL saline group, i.t. administration of MK-801 (5 µl of 3, 10, and 30 µg/kg) all significantly elevated the PWTs (Figure 3(a); P < 0.05). Based on the values of AUCs and PWTs, the analgesic effects of i.t. MK-801 treatment presented a significant group difference among the three dose regimes with a dose-dependent manner (Figure 3(b) and (c)). The effects of different doses of i.t. MK-801 on the PWTs were then calculated as log (dose) versus response curve (Figure 3(d)), from which the ED50 of i.t. MK-801 analgesia was calculated as 31.52 µg/kg.
Figure 3.
MK-801 dose dependently inhibited SNL-induced mechanical allodynia of the ipsilateral hind paw. (a) The analgesia effects of different doses of i.t. MK-801. (b) The AUCs for the different groups were calculated to perform a statistical analysis. (c–d) The dose-effect and log (dose)-effect curves for the analgesic effects in attenuating SNL-induced mechanical allodynia after i.t. saline and MK-801, respectively. ⋆P < 0.001, SNL saline group versus sham saline group; *P < 0.05, **P < 0.01, and ***P < 0.001, SNL-MK-801 groups versus SNL saline group; ##P < 0.01, SNL-MK-801 (30 µg/kg) versus SNL-MK-801 (10 µg/kg) group; ♦P < 0.05, SNL-MK-801 (90 µg/kg) versus SNL-MK-801 (30 µg/kg) group; ★★★P < 0.001, SNL-MK-801 (90 µg/kg) versus SNL-MK-801 (10 µg/kg) group. AUC: area under curve; SNL: spinal nerve ligation; ED50: effective dose 50.
Taken together, these results demonstrate that i.t. administration of T10 at 10 and 30 µg/kg or MK-801 at 3, 10, and 30 µg/kg has analgesic effects.
Effects of i.t. administration of T10 and MK-801 on SNL-induced mechanical allodynia
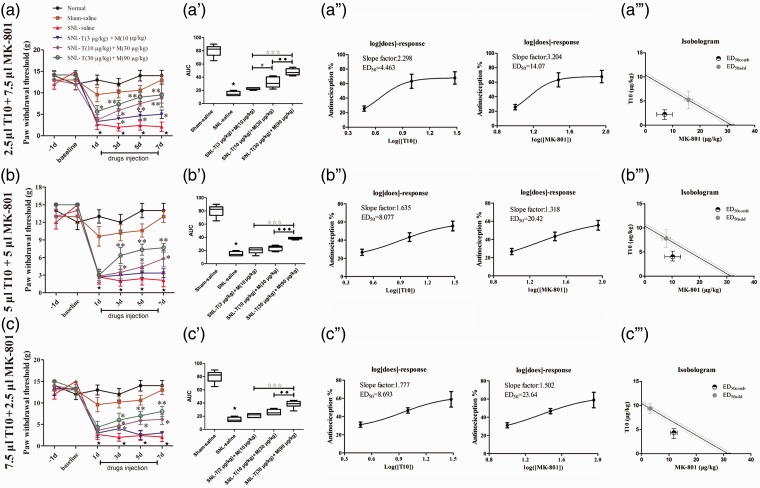
Due to the different analgesic profiles of i.t. T10 and MK-801 administration, interaction parameters were calculated based on the antinociceptive effects. The middle dose-response curves of both compounds were linear. Thus, a composite additive curve was constructed (Figure 4(a) to (c)). Additive regression allowed us to calculate the theoretical ED50 (ED50add) for a fixed ratio (1:1) of T10 and MK-801 (ED50add = 5.19 µg/kg T10 + 15.76 µg/kg MK-801). To investigate the experimental ED50 (ED50comb) of the combined T10 and MK-801, the experimental dose-response curves were obtained by treating animals with one of the following combination doses: ED50add×0.3 (3.47 µg/kg T10 + 10.51 µg/kg MK-801), ED50add×1 (10.37 µg/kg T10 + 31.52 µg/kg MK-801), or ED50add×3 (31.11 µg/kg T10 + 94.56 µg/kg MK-801) in ratio of 1:1 for T10 and MK-801. Based on the different combination doses, different ratio of the volume (ν/ν) of T10 and MK801 (T/M = 1:3, 2.5 µl T10 + 7.5 µl MK-801, T/M = 1:1, 5 µl T10 + 5 µl MK-801 or T/M = 3:1, 7.5 µl T10 + 2.5 µl MK-801) was also applied (Figure 4(a) to (c)).
Figure 4.
Combination treatment of T10 or MK-801 with different ratios. (a, b, and c) The analgesia effect of different volumes of i.t. T10 or MK-801 with different dose ratios. (a′, b′, and c′) The AUCs for different groups were calculated to perform statistical analysis. (a′′, b′′, and c′′) The dose-effect and log (dose)-effect curves for the analgesic effects in attenuating SNL-induced mechanical allodynia after i.t. saline, T10 and/or MK-801. (a′′′, b′′′, and c′′′) The iosobolograms for the combination analgesia. ⋆P < 0.001, SNL saline group versus sham saline group; *P < 0.05, **P < 0.01, SNL-T+M groups versus SNL saline group; #P < 0.05, SNL-T (10 µg/kg)+M (30 µg/kg) groups versus SNL-T (3 µg/kg)+M (10 µg/kg) groups; ♦♦P < 0.01 and ♦♦♦P < 0.001, SNL-T (30 µg/kg)+M (90 µg/kg) groups versus SNL-T (10 µg/kg)+M (30 µg/kg) groups; ★★★P < 0.001, SNL-T (30 µg/kg)+M (90 µg/kg) groups versus SNL-T (3 µg/kg)+M (10 µg/kg) groups. AUC: area under curve; SNL: spinal nerve ligation; ED50: effective dose 50.
The i.t. T10 and MK-801 combination significantly elevated the PWTs in a dose-dependent manner (Figure 4(a) to (c); P < 0.01). As summarized by the AUC values of the PWTs, the analgesic effects of the i.t. T10 and MK-801 combinations presented a significant group difference among the three dose regimes (Figure 4(a′) to (c′); P < 0.01). The experimental ED50 of T10 and MK801 that were calculated from these dose-response curves were shown in Figure 4(a′′) to (c′′). Isobolographic analysis of the combined T10 and MK-801 effect during the nociceptive test showed that the ED50comb were 2.23 µg/kg T10 + 7.04 µg/kg MK-801 (T/M = 1:3), 4.04 µg/kg T10 + 10.21 µg/kg MK-801 (T/M = 1:1), and 4.35 µg/kg T10 + 11.82 µg/kg MK-801 (T/M = 3:1) (Figure 4(a′′′) to (c′′′)). With 1:3 T/M ratio, the ED50comb was lower than the lower (95%) range of ED50add, suggesting that the interaction between the two drugs was synergistic (Figure 4(a′′′)).
These results revealed that the 1:3 ratio of T10 and MK-801 synergistic analgesia enhanced analgesic intensity with significantly reduced doses of T10 and MK-801.
Effective drug duration of i.t. T10, MK-801 or their combination on SNL-induced mechanical allodynia after drug withdrawal
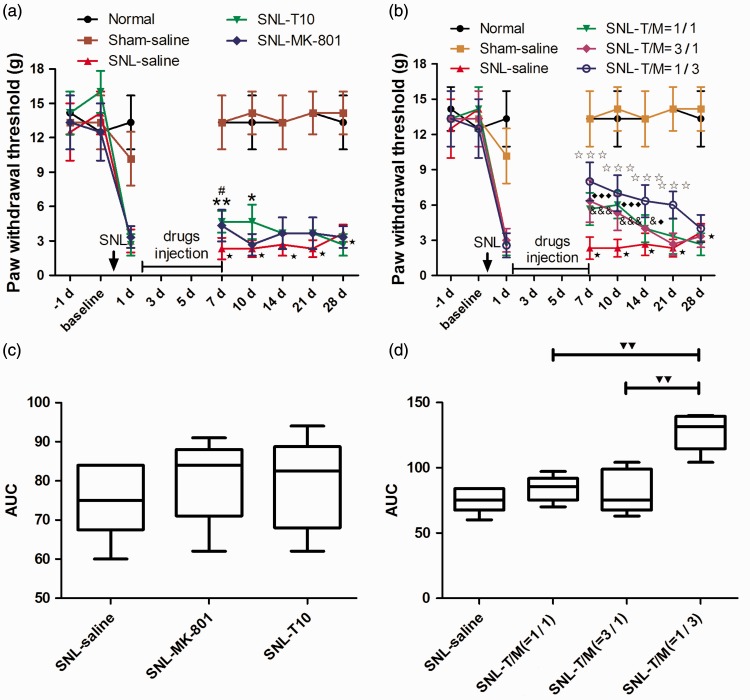
To determine the long-term effect after drug withdrawal, we continually to observe the possible analgesia effect for another three weeks after injecting drugs for one week (from POD 1 to POD 7). After drug withdrawal, the PWTs in the combination drug administration groups (Figure 5(b)) were significantly higher than those in the single drug administration groups (Figure 5(a)) and saline administration group. As summarized by the AUC values of the PWTs, the analgesic effects of i.t. T10 (10 µg/kg) and MK-801 (30 µg/kg) treatments have no significant difference, while the combination groups all greatly increase the AUC (Figure 5(c) and (d)). Among the combination groups, the anti-nociceptive effect with the ratio = 3:1 of MK-801 and T10 was more pronounced than the ratio was 1:1 and 1:3 (Figure 5(d)).
Figure 5.
Repeated administration of T10, MK-801, or their combination reversed SNL-induced mechanical allodynia. (a–b) The continual analgesia duration of i.t. administration of T10, MK-801 or their combination after terminating drug treatment. (c–d) The AUCs for the different groups were calculated to perform statistical analyses. ⋆P < 0.001, SNL saline group versus sham saline group; *P < 0.05 and **P < 0.01, SNL-T10 group versus SNL saline group; #P < 0.05, SNL-MK-801 group versus SNL saline group; ★★★P < 0.001, SNL-T/M (1/3) group versus SNL saline group; ♦P < 0.05 and ♦♦♦P < 0.001, SNL-T/M (1/1) group versus SNL saline group; &P < 0.05 and &&&P < 0.001, SNL-T/M (3/1) group versus SNL saline group; ▾▾P < 0.01, SNL-T/M (1/3) group versus SNL-T/M (3/1) group and SNL-T/M (1/1) group, respectively. AUC: area under curve; SNL: spinal nerve ligation.
Effect of i.t. administration of T10, MK-801, or their combination on SNL-induced glial activation
Our previous studies have suggested that microglia and astrocytic activation was involved in the development and maintenance of chronic pain. Thus, we questioned whether microglial or astrocytic inhibition contributed to synergistic analgesia following the co-administration of T10 and MK-801. To test this hypothesis, we used ionized calcium-binding adapter molecule 1 (Iba-1, a marker of microglia) to detect microglial activation on POD3 and GFAP (a marker of astrocytes) to observe astrocyte activation on POD 3, 7, 14, and 21.
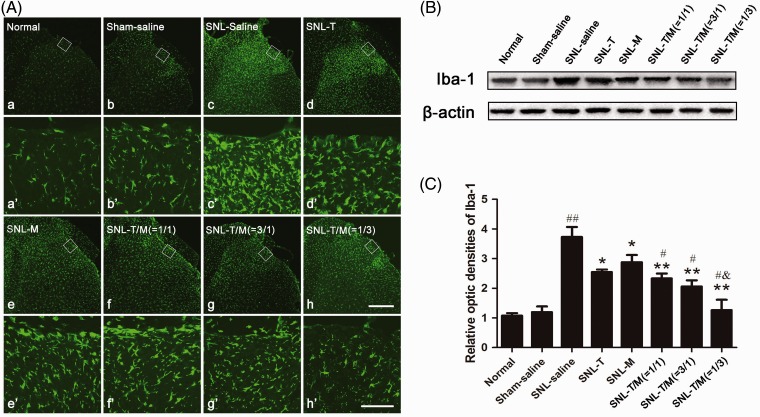
In our present study, the results of immunofluorescence histochemistry for Iba-1 and Western blot on POD3 showed that SNL evoked significant microglial activation (Figure 6(A: c and c′)) and that the combination of T10 and MK-801 significantly inhibited the expression and activation of Iba-1 in the ipsilateral SDH (Figure 6(A: f, f′, g, g′, h, and h′), (B), and (C)) (##P < 0.01). In addition, T10 and MK-801 administration alone could down-regulate the expression of Iba-1 when compared with the SNL saline group (Figure 6(B) and (C); *P < 0.05). When compared with the single medication group, the drug combination had stronger inhibition on the Iba-1 expression (Figure 6(B) and (C); **P < 0.01). There was no significant difference among the intervention groups when comparing the SNL-T10 and SNL-MK-801 groups. However, among the combination groups, Iba-1 expression was significantly reduced when the ratio of T10 and MK-801 was 1:3 (Figure 6(B) and (C); &P < 0.05).
Figure 6.
Administration of T10, MK-801, or their combination on SNL-induced microglial activation. Immunofluorescent results showed that SNL induced up-regulation of Iba-1 in the ipsilateral SDH (A; c and c′). Administration of individual or concomitant drugs down-regulated Iba-1 expression in the ipsilateral SDH on day 3 (d–h and d′-h′ ). The rectangle areas in a–h are enlarged in a′-h′, respectively. (B) Western blot of Iba-1 expression at day 3 in groups with saline or drug treatment. (C) The relative optic densities of Iba-1 shown in (B). ##P < 0.001, SNL saline group versus sham saline or normal group; *P < 0.05, **P < 0.01, SNL-T group, SNL-M group, SNL-T/M (1/1) group, SNL-T/M (3/1) group, SNL-T/M (1/3) group versus sham saline group, respectively; #P < 0.05, SNL-T/M (1/1) group, SNL-T/M (3/1) group, SNL-T/M (1/3) group versus SNL-T group and SNL-M group, respectively; &P < 0.05, SNL-T/M (1/3) group versus other mixed drugs groups. Scale bars = 40 µm in a–h and 200 µm in a′-h′. AUC: area under curve; SNL: spinal nerve ligation.
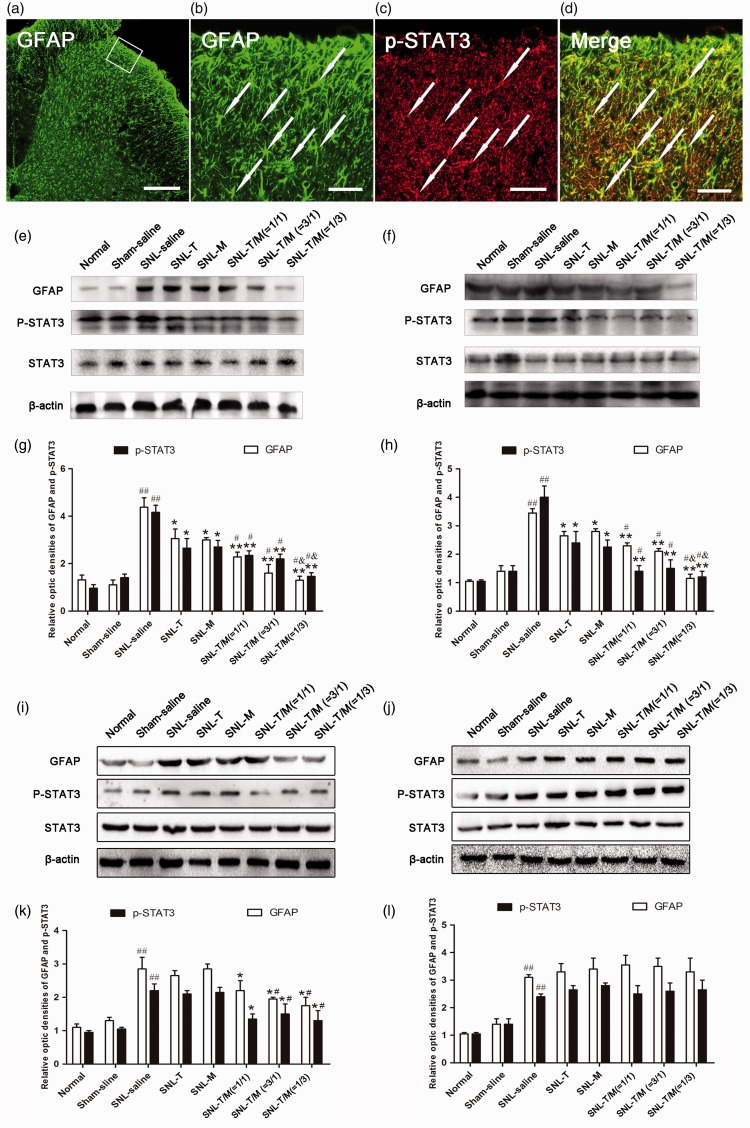
Our previous studies have also showed that SNL rats exhibited GFAP up-regulation, which is significant on day 3 and reaches the peak on day 7 after SNL. In the present study, we also found the activated astrocytes, presenting as hypertrophied cell bodies and thickened processes with enhanced GFAP-immunoreactivity (IR) (Figure 7(a) and (b)). Western blot analysis showed that the GFAP and p-STAT3 expression levels were significantly increased after SNL. However, treatment with T10 or MK-801 decreased the GFAP and p-STAT3 expression on POD 3 (Figure 7(e) and (g)) and POD 7 (Figure 7(f) and (h)), but not on POD 14 (Figure 7(i) and (k)) and POD 21 (Figure 7(j) and (l)), in comparison with the SNL saline group. The combined T10 and MK-801 treatment groups showed stronger reversal effects of the GFAP expression than T10 or MK-801 single treatment groups, except on POD 21 (Figure 7(g), (h) and (k), (l)). When the ratio of T10:MK-801 was 3:1, the expression levels of GFAP and p-STAT3 were inhibited maximally (Figure 7(g), (h), and (k)).
Figure 7.
Administration of T10, MK-801, or their combination on SNL-induced astrocytic activation and STAT3 phosphorylation. (a–d) Immunohistochemistry indicated that activated astrocytes presented as hypertrophied cell bodies and thickened processes with enhanced GFAP immunoreactivity (IR) and many of the p-STAT3-IRs was co-labeled with GFAP-IR. Western blot analysis showed that the GFAP and p-STAT3 expression levels were significantly decreased on days 3 (e and g) and 7 (f and h) but not on days 14 (i and k) and 21 (j and l), in all intervention groups compared with the SNL saline group. However, the combined treatment groups experienced better outcomes than the single treatment groups except on day 21 (g, h and k, l); When the ratio of T10 and MK-801 was 1/3, the expression levels of GFAP and p-STAT3 were significantly reduced (g, h, and k). ##P < 0.001, SNL saline group versus sham saline or normal group; *P < 0.05, **P < 0.01, medication groups versus SNL saline group, #P < 0.05, mixed drugs administration groups versus single drug injection groups, &P < 0.05, SNL-T/M (1/3) group versus SNL-T/M (3/1) group and SNL-T/M (1/1) group, respectively. Scale bar = 120 µm in a, c and 40 µm in b, d. SNL: spinal nerve ligation; SDH: spinal dorsal horn; GFAP: glial fibrillary acidic protein.
Effect of i.t. T10, MK-801, or their combination on SNL-induced activation of NMDAR
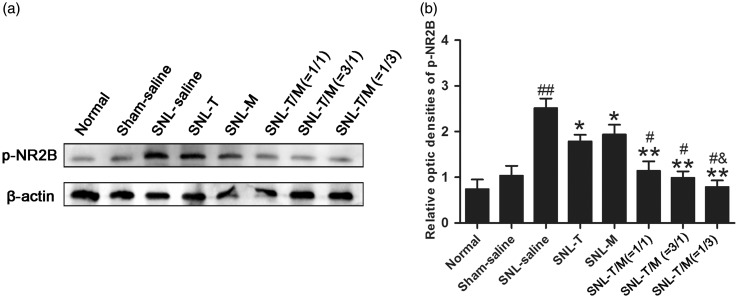
Activation of NMDARs, especially the activated subtype 2B of NMDAR (NR2B), is widely reported in rodents' SDH in chronic pain condition, we thus wanted to check whether i.t. T10 and/or MK-801 affected the expression of phosphorylated NR2B (pNR2B). The expression of pNR2B in each group on POD 7 was detected by Western blot analysis (Figure 8(a) and (b)). We observed a significant increase in pNR2B in the SDH of rat ipsilateral to the nerve ligation side (##P < 0.01). Furthermore, the increased pNR2B expression in SNL rats was markedly suppressed by treatment with T10, MK-801 (*P < 0.05), or their combination (**P < 0.01). When the ratio of T10:MK-801 was 1:3, the expression level of pNR2B was inhibited maximally (&P < 0.05).
Figure 8.
Administration of T10, MK-801, or their combination on SNL-induced expression of pNR2B. SNL injury resulted in increased levels of pNR2B in the ipsilateral SDH seven days after nerve ligation. Administration of T10, MK-801, or their combination could prevent SNL-evoked pNR2B accumulation. Combination groups have better effects than single drug groups. ##P < 0.001, SNL saline group versus sham saline or normal group; *P < 0.05, **P < 0.01, medication groups versus SNL saline group, #P < 0.05, mixed drugs administration groups versus single drug injection groups, &P < 0.05, SNL-T10/MK-801 (1/3) group versus other mixed drugs groups.
Discussion
To our knowledge, this is the first study that explores the synergistic analgesia of T10 and MK-801 in chronic NP rat. Our data demonstrate that i.t. co-administration of T10 and MK-801 in a fixed ratio of 1:3 is able to yield strongest synergistic analgesia effects in rats with SNL model by inhibiting neuroinflammation associated with microglial and astrocytic activation and down-regulating the phosphorylation of STAT3 and NR2B in the SDH.
Administration of T10 and MK-801 had synergistic analgesic effect but with weak side effects
The interest in exploiting traditional Chinese medicine for the prevention or treatment of chronic pain has been increased greatly.8,18,19 T10 has an important role in modulating immune function both through and independent of the nervous system. It is reported that T10 can inhibit neurotoxic and proinflammatory cytokine, such as inducible nitric oxide synthase and cyclooxygenase-2 in microglia.10,20 Furthermore, T10 also exerts protective effects on neurons suffering from inflammation-mediated damage.21 A recent study has demonstrated that T10 suppresses the development of NP and T-cell activation.22,23
NMDARs are involved in the development and regulation of chronic pain.24,25 MK-801, also called dizocilpine, displays a wide array of biological properties, including anticonvulsant and anesthetic properties, due to its activity as a noncompetitive NMDAR antagonist. It is reported that i.t. injection of MK-801 can significantly block SNL-induced mechanical allodynia.26,27
In our present study, we found that the alone administration of T10 or MK-801 can inhibit SNL-induced mechanical allodynia. Based upon previous studies and our present experimental data, the anti-nociceptive effect of T10 or MK-801 is dose dependent. However, high dose of T10 or MK-801 will induce accompanying toxic side effects. For example, T10 accumulates in the testis and liver will damage the normal functions of these organs. A large dose of MK-801 impairs locomotion.13 Therefore, we checked whether i.t. co-administration of T10 and MK-801 can superimpose their anti-nociceptive effects and reduce the dosage of each drug. Our results clearly show that co-administration of low dose of MK-801 and T10 have strong synergistically analgesic effect on the chronic pain. We also found that combination of T10 and MK-801 at the ratio of 1:3 is more effective than the other combination ratios. Meanwhile, the normal locomotion, which is always reported to be damaged by application of high dose of MK801, is not affected in the present used dose.
Administration of T10 and MK-801 inhibited activation of glial cells and NR2B in the spinal cord
Our previous study indicated that i.t. T10 significantly attenuates mechanical allodynia, glial cell activation, and the JAK-STAT3 pathway in SNL animal.8 In addition, spinal NMDAR activation induced by peripheral nerve injury can increase astrocytic JNK phosphorylation, which suggests that NMDAR antagonists may inhibit the activation of glial cells in chronic pain condition.28 Consistent with the abovementioned observations, i.t. alone administration of T10, MK-801, or their combination significantly reduced the expression and activation of both microglia and astrocyte and the expression of phosphorylated STAT3. T10 might directly inhibit microglial and astrocytic activation and then indirectly inhibit neuronal activation.8 In contrast, MK-801 might indirectly inhibit glial activation through the glutamate-cytokine pathway, which requires further exploration in the future works.
Modification of synaptic NMDAR expression will influence NMDAR-mediated synaptic function and associated physiological and pathological effects. Membrane NMDARs is dynamic, especially the NR2B subunit. NR2B binds to the membrane-associated guanylate kinases that regulate surface and synaptic NMDAR trafficking29. It is reported that NR2B plays role in the transmission of nociceptive information and development of chronic pain.30,31 The expression of NR2B in SDH increases 48 h after SNL in rats. This increase reaches its peak at 3 days, lasts for 14 days, and returns to pre-operational levels 28 days after SNL.32 The activation of astrocytes leads to a consequent release of neuro-excitatory substances, including prostaglandins, IL-1, IL-6, and subsequently induces the phosphorylation of NR2B subunit in spinal neurons. In this study, Western blot analysis revealed that i.t. mixed drugs can inhibit the SNL-induced elevation of pNR2B expression more obviously than i.t. alone administration. Therefore, we speculate that the regulation of the expression of pNR2B after SNL by mixed drugs may be correlated with the inhibition of astrocytic activation and the antagonism of MK-801.
Based upon these previous studies and our experimental data, we hypothesize that the synergistic analgesic mechanisms of i.t. co-administration of T10 and MK-801 might include the following possibilities. (i) Both T10 and MK-801 may be able to inhibit glutamate release, which is otherwise induced by central sensitization to pain during the “initiative phase”. (ii) T10 might directly inhibit astrocyte activation and interrupt the neuronal-astrocyte cross-talk during pain's “maintenance phase”. (iii) MK-801 might indirectly inhibit microglial or astrocytic activation due to blockage of neuronal sensitization, thereby achieving synergistic analgesia by interrupting the neuron-glial cell cross-talk. However, the underlying mechanisms remain to be clarified.
Administration of T10 as an adjuvant could be a new analgesic strategy
Clinical cases have shown that drug combinations can provide better pain relief and minimize side effects compared with singly groups. However, most analgesic methods focus on neuronal participation during chronic pain progression. In consideration of the pivotal role of glial cells during the initiation and maintenance of chronic pain, the combination of a neuronal inhibitor and a glial inhibitor may represent a new strategy for a more effective treatment of chronic pain.
Our present results showed that the co-administration of T10 and MK-801 can enhance the synergistic analgesic effects on NP, compared with the single administration of either drug. The experimental ED50 (ED50comb) of the drug combination was lower than that of the theoretical ED50 (ED50add). Moreover, the present study showed that the combination of MK-801 with T10 could induce a long-term effect even at 7–14 days after drug injection, particularly in the group with MK801:T10 equals to 3:1. Although the lifetime of MK-801 is short (approximately three days), the sedative and analgesic properties of MK-801 might contribute to this long-lasting and accumulative analgesic effect. In contrast, the lifetime of T10 is long (approximately seven days). Therefore, a combination of T10 and MK-801 could compensate the short lifetime of MK-801. More importantly, in the combination groups, the dosages of ED50comb of T10 and MK801 are all decreased but the analgesic effect are all potentiated, compared with the single application groups. Therefore, these combined groups reduce T10 and MK801 administration, indicating less potential neurotoxicity and a possible reduction in additional side effects.
Conclusions
These results demonstrate that the i.t. injection of the traditional Chinese medicine T10 and the neuronal activity inhibitor MK-801 can effectively reduce NP. Moreover, the combination of T10 and MK-801 results in synergistic effects on NP by inhibiting the neuroinflammation associated with microglial and astrocyte activation. Comparatively, T10 has a slow-acting but long-term effect, while MK-801 has a fast-acting and short-term effect in treating pain process. Therefore, when combined, these two drugs could treat various types of NP in which the spinal “glial activation” and “phosphorylation level of NMDAR-2B subunit” mechanism may be involved in. Our results thus provide a more potent medication strategy for the treatment of NP by combining a modern analgesic with traditional Chinese medicine.
Supplementary Material
Author contributions
J Wang, Y Qiao, and R-S Yang contributed equally to this work. Y-Q Li, Y-L Dong, and J-L Li corresponded equally to this work in designing and supervising the project. J Wang, Y Qiao, R-S Yang, and C-K Zhang performed the animal surgery, carried out the immunofluorescence and Western blot study, and drafted the manuscript. J-J Lin and T Zhang performed the behavioral test. H-H Wu participated in producing graphics and performed the statistical analysis. J Wang, Y Qiao, R-S Yang, and C-K Zhang conceived the study and participated in its design and coordination. T Chen, Y-Q Li, Y-L Dong, and J-L Li designed the experiment. All authors read and approved the final manuscript.
Declarations of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81571074, 31671087, 31371126 and 81671095), the National Key Scientific Instrument & Equipment Development Program of China (2012YQ03026009 to Y-Q Li), and an intramural grant of the Fourth Military Medical University (4139C4IAA1).
Supplemental Material
Supplementary material is available for this article online.
References
- 1.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 2009; 32: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol 2003; 60: 1524–1534. [DOI] [PubMed] [Google Scholar]
- 3.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei XP, Zhang H, Wang W, et al. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J Neuroinflammation 2011; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci 2005; 28: 101–107. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain 2011; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hailong G, Yujie Z, Hanying M, et al. Effectiveness of triptolide-coated stent on decreasing inflammation and attenuation of intimal hyperplasia in a pig after coronary angioplasty. Angiology 2011; 62: 265–269. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Li ZH, Ge SN, et al. The inhibition of spinal astrocytic JAK2-STAT3 pathway activation correlates with the analgesic effects of triptolide in the rat neuropathic pain model. Evid Based Complement Alternat Med 2012; 2012: 185167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang F, Bai XJ, Hu D, Li ZF, Liu KJ. Effect of triptolide on secretion of inflammatory cellular factors TNF-alpha and IL-8 in peritoneal macrophages of mice activated by lipopolysaccharide. World J Emerg Med 2010; 1: 70–74. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang T, Gong X, Hu G, Wang X. EP2-PKA signaling is suppressed by triptolide in lipopolysaccharide-induced microglia activation. J Neuroinflammation 2015; 12: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou HF, Niu DB, Xue B, et al. Triptolide inhibits TNF-alpha, IL-1 beta and NO production in primary microglial cultures. Neuroreport 2003; 14: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 12.Suhs KW, Fairless R, Williams SK, et al. N-methyl-D-aspartate receptor blockade is neuroprotective in experimental autoimmune optic neuritis. J Neuropathol Exp Neurol 2014; 73: 507–518. [DOI] [PubMed] [Google Scholar]
- 13.Vandame D, Ulmann L, Teigell M, et al. Development of NMDAR antagonists with reduced neurotoxic side effects: a study on GK11. PLoS One 2013; 8: e81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther 2001; 298: 865–872. [PubMed] [Google Scholar]
- 15.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 16.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992; 50: 355–363. [DOI] [PubMed] [Google Scholar]
- 17.Wu HH, Yin JB, Zhang T, et al. Inhibiting spinal neuron-astrocytic activation correlates with synergistic analgesia of dexmedetomidine and ropivacaine. PLoS One 2014; 9: e92374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Mei XP, Chen L, et al. Triptolide prevents and attenuates neuropathic pain via inhibiting central immune response. Pain Physician 2012; 15: E995–1006. [PubMed] [Google Scholar]
- 19.Hu JY, Li CL, Wang YW. Intrathecal administration of triptolide, a T lymphocyte inhibitor, attenuates chronic constriction injury-induced neuropathic pain in rats. Brain Res 2012; 1436: 122–129. [DOI] [PubMed] [Google Scholar]
- 20.Gong Y, Xue B, Jiao J, Jing L, Wang X. Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappaB and JNK in LPS-treated microglia. J Neurochem 2008; 107: 779–788. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Li F, Wang X. Novel anti-inflammatory and neuroprotective agents for Parkinson's disease. CNS Neurol Disord Drug Targets 2010; 9: 232–240. [DOI] [PubMed] [Google Scholar]
- 22.Yan YH, Shang PZ, Lu QJ, Wu X. Triptolide regulates T cell-mediated immunity via induction of CD11c(low) dendritic cell differentiation. Food Chem Toxicol 2012; 50: 2560–2564. [DOI] [PubMed] [Google Scholar]
- 23.Taguchi T, Yasui M, Kubo A, et al. Nociception originating from the crural fascia in rats. Pain 2013; 154: 1103–1114. [DOI] [PubMed] [Google Scholar]
- 24.Zhou XL, Zhang CJ, Wang Y, et al. EphrinB-EphB signaling regulates spinal pain processing via PKCgamma. Neuroscience 2015; 307: 64–72. [DOI] [PubMed] [Google Scholar]
- 25.Xia T, Cui Y, Shi H, Ma Z, Gu X. The effect of NR2B subunit palmitoylation at the spinal level after chronic dorsal root ganglia compression in rats. Anesth Analg 2014; 119: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 26.Srebro DP, Vuckovic SM, Savic Vujovic KR, Prostran MS. TRPA1, NMDA receptors and nitric oxide mediate mechanical hyperalgesia induced by local injection of magnesium sulfate into the rat hind paw. Physiol Behav 2015; 139: 267–273. [DOI] [PubMed] [Google Scholar]
- 27.Cavalcante AL, Siqueira RM, Araujo JC, et al. Role of NMDA receptors in the trigeminal pathway, and the modulatory effect of magnesium in a model of rat temporomandibular joint arthritis. Eur J Oral Sci 2013; 121: 573–583. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, Zhu C, Li ZH, et al. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J Neuroinflammation 2015; 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao YX, Rumbaugh G, Wang GD, et al. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 2003; 23: 6703–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda A, Mickle A, Bruckert M, Kannampalli P, Banerjee B, Sengupta JN. NMDA receptor mediates chronic visceral pain induced by neonatal noxious somatic stimulation. Eur J Pharmacol 2014; 744: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Mei XP, Wei YY, et al. Neuronal NR2B-containing NMDA receptor mediates spinal astrocytic c-Jun N-terminal kinase activation in a rat model of neuropathic pain. Brain Behav Immun 2011; 25: 1355–1366. [DOI] [PubMed] [Google Scholar]
- 32.Geng SJ, Liao FF, Dang WH, et al. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 2010; 222: 256–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.