Abstract
Creutzfeldt–Jakob disease accounts for more than 90% of all sporadic prion disease cases. The molecular MM2 genotype has been divided into cortical and thalamic subtypes based on structures involved and is characterized clinically by progressive dementia without ataxia or typical electroencephalography changes. Proposed diagnostic criteria for MM2 cortical type sporadic Creutzfeldt–Jakob disease include progressive dementia, cortical hyper-intensity on diffusion-weighted magnetic resonance imaging, increased cerebrospinal fluid 14-3-3 protein level, and the exclusion of other types of dementia. The presence of periodic discharges on electroencephalography in MM2 cortical type were reported in 42% of the cases. We are reporting a case of sporadic Creutzfeldt–Jakob disease cortical MM2-type presenting with rapid cognitive decline, who survived 8 months since symptom onset. Brain imaging, cerebrospinal fluid analysis, and long-term electroencephalography monitoring were obtained and diagnosis was confirmed by autopsy. Short-term electroencephalography recording, performed 5 months after symptom onset, demonstrated diffuse background slowing without epileptiform activity. Long-term video electroencephalography monitoring demonstrated generalized slowing, maximum in bilateral frontal areas, which intermittently would become rhythmic (1–2 Hz) without hemispheric predominance. If the findings do not clearly meet the proposed clinical criteria for sporadic Creutzfeldt–Jakob disease, the use of long-term electroencephalography could increase the sensitivity. We question whether the lack of the characteristic findings on electroencephalography in some cases could be due to insufficient time of recording. Application of long-term electroencephalography monitoring increases the sensitivity of electroencephalography and the certainty of pre-mortem diagnosis of sporadic Creutzfeldt–Jakob disease.
Keywords: Neurology, prion disease, cognitive neurology, diagnostic criteria, Creutzfeldt–Jakob disease, sporadic Creutzfeldt–Jakob disease, long-term electroencephalography
Case description
A 49-year-old man presented to the cognitive and memory disorder outpatient clinic for work up of rapid cognitive decline. Patient’s family reported that his symptoms started with confusion, memory, and language impairment (naming errors and comprehension difficulties) 5–6 months prior to presentation. Soon after developing cognitive impairment, he developed gait abnormalities and action tremor. The symptoms were worsening. His decision-making became impaired and he had to retire from work 1 month after the onset of his symptoms. His medical history was remarkable for childhood dyslexia and lymphoma treated with chemotherapy more than a decade ago. There was no family history of neurological disorders. He was a former smoker with no history of alcohol or drug use. There was no recent travel history. The patient has never had any neurosurgical or ophthalmological procedures done.
On the initial evaluation, the patient was alert and awake but only oriented to person. He appeared emotionally labile and demonstrated frequent verbal and motor perseverations, echolalia, and echopraxia. Processing time was prolonged. Working memory and visuospatial functions were impaired and he had difficulty with object naming and recognition. General neurologic examination did not demonstrate myoclonus or other types of adventitious movements. His muscle tone was normal and gait evaluation demonstrated mild ataxia. Deep tendon reflexes were brisk throughout.
The patient had two magnetic resonance imaging (MRIs) of the brain performed: the first, 1 month into the disease and the second, 5 months later. Both brain scans demonstrated restricted diffusion in a gyral pattern along the cortical gray matter in bilateral parietal and occipital lobes, with lesser involvement of prefrontal cortical gray matter. Primary motor and sensory cortices were spared. There were mild signal changes in the corresponding regions on fluid attenuation inversion recovery (FLAIR) sequences. There were no signal changes in subcortical structures and no gadolinium enhancement present. Fluorodeoxyglucose-positron emission tomography (FDG-PET) scan of the brain showed diminished metabolism, predominantly in the posterior brain regions.
Cerebrospinal fluid analysis was performed twice. Concentrations of amyloid-β (Aβ42), total-tau (t-tau), phospho-tau181 (p-tau), and Aβ42-Tau Index (ATI) were measured by commercial assay (Athena) on the first cerebrospinal fluid (CSF) specimen. It was negative for protein 14-3-3 (Aβ42—498.95 pg/ml, t-tau—654.45 pg/ml, p-tau—43.4 pg/ml, ATI—0.49).
The second CSF analysis performed 4 months later demonstrated t-tau protein of 1179 pg/mL (>1150: 76% probability of prion disease), negative for 14-3-3 protein, and negative real-time quaking-induced conversion (RT-QuIC). Neuron-specific enolase was within normal limit (1.6 μg/L), with reference range of 1.0–7.0).
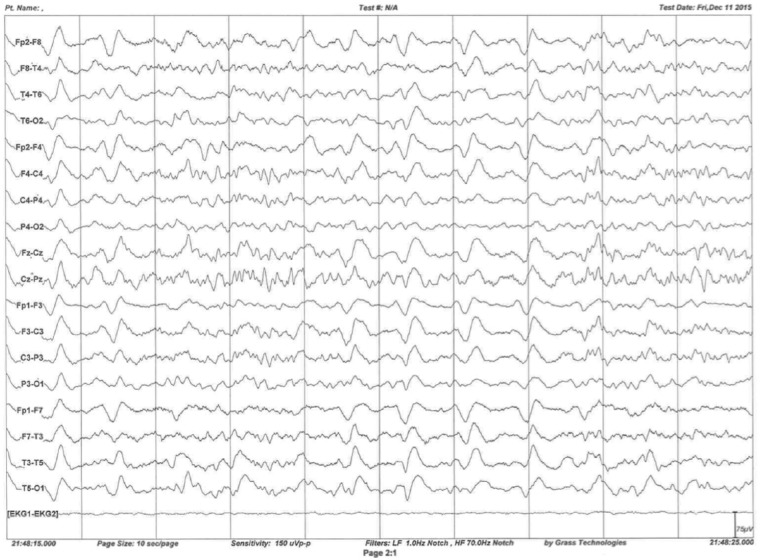
A routine 45-min electroencephalography (EEG) was obtained. It demonstrated diffuse background slowing at 2–5 Hz without epileptiform features. As part of a comprehensive evaluation of the patient, he was admitted to the inpatient neurology service and continuous video EEG monitoring was started. The long-term monitoring went on for almost 38 h and demonstrated generalized medium-to-high amplitude slowing, more prominent in bilateral frontal regions at 2–6 Hz frequencies. At times, it demonstrated rhythmic 1–2 Hz discharges. Although a normal posterior dominant rhythm with a frequency of 8–9 Hz in the most alert state with attenuation with eye opening was identified, the study was considered abnormal due to generalized, intermittent rhythmic slowing and absence of normal sleep structures. The routine and long-term EEGs were performed approximately 6 months after emerging of the initial symptoms (Figure 1).
Figure 1.
Long-term EEG monitoring demonstrated intermittent rhythmic generalized slowing (1–2 Hz).
The patient rapidly deteriorated and developed severe aphasia, apraxia, agnosia, and abulia. He died 8 months after developing the initial clinical symptoms.
The patient underwent an autopsy. The brain tissue was sent for special analysis for confirmation of the diagnosis. Western blot, histopathological, and immunohistochemical examination of the autopsied tissue demonstrated the presence of abnormal protease-resistant prion protein PrPSc (PrP 27-30) confirming the diagnosis of MM2 subtype of sporadic Creutzfeldt–Jakob disease (sCJD) according to the old and current criteria for classification of sporadic prion disease.1,2 The prion protein (PRNP) gene sequencing analysis did not demonstrate pathogenic mutation at the coding region of the PRNP gene, therefore a familial prion disease was ruled out based on the current criteria.
Discussion
Prion diseases or transmissible spongiform encephalopathies are a group of fatal neurodegenerative disorders caused by the conversion of the normal prion protein (PrPC) with a primarily α-helical structure into an abnormal form of the protein called the scrapie prion protein (PrPSc).3,4 Human prion diseases can occur as spontaneous (sporadic), genetic (familial), and acquired (infectious/transmitted). The 80%–95% of cases are sCJDs, 10%–15% are genetic (often familial), and less than 1% are acquired.5
Sporadic forms of prion disease include sCJD, fatal insomnia, and the recently identified variably protease-sensitive prionopathy.6
More than 90% of all sporadic forms are Creutzfeldt–Jakob disease (CJD). Based on the predominant clinical signs, histological and molecular findings, sCJD is commonly categorized into five subtypes with three affecting prominently cognitive functions and the other two—cerebellar motor activities.5
The codon 129 polymorphism and the existence of one of the two types of protease-resistant PrP are the major determinants of phenotypic heterogeneity in sCJD. On the basis of these characteristics, six molecular subtypes of the sCJD can be identified: MM1, MV1 (both are also called Typical), VV1 (Early onset), MM2 (Long duration), MV2 (Kuru plaques), and VV2 (Ataxic).7 Each genotype corresponds to a different clinical and neuropathological phenotype. The MM2 genotype is characterized clinically by progressive dementia with ataxia and typical EEG changes being less common.8
The sCJD MM2 subtype (combination of methionine homozygosity and PrPsc type 2) has a thalamic form (MM2T) and a cortical form (MM2C). Pathologically, large confluent vacuoles and perivacuolar labeling for PrP in all cortical layers characterizes the MM2C subtype.2
MM2T and MM2C subtypes are rare (only 1% of sCJD cases each). A phenotype with combined features of both subtypes (MM2T + C) has been rarely described as well.9
MM2C subtype is a late-onset, slowly progressive form frequently associated with cortical hyperintense signals on diffusion-weighted magnetic resonance imaging (DW-MRI) sequences and elevated CSF levels of 14-3-3 protein.
Cortical hyperintense signals present on diffusion-weighted sequences of brain MRI are useful for the diagnosis of the cortical form. Whereas, in the thalamic form, thalamic hypoperfusion, and hypometabolism can be demonstrated using brain single photon emission computed tomography (SPECT) and FDG-PET scans, respectively.
Hamaguchi et al.10 proposed a diagnostic criteria for MM2C sCJD—(1) progressive dementia, (2) cortical hyperintense signal changes on diffusion-weighted imaging (DWI), and (3) increased CSF 14-3-3 protein level—and exclusion of other types of cognitive disorders. Other neuropsychiatric abnormalities or periodic sharp wave complexes seen on EEG are not necessarily required.10
EEG in CJD
Periodic sharp wave complexes (PSWCs) are symmetrical generalized triphasic, biphasic, or mixed complexes occurring approximately every second on the EEG. This periodic EEG pattern is characteristic and, in the right clinical setting, consistent with CJD but is not pathognomonic. Detection of PSWCs during EEG recording is associated with a sensitivity of 67% and specificity of 86% for detection of CJD.11
EEG changes evolve during the disease.12 Lateralized PSWCs have been described early in the disease course. However, nonspecific diffuse slowing background or other nonspecific signs of cerebral dysfunction are most commonly detected early EEG abnormalities.13 Lateralized PSWCs sometimes progress to more symmetrical and generalized complexes. The characteristic PSWCs are commonly seen during the second phase of the disease, usually about 3 months after the initial presentation. With time, the background amplitude becomes attenuated and the frequency of PSWCs starts declining.
Different studies have reported that majority CJD patients would develop PSWCs at some point during the disease; however, this finding is neither 100% specific nor sensitive.
EEG findings are being studied in patient groups classified according to PRNP gene polymorphism. In a study by Iwasaki et al. among 18 MM1-type cases, PSWCs were observed in 17 (94.4%) cases. Meanwhile, in the two thalamic MM2-type cases, PSWCs were not observed. Parchi et al.1 did not find PSWCs in six cortical and six thalamic MM2 CJD patients.
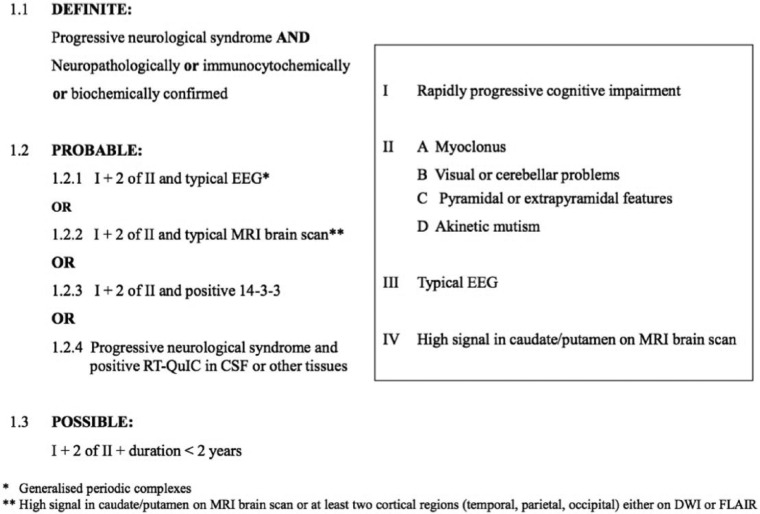
In a study by Jansen et al., all five MM1 patients showed typical EEG findings, but the only patient with MM2 subtype was found to have atypical EEG findings, which were nonspecific in nature. PSWCs are reported to be detected in 42% of MM2 patients. Based on the proposed criteria, detection of PSWCs in routine EEG helps with diagnosing the patient with probable CJD (Figure 2).2,14
Figure 2.
Sporadic CJD diagnostic criteria (January 2017).
Conclusion
However, there is no data available to date to demonstrate whether increasing the duration of EEG recording could improve the sensitivity of detection of abnormalities associated with sCJD. It is also not known whether topographical localization of PSWCs correlates with the regions of signal abnormalities detected on DWI imaging of the brain.
In the absence of other findings, the use of long-term EEG could be implemented to increase the sensitivity of EEG in diagnosing sCJD.
The lack of the PSWCs on EEG in some cases could be due to insufficient time of recording. Increasing the duration of EEG monitoring can increase the diagnostic sensitivity by capturing episodes of PSWCs if the clinical picture and supporting data are otherwise equivocal.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Verbal and written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Parchi P, Giese A, Capellari S, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 1999; 46(2): 224–233. [PubMed] [Google Scholar]
- 2. http://www.cjd.ed.ac.uk/ https://www.cjd.ed.ac.uk/sites/default/files/diagnostic%20criteria.pdf
- 3. Geschwind MD. Prion diseases. Continuum 2015; 21(6): 1612–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansen C, Head MW, Rozemuller AJ, et al. Panencephalopathic Creutzfeldt-Jakob disease in The Netherlands and the UK: clinical and pathological characteristics of nine patients. Neuropathol Appl Neurobiol 2009; 35(3): 272–282. [DOI] [PubMed] [Google Scholar]
- 5. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 2012; 11(7): 618–628. [DOI] [PubMed] [Google Scholar]
- 6. Zou WQ, Puoti G, Xiao X, et al. Variably protease-sensitive prionopathy: a new sporadic disease of the prion protein. Ann Neurol 2010; 68(2): 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gambetti P, Kong Q, Zou W, et al. Sporadic and familial CJD: classification and characterisation. Br Med Bull 2003; 66: 213–239. [DOI] [PubMed] [Google Scholar]
- 8. Krasnianski A, Meissner B, Schulz-Schaeffer W, et al. Clinical features and diagnosis of the MM2 cortical subtype of sporadic Creutzfeldt-Jakob disease. Arch Neurol 2006; 63(6): 876–880. [DOI] [PubMed] [Google Scholar]
- 9. Grau-Rivera O, Sanchez-Valle R, Bargallo N, et al. Sporadic MM2-thalamic + cortical Creutzfeldt-Jakob disease: utility of diffusion tensor imaging in the detection of cortical involvement in vivo. Neuropathology 2016; 36(2): 199–204. [DOI] [PubMed] [Google Scholar]
- 10. Hamaguchi T, Kitamoto T, Sato T, et al. Clinical diagnosis of MM2-type sporadic Creutzfeldt-Jakob disease. Neurology 2005; 64(4): 643–648. [DOI] [PubMed] [Google Scholar]
- 11. Steinhoff BJ, Racker S, Herrendorf G, et al. Accuracy and reliability of periodic sharp wave complexes in Creutzfeldt-Jakob disease. Arch Neurol 1996; 53(2): 162–166. [DOI] [PubMed] [Google Scholar]
- 12. Chiofalo N, Fuentes A, Galvez S. Serial EEG findings in 27 cases of Creutzfeldt-Jakob disease. Arch Neurol 1980; 37(3): 143–145. [DOI] [PubMed] [Google Scholar]
- 13. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus 2015; 39(5): E2. [DOI] [PubMed] [Google Scholar]
- 14. Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009; 132(Pt 10): 2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]