Abstract
Background:
Capitellar osteochondritis dissecans (OCD) in skeletally immature athletes has often been seen in baseball players and gymnasts. The choice of surgical procedure for unstable lesions in skeletally immature athletes remains controversial.
Purpose:
To investigate functional outcomes and radiographic changes in the midterm to long-term postoperative period after arthroscopic (AS) resection for small to large capitellar OCD lesions in skeletally immature athletes.
Study Design:
Case series; Level of evidence, 4.
Methods:
A total of 38 elbows in 38 patients (33 boys, 5 girls; mean age, 14 years [range, 13-15 years]) with skeletally immature elbows underwent AS resection for capitellar OCD. Patients were observed for at least 5 years (mean, 8 years [range, 5-12 years]). Elbows with a lesion width that did not exceed one-half of the radial head diameter were assigned to group 1 (n = 17 elbows), and larger lesions were assigned to group 2 (n = 21 elbows). Functional scores, patient satisfaction, range of motion (ROM), and osteoarthritis (OA) grades were evaluated between the groups.
Results:
All patients returned to sports activity. Functional scores at the final follow-up were not significantly different between the groups. Patient satisfaction scores were significantly higher in group 1 than in group 2. There was significant improvement in flexion ROM at the final follow-up compared with preoperative values in group 1 (P = .017), and there was a significant between-group difference (group 1: 141°; group 2: 133°; P = .002). Extension ROM showed significant improvement in both groups (group 1: from –8° to 3°; group 2: from –17° to –1°; P < .001 for both). Group 1 tended to have better extension than group 2, but the difference was not significant. There were no elbows with severe OA in either group, but the OA grade progressed in 5 elbows (29%) in group 1 and 9 elbows (43%) in group 2, and this rate of OA progression was statistically significant between groups (P = .005).
Conclusion:
Both functional outcomes and radiological findings after AS fragment resection were excellent in elbows with small lesions. Although overall outcomes were acceptable in elbows with larger lesions, flexion ROM and patient satisfaction scores were significantly inferior to those in elbows with smaller lesions.
Keywords: elbow, osteochondritis dissecans, adolescent, arthroscopic fragment resection, baseball, gymnast
Capitellar osteochondritis dissecans (OCD) in skeletally immature athletes is often seen in baseball players and gymnasts. Repetitive microtrauma and poor blood supply have been proposed as the causes of this disorder.10,33 Conservative treatment is usually indicated for stable lesions with an open capitellar growth plate, and many studies have reported good outcomes after nonoperative treatment.11,15,26–29 Surgery is indicated for unstable lesions with a range of motion (ROM) loss of >20°, a closed capitellar growth plate, and high intensity in the capitellum on T2-weighted magnetic resonance imaging.26 There are a large variety of surgical procedures for unstable capitellar OCD, including fragment resection by open or arthroscopic (AS) procedures, fragment fixation with bone pegs or Herbert screws, and osteochondral grafting; however, the choice of surgical procedure for unstable lesions in skeletally immature athletes remains controversial.‡
AS fragment resection has an advantage in terms of minimum invasion and rapid recovery of function in small lesions.1,3,8,18,22,23 Some have reported good short-term clinical outcomes after AS fragment resection for larger lesions,1,3,22 while others have reported poor outcomes after AS fragment resection with open physis of the radial head.18 To date, there has been no information regarding longer term clinical outcomes after AS fragment resection for small to large lesions.
Before 2007, we exclusively performed AS fragment resection for all inviable lesions regardless of the lesion size. However, we began to perform osteochondral grafting for selected larger lesions in 2007. Then, the indication for grafting was gradually expanded until 2010. Since 2010, we have been performing AS fragment resection for small lesions (<50% of the capitellar articular width) and osteochondral grafting for larger lesions (>50% of the capitellar articular width), and we have been satisfied with the clinical outcomes based on our current surgical indication.25 However, our concern was that patients who underwent AS fragment resection for larger lesions before 2010 may have progressed to osteoarthritis (OA) or severe motion loss. Therefore, the purpose of this retrospective study was to investigate functional outcomes and radiographic changes in the midterm to long-term postoperative period after AS resection for small to large capitellar OCD lesions in skeletally immature athletes. We hypothesized that, although functional and radiological outcomes of small lesions would remain acceptable, those for larger lesions would not be acceptable in the longer term.
Methods
We received institutional review board approval before initiating this study and obtained informed consent from all participating patients.
Patient Selection
Between April 2002 and May 2010, a total of 77 elbows in 76 consecutive patients with skeletally immature elbows, in which there were open epiphyseal lines of the lateral epicondyle in both affected and contralateral elbows, underwent AS fragment resection for unstable capitellar OCD lesions. In all cases, OCD fragments were symptomatic and displaced or detached, which corresponded to grade III in the radiographic classification of Takahara et al.26 These patients were selected as candidates for this retrospective follow-up study, but only 38 elbows had >5-year follow-up. Thus, this study included 38 elbows in 38 patients (33 male and 5 female) with a mean age of 14 years (range, 13-15 years) at the time of surgery. The mean follow-up was 8 years (range, 5-12 years). Twenty-seven patients were engaged in baseball, 7 in gymnastics, and 4 in other sports (handball, basketball, badminton, and judo).
Surgical Procedures
All surgical procedures were performed by 1 of 2 senior authors (H.S., N.T.) in the prone position under general anesthesia. First, the arthroscope was introduced from an anteromedial portal, and an anterolateral portal was created using the outside-in technique. Routine diagnostic arthroscopic surgery was carried out in the anterior compartment, and synovial debridement and removal of loose bodies were performed if present (Figure 1A). Next, a posterolateral portal was established in the lateral side of the soft spot, which was the posterior radiocapitellar joint, followed by the establishment of a medial soft-spot portal, which was located at the superomedial corner of the soft spot; resection of unstable fragments was performed, and the lesion was debrided until bleeding was detected from the bone marrow using these 2 portals (Figure 1B). Drilling was not performed. Then, the arthroscope was introduced to the posterior compartment through the lateral gutter, and 1 or 2 posterior portals were created in the olecranon fossa. Finally, loose body resection was carried out using these posterior portals.
Figure 1.
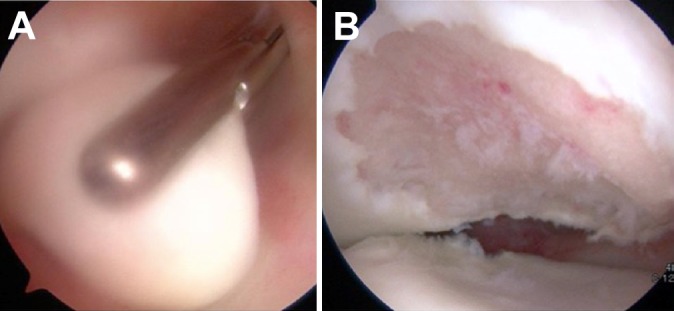
(A) Loose body removal in the anterior compartment. (B) Posterior view of the radiocapitellar joint after debridement of a large capitellar osteochondritis dissecans lesion.
Postoperative Procedures
Postoperatively, an arm sling was used for 2 days, and all patients were allowed to use their arm for activities of daily living after stitch removal. Physical therapy to resume elbow ROM and correct scapular dyskinesis was initiated right after surgery. Most of the patients returned to their sporting activity 1 month after surgery and returned to competition 3 months after surgery.
Clinical Evaluation
Patients were preoperatively and postoperatively evaluated with the Japanese Orthopaedic Association–Japan Elbow Society (JOA-JES) elbow function score for sports injuries (Table 1),20 the Disabilities of the Arm, Shoulder and Hand (DASH) score,4 and flexion and extension ROM. Postoperative return to sports and patient satisfaction (0-100 scale) were also assessed. The pain subset of the JOA-JES score was used for the evaluation of preoperative and postoperative pain.
TABLE 1.
Japanese Orthopaedic Association–Japan Elbow Society (JOA-JES) Elbow Function Score for Sports Injuries
| Variable | Points |
|---|---|
| Pain | |
| None | 30 |
| Mild | 20 |
| Moderate | 10 |
| Severe | 0 |
| Total arc of flexion/extension | |
| ≥136° | 22 |
| 121°-135° | 18 |
| 91°-120° | 15 |
| 61°-90° | 10 |
| 31°-60° | 5 |
| 16°-30° | 3 |
| ≤15° | 0 |
| Total arc of supination/pronation | |
| ≥151° | 8 |
| 121°-150° | 6 |
| 91°-120° | 4 |
| 31°-90° | 2 |
| ≤30° | 0 |
| Instability | |
| No instability | 10 |
| ≤10° | 5 |
| ≥11° | 0 |
| Sports performance | |
| Full performance | 30 |
| Mild impairment | 20 |
| Moderate impairment | 10 |
| Severe impairment | 5 |
| Unable to play | 0 |
| Nerve disorder | |
| Complete paralysis | –20 |
| Incomplete paralysis | –10 |
| None | 0 |
Radiographic Evaluation
Patients underwent bilateral radiography preoperatively and postoperatively including anteroposterior and lateral views as well as a tangential view, which was an anteroposterior view with 45° of elbow flexion. Multislice computed tomography (CT) was also performed preoperatively with a 0.5-mm slice thickness (Alexion; Toshiba). Coronal and sagittal images were subsequently reconstructed from the axial data, and then, a 3-dimensional CT image of the distal humerus was created with the radius and ulna digitally subtracted. The OCD lesion size was determined with preoperative CT images utilizing the classification of Takahara et al.27 The radial head diameter was measured with coronal images, and the lesion width was measured with 3-dimensional CT at the maximum width. Lesions whose width did not exceed one-half of the radial head diameter were assigned to group 1 (17 elbows), and the larger lesions were assigned to group 2 (21 elbows).
OA was evaluated with tangential and lateral views according to the Kellgren-Lawrence grade (grade 0, no radiographic features of OA; grade 1, doubtful joint space narrowing [JSN] and possible osteophytic lipping; grade 2, definite osteophytes and possible JSN; grade 3, multiple osteophytes, definite JSN, sclerosis, and possible bony deformities; grade 4, large osteophytes, marked JSN, severe sclerosis, and definite bony deformities).9
Enlargement and superior migration of the radial head were evaluated with anteroposterior views. Enlargement of the radial head was defined as a >2-mm difference of the head diameter between the affected and nonaffected elbows. A line was drawn through the proximal end of the radius, and the distance between the line and the tip of the coronoid process was measured (Figure 2). Superior migration of the radial head was defined as a >2-mm side-to-side difference of the distance.
Figure 2.
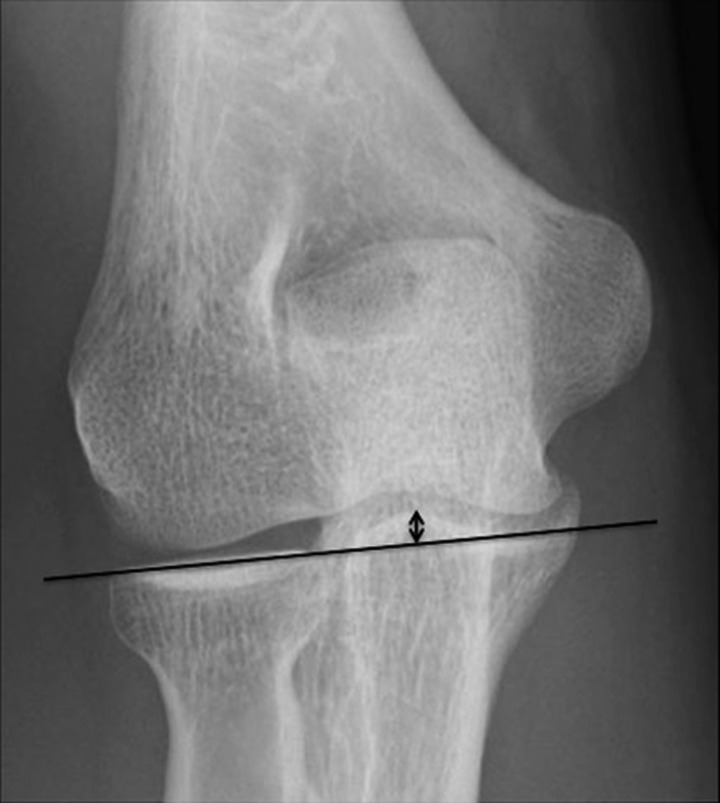
Evaluation of superior migration of the radial head. A line was drawn through the proximal end of the radius, and the distance between the line and the tip of the coronoid process was measured (arrow). Superior migration of the radial head was defined as a >2-mm side-to-side difference of this distance.
Statistical Analysis
Statistical analysis was conducted with the use of Statcel software (version 3; OMS Institute). The Mann-Whitney U test was used for comparison of OA grades between groups 1 and 2. The paired t test was used for comparison of preoperative and postoperative clinical scores, pain, and ROM. The Welch T test was used for comparison of satisfaction, clinical scores, pain, and ROM between the groups. The level of significance was set at P < .05.
Results
Clinical Evaluation
The JOA-JES and DASH scores at the final follow-up were significantly improved in both groups compared with preoperative scores (P < .001 for both). There were no significant differences in the scores between the groups. Pain scores at the final follow-up were significantly improved in both groups compared with preoperative values (Table 2). There were no significant differences in either preoperative or postoperative pain scores between the groups. The patient satisfaction score was significantly lower in group 2 than in group 1 (P = .006) (Table 2). All patients returned to sports activity.
TABLE 2.
Comparison of Clinical Evaluation Scoresa
| JOA-JES Score | DASH Score | JOA-JES Pain Subscore | Patient Satisfaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Final | P Value | Preoperative | Final | P Value | Preoperative | Final | P Value | ||
| Group 1 | 66 (40-90) | 94 (86-100) | <.001 | 46 (22-74) | 3 (0-14) | <.001 | 16 (5-20) | 26 (20-30) | <.001 | 92 (70-100) |
| Group 2 | 60 (28-78) | 91 (82-100) | <.001 | 49 (21-76) | 3 (0-14) | <.001 | 18 (0-30) | 25 (20-30) | <.001 | 80 (50-100) |
| P value | .2 | .1 | .7 | .9 | .4 | .2 | .006 | |||
aValues are presented as mean (range). DASH, Disabilities of the Arm, Shoulder and Hand; JOA-JES, Japanese Orthopaedic Association–Japan Elbow Society.
Range of Motion
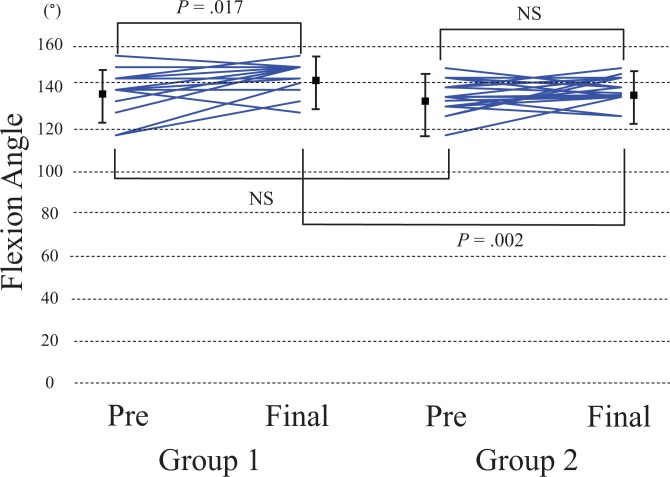
In group 1, flexion ROM was significantly improved at the final follow-up compared with preoperative values, from a mean of 135° (range, 115°-150°) to 141° (range, 125°-150°) (P = .017). Preoperative and postoperative flexion ROM were 131° (range, 110°-145°) and 133° (range, 120°-145°), respectively, in group 2, and the change was not significant (P = .1). There was no significant difference in preoperative flexion ROM between the groups (P = .4), while a significant difference was detected at the final follow-up (P = .002) (Figure 3).
Figure 3.
Individual changes in the elbow flexion angle. The dots and bars represent means and SDs.
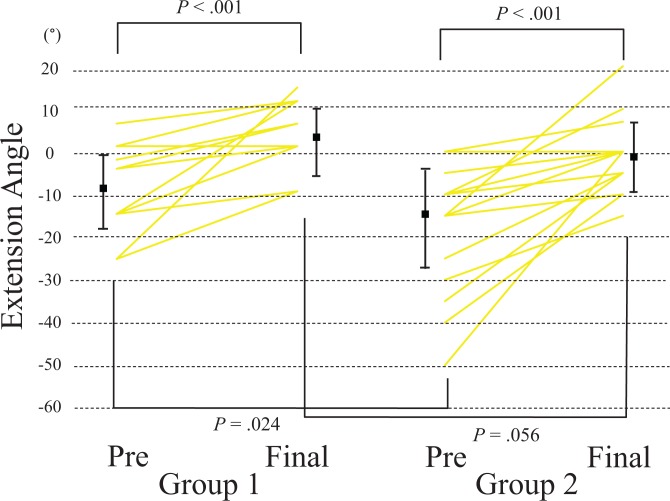
Extension ROM was significantly improved in both groups, from a mean of –8° (range, –25° to 3°) to 3° (range, –10° to 13°) in group 1 (P < .001) and from –17° (range, –50° to 0°) to –1° (range, –10° to 20°) in group 2 (P < .001) (Figure 4). Group 1 had better extension than group 2 preoperatively (P = .024), but the difference was not significant at the final follow-up (P = .056) (Figure 4).
Figure 4.
Individual changes in the elbow extension angle. The dots and bars represent means and SDs.
Radiographic Evaluation
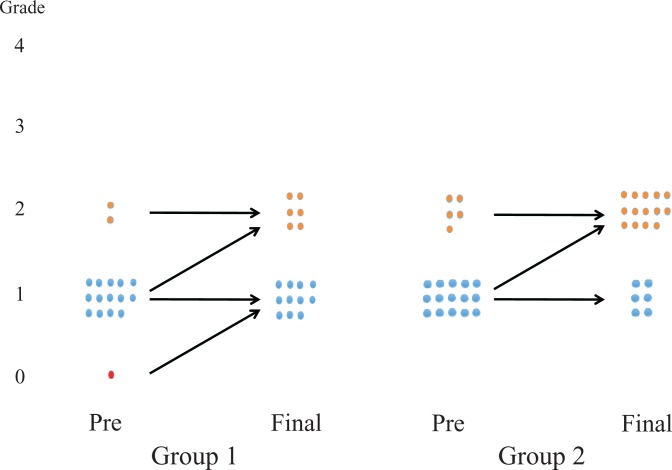
In group 1, preoperative OA was grade 0 in 1 elbow, grade 1 in 14 elbows, and grade 2 in 2 elbows; in group 2, there were 15 elbows with grade 1 and 6 with grade 2 OA (Figure 5). There was no difference in preoperative OA grades between the groups (P = .3). OA progressed postoperatively by 1 grade in 5 elbows (29%) in group 1 and 9 elbows (43%) in group 2, but there were no grade 3 or 4 OA elbows in either group. Group 2 had a significantly higher rate (43%) in radiographic OA progression than group 1 (29%) (P = .005).
Figure 5.
Diagram comparing preoperative and final Kellgren-Lawrence osteoarthritis (OA) grades. OA progressed by 1 grade in 5 elbows in group 1 and 9 elbows in group 2; the change was significant in group 2 (P = .005).
Radial head enlargement was found preoperatively in 19 elbows (100%) in group 1 and 23 elbows (96%) in group 2 and postoperatively in all elbows of both groups. No elbows showed superior migration of the radial head preoperatively (Figures 6 and 7). One elbow, from a 14-year-old patient in group 2, showed a postoperative 2.5-mm superior migration with grade 2 OA change at the final follow-up (10 years after surgery) (Figure 8).
Figure 6.
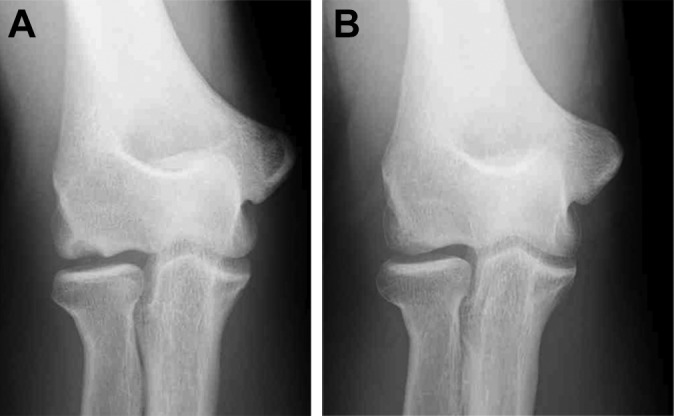
A 14-year-old male baseball player with a small capitellar osteochondritis dissecans lesion. (A) Preoperative anteroposterior tangential view. (B) Tangential view at the age of 23 years. The lesion was well remodeled.
Figure 7.
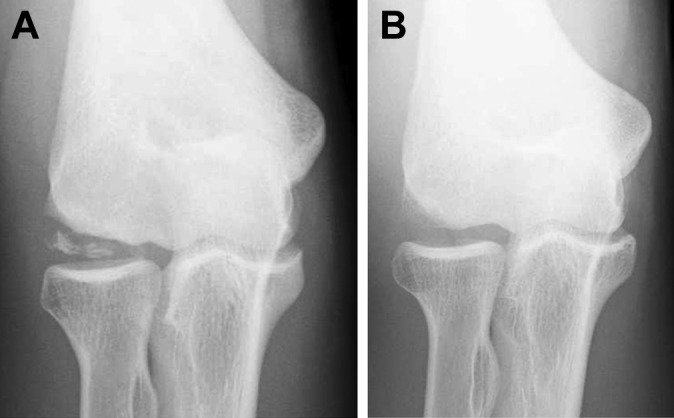
A 14-year-old male baseball player with a large capitellar osteochondritis dissecans lesion. (A) Preoperative anteroposterior tangential view. (B) Tangential view at the age of 23 years. The bony defect diminished with remodeling of the capitellum.
Figure 8.
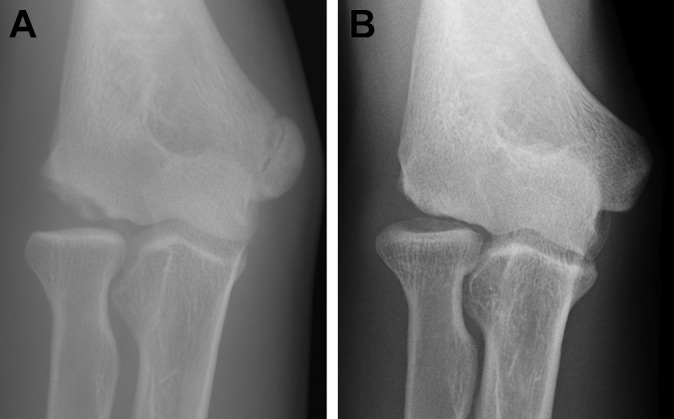
A 14-year-old male baseball player with a large capitellar osteochondritis dissecans lesion. (A) Preoperative anteroposterior tangential view. (B) Tangential view at 10 years after surgery. Although the capitellum was remodeled, the radial head was migrated superiorly.
Discussion
This study assessed midterm to long-term outcomes after AS fragment resection for capitellar OCD lesions in a considerable number of adolescent athletes with an open epiphyseal line and revealed that both functional outcomes and radiological findings were excellent in elbows with a small OCD lesion. Despite similar clinical and pain scores, patients with larger lesions had significantly decreased ROM and lower satisfaction scores.
Generally, microfracture or drilling is performed in addition to resection of fragments for OCD lesions.2,32 We performed debridement of the lesion down to bleeding bone instead of these procedures. Microfracture or drilling causes the release of multipotent mesenchymal stem cells from the bone marrow, and the cells enhance tissue healing.16 As our procedure also promotes bleeding from the bone marrow, it should have a similar effect as microfracture or drilling.
In previous studies with a small number of patients and short follow-up, both functional and radiographic outcomes after AS fragment resection were excellent in small OCD lesions, and the authors recommended AS fragment resection for small lesions.1,3,8,18,22 The results of this study also indicated that functional outcomes and patient satisfaction were excellent in small OCD lesions even with midterm to long-term follow-up. For small OCD lesions in adolescent athletes, AS fragment resection can be the first choice of surgical treatment, and additional procedures such as osteochondral grafting may not be required.
Surprisingly, shoulders with a larger OCD lesion also showed good outcomes in this study. AS resection can be a treatment option for elbows with a large OCD lesion as well as for those with a small lesion. Although osteochondral grafting is generally recommended for larger lesions,7,14,19,24,34 no studies have proven that osteochondral grafting will produce better results for larger OCD lesions than AS resection. We may need a comparative study of the 2 procedures to examine whether osteochondral grafting is necessary for larger lesions.
Radial head enlargement can cause limitations in elbow flexion,18,22 but this may not be the reason for the flexion limitation in elbows with a large lesion because head enlargement was seen in all elbows of both groups. We presumed that the limited flexion might be associated with poor congruity of the radiohumeral joint and may be responsible for lower satisfaction. Several authors have reported the importance of restoring the lateral margin of the capitellum for good outcomes,6,31 and osteochondral grafting has been recommended for larger lesions to restore joint congruity.7,14,19,24,34
Several studies have reported severe OA or radial head superior migration after AS resection for capitellar OCD.14,18,20 In this study, no grade 3 or 4 OA elbows were found on postoperative radiographs, although OA progressed postoperatively by 1 grade in 29% of elbows in group 1 and 43% in group 2. However, we found 1 patient from group 2 who demonstrated severe postoperative progression of radial head superior migration. In this case, poor joint congruity was already observed on preoperative radiographs (see Figure 8). Therefore, we suppose that poor joint congruity might be the cause of the postoperative exacerbation of malalignment and OA.
This study had several limitations, one of which was that it was a retrospective case-series study. Second, the mean follow-up may not have been long enough for the evaluation of OA progression, particularly in such a young patient population. Third, we were unable to compare our results with those after microfracture or osteochondral grafting. Last, the follow-up rate was relatively low. However, this is the first study to report longer term postoperative outcomes after AS resection of OCD fragments, including outcomes for larger lesions.
Conclusion
Both functional outcomes and radiological findings after AS fragment resection were excellent in elbows with a small OCD lesion. Although overall outcomes were also excellent in elbows with a larger lesion, flexion ROM and patient satisfaction scores were lower than those for elbows with a small lesion. AS resection can be an effective treatment option for elbows with large as well as small OCD lesions; however, reconstructive procedures such as osteochondral grafting may also need to be considered for elbows with a larger lesion.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.
Ethical approval for this study was obtained from the institutional review board of Funabashi Orthopaedic Hospital (proposal No. 2015007).
References
- 1. Baumgarten TE, Andrews JR, Satterwhite YE. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am J Sports Med. 1998;26(4):520–523. [DOI] [PubMed] [Google Scholar]
- 2. Bojanic I, Ivkovic A, Boric I. Arthroscopy and microfracture technique in the treatment of osteochondritis dissecans of the humeral capitellum: report of three adolescent gymnasts. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):491–496. [DOI] [PubMed] [Google Scholar]
- 3. Byrd JW, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474–478. [DOI] [PubMed] [Google Scholar]
- 4. Germann G, Wind G, Harth A. [The DASH (Disability of Arm-Shoulder-Hand) questionnaire: a new instrument for evaluating upper extremity treatment outcome]. Handchir Mikrochir Plast Chir. 1999;31(3):149–152. [DOI] [PubMed] [Google Scholar]
- 5. Harada M, Ogino T, Takahara M, Ishigaki D, Kashiwa H, Kanauchi Y. Fragment fixation with a bone graft and dynamic staples for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2002;11(4):368–372. [DOI] [PubMed] [Google Scholar]
- 6. Iwahori Y, Katoho M, Ooshuga T. Surgical treatment for osteochondritis dissecans of humeral capitellum: clinical results and role of arthroscopy. J Jpn Soc Surg Elbow. 2006;13:67–68. [Google Scholar]
- 7. Iwasaki N, Kato H, Ishikawa J, Saitoh S, Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34(8):1233–1239. [DOI] [PubMed] [Google Scholar]
- 8. Jones KJ, Wiesel BB, Sankar WN, Ganley TJ. Arthroscopic management of osteochondritis dissecans of the capitellum: mid-term results in adolescent athletes. J Pediatr Orthop. 2010;30(1):8–13. [DOI] [PubMed] [Google Scholar]
- 9. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lyman S, Fleisig GS, Waterbor JW, et al. Longitudinal study of elbow and shoulder pain in youth baseball pitchers. Med Sci Sports Exerc. 2001;33(11):1803–1810. [DOI] [PubMed] [Google Scholar]
- 11. Matsuura T, Kashiwaguchi S, Iwase T, Takeda Y, Yasui N. Conservative treatment for osteochondrosis of the humeral capitellum. Am J Sports Med. 2008;36(5):868–872. [DOI] [PubMed] [Google Scholar]
- 12. McManama GB, Jr, Micheli LJ, Berry MV, Sohn RS. The surgical treatment of osteochondritis of the capitellum. Am J Sports Med. 1985;13(1):11–21. [DOI] [PubMed] [Google Scholar]
- 13. Menche DS, Vangsness CT, Jr, Pitman M, Gross AE, Peterson L. The treatment of isolated articular cartilage lesions in the young individual. Instr Course Lect. 1998;47:505–515. [PubMed] [Google Scholar]
- 14. Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31–37. [DOI] [PubMed] [Google Scholar]
- 15. Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med. 2009;37(2):298–304. [DOI] [PubMed] [Google Scholar]
- 16. Min BH, Truong MD, Song HK, et al. Development and efficacy testing of a “hollow awl” that leads to patent bone marrow channels and greater mesenchymal stem cell mobilization during bone marrow stimulation cartilage repair surgery. Arthroscopy. 2017;33(11):2045–2051. [DOI] [PubMed] [Google Scholar]
- 17. Mitsunaga MM, Adishian DA, Bianco AJ., Jr Osteochondritis dissecans of the capitellum. J Trauma. 1982;22(1):53–55. [DOI] [PubMed] [Google Scholar]
- 18. Miyake J, Masatomi T. Arthroscopic debridement of the humeral capitellum for osteochondritis dissecans: radiographic and clinical outcomes. J Hand Surg Am. 2011;36(8):1333–1338. [DOI] [PubMed] [Google Scholar]
- 19. Oka Y, Ohta K, Fukuda H. Bone-peg grafting for osteochondritis dissecans of the elbow. Int Orthop. 1999;23(1):53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onomura T, Ishii S. Elbow Evaluation Sheet: Japanese Orthopaedic Association. J Jpn Orthop Assoc. 1992;66(5):591–595. [Google Scholar]
- 21. Peterson RK, Savoie FH, 3rd, Field LD. Osteochondritis dissecans of the elbow. Instr Course Lect. 1999;48:393–398. [PubMed] [Google Scholar]
- 22. Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 1998;14(8):797–803. [DOI] [PubMed] [Google Scholar]
- 23. Schoch B, Wolf BR. Osteochondritis dissecans of the capitellum: minimum 1-year follow-up after arthroscopic debridement. Arthroscopy. 2010;26(11):1469–1473. [DOI] [PubMed] [Google Scholar]
- 24. Shimada K, Tanaka H, Matsumoto T, et al. Cylindrical costal osteochondral autograft for reconstruction of large defects of the capitellum due to osteochondritis dissecans. J Bone Joint Surg Am. 2012;94(11):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sugaya H, Takahashi N, Kawai N, Nagai H, Kajiyama S, Tanaka M. Autologous osteochondral transplantation for osteochondritis dissecans of the humeral capitellum in young athletes: timing for surgery and clinical outcomes. J Jpn Soc Surg Elbow. 2013;19:98–101. [Google Scholar]
- 26. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205–1214. [DOI] [PubMed] [Google Scholar]
- 27. Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;(363):108–115. [PubMed] [Google Scholar]
- 28. Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207–212. [DOI] [PubMed] [Google Scholar]
- 29. Takahara M, Shundo M, Kondo M, Suzuki K, Nambu T, Ogino T. Early detection of osteochondritis dissecans of the capitellum in young baseball players: report of three cases. J Bone Joint Surg Am. 1998;80(6):892–897. [DOI] [PubMed] [Google Scholar]
- 30. Takeda H, Watarai K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30(5):713–717. [DOI] [PubMed] [Google Scholar]
- 31. Tomatsuri M, Tanaka J, Oomukai T, Okuno H. Osteochondral graft for osteochondritis dissecans of the humeral capitellum. J Jpn Elbow Soc. 2003;10:33–34. [Google Scholar]
- 32. Wulf CA, Stone RM, Giveans MR, Lervick GN. Magnetic resonance imaging after arthroscopic microfracture of capitellar osteochondritis dissecans. Am J Sports Med. 2012;40(11):2549–2556. [DOI] [PubMed] [Google Scholar]
- 33. Yamaguchi K, Sweet FA, Bindra R, Morrey BF, Gelberman RH. The extraosseous and intraosseous arterial anatomy of the adult elbow. J Bone Joint Surg Am. 1997;79(11):1653–1662. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714–720. [DOI] [PubMed] [Google Scholar]