Abstract
Recently, heart rate variability (HRV) analysis has been used as an indicator of epileptic seizures. As women have a lower sudden, unexpected death in epilepsy risk and greater longevity than men, the authors postulated that there are significant gender-related differences in heart rate dynamics of epileptic patients. The authors analyzed HRV during 5-minute segments of continuous electrocardiogram recording of age-matched populations. The middle-aged epileptic patients included males (n = 12) and females (n = 12), ranging from 41 to 65 years of age. Relatively high- (0.15 Hz-0.40 Hz) and low-frequency (0.01 Hz-0.15 Hz) components of HRV were computed using spectral analysis. Poincaré parameters of each heart rate time series were considered as nonlinear features. The mean heart rate markedly differed between gender groups including both right- and left-sided seizures. High-frequency heart rate power and the low-frequency/high-frequency ratio increased in the pre-ictal phase of both male and female groups (p < .01), but men showed more increase especially in right-sided seizures. The standard deviation ratio, SD2/SD1, of pre-ictal phase was greater in males than females (p < .01). High-frequency spectral power and parasympathetic activity were higher in the female group with both right- and left-sided seizures. Men showed a sudden increase in sympathetic activity in the pre-ictal phase, which might increase the risk of cardiovascular disease in comparison to women. These complementary findings indicate the need to account for gender, as well as localization in HRV analysis.
Keywords: epilepsy, gender, lateralization, HRV
Introduction
Studies of heart rate variability (HRV) confirm that this signal is an appealing noninvasive and quantitative research tool in cardiology to investigate autonomic effects on the heart. HRV refers to the compound beat-to-beat variation in heart rate (HR), produced by the interaction of sympathetic and parasympathetic activity at the sinus node of the heart (Levy, 1990; Stein, Bosner, Kleiger, & Conger, 1994). HRV expresses variations of instantaneous HR and R-R intervals (RR). The use of this technique in describing the physiological response in normal subjects, as well as for the case of patients with various cardiovascular disorders, has been studied extensively. Previous research has shown that decreased fluctuation of RR should not be confused with noise. It implicates an increased risk of arrhythmic events and mortality rate in patients with myocardial infarction (Bigger et al., 1992).
Power spectral analysis of interbeat interval time series can distinguish among the intrinsic sources of HRV as these rhythms occur at different frequencies. Typically, the power spectrum of HRV as a time series contains two major components. A relative power in high frequency (HF; 0.15 Hz-0.40 Hz) has been used to reflecting the cardiac vagal tone. Low frequency (LF; 0.01 Hz-0.15 Hz) indicates the inputs from both branches of autonomic nervous system and reflects the mixture of vagal and sympathetic influences (Malliani, Pagani, Lombardi, & Cerutti, 1991; Parati, Saul, Di Rienzo, & Mancia, 1995). The ratio of the LF to HF components represents a measure of sympathovagal balance (Pagani & Malliani, 2000).
The nonlinear analysis of HRV, in addition to the time– and frequency–domain analyses, has been suggested to provide more valuable information as to the physiological interpretation of HR fluctuation and risk assessments (Task Force of the European Society of Cardiology & the North American Society of Pacing and Electrophysiology, 1996). Poincaré plot analysis of RR enables displaying information about beat-to-beat variability and autonomic control of the HR in a compact visual format, which is easy to read. Two standard descriptors of Poincaré plot, namely, SD1 and SD2, are commonly used in the nonlinear analysis (Bergfeldt & Haga, 2003; Brennan, Palaniswami & Kamen, 2001; Brennan, Palaniswami & Kamen, 2002; Guzik, Piskorski, Krauze, Wykretowicz, & Wysocki, 2005; Tulppo et al., 2001). SD2 is a measure of long- and short-term HRV, and SD1 is a measure of short-term HRV. Moreover, the combination of SD1 and SD2 provides additional description of Poincaré plots. The S value corresponds to the area of the imaginary ellipse on the Poincaré plot; the SD2/SD1 ratio has an analogy with the LF/HF ratio from the spectral HRV analysis. There are reasons to postulate that S illustrates the total HRV and the SD2/SD1 ratio describes the sympathovagal balance (Guzik et al., 2005).
There are a few reports on gender-related differences in cardiac autonomic tone. In healthy subjects, using short- and long-term recordings, a predominance of parasympathetic over sympathetic tone was found in women, and vice versa in men (Evans et al., 2001; Rossy & Thayer, 1998; Ryan, Goldberger, Pincus, Mietus, & Lipsitz, 1994). It was also demonstrated that the gender-related difference in parasympathetic regulation diminishes after the age of 50 while sympathetic dominance in men disappears significantly later (Kuo et al., 1999). A higher vagal tone in women may protect them against fatal arrhythmias and sudden cardiac death and potentially explains their lower risk for these conditions compared to men (Kuo et al., 1999). Sympathetic responses predominate during most seizures, causing tachycardia, tachypnea, increased blood pressure, and pupillary dilatation. From infancy to adulthood, complex partial seizures of temporal or extratemporal origin often lead to sympathetic activation. However, ictal parasympathetic activity or sympathetic inhibition can predominate, causing increased salivation, gastric acid secretion, reduced heart and respiratory rates, and decreased blood pressure (Kannel, Wilson, D’Agostino, & Cobb, 1998).
Partial and generalized epilepsies, which are two prevalent types of epileptic seizures, alter autonomic function during install, postictal, and intellectual states. All aspects of autonomic function can be affected, including the parasympathetic, sympathetic, and adrenal medullary systems. Autonomic changes are the most common symptoms of simple partial seizures but may go unrecognized. Seizures typically activate sympathetic nervous activity, increasing the HR and blood pressure, although parasympathetic activation or sympathetic inhibition may predominate during partial seizures. Seizure-induced cardiovascular dysfunction, pulmonary edema, postictal depression of autonomic respiratory reflexes, and cardiovascular function may contribute to SUDEP (sudden, unexpected death in epilepsy; Devinsky, 2004). Moreover, several studies suggest hemispheric lateralization of autonomic cardiovascular control. The right hemisphere may predominantly modulate sympathetic tone, whereas the left hemisphere modulates parasympathetic tone (Van Buren, 1958).
According to the reported results of existing research, no study has explored the influence of gender on the HRV of epileptic patients. There is some evidence confirming the right hemispheric lateralization of sympathetic cardiac control in epileptic patients (Mandic et al., 2010; Mayer et al., 2004; McKee & Bodfish, 2000). Therefore, the primary goal of this study was to investigate whether there is any different behavior in HRV of epileptic patients based on their gender. In the current study, the lateralization of epileptic seizures, which may have influenced HRV indices, was considered in both groups of males and females. Left- and right-sided males were compared to left- and right-sided females, respectively.
The long-term goal of this study was to provide an algorithm to predict epileptic seizures based on HRV. Therefore, as much as possible, factors that may contribute to the success of these algorithms have been studied (Behbahani, Dabanloo, Nasrabadi, & Dourado, 2015; Behbahani, Dabanloo, Nasrabadi, Teixeira, & Dourado, 2012, 2013a, 2013b). In previous research, the behavior of HRV was evaluated in different periods from 90 minutes to 5 minutes before the onset of the seizure (Behbahani et al., 2013). The results showed that segments closer to the seizure represent a continuum of increasing sympathetic dominance over those segments away from it. Increase of sympathetic and inhabitation of parasympathetic function occurs in most epileptic seizures regardless of the type of epilepsy, especially in the 5 minutes before the seizure. Thus this period is highlighted as a target interval in the current research.
Materials and Method
Data Description
One-lead electrocardiogram (ECG) recordings of patients with pharmacoresistant focal epilepsy were compiled as a part of the EPILEPSIAE project (Teixeira et al., 2011). Long-term, continuous electroencephalogram and ECG data were managed in this database as a joint effort in three epilepsy centers including Portugal (Coimbra), France (Paris), and Germany (Freiburg). The data include standardized annotations. The sampling rates of the data were 256 Hz, 512 Hz, and 1,024 Hz and filtered for the line noise of 50 Hz. Table 1 lists the clinical features of epileptic patients.
Table 1.
The Clinical Features of 24 Studied Patients.
| Gender | Age, years | Seizure type | Localization | Lateralization | No. of seizures |
|---|---|---|---|---|---|
| Male | 47.58 ± 5.97 | CP: 11 | R: 6 | F: 2 | 133 |
| SP: 5 | L: 6 | T: 10 | SD: 4.99 | ||
| SG: 2 | |||||
| UC: 8 | |||||
| Female | 50.91 ± 8.07 | CP: 11 | R: 6 | F: 1 | 99 |
| SP: 4 | L: 6 | T: 11 | SD: 4.44 | ||
| SG: 4 | |||||
| UC: 8 |
Note. CP = complex partial; SP = simple partial; SG = secondarily generalized; UC = unclassified; R = right-sided; L = left-sided; T = temporal; F = frontal; SD = standard deviation.
In the current research, a total number of 232 seizures from 24 patients were selected for the HRV analysis. The patients composed 12 males with the mean age of 47.58 years (SD = 5.97), and 12 females with the average age of 50.91 years (SD = 8.07). All cases included the electroencephalogram signal to confirm the seizure onset. As there is some evidence that lateralization of epileptic seizure might have effects on HRV, the authors made the balance between right- and left-sided seizures to avoid the possible effect on results. The primary criterion for selecting these patients was based on analyzing the HRV during the day and night. As HR variables have different values during the day and night, only patients with day seizures were selected. Even the seizure-free HRV segments were chosen for the day parts of ECG recordings.
Statistical Analysis
The results are presented as mean ± standard deviation and percentages. The differences of all variables between males and females were compared with paired sample t tests. Statistical analyses were performed using IBM SPSS Statistics Version 19 (IBM, Armonk, NY), and differences were considered statistically significant at p < .05 and p < .01.
Results
The data consist of two sets: seizure-free and 5 minutes before the seizure. The seizure-free intervals were randomly selected within 4 to 5 hours before the onset of seizure to ensure they stay far from it.
Peer to peer comparison of both groups showed that independent of being left- or right-sided, all HRV indices had greater percentages of changes in males rather than in females (Tables 2 and 3). The results are presented separately for left- and right-sided seizures in males and females. Results are reported in Figure 1a, b, c, d (left parts), which represent the comparison of males and females with left-sided seizures. Mean HR (SD) increased from 63.35 (6.23) to 100.32 (2.42) beats/minute in males and from 69.22 (4.3) to 94.43 (4.8) beats/minute in females (Figure 1a [left]). These results show the increase of 58.23% and 36.42% in HR of males and females, respectively.
Table 2.
Comparison of HR Variables Between Seizure-Free and Pre-Ictal Signals in Females With Left- and Right-Sided Seizures.
| Feature | Right-sided |
Left-sided |
||||
|---|---|---|---|---|---|---|
| Seizure-free | 5 Minutes before | % Increase or decrease | Seizure-free | 5 Minutes before | % Increase or decrease | |
| RR (ms) | 801.45 ± 51.33 | 538.47 ± 45.94** | −32.81 | 868.29 ± 49.3 | 635.9 ± 30.73** | −26.76 |
| Mean HR | 77.51 ± 5.3 | 117.53 ± 6.4** | +51.63 | 69.22 ± 4.3 | 94.43 ± 4.8** | +36.42 |
| LF (ms2) | 180.1 ± 33.13 | 290.25 ± 15.17** | +61.16 | 154.77 ± 29.2 | 239.33 ± 26.13** | +54.63 |
| HF (ms2) | 144.17 ± 34.09 | 166.42 ± 29.07* | +15.43 | 142.89 ± 42 | 158.12 ± 18.15** | +10.65 |
| LF/HF | 1.18 ± 0.2 | 1.89 ± 0.58** | + 60.16 | 1.16 ± 0.29 | 1.64 ± 0.32* | +41.37 |
| SD1 | 42.46 ± 9.93 | 29.3 ± 1.05* | −30.99 | 38.2 ± 10.1 | 32.04 ± 2.24* | −16.12 |
| SD2 | 50.11 ± 10.35 | 55.25 ± 1.26* | +10.25 | 40.61 ± 11.9 | 46.63 ± 2.13* | +14.82 |
| SD2/SD1 | 1.13 ± 0.23 | 1.75 ± 0.19** | +54.86 | 1.02 ± 0.46 | 1.37 ± 0.34** | +34.31 |
| S | 8.96 ± 0.78 | 3.67 ± 0.18** | −57.76 | 5.19 ± 1.2 | 2.57 ± 0.5* | −50.48 |
Note. HR = heart rate; RR = R-R intervals; LF = low frequency; HF = high frequency; SD = standard deviation. Paired sample t test.
p < .05. **p < .01.
Table 3.
Comparison of HR Variables Between Seizure-Free and Pre-Ictal Signals in Males With Left- and Right-Sided Seizures.
| Feature | Right-sided |
Left-sided |
||||
|---|---|---|---|---|---|---|
| Seizure-free | 5 Minutes before | % Increase or decrease | Seizure-free | 5 Minutes before | % Increase or decrease | |
| RR (ms) | 831.22 ± 50.23 | 501.47 ± 40.24** | 39.67 | 905.95 ± 20.6 | 598.16 ± 42.7** | −33.97 |
| Mean HR | 70.21± 2.3 | 125.03 ± 11.4** | +78.08 | 63.35 ± 6.23 | 100.24 ± 7.3** | +58.23 |
| LF (ms2) | 189.63 ± 24.14 | 348.2 ± 21.37** | +83.62 | 163.75 ± 30.2 | 290.11 ± 21.13** | +77.16 |
| HF (ms2) | 143.87 ± 44.79 | 157.92 ± 19.09** | +9.76 | 152.21 ± 53 | 163.52 ± 12.15* | +7.43 |
| LF/HF | 1.34 ± 0.31 | 2.34 ± 0.23** | +74.62 | 1.27 ± 0.43 | 1.87 ± 0.42** | +47.24 |
| SD1 | 51.46 ± 11.91 | 35.42 ± 5.71* | −31.16 | 42.3 ± 8.9 | 32.33 ± 2.24* | −23.65 |
| SD2 | 60.3 ± 12.3 | 69.58 ± 7.56* | +15.38 | 46.51 ± 7.2 | 51.27 ± 2.13* | +10.23 |
| SD2/SD1 | 1.33 ± 0.24 | 2.13 ± 0.13** | +60.15 | 1.09 ± 0.41 | 1.59 ± 0.54** | +50 |
| S | 7.87 ± 0.72 | 3.11 ± 0.27** | −60.48 | 4.21 ± 0.93 | 2.01 ± 0.72* | −52.25 |
Note. HR = heart rate; RR = R-R intervals; LF = low frequency; HF = high frequency; SD = standard deviation. Paired sample t test.
p < .05. **p < .01.
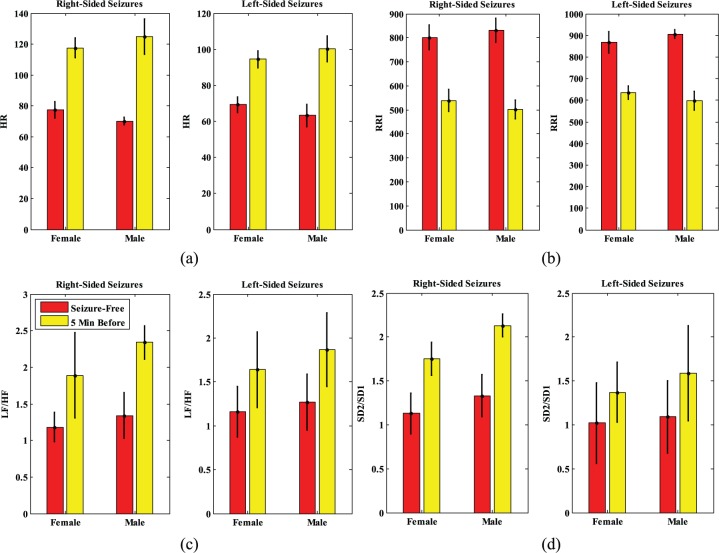
Figure 1.
Comparison of (a) HR, (b) RR, (c) LF/HF ratio, and (d) SD2/SD1 ratio, between males and females (right and left-sided seizures)
Note. HR = heart rate; RR = R-R intervals; LF = low frequency; HF = high frequency; SD = standard deviation. Values are mean p < .01.
The RR intervals show a decline from 895.95 (20.6) to 598.16 (42.7) in males and from 868.29 (49.3) to 635.9 (30.73) in females, showing a 33.97% and 26.76% decrease in males and females, respectively (Figure 1b [left]). The SD of both groups increased in pre-ictal phase, which might be interpreted as alternations before the occurrence of the seizure. The LF/HF ratio shows an increment of 1.27 (0.43) to 1.87 (0.42) in males and 1.16 (0.29) to 1.64 (0.32) in females, which displays the increase of 47.24% in men and 41.37% in women (Figure 1c [left]).
As reported in Figure 1d (left), SD2/SD1 showed a lower ratio in males than in females. A higher SD2/SD1 ratio might reflect a decrease in SD1, an increase in SD2, or both. In the present study, the SD2/SD1 ratio increased from 1.09 (0.41) to 1.59 (0.54) in males and from 1.02 (0.46) to 1.37 (0.34) in females, which indicates a rise by 50% in males and by 34.31% in females with left-sided seizures. Finally, the S value shows a decrease of 5.19 (1.2) to 2.57 (0.5) in females and 4.21 (0.93) to 2.01 (0.72) in males with left-sided seizures. This result shows the decrease of 52.25% in males and 50.48% for females.
In Figure 1a, b, c, and d (right parts) the same indices are reported in the right-sided seizures of males and females. Mean HR increased from 70.21 (2.3) to 125.03 (11.4) beats/minute in males and from 77.51 (5.3) to 117.53 (6.4) beats/minute in females with right-sided seizures (Figure 1a [right]). This result represents the increase of 78.08% and 51.63% in HR of males and females respectively.
The RR intervals decline from 831.22 (50.23) to 501.47 (40.24) in males and from 801.45 (51.33) to 538.47 (45.94) in females, which shows a 39.67% and 32.81% decrease in males and females, respectively (Figure 1b [right]). The SD of both groups increased in episodes close to the seizure. The LF/HF ratio shows the increase from 1.34 (0.31) to 2.34 (0.23) in males and 1.18 (0.2) to 1.89 (0.58) in females, which displays the increase of 74.62% in men and 60.16% in females with right-sided seizures (Figure 1c [right]). The SD2/SD1 increased from 1.33 (0.24) to 2.13 (0.13) in males and from 1.13 (0.23) to 1.75 (0.19) in females. The SD2/SD1 of men and women with right-sided seizure increased by 60.15% and 54.86%, respectively. The S value shows a decrease of 7.87 (0.72) to 3.11 (0.27) for males and 8.96 (0.78) to 3.67 (0.18) in females with right-sided seizures that is decreased by 60.48% in males and 57.76% in females.
The statistical analysis was applied to both groups of males and females regardless of them being affected by a right- or left-sided seizure. The intent was to recognize the difference between the HR parameters in two genders. Table 4 represents the gender comparison of HRV indices. Mean HR of males shows an increase from 66.76 (4.3) to 112.63 (9.34) and of females from 73.36 (4.8) to 105.98 (5.6). These changes represent an increase of 68.65% in mean HR of males and 44.46% in females. The RR intervals decline from 918.58 (35.95) to 549.81 (44.08) in males and from 834.87 (50.31) to 587.18 (38.33) in females. The RR intervals show 40.14% and 29.66% of a decrease in males and females, respectively. Similarly, the LF/HF ratio shows an increase from 1.3 (0.37) to 2.1 (0.32) in males and from 1.12 (0.24) to 1.63 (0.45) in females, which is equivalent to 61.53% and 45.53% of the increase in males and females, respectively. The SD2/SD1 increased from 1.11 (0.32) to 1.81 (0.33) in males and from 1.14 (0.34) to 1.76 (0.26) in females, which means this ratio has an increment of 63.06% in males and 54.38% in females.
Table 4.
Comparison of HR Variables Between Seizure-Free and Pre-Ictal Signals in Females and Males.
| Feature | Right-sided |
Left-sided |
||||
|---|---|---|---|---|---|---|
| Seizure-free | 5 Minutes before | % Increase or decrease | Seizure-free | 5 Minutes before | % Increase or decrease | |
| RR (ms) | 834.87 ± 50.31 | 587.18 ± 38.33** | −29.66 | 918.58 ± 35.95 | 549.81 ± 44.08** | −40.14 |
| Mean HR | 73.36 ± 4.8 | 105.98 ± 5.6** | +44.46 | 66.78 ± 4.3 | 112.63 ± 9.35** | +68.65 |
| LF (ms2) | 167.43 ± 31.16 | 264.79 ± 20.65** | +58.14 | 176.69 ± 27.17 | 319.15 ± 21.25** | +80.62 |
| HF (ms2) | 143.53 ± 38.04 | 162.27 ± 23.56* | +13.05 | 148.04 ± 48.89 | 160.72 ± 15.62** | +8.56 |
| LF/HF | 1.12 ± 0.24 | 1.63 ± 0.45** | +45.53 | 1.3 ± 0.37 | 2.1 ± 0.32* | +61.53 |
| SD1 | 40.33 ± 10.01 | 30.67 ± 1.64* | −23.95 | 46.9 ± 10.4 | 33.87 ± 3.97* | −27.78 |
| SD2 | 45.36 ± 11.12 | 50.94 ± 1.69* | +12.3 | 53.4 ± 9.75 | 60.42 ± 4.84* | +13.14 |
| SD2/SD1 | 1.14 ± 0.34 | 1.76 ± 0.26** | +54.38 | 1.11 ± 0.32 | 1.81 ± 0.33** | +63.06 |
| S | 7.07 ± 0.99 | 3.12 ± 0.34** | −55.86 | 6.04 ± 0.82 | 2.56 ± 0.49* | −57.61 |
Note. HR = heart rate; RR = R-R intervals; LF = low frequency; HF = high frequency; SD = standard deviation. Paired sample t test.
p < .05. **p < .01.
The percentages of increase of other indices LF and SD2 were apparently greater in men rather than in women (Table 4). Additionally, the HF showed more increase in females, which implies that parasympathetic activity has a bigger influence in females rather than in males. Figure 2 illustrates the percentages of changes in males and females in the transition from the seizure-free episode to 5 minutes before the seizure. The RR intervals (ms), SD1, and S values were decreased in the 5 minutes before the seizure, and the amount of reduction was higher in men. All other indices had increased. Except HF value that had a higher increase in women, others showed a greater percentage of increase in men.
Figure 2.
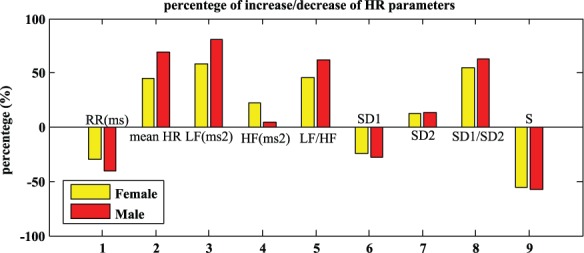
Percentage of increase/decrease of HR parameters in males and females in transition from seizure-free episode of 5 minutes before seizure.
Note. RR = R-R intervals; HR = heart rate; LF = low frequency; HF = high frequency; SD = standard deviation.
Discussion
HRV is considered as a marker of cardiac autonomic function and an index of cardiovascular disease. Before any discussion concerning the results obtained in this article, it is better to make a brief review of some of the physiological differences between men and women. In healthy subjects, the HR of men is lower than that of women because the size of the heart in men is 25% larger. The larger heart can pump more blood than the smaller heart, in one beat. Another reason for men to have a lower HR is the capacity of their lungs, which is 25% to 30% higher than women. Women not only develop cardiovascular diseases at older ages than men but also live longer, as higher HR fluctuations have not been reported in females (Bradshaw & Thompson, 2007; Hamm et al., 2011). Factors including age, body mass index, alcohol consumption, cigarette smoking, and even ethnicity can affect autonomic function. Mentioned factors lead to different HRV in both groups (Choi et al., 2006; Elsenbruch, Harnish, & Orr, 1999; Hogarth, Graham, Mary David, & Greenwood, 2009; Kuo et al., 1999; Moodithaya & Avadhany, 2012; Palisso et al., 1999; Regitz-Zagrosek, Lehmkuhl, & Weickert, 2006; Tanaka, Kimura, Goyagi, & Nishikawa, 2004; Uusitalo et al., 2007).
In the current research, the focus was on possible effects of gender on HRV and autonomic dysfunction in epileptic patients. Heart rate dynamics were analyzed during 5-minute segments of continuous ECG recording in middle-aged epileptic patients (41-65 years old) in an equal number of male and female subjects (n = 12). As previous research had confirmed the effect of lateralization on control of autonomic function, subjects were selected equally from the side of lateralization to avoid its impact on the results. In the first step, the comparison was made, with a focus on lateralization, and then it was made between males and females regardless of them having right- or left-sided seizures.
The current results showed that the greater percentage of change in HR indices from seizure-free episodes to 5 minutes before seizure including HR, LF, LF/HF, SD2/SD1 were apparently greater in men. HF indicated a higher percentage of change in the transition from seizure-free with the pre-actual status of women, which represents the lower sympathetic influence on the HR of females compared to males. Being right- or left-sided seizure, the HRV fluctuations in the pre-ictal segments were smaller in women than in men. Statistical analysis showed that sudden increases in HR indices in the episode of 5 minutes before seizures were higher in men with or without a focus on lateralization of seizures (p < .01).
The reactivity to increase the HR, LF, LF/HF, SD2/SD1, and decrease HF were more prominent in right-sided patients (males and females). Indeed, both males and females with right-sided seizures have significantly higher HRV fluctuations related to their baseline values (p < .01) as compared to the left-sided group. This finding supports previously noted lateralization differences in autonomic cardiovascular control of epileptic patients (Mandic et al., 2010; Mayer et al., 2004; McKee & Bodfish, 2000).
The standpoints on gender-related differences in HRV are diverse in the literature. The discrepancy between the studies may be partly due to the different experimental variables and conditions. Some research reported that men have larger LF than women, but they have similar HF (Bigger et al., 1995). There is another report that shows that men have higher LF and LF/HF but smaller HF (Montano et al., 1994). Later studies indicated that HRV for all time–domain measurements is lower in women, especially at the age of 50 or below. They believe the level of parasympathetic activity is lower in younger women (Umetani, Singer, McCraty, & Atkinson, 1998).
The results obtained for middle-aged epileptic patients conform to some previous reports (Huikuri et al., 1996; Park, Lee & Jeong, 2007). In the current study, regardless of having left- or right-sided seizures, men exhibited larger LF/HF, LF, and HR than women in pre-ictal phase. However, women had the higher HF in pre-ictal phase (p < .01). Although both groups show the increase or decrease of HRV indices in pre-ictal phase, the percentage of changes are greater in males, which confirms the considerable influence of sympathetic activity in males as compared to females. The sympathetic dominance of males could be demonstrated by LF/HF and LF in different age stages.
Another aspect of gender-related differences of HRV in epileptic patients that can be considered is SUDEP. However, it is not yet ascertained whether there are sex-related differences in the incidence of sudden epileptic death (Lhatoo et al., 2001). Even though sex-related differences do exist, it is yet to be known whether this syndrome is the outcome of central apnea or rather of central autonomic deregulation (Langan, Nashef, & Sander, 2000).
The presented results showed that in analyzing middle-aged epileptic patients in the current research, autonomic activity was higher in men compared to women with both right- and left-sided seizures. It can be explicated that male epileptic patients have an overall higher sympathetic drive, which might lead to greater susceptibility to fatal arrhythmia, coronary artery disease, and other cardiac disorders (Schwartz, La Rovere, & Vanoli, 1992).
The current results indicated that the sudden increase in HRV in men was significantly higher than in women. Some reports had emphasized that most of the patients with SUDEP show symptoms of tachycardia with the sudden increase in HR. Therefore, the results obtained in this study can strengthen the hypothesis that being a male is a risk factor of SUDEP and reduced LF power in women could protect them against cardiac disease and arrhythmia.
Some of the limitations of the current study should be investigated. The main limitation was a small number of subjects due to the rarity of matched epileptic patients. The specific nature of the heart and influence of the circadian cycle on HRV implied that several factors should be considered to avoid possible effects on the results. Although more data were available, finding subjects that have similar characteristics such as the type of seizure, lateralization, age, and gender that have similar circadian times for seizures was a blocking factor. Therefore, the results of the current research could not be generalized to other age-groups due to the small number of evaluated subjects. Another limitation was the lack of knowledge of some factors that could affect the HRV. The type of medication used in patients and the family history of cardiac disease were missing links in this research.
Conclusion
To summarize, this article describes that in the pre-ictal phase of epileptic patients, gender difference in HRV has an impact. The indices of HRV indicate that vagal activity significantly differed in men and women with right- and left-sided seizures. The results reflect a higher sympathetic activity in men, especially with right-sided seizures. Cardiac autonomic modulation, which is determined by HRV, is significantly lower in epileptic women. It is presumed that this may provide protection against arrhythmia and SUDEP in epileptic women. Awareness of gender differences in pre-ictal HRV not only can provide a better choice of treatment for patients but also can be an important factor in the success of the epileptic seizure prediction algorithm.
Other factors that might have an effect on HRV such as body mass index, alcohol consumption, cigarette smoking, and so on were not recorded in the database. Therefore, it cannot be concluded that the increase or decrease of HRV indices is the only effect of decrease or increase of vagal activity. Furthermore, there was no information about the subject’s lifestyle, such as quantification of exact physical activity or the exercise capacity of the participants. Even moderate levels of physical significantly affect HRV.
To eliminate the confounding effects of aging and circadian changes in HRV, only middle-aged epileptic patients with only daily seizures were selected. Future studies should consider gender-related differences of HRV in other age-groups of epileptic patients as there are increasing reports that women and men undergo marked autonomic changes in different age stages. Due to the small amount of data examined in this study, more subjects should be examined. Seizures occuring during sleep and nighttime could be topics for evaluation in future works.
Acknowledgments
The authors would like to extend their gratitude to Dr. Cesar A. Teixeira for his support of this research.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest in the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: European Project EPILEPSIAE (EU FP7 Grant 211713) and ICIS project CENTRO-07-0224-FEDER-002003 partially funded this work.
References
- Behbahani S., Dabanloo N. J., Nasrabadi A. M., Dourado A. (2015). Classification of ictal and seizure-free HRV signals with a focus on lateralization of epilepsy. Technology and Health Care, 1-14. [DOI] [PubMed] [Google Scholar]
- Behbahani S., Dabanloo N. J., Nasrabadi A. M., Teixeira C. A., Dourado A. (2012). Epileptic seizure behavior from the perspective of heart rate variability. Computing in Cardiology, 39, 117-120. [Google Scholar]
- Behbahani S., Dabanloo N. J., Nasrabadi A. M., Teixeira C. A., Dourado A. (2013. a). Ictal heart rate variability assessment with focus on secondary generalized and complex partial epileptic seizures. Advances in Bioresearch, 4(1), 50-58. [Google Scholar]
- Behbahani S., Dabanloo N. J., Nasrabadi A. M., Teixeira C. A., Dourado A. (2013. b). Pre-ictal heart rate variability assessment of epileptic seizures by means of linear and nonlinear analyses. Anatolian Journal of Cardiology, 13, 797-803. [DOI] [PubMed] [Google Scholar]
- Bergfeldt L., Haga Y. (2003). Power spectral and Poincare plot characteristics in sinus node dysfunction. Journal of Applied Physiology, 94, 2217-2224. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr., Fleiss J. L., Steinman R. C., Rolnitzky L. M., Kleiger R. E., Rottman J. N. (1992). Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation, 85, 164-171. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr., Fleiss J. L., Steinman R. C., Rolnitzky L. M., Schneider W. J., Stein P. K. (1995). RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation, 91, 1936-1943. [DOI] [PubMed] [Google Scholar]
- Bradshaw P. J., Thompson P. L. (2007). Sex in the CCU: Women with non-ST-segment elevation acute coronary syndrome may do no worse despite less intervention. Heart, 93, 1327-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M., Palaniswami M., Kamen P. (2001). Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Transactions on Biomedical Engineering, 48, 1342-1347. [DOI] [PubMed] [Google Scholar]
- Brennan M., Palaniswami M., Kamen P. (2002). The Poincaré plot interpretation using a physiological model of HRV based on a network of oscillators. American Journal of Physiology, 283, H1873-H1886. [DOI] [PubMed] [Google Scholar]
- Choi J. B., Hong S., Nelesen R., Bardwell W. A., Natrajan L., Schubert C., Dimsdale J. E. (2006). Age and ethnicity differences in short-term heart-rate variability. Psychosomatic Medicine, 68, 421-426. [DOI] [PubMed] [Google Scholar]
- Devinsky O. R. (2004). Effects of seizures on autonomic and cardiovascular function. Epilepsy Currents, 4, 43-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S., Harnish M. J., Orr W. C. (1999). Heart rate variability during waking and sleep in healthy males and females. Sleep, 22, 1067-1071. [DOI] [PubMed] [Google Scholar]
- Evans J. M., Ziegler M. G., Patwardhan A. R., Ott J. B., Kim C. S., Leonelli F. M., Knapp C. F. (2001). Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. Journal of Applied Physiology, 91, 2611-2618. [DOI] [PubMed] [Google Scholar]
- Guzik P., Piskorski J., Krauze T., Wykretowicz A., Wysocki H. (2005, June). The autonomic modulating effects of changing respiratory rate on HRV are portrayed by a novel description of the Poincaré plot analysis. Paper presented at the Joint ISE and ISHNE Congress, Gdansk, Poland. [Google Scholar]
- Hamm C. W., Bassand J. P., Agewall S., Bax J., Boersma E., Bueno H., . . . Zahger D. (2011). ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the Management of Acute Coronary Syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 32, 2999-3054. [DOI] [PubMed] [Google Scholar]
- Hogarth A. J., Graham L. N., Mary David A. S. G., Greenwood J. P. (2009). Gender differences in sympathetic neural activation following uncomplicated acute myocardial infarction. European Heart Journal, 30, 1764-1770. [DOI] [PubMed] [Google Scholar]
- Huikuri H. V., Pikkujamsa S. M., Airaksinen K. E., Ikaheimo M. J., Rantala A. O., Kauma H., . . . Kesaniemi Y. A. (1996). Sex-related differences in autonomic modulation of heart rate in middle-aged subjects. Circulation, 94, 122-125. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Wilson P. W., D’Agostino R. B., Cobb J. (1998). Sudden coronary death in women. American Heart Journal, 136, 205-212. [DOI] [PubMed] [Google Scholar]
- Kuo T. B., Lin T., Yang C. C., Li C. L., Chen C. F., Chou P. (1999). The effect of aging on gender differences in neural control of heart rate. American Journal of Physiology, 277, H2233-H2239. [DOI] [PubMed] [Google Scholar]
- Langan Y., Nashef L., Sander J. W. (2000). Sudden, unexpected death in epilepsy: A series of witnessed deaths. Journal of Neurology, Neurosurgery & Psychiatry, 68, 211-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M. N. (1990). Autonomic interactions in cardiac control. Annals of the New York Academy of Sciences, 601, 209-221. [DOI] [PubMed] [Google Scholar]
- Lhatoo S. D., Johnson A. L., Goodridge D. M., MacDonald B. K., Sander J. W., Shorvon S. D. (2001). Mortality in epilepsy in the first 11 to 14 years after diagnosis: A multivariate analysis of a long-term, prospective, population-based cohort. Annals of Neurology, 49, 336-344. [PubMed] [Google Scholar]
- Malliani A., Pagani M., Lombardi F., Cerutti S. (1991). Cardiovascular neural regulation explored in the frequency domain. Circulation, 84, 482-492. [DOI] [PubMed] [Google Scholar]
- Mandic S., Fonda H., Dewey F., Le V., Stein R., Wheeler M., . . . Froelicher V. F. (2010). Effect of gender on computerized electrocardiogram measurements in college athletes. Physician and Sportsmedicine, 2, 1-9. [DOI] [PubMed] [Google Scholar]
- Mayer H., Benninger F., Urak L., Plattner B., Geldner J., Feucht M. (2004). EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology, 63, 324-328. [DOI] [PubMed] [Google Scholar]
- McKee J. R., Bodfish J. W. (2000). Sudden unexpected death in epilepsy in adults with mental retardation. American Journal of Mental Retardation, 105, 229-235. [DOI] [PubMed] [Google Scholar]
- Montano N., Ruscone T. G., Porta A., Lombardi F., Pagani M., Malliani A. (1994). Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation, 90, 1826-1831. [DOI] [PubMed] [Google Scholar]
- Moodithaya S. H., Avadhany S. T. (2012). Gender differences in age-related changes in cardiac autonomic nervous function. Journal of Aging Research, 2012, 679345. doi: 10.1155/2012/679345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M., Malliani A. (2000). Interpreting oscillations of muscle sympathetic nerve activity and heart rate variability. Journal of Hypertension, 18, 1709-1719. [DOI] [PubMed] [Google Scholar]
- Palisso G., Manzella D., Barbieri M., Rizzo M. R., Gambardella A., Varrcchio M. (1999). Baseline heart rate variability in healthy centenarians: Differences compared with aged subjects (>75 years old). Clinical Science, 97, 579-584. [PubMed] [Google Scholar]
- Parati G., Saul J. P., Di Rienzo M., Mancia G. (1995). Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: A critical appraisal. Hypertension, 25, 1276-1286. [DOI] [PubMed] [Google Scholar]
- Park S. B., Lee B. C., Jeong K. S. (2007). Standardized tests of heart rate variability for autonomic function tests in healthy Koreans. International Journal of Neuroscience, 117, 1707-1717. [DOI] [PubMed] [Google Scholar]
- Regitz-Zagrosek V., Lehmkuhl E., Weickert M. O. (2006). Gender differences in the metabolic syndrome and their role in cardiovascular disease. Clinical Research in Cardiology, 95, 136-147. [DOI] [PubMed] [Google Scholar]
- Rossy L. A., Thayer J. F. (1998). Fitness and gender-related differences in heart period variability. Psychosomatic Medicine, 60, 773-781. [DOI] [PubMed] [Google Scholar]
- Ryan S. M., Goldberger A. L., Pincus S. M., Mietus J., Lipsitz L. A. (1994). Gender- and age-related differences in heart rate dynamics: Are women more complex than men? Journal of the American College of Cardiology, 24, 1700-1707. [DOI] [PubMed] [Google Scholar]
- Schwartz P. J., La Rovere M. T., Vanoli E. (1992). Autonomic nervous system and sudden cardiac death: Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation, 85, 177-191. [PubMed] [Google Scholar]
- Stein P. K., Bosner M. S., Kleiger R. E., Conger B. M. (1994). Heart rate variability: A measure of cardiac autonomic tone. American Heart Journal, 127, 1376-1381. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Kimura T., Goyagi T., Nishikawa T. (2004). Gender differences in baroreflex response and heart rate variability in anesthetized humans. British Journal of Anaesthesia, 92, 831-835. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology & the North American Society of Pacing and Electrophysiology. (1996). Heart rate variability: Standard of measurement, physiological interpretation, and clinical use. Circulation, 93, 1043-1065. [PubMed] [Google Scholar]
- Teixeira C. A., Direito B., Feldwisch-Drentrup H., Valderrama M., Costa R. P., Alvarado-Rojas C., . . . Dourado A. (2011). EPILAB: A software package for studies on the prediction of epileptic seizures. Journal of Neuroscience Methods, 200, 257-271. [DOI] [PubMed] [Google Scholar]
- Tulppo M. P., Makikallio T. H., Seppanen T., Shoemaker K., Tutungi E., Hughson R. L., Huikuri H. V. (2001). Effects of pharmacological adrenergic and vagal modulation on fractal heart rate dynamics. Clinical Physiology, 21, 515-523. [DOI] [PubMed] [Google Scholar]
- Umetani K., Singer D. H., McCraty R., Atkinson M. (1998). Twenty-four-hour-time domain heart rate variability and heart rate: Relations to age and gender over nine decades. American College of Cardiology, 31, 593-601. [DOI] [PubMed] [Google Scholar]
- Uusitalo A. L. T., Vanninen E., Levälahti E., Battie M. C., Videman T., Kaprio J. (2007). The role of genetic and environmental influences on heart rate variability in middle-aged men. American Journal of Physiology, 293, H1013-H1022. [DOI] [PubMed] [Google Scholar]
- Van Buren J. M. (1958). Some autonomic concomitants of ictal automatism. Brain, 81, 505-529. [DOI] [PubMed] [Google Scholar]