Abstract
Growing evidence suggests that respiratory and limb muscle function may be impaired in patients with interstitial lung disease (ILD). Importantly, muscle dysfunction could promote dyspnoea, fatigue and functional limitation all of which are cardinal features of ILD. This article examines the risk factors for skeletal muscle dysfunction in ILD, reviews the current evidence on overall respiratory and limb muscle function and focuses on the occurrence and implications of skeletal muscle dysfunction in ILD. Research limitations and pathways to address the current knowledge gaps are highlighted.
Keywords: Interstitial lung disease, risk factors, respiratory muscles, skeletal muscle, muscle dysfunction
Introduction
More than 300 different conditions are encompassed under the umbrella term of interstitial lung disease (ILD).1 Some ILD subtypes such as sarcoidosis and connective tissue disease-associated ILD are secondary to systemic disease (secondary ILD) rather being specific to the lung and may have primary muscle involvement. However, skeletal muscle seems to be affected even in lung-specific idiopathic interstitial pneumonias (IIPs).2 The most common types of IIPs are idiopathic pulmonary fibrosis (IPF) and non-specific interstitial pneumonia (NSIP) accounting for approximately 55% and 25% of all IIPs.1 Overall, IPF and sarcoidosis are the most frequent types of ILD and together comprise more than half of all cases.1
Dyspnoea and lower limb fatigue leading to reduced functional capacity are key features of ILD and have long been associated with reduced quality of life and poor survival.3–5 Although both phenomena have been primarily linked to respiratory impairment and circulatory limitation,6 skeletal muscle dysfunction per se is increasingly implicated in these clinical manifestations.7 Albeit commonly unrecognized in clinical practice, respiratory and limb muscle dysfunction in ILD calls for a wider appreciation and deeper understanding. It is through a comprehensive and collective appreciation of all the components of ILD that patients can benefit from better-targeted interventions and higher levels of care.
This narrative literature review examines the risk factors for skeletal muscle dysfunction in ILD, discusses the evidence on overall respiratory and limb muscle function and focuses on the occurrence and implications of skeletal muscle dysfunction in ILD. IIPs – the primary focus of this review – and secondary ILD are examined separately. Research limitations and pathways to address the current knowledge gaps are also highlighted. Allowing for the changeful terminology that applies to ILD and the numerous encompassed conditions, the presented evidence was identified by searching PubMed for published articles in English language reporting on the skeletal muscle function in ILD through June 2015. All identified studies are presented and discussed in a non-selective manner. To our knowledge, this is the first literature review describing research into skeletal muscle dysfunction in ILD.
Risk factors for muscle dysfunction in ILD
Although evidence on causal relationship is still missing, several well-established muscle dysfunction-promoting factors such as chronic hypoxaemia, inflammatory and oxidative stress, corticosteroid use, physical inactivity and malnutrition exist in ILD. These factors may exert a synergistic, deleterious effect on muscle function. In brief, the coupling of resting and/or exertional hypoxaemia5 with increased work of breathing8 in ILD renders the respiratory muscles prone to hypoxia. The latter promotes inspiratory (diaphragm), expiratory (abdominal muscles)9 and limb (quadriceps)10 muscle fatigability.
Pulmonary inflammation and oxidative stress are thought to be pivotal in the pathogenesis of IIPs.11–13 A systemic inflammatory and oxidative response has also been well documented in various types of ILD including IPF11 and ILD associated with collagen vascular diseases.14 This is in line with findings in chronic obstructive pulmonary disease (COPD) and other inflammatory lung conditions such as asthma and bronchiectasis.14 Importantly, inflammation and oxidative stress disrupt the balance between degradation and resynthesis of skeletal muscle proteins and promote muscle wasting.15 It is, therefore, possible that ILD follows the paradigm of COPD by exerting pathological effects on skeletal muscle through systemic transmission of inflammatory and oxidative stress.14,15 However, further study is required to determine if this indeed is the case in ILD.
Glucocorticoids are also associated with muscle dysfunction.15 Glucocorticoid treatment increases oxidative stress and induces mitochondrial dysfunction in cultured human muscle cells,16 whilst physiological hypercortisolemia increases proteolysis.17 Glucocorticoids are also thought to be important mediators of cachexia and physical inactivity-induced muscle atrophy,14 which are likely to be relevant to ILD (see below). These findings are supported by long-established evidence of clinically significant corticosteroid-induced myopathy (involving both respiratory and limb muscles) in COPD.18 In a cohort of 60 patients with various chronic diseases,19 long-term treatment with high doses of prednisolone (exceeding 50 mg daily) was associated with significant overproduction of serum lactate during aerobic exercise as well as decreased aerobic (complex I) mitochondrial enzyme activity and increased levels of oxidative damage of mitochondrial and nuclear DNA in quadriceps muscles.19 These findings provide evidence that chronic corticosteroid administration induces mitochondrial dysfunction and oxidative damage in skeletal muscles, which may be the pathogenesis, at least in part, of corticosteroid-induced myopathy.19 However, whether a minimum threshold for steroid usage causing clinical muscle dysfunction exists remains to be defined.
Two studies reported on the impact of steroid use in muscle function in ILD. In 45 patients with advanced IPF, there was an inverse relationship between the mean daily dose of oral corticosteroids (22 mg of prednisolone) and improvement in quadriceps force derived from a pulmonary rehabilitation program; the improvement in quadriceps force was less in subjects taking oral corticosteroids than those not receiving corticosteroids.20 Also, in 25 patients with sarcoidosis-associated ILD, the mean daily dose of long-term oral corticosteroids (5 mg methylprednisolone equivalents) in the 6-month period before testing was inversely associated with the quadriceps force.21 Taken together, the evidence suggests that steroid myopathy is likely to be clinically relevant to patients with ILD; in particular, those who receive intensified corticosteroid treatment.
Patients with ILD often adopt a sedentary lifestyle, which may also exert detrimental effects on muscle function. Assessed by accelerometry, lung transplant candidates with ILD (half of which had IPF) were shown to be markedly inactive, achieving less than a third of the levels of a general population.22 Accelerometer-assessed physical activity was also reduced in subjects with mild IPF.23 Physical inactivity, in turn, leads to skeletal muscle deconditioning and a vicious cycle of worsening exercise capacity and increasing symptoms.7 Importantly, skeletal muscle disuse leads to muscle atrophy (characterized by a decrease in protein content, fibre diameter, force production, and fatigue resistance) through alterations in myofibrillar protein synthesis and proteolysis, gene expression24 (including Muscle Ring Finger 1 and Muscle Atrophy F-box/atrogin-125) and activation of molecular mechanisms (such as nuclear factor κB signalling pathways26). Work in COPD has shown a negative association between physical activity, exercise capacity, type I fibre proportion, and percentage of intramuscular fat and quadriceps muscle attenuation.27 Given the link between physical inactivity and muscle fibre shift, this probably reflects reduced quadriceps oxidative capacity and endurance, leading to early fatigue.27 However, these possibilities remain to be explored in ILD as direct (biopsy) measurements of such muscle parameters at the tissue, cellular and molecular level have yet to be undertaken in ILD populations.
Patients with ILD are often clinically malnourished. In IPF, nutritional depletion as assessed by body mass index is a common finding and an independent predictor of mortality.28,29 Malnutrition causes changes in the myosin content and muscle atrophy, alters muscle bioenergetics and downregulates energy-dependent cellular membrane pumping of the muscle fibres; it ultimately leads to an altered pattern of muscle contraction and relaxation, reduced force-generating capacity and increased fatiguability.30–32
Ageing impacts significantly on muscle performance. Skeletal muscle force is known to naturally decrease by 30–40% between 30 and 80 years of age, and this correlates well with the decrease in muscle mass.33 By 60–70 years of age, muscle mass in humans decreases by 25–30% as a consequence of a reduction in fibre cross-sectional area, number and size predominantly involving type II fibres; specific muscle force (i.e. muscle force per unit cross-sectional area) also decreases with increasing age.33 Accordingly, inspiratory muscle force in ILD has been shown to decrease with age,34 although whether this is over and above, the effect of normal ageing has not been tested. In any case, as the incidence of ILD increases with older age,1,5 the effect of ageing on muscle function should be taken into account.
The chronically increased work of breathing due to mechanical restraints (i.e. stiffness of the lung) is commonly considered to exert a ‘training effect’ on the diaphragm.8,34–36 However, it is possible that excessive chronic overload could represent an additional adverse factor for respiratory muscle function in advanced ILD.
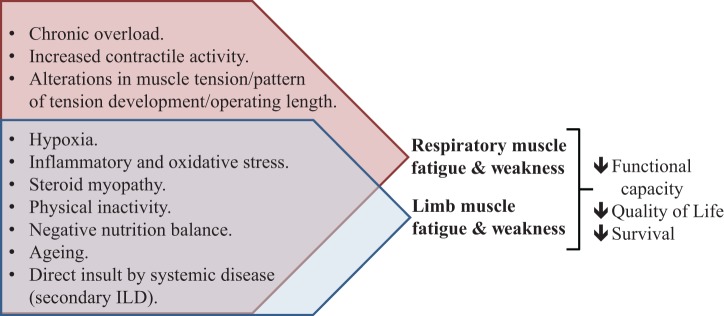
A conceptual model of systemic and local factors that may promote respiratory and limb muscle dysfunction in ILD and their implications is presented in Figure 1.
Figure 1.
A conceptual model of systemic and local factors that may promote respiratory and limb muscle dysfunction in ILD and their implications. ILD: interstitial lung disease.
Idiopathic interstitial pneumonias
Respiratory muscle function
Despite the aforementioned adverse factors, patients with ILD often exhibit a preserved force-generating capacity of the inspiratory muscles.2,8,36–40 This may reflect the mechanical advantage of inspiratory force generation at lower operating lung volumes (improved muscle length–tension relationship) but also the absence of impairments in inspiratory threshold and resistive loading.8,34,35 Indeed, maximal inspiratory mouth pressures showed a significant inverse correlation with lung volume in a cohort of 15 IPF patients with preserved inspiratory capacity.36 In theory, preservation of the inspiratory force-generating capacity may also be aided by a decrease in the radius of the diaphragmatic curvature allowing for an increase in transdiaphragmatic pressure according to Laplace’s law.36
In contrast, impaired maximal inspiratory mouth pressures have been shown in 12 patients with various types of mild-to-moderate ILD.41 Also, in the only study in moderately severe ILD, non-volitional measurements in a heterogeneous cohort of 25 patients showed a notable reduction in diaphragmatic muscle strength.40 This suggests that due to a substantial diaphragmatic reserve as a pressure generator, it may not be until advanced stages of the disease that diaphragm impairment becomes clinically important. Therefore, although most of the studies suggest preserved static inspiratory muscle strength in ILD, the evidence is not unanimous.
Whether expiratory muscle strength is impaired in ILD also remains controversial. Preserved expiratory pressures were reported in patients with various ILD subtypes in both mild–moderate8,38 and severe disease.40 However, low maximal expiratory pressures were documented in patients with mild-to-moderate IPF2,36 and fibrotic NSIP.39 This may due to the expiration being passive under almost all conditions resulting in relative deconditioning of the expiratory muscles.36 Also, a decrease in lung volume, albeit beneficial for inspiratory muscles would be expected to contribute to a decrease in tension produced by expiratory muscles. However, no correlation was identified between maximal expiratory mouth pressures and lung volume in 41 patients with IPF with reduced expiratory muscle strength.2
The role of the abdominal muscles in ILD is not well understood. In a model of ILD, externally added inspiratory elastic mechanical loading in resting, seated (but not supine) healthy subjects provoked recruitment of the abdominal muscles.42 However, abdominal muscles are considered to play an important role in high-intensity exercise43 rather than rest. In both health43 and ILD,44 recruitment of the abdominal muscles during moderate-to-heavy exercise has been shown to facilitate lung emptying and reduce the end-expiratory lung volume (EELV), thus contributing to the increase in inspiratory capacity. Active contraction of the abdominal muscles increases expiratory flow rate by raising the intra-abdominal pressure and shifting the abdominal contents and diaphragm cephalically, whereas their relaxation aids inspiration by allowing the abdominal contents to fall.44 Recruitment of the abdominal muscles also improves the pressure-generating capacity of the diaphragm by increasing its length42 as well as its rib cage expanding action.45
On a clinical level, fatigue of the abdominal muscles might limit ventilatory capacity in healthy subjects.46 Specific abdominal muscle fatigue (defined as having a ≥10% fall in gastric pressure at twitch T10 nerve roots) has been demonstrated in 9 of 16 (56%) patients with mild-to-moderate ILD (primarily connective tissue disease-associated ILD) who underwent symptom-limited (maximal) exercise on a cycle ergometer.44 These patients (fatiguers) displayed greater oxygen desaturation and reduced exercising EELV consistent with increased abdominal muscle recruitment and shortening compared to non-fatiguers. The latter contradicts the established notion derived from healthy and COPD subjects47,48 that muscle shortening exerts an anti-fatigue effect, possibly, via alterations in the intracellular propagation of the depolarization.49 It rather suggests that factors other than muscle length such as contractile activity (respiratory frequency) and hypoxia may be additional and/or more important determinants of the likelihood of abdominal fatigue in ILD.44 Finally, resting twitch transdiaphragmatic pressure was lower in fatiguers raising the possibility of an increased contribution of the abdominal muscle to ventilation to compensate for diaphragm dysfunction. However, it should be noted that maximum inspiratory pressures were in the normal range.44
In summary, the current evidence on respiratory muscle dysfunction in ILD is inconclusive. Whether this reflects the absence of clinically significant muscle impairment or it is accounted by research limitations (see below) remains to be proven. The methodology and findings of the main studies on skeletal muscle function in ILD are summarized in a chronological order in Table 1.
Table 1.
Clinical studies on muscle dysfunction in ILD.a
| Author, year | Study design | Cases/controls | Disease [n]. Restrictive defect | Muscle strength | Findings in ILD | Limitations |
|---|---|---|---|---|---|---|
| De Troyer et al.,34 1980 | Prospective case control | 12/135 | Sarcoidosis-assoc. ILD [1], asbestosis [2], CTD [3] and HP [1]. TLC 75% pred. | Ppl min. | Ppl min. normal relative to lung volume over much of the inspiratory capacity suggesting preservation of the inspiratory muscle strength. | Volitional tests. Heterogeneity of the study population. |
| Gorini et al.,41 1989 | Prospective case control | 12/18 | IPF [8] and CTD [1]. TLC 67% pred. | PImax | Reduced PImax %pred. (69%). | Volitional tests. Results might have been confounded by chronic steroid treatment (received by 10 patients). |
| Nishimura et al.,36 1989 | Prospective cross section | 15/0 | IPF. TLC 71% pred. | PImax and PEmax | Preserved PImax. Reduced PEmax %pred. (57%). Significant negative correlation between lung volumes %pred. and PImax %pred. but not with PEmax %pred. Respiratory muscle strength had no correlation with PaO2 or PaCO2. | Volitional tests. PImax and PEmax were overestimated (not corrected for lung volume). The method of diagnosis of IPF is unclear. |
| O’Donnell et al.,8 1998 | Prospective case control | 12/12 | IPF [3], Sarcoidosis [2], CTD [1] and other [3].TLC 70% pred. | PImax and PEmax | Preserved PImax and PEmax. | Volitional tests. Heterogeneity of the study population. |
| Baydur et al.,50 2001 | Prospective case control | 36/25 | Sarcoidosis. TLC 84% pred. | PImax and PEmax | Reduced PImax (37% less) and PEmax (39% less). Strong inverse relationships between PEmax and PImax with dyspnoea/activity. | Volitional tests. Only 24 patients had ILD. No restrictive defect. |
| Garcia-Rio et al.,37 2003 | Prospective case control | 14/11 | UIP. TLC 68% pred. | PImax, PI mean, Pdi and Pes | Increased PI mean. Preserved PImax, Pdi and Pes. | Volitional tests. The method of estimation may have led to an overestimation of PI mean. |
| Nishiyama et al.,2 2005 | Retrospective cross section | 41/0 | IPF. TLC 77% pred. | PImax, PEmax, QF and HF | Preserved PImax and HF. Reduced PEmax (68% pred.) and QF (65% pred.). QF was independent predictor of V▪O2max in patients who discontinued exercise because of dyspnoea and/or leg fatigue. | Retrospective. Volitional tests. |
| Walterspacher et al.,40 2013 | Prospective case control | 25/24 | UIP [16], NSIP [3], HP [1], AIP [1] and unspecified [4]. TLC 55% pred. | PImax, PEmax, SNiP, TwPmo and TwPdi | Reduced TwPmo (35% less) and TwPdi (29% less). Preserved PImax, SNiP and PEmax. | Heterogeneity of the study population. Results might have been confounded by chronic steroid treatment (received by 19 patients). |
| Elia et al.,44 2013 | Prospective cross section | 16/0 | IPF [1], HP [3] and CTD [9]. TLC 74% pred. | PImax, PEmax, TwPdi, TwT10Pga, SNiP, SnPdi and cough Pga | During maximal exercise, TwPdi did not change but TwT10Pga fell by 12%. The fall in TwT10Pga correlated with V▪O2max and the decrease in EELV. Fatiguers (defined as having a ≥10% fall in TwT10Pga) had a fall in EELV and lower pre-exercise TwPdi. | Results might have been confounded by chronic steroid treatment (received by all patients). |
| Watanabe et al.,39 2013 | Retrospective cross section | 30/0 | f-NSIP. VC 75% pred. | PImax, PEmax, QF and HF | Preserved PImax and HF. Decreased PEmax (75% pred.) and QF (82% pred.). Adjusted for sex, QF was an independent predictor of 6MWD. | Retrospective. Volitional tests. Mild disease. PImax, PEmax and HF were related to predicted values derived from populations different from the study population. |
| Mendoza et al.,38 2014 | Prospective case control | 25/33 | IPF and f-NSIP. TLC 68% pred. | PImax, PEmax, SNiP, QMVC, TwQ and QE | Reduced TwQ (20% less) and QE. Preserved PImax, PEmax, and SNiP. PImax correlated with 6MWD. | Use of 6MWT instead of formal CPET for the assessment of exercise capacity. |
ILD: interstitial lung disease; SNiP: sniff nasal inspiratory pressure; Pes: oesophageal pressure; HF: handgrip peak voluntary force; QF: quadriceps peak voluntary force; QMVC: quadriceps maximum voluntary contraction; TwQ: quadriceps twitch force in response to magnetic femoral nerve stimulation; QE: quadriceps endurance using the decay in force in response to repetitive magnetic stimulation of the quadriceps over 5 min; EELV: end-expiratory lung volume; VC: vital capacity; SVC: slow vital capacity; FVC: forced vital capacity; TLC: total lung capacity; PaO2/PaCO2: partial pressure of oxygen/carbon dioxide in the arterial blood; V▪O2max: maximum oxygen uptake; 6MWD: 6-minute walk distance; CPET: cardiopulmonary exercise test; f-NSIP: fibrotic non-specific interstitial pneumonia, HP: hypersensitivity pneumonitis; AIP: acute interstitial pneumonia; CTD: connective tissue disease; PImax/PEmax: maximum inspiratory/expiratory mouth pressure; Ppl min.: minimum (greatest negative) pleural pressure; Pdi: maximum transdiaphragmatic pressure during volitional manoeuvers; SnPdi: sniff transdiaphragmatic pressure; TwPmo/TwPdi: twitch mouth/transdiaphragmatic pressure during bilateral magnetic phrenic nerve stimulation; TwT10Pga: gastric pressure at twitch T10 nerve roots; IPF: idiopathic pulmonary fibrosis; UIP: usual interstitial pneumonia; assoc.: associated; pred.: predicted.
aAll studies are single centred. Data are presented as n or mean unless otherwise stated.
Clinical implications of respiratory muscle dysfunction
Dyspnoea intensity is increased in patients with ILD at any given absolute exercise intensity compared with healthy controls and extend to the resting state in advanced disease.8 Slopes of Borg ratings over oxygen uptake were greater and the onset of dyspnoea occurred at a much lower oxygen uptake in 12 exercising patients with ILD than in normal subjects.8 Also, at an oxygen uptake equivalent to the breakpoint of exercise, patients with ILD experienced severe dyspnoea, whereas normal subjects had not yet attained a noticeable level of respiratory difficulty.8 In fact, activity-related dyspnoea appears to be the most common, earliest and dominant exercise-limiting symptom in the majority of patients with ILD.35 Functional weakening of the ventilatory muscles, along with increased ventilatory demand relative to capacity and impediment of the action of the ventilatory muscles may provoke dyspnoea.35 The respiratory muscles may also modify dyspnoea sensation by fatigue-independent mechanisms associated with alterations in muscle tension and the pattern of tension development. Muscular tension is affected by velocity (airflow), frequency of contraction (respiratory frequency) and the duty cycle (the ratio of inspiratory time to total breath duration),51 which are all altered in ILD.
Few studies have associated respiratory muscle performance with functional impairment. Abdominal muscle strength has been correlated with peak oxygen uptake in 25 patients with ILD, although fatigue of the abdominal muscles was not associated with exertional dyspnoea or reduced exercise tolerance.40 Elsewhere, maximal inspiratory and expiratory mouth pressures demonstrated a more consistent, graded decline with increasing dyspnoea/reducing activity levels than lung volumes and gas transfer in 36 patients with ILD due to sarcoidosis.50 Also, maximal inspiratory pressures, along with hypoxia, correlated with 6-minute walk distance (6MWD), whereas quadriceps strength and quadriceps endurance failed to show a significant correlation with 6MWD in 25 patients with ILD; in contrast, quadriceps parameters correlated with 6MWD in healthy controls.38
Lower limb muscle function
Volitionally assessed quadriceps force was shown to be reduced (65% predicted) in 41 patients with mild-to-moderate IPF2 and it correlated well with lung function impairment. Similar data exist for patients with other IIPs. In a retrospective study of 30 patients with fibrotic NSIP, 16 patients showed significantly decreased volitional quadriceps force with the mean value for all patients being 82% predicted.39 Mendoza et al. employed non-volitional tests to assess muscle parameters to show that quadriceps strength and endurance in 25 patients with IPF and fibrotic NSIP were reduced compared with healthy controls.38 Finally, adjusted for body weight, quadriceps force was lower in IPF patients compared to COPD patients.20
Clinical implications of lower limb muscle dysfunction
Evidence suggests a close relationship between functional impairment and limb muscle strength. Adjusted for sex, the reduced quadriceps force in 30 patients with fibrotic NSIP showed a significant correlation to 6MWD, whereas pulmonary function parameters such as vital capacity showed only marginal correlations. In stepwise multiple regression analysis, only quadriceps force was an independent predictor of exercise performance.39 In a cohort of 41 IPF patients with quadriceps weakness, quadriceps force and vital capacity were the only independent predictors of exercise capacity in a stepwise multiple regression analysis that also included maximum expiratory and inspiratory pressures, total lung capacity, diffusion capacity and systemic oxygenation indices.2 Interestingly, quadriceps force was a significant factor in patients who discontinued exercise, irrespectively of whether their exercise-limiting symptom was leg fatigue or dyspnoea. In contrast, respiratory function correlated with exercise capacity only in patients who terminated the exercise due to dyspnoea but not in those who stopped due to leg fatigue. Therefore, quadriceps force may be the strongest predictor of exercise capacity in both dyspnoea- and leg fatigue–exercise-limited patients.2 In 24 lung transplant candidates with ILD peak, quadriceps torque was moderately correlated with physical activity (total daily steps), whereas measures of pulmonary function, age, or measures of gas exchange failed to show such correlation.22 Finally, in a group of 45 patients with advanced IPF and reduced quadriceps force, the improvement in 6MWD following pulmonary rehabilitation was positively correlated with the improved quadriceps force,20 suggesting that limb muscle strength and endurance are potential targets for meaningful interventions.
Secondary ILD
The heterogeneity of patients with systemic diseases that may cause ILD who may or may not have lung parenchymal involvement and/or muscle impairment makes this population a difficult target for study. Alternatively, unless the exact combination of lung parenchymal involvement and muscle performance is known, findings in populations with systemic diseases that may cause ILD should be interpreted with caution. Findings should also not be extrapolated to other types of ILD.
Sarcoidosis is the best-studied example of secondary ILD. Muscle involvement in the rare form of myositis and chronic myopathy is largely symptomatic.52 Clinically significant involvement of respiratory and limb muscle by sarcoid myositis has been described in case reports.53 In contrast, granulomatous involvement of the skeletal muscles that occurs in 50–80% of sarcoidosis patients is almost always symptomless and resolves during the natural course of the disease.52 However, it has still been identified as a rare cause of diaphragmatic weakness54 and patients with (but also without) quadriceps granulomatous involvement may show respiratory muscle weakness.55 Maximal inspiratory and expiratory muscle strength were found to be decreased in 36 patients with sarcoidosis, two-thirds of whom had sarcoidosis-associated ILD.50 The clinical impact of these phenomena is debatable: Some studies claim that impaired inspiratory muscle strength is correlated with exertional dyspnoea and reduced walking capacity (only reliably detectable when using non-volitional tests of inspiratory muscle strength),56 whereas others (using volitional tests) failed to confirm this association.57 Finally, chronic fatigue, a frequent and commonly debilitating co-morbidity of sarcoidosis, even during clinical remission, has been associated with upper and lower limb muscle weakness and reduced physical activity,58 exercise tolerance and health status.21 This suggests that many patients with sarcoidosis may indeed benefit by an exercise program.
In scleroderma, direct insult of the diaphragm and intercostal muscles inducing inflammatory, fibrotic and atrophic changes has been histologically demonstrated and associated with fatal respiratory failure in case reports, in both the presence59 and absence60 of associated ILD. Patients with idiopathic inflammatory myopathies may also demonstrate diaphragmatic and intercostal muscle weakness that may rarely lead to respiratory failure.61 In a study of 23 patients with idiopathic inflammatory myopathies, 18 patients were diagnosed with diaphragm weakness; however, only six had associated ILD.62
Research considerations
Some facts should be taken into consideration on attempting to interpret the evidence on skeletal muscle function in ILD. First, most of the studied ILD populations were heterogeneous with regard to age, sex, disease severity and, importantly, specific underlying pathology, all of which are potential confounding factors in the assessment of muscle strength.34,41 Second, it is unlikely that the numerous conditions encompassed under the umbrella term of ILD share the same systemic and local determinants of muscle performance. Of course, the rarity of some of the subtypes of ILD poses an essential research obstacle. Third, the participants are commonly carefully selected to be clinically stable and free from comorbidities with only mild-to-moderate lung disease and reasonable exercise capacity. Thus, although muscle function is more likely to be impaired in advanced ILD compared with early disease, the evidence in this patient population is scarce. Fourth, the relatively small sample size of most of the studies does not assure confidence in generalizing the findings and conclusions to the broader ILD populations. Finally, study results may be confounded by the methods of investigation (volitional vs. non-volitional tests and non-invasive vs. invasive pressure measurements).63 Of note, some of the referenced studies34,36,41 were conducted in the 1980’s, and their results should be interpreted in the context of the different ILD classification, assessment methodology and research equipment compared to current guidelines and practices. For these reasons, the current knowledge cannot provide a sufficient evidence base for the different subtypes of ILD and, especially, ILD as a whole.
Future directions
The evidence on muscle dysfunction in ILD calls for further study. The pathogenesis, molecular basis and the extent of muscle dysfunction need to be further explored. Histological examination and computed tomography/magnetic resonance imaging measurements of muscle parameters would provide direct insights into the morphology, metabolism and function of respiratory and limb muscles. Concomitant assessment of respiratory and limb muscles is required to establish whether both muscle groups are mutually affected. The potential associations between identified muscle abnormalities and candidate etiological factors such as hypoxia, systemic inflammation, inactivity and malnutrition should also be investigated.
The interrelationship between muscle abnormalities, physiological parameters (e.g. muscle strength and endurance, pulmonary function tests and arterial blood gas tensions) and clinical features (e.g. exercise capacity, dyspnoea, quality of life and mortality) need to be further explored. This would clarify whether muscle dysfunction in ILD is a true mediator of the disease process. Future studies need to include larger and more homogenous populations in terms of ILD subtype, disease severity and duration, age, nutritional status, comorbidities and treatments and, albeit challenging, extend to severe ILD. This would help highlight possible heterogeneity in muscle function and draw the attention to the most severely affected populations. Finally, more studies are required to investigate the impact of exercise training and contemporary treatment regimens for ILD on muscle function.
Conclusions
The current evidence on respiratory and limb muscle dysfunction in ILD is inconclusive with methodological limitations in the existing literature. However, absence of strong evidence is not the evidence of absence. Multiple risk factors exist and evidence from small series of mixed groups of patients suggests that muscle dysfunction occurs in ILD. Nevertheless, the strong interrelationship between muscle function, functional capacity, quality of life and survival is clear; and, interventions that benefit this sequence are highly desirable. In this framework, muscle function, a variable not currently targeted by most treatment interventions in ILD, requires more research and focus.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Interstitial lung disease. In: Gibson GJ, Loddenkemper R, Sibille Y, et al. (eds) European Lung White Book. 2nd ed Sheffield: European Respiratory Society, 2013, pp. 256–269. [Google Scholar]
- 2. Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest 2005; 127: 2028–2033. [DOI] [PubMed] [Google Scholar]
- 3. Chang JA, Curtis JR, Patrick DL, et al. Assessment of health-related quality of life in patients with interstitial lung disease. Chest 1999; 116: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 4. Caminati A, Bianchi A, Cassandro R, et al. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med 2009; 103: 117–123. [DOI] [PubMed] [Google Scholar]
- 5. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest 1996; 109: 1566–1576. [DOI] [PubMed] [Google Scholar]
- 7. Holland AE. Exercise limitation in interstitial lung disease - mechanisms, significance and therapeutic options. Chron Respir Dis 2010; 7: 101–111. [DOI] [PubMed] [Google Scholar]
- 8. O’Donnell DE, Chau LK, Webb KA. Qualitative aspects of exertional dyspnea in patients with interstitial lung disease. J Appl Physiol (1985) 1998; 84: 2000–2009. [DOI] [PubMed] [Google Scholar]
- 9. Verges S, Bachasson D, Wuyam B. Effect of acute hypoxia on respiratory muscle fatigue in healthy humans. Respir Res 2010; 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katayama K, Amann M, Pegelow DF, et al. Effect of arterial oxygenation on quadriceps fatigability during isolated muscle exercise. Am J Physiol Regul Integr Comp Physiol 2007; 292: R1279–R1286. [DOI] [PubMed] [Google Scholar]
- 11. Rahman I, Skwarska E, Henry M, et al. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med 1999; 27: 60–68. [DOI] [PubMed] [Google Scholar]
- 12. Bringardner BD, Baran CP, Eubank TD, et al. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 2008; 10: 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal 2008; 10: 303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hiraiwa K, van Eeden SF. Nature and consequences of the systemic inflammatory response induced by lung inflammation. In: Ong KC. (ed) Lung Inflammation, http://intechopen.com/books/lung-inflammation/nature-and-consequences-of-the-systemic-inflammatory-response-induced-by-lung-inflammation (2014, accessed 18 July 2015).
- 15. Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007; 35: 411–429. [DOI] [PubMed] [Google Scholar]
- 16. Oshima Y, Kuroda Y, Kunishige M, et al. Oxidative stress-associated mitochondrial dysfunction in corticosteroid-treated muscle cells. Muscle Nerve 2004; 30: 49–54. [DOI] [PubMed] [Google Scholar]
- 17. Darmaun D, Matthews DE, Bier DM. Physiological hypercortisolemia increases proteolysis, glutamine, and alanine production. Am J Physiol 1988; 255: E366–E373. [DOI] [PubMed] [Google Scholar]
- 18. Decramer M, Lacquet LM, Fagard R, et al. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150: 11–16. [DOI] [PubMed] [Google Scholar]
- 19. Mitsui T, Azuma H, Nagasawa M, et al. Chronic corticosteroid administration causes mitochondrial dysfunction in skeletal muscle. J Neurol 2002; 249: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 20. Kozu R, Senjyu H, Jenkins SC, et al. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration 2011; 81: 196–205. [DOI] [PubMed] [Google Scholar]
- 21. Spruit MA, Thomeer MJ, Gosselink R, et al. Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax 2005; 60: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wickerson L, Mathur S, Helm D, et al. Physical activity profile of lung transplant candidates with interstitial lung disease. J Cardiopulm Rehabil Prev 2013; 33: 106–112. [DOI] [PubMed] [Google Scholar]
- 23. Nakayama M, Bando M, Araki K, et al. Physical activity in patients with idiopathic pulmonary fibrosis. Respirology 2015; 20: 640–646. [DOI] [PubMed] [Google Scholar]
- 24. Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 2004; 287: C834–C843. [DOI] [PubMed] [Google Scholar]
- 25. Bodine SC. Disuse-induced muscle wasting. Am J Physiol Cell Physiol 2013; 45: 2200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl) 2008; 86: 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maddocks M, Shrikrishna D, Vitoriano S, et al. Skeletal muscle adiposity is associated with physical activity, exercise capacity and fibre shift in COPD. Eur Respir J 2014; 44: 1188–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alakhras M, Decker PA, Nadrous HF, et al. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007; 131: 1448–1453. [DOI] [PubMed] [Google Scholar]
- 29. Kim JH, Lee JH, Ryu YJ, et al. Clinical predictors of survival in idiopathic pulmonary Fibrosis. Tuberc Respir Dis (Seoul) 2012; 73: 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saunders J, Smith T. Malnutrition: causes and consequences. Clin Med 2010; 10: 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jeejeebhoy KN. Muscle function and nutrition. Gut 1986; 27: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopes J, Russell DM, Whitwell J, et al. Skeletal muscle function in malnutrition. Am J Clin Nutr 1982; 36: 602–610. [DOI] [PubMed] [Google Scholar]
- 33. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med 1999; 159: S1–S40. [DOI] [PubMed] [Google Scholar]
- 34. De Troyer A, Yernault JC. Inspiratory muscle force in normal subjects and patients with interstitial lung disease. Thorax 1980; 35: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laveneziana P. Qualitative aspects of exertional dyspnea in patients with restrictive lung disease. Multidiscip Respir Med 2010; 5: 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishimura Y, Hida W, Taguchi O, et al. Respiratory muscle strength and gas exchange in neuromuscular diseases: comparison with chronic pulmonary emphysema and idiopathic pulmonary fibrosis. Tohoku J Exp Med 1989; 159: 57–68. [DOI] [PubMed] [Google Scholar]
- 37. Garcia-Rio F, Pino JM, Ruiz A, et al. Accuracy of noninvasive estimates of respiratory muscle effort during spontaneous breathing in restrictive diseases. J Appl Physiol (1985) 2003; 95: 1542–1549. [DOI] [PubMed] [Google Scholar]
- 38. Mendoza L, Gogali A, Shrikrishna D, et al. Quadriceps strength and endurance in fibrotic idiopathic interstitial pneumonia. Respirology 2014; 19: 138–143. [DOI] [PubMed] [Google Scholar]
- 39. Watanabe F, Taniguchi H, Sakamoto K, et al. Quadriceps weakness contributes to exercise capacity in nonspecific interstitial pneumonia. Respir Med 2013; 107: 622–628. [DOI] [PubMed] [Google Scholar]
- 40. Walterspacher S, Schlager D, Walker DJ, et al. Respiratory muscle function in interstitial lung disease. Eur Respir J 2013; 42: 211–219. [DOI] [PubMed] [Google Scholar]
- 41. Gorini M, Spinelli A, Ginanni R, et al. Neural respiratory drive and neuromuscular coupling during CO2 rebreathing in patients with chronic interstitial lung disease. Chest 1989; 96: 824–830. [DOI] [PubMed] [Google Scholar]
- 42. Martin JG, De Troyer A. The behaviour of the abdominal muscles during inspiratory mechanical loading. Respir Physiol 1982; 50: 63–73. [DOI] [PubMed] [Google Scholar]
- 43. Abraham KA, Feingold H, Fuller DD, et al. Respiratory-related activation of human abdominal muscles during exercise. J Physiol 2002; 541: 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elia D, Kelly JL, Martolini D, et al. Respiratory muscle fatigue following exercise in patients with interstitial lung disease. Respiration 2013; 85: 220–227. [DOI] [PubMed] [Google Scholar]
- 45. De Troyer A. Cournand lecture: mechanical action of the abdominal muscles. Bull Eur Physiopathol Respir 1983; 19: 575–581. [PubMed] [Google Scholar]
- 46. Kyroussis D, Mills GH, Polkey MI, et al. Effect of maximum ventilation on abdominal muscle relaxation rate. Thorax 1996; 51: 510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Polkey MI, Kyroussis D, Hamnegard CH, et al. Diaphragm performance during maximal voluntary ventilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 155: 642–648. [DOI] [PubMed] [Google Scholar]
- 48. Polkey MI, Kyroussis D, Keilty SE, et al. Exhaustive treadmill exercise does not reduce twitch transdiaphragmatic pressure in patients with COPD. Am J Respir Crit Care Med 1995; 152: 959–964. [DOI] [PubMed] [Google Scholar]
- 49. Sacco P, McIntyre DB, Jones DA. Effects of length and stimulation frequency on fatigue of the human tibialis anterior muscle. J Appl Physiol (1985) 1994; 77: 1148–1154. [DOI] [PubMed] [Google Scholar]
- 50. Baydur A, Alsalek M, Louie SG, et al. Respiratory muscle strength, lung function, and dyspnea in patients with sarcoidosis. Chest 2001; 120: 102–108. [DOI] [PubMed] [Google Scholar]
- 51. Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol (1985) 2008; 104: 879–888. [DOI] [PubMed] [Google Scholar]
- 52. Costabel U. Skeletal muscle weakness, fatigue and sarcoidosis. Thorax 2005; 60: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pringle CE, Dewar CL. Respiratory muscle involvement in severe sarcoid myositis. Muscle Nerve 1997; 20: 379–381. [DOI] [PubMed] [Google Scholar]
- 54. Dewberry RG, Schneider BF, Cale WF, et al. Sarcoid myopathy presenting with diaphragm weakness. Muscle Nerve 1993; 16: 832–835. [DOI] [PubMed] [Google Scholar]
- 55. Baydur A, Pandya K, Sharma OP, et al. Control of ventilation, respiratory muscle strength, and granulomatous involvement of skeletal muscle in patients with sarcoidosis. Chest 1993; 103: 396–402. [DOI] [PubMed] [Google Scholar]
- 56. Kabitz HJ, Lang F, Walterspacher S, et al. Impact of impaired inspiratory muscle strength on dyspnea and walking capacity in sarcoidosis. Chest 2006; 130: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 57. Brancaleone P, Perez T, Robin S, et al. Clinical impact of inspiratory muscle impairment in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004; 21: 219–227. [PubMed] [Google Scholar]
- 58. Korenromp IH, Heijnen CJ, Vogels OJ, et al. Characterization of chronic fatigue in patients with sarcoidosis in clinical remission. Chest 2011; 140: 441–447. [DOI] [PubMed] [Google Scholar]
- 59. Iliffe GD, Pettigrew NM. Hypoventilatory respiratory failure in generalised scleroderma. BMJ 1983; 286: 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Russell DC, Maloney A, Muir AL. Progressive generalized scleroderma: respiratory failure from primary chest wall involvement. Thorax 1981; 36: 219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kalluri M, Oddis CV. Pulmonary manifestations of the idiopathic inflammatory myopathies. Clin Chest Med 2010; 31: 501–512. [DOI] [PubMed] [Google Scholar]
- 62. Teixeira A, Cherin P, Demoule A, et al. Diaphragmatic dysfunction in patients with idiopathic inflammatory myopathies. Neuromuscul Disord 2005; 15: 32–39. [DOI] [PubMed] [Google Scholar]
- 63. American Thoracic Society/European Respiratory S. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]